Abstract
In resource-limited savannas, the distribution and abundance of fine roots play an important role in acquiring essential resources and structuring vegetation patterns and dynamics. However, little is known regarding the three-dimensional distribution of fine roots in savanna ecosystems at the landscape scale. We quantified spatial patterns of fine root density to a depth of 1.2 m in a subtropical savanna landscape using spatially specific sampling. Kriged maps revealed that fine root density was highest at the centers of woody patches, decreased towards the canopy edges, and reached lowest values within the grassland matrix throughout the entire soil profile. Lacunarity analyses indicated that spatial heterogeneities of fine root density decreased continuously to a depth of 50 cm and then increased in deeper portions of the soil profile across this landscape. This vertical pattern might be related to inherent differences in root distribution between trees/shrubs and herbaceous species, and the presence/absence of an argillic horizon across this landscape. The greater density of fine roots beneath woody patches in both upper and lower portions of the soil profile suggests an ability to acquire disproportionately more resources than herbaceous species, which may facilitate the development and persistence of woody patches across this landscape.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Savanna ecologists have long been interested in root distribution patterns, and have invoked niche partitioning to explain the mechanisms that permit co-existence of growth-form divergent trees and grasses (Walter 1971). It is well recognized that the characterization of root distribution in savannas is relevant not only for explaining species co-existence and community dynamics (Walter 1971; February and Higgins 2010; Holdo 2013; Kambatuku et al. 2013), but also for understanding the hydrology and biogeochemistry of these ecosystems (Oliveira et al. 2005; Boutton et al. 2009), and anticipating the responses of savannas to potential changes in rainfall regimes associated with climate change (Kulmatiski and Beard 2013). Previous studies leave little doubt that the size and shape of root systems differ between woody and herbaceous species in savanna ecosystems (Jackson et al. 1996; Schenk 2006). Compared to grasses that have comparatively shallow and laterally constrained root systems, trees/shrubs generally have deeper root systems with significant lateral extent (Schenk 2006). This difference in size and structure could potentially enable trees/shrubs to acquire disproportionately more resources, conferring a competitive advantage relative to grasses (Schwinning and Weiner 1998; Rajaniemi 2003; Schenk 2006; DeMalach et al. 2016), and facilitating their development and persistence. However, the question remains whether and to what extent this size-asymmetric advantage might change along the soil profile.
Fine roots (< 2 mm) are extremely important functional components of plants, as they not only acquire essential resources from highly heterogeneous soils (Pregitzer 2002), but also provide a pathway for carbon and energy transfer from plants to soils (Matamala et al. 2003). Exceptionally high spatial heterogeneity in soil resources is an intrinsic feature of arid and semiarid ecosystems (including many savannas, Schlesinger et al. 1996) that can structure and constrain the growth and elongation of fine roots (Caldwell et al. 1996; Hodge 2004; Loiola et al. 2016), making it more difficult to quantitatively assess fine root distribution based on non-spatial sampling approaches. Therefore, quantifying fine root distribution in a spatially specific manner in savannas could provide critical insights towards understanding plant functional strategies for soil resource acquisition and also soil biogeochemical processes that are related to fine root turnover. Despite this, most empirical and modeling studies on root distribution patterns in savannas have been confined to the individual tree or woody patch scale (e.g. Hipondoka and Versfeld 2006; Koteen et al. 2015; O’Donnell et al. 2015), and have not been spatially explicit at the landscape scale. There is increasing recognition of the fundamental need for the application of landscape-scale quantitative spatial analyses to enhance our understanding of community and ecosystem processes in savannas (Jackson and Caldwell 1993; Bai et al. 2009; Liu et al. 2011; Mudrak et al. 2014; Zhou et al. 2017a). Landscape scale topoedaphic drivers could interact with the size, species composition, and distribution of woody patches to affect patterns of spatial heterogeneity of fine root distribution throughout the soil profile in savannas, though this has not been explicitly investigated.
To address this, we quantified landscape-scale three-dimensional fine root distribution patterns in a subtropical savanna by spatially explicit soil sampling to a depth of 1.2 m across a 160 m × 100 m landscape. This landscape, consisting of different sizes of woody patches interspersed within a grassland matrix, is uniform with respect to topographic features (e.g. slope, aspect, elevation, and exposure) and land use history, but varies with respect to subsurface soil texture with non-argillic inclusions embedded within an otherwise continuous argillic horizon (Archer 1995; Zhou et al. 2017b). Therefore, in this study, we examined how landscape scale patterns of spatial heterogeneity in fine root density change throughout the soil profile. We hypothesized that landscape scale fine root distribution would be primarily controlled by (1) the spatial distribution of woody vs. herbaceous vegetation, and (2) edaphic heterogeneity related to the presence/absence of a subsurface argillic horizon.
Methods and materials
Study site
Research was conducted at the Texas A&M AgriLife La Copita Research Area (27°40′N, 98°12′W) in the Rio Grande Plains, Texas, USA. Climate is subtropical, with a mean annual temperature of 22.4 °C and mean annual precipitation of 680 mm. Although rainfall generally peaks in May and September, there are no distinct dry and wet seasons in this study site (Fig. S1). In 2014, the cumulative rainfall from January to June prior to the sampling time (i.e. July) was 48% less than the 30-year average due to a dry winter (Fig. S1). However, at the beginning of the growing season in May 2014, the study site received approximately 47% more rainfall than the 30-year average (Fig. S1). Topography consists of gently sloping (1–3%) uplands, surrounded by lower-lying ephemeral drainages and playas. Elevation ranges from 75 to 90 m above sea level across the entire research station. This site was continuously grazed by cattle from the late 1800s until it became a research area in 1984 (Scifres and Koerth 1987). The portion of the La Copita Research Area where our research was conducted has not been grazed since 2000.
This study was conducted on an upland portion of the landscape where soils are sandy loams (Typic and Pachic Argiustolls) derived from the Goliad formation (NRCS 1979) and characterized by a nearly continuous argillic (Bt) horizon with non-argillic inclusions distributed randomly across the landscape (Fig. S2) (Zhou et al. 2018). Multiple lines of evidence (i.e. tree ring analysis, historical aerial photographs, and coupled δ13C–14C analyses of soil organic matter) show that uplands were once almost exclusively dominated by C4 grasses, and woody encroachment into grasslands has occurred during the past century (Archer et al. 1988; Archer 1995; Boutton et al. 1999). Woody encroachment is initiated by the colonization of N2-fixing honey mesquite (Prosopis glandulosa) trees, which serve as nurse plants that facilitate the establishment of other understory tree/shrub species to form discrete woody clusters (< 100 m2) (Archer et al. 1988). Discrete clusters occupying non-argillic inclusions expand laterally and often coalesce to form large groves (> 100 m2) (Archer 1995; Bai et al. 2012; Zhou et al. 2017b). Current vegetation structure on uplands of this study site has a two-phase pattern (Whittaker et al. 1979), with discrete woody clusters and groves embedded within a nearly continuous grassland matrix (Fig. S2). Common understory shrub/tree species in clusters and groves include Zanthoxylum fagara, Schaefferia cuneifolia, Celtis pallida, and Ziziphus obtusifolia. In the herbaceous matrix, dominant C4 grasses include Paspalum setaceum, Setaria geniculata, Bouteloua rigidiseta and Chloris cucullata, and dominant C3 forbs include Croton texensis, Wedelia texana, Ambrosia confertiflora, and Parthenium hysterophorus (Boutton et al. 1998).
Field sampling and lab analyses
A 160 m × 100 m landscape consisting of three landscape elements (grasslands, clusters, and groves) was established on an upland in January 2002 (Fig. 1a) (Bai et al. 2009; Liu et al. 2011). Elevations within this sample area were measured directly by terrain surveying, GIS, and kriging interpolation (Bai et al. 2009). The highest elevation within plot is the northeast corner (90.67 m) and the lowest is the southwest corner (87.93 m), resulting in a gentle northeast to southwest slope of 1.4%. A color-infrared aerial photograph (6 cm × 6 cm resolution) of this landscape was acquired in July 2015.
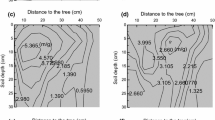
a Aerial photograph of the 160 m × 100 m study area. Red dots indicate 320 random soil sampling points and red lines indicate the edge of groves. Dark green patches are shrub clusters and groves, while light grey color indicates open grassland. b Kriged maps of fine root density (kg m−3) along the soil profile based on 320 random soil sampling points. (Color figure can be viewed in the online issue)
The 160 m × 100 m landscape was subdivided into 10 m × 10 m grid cells, and the corners of each grid cell were georeferenced based on UTM coordinates system (14 North, WGS 1984). Within each grid cell, two randomly located sampling points were selected in July 2014 (320 points in total) (Fig. 1a). The distances from each sampling point to two georeferenced cell corners were recorded to calculate the exact location of each sampling point. The landscape element present at each sampling point was classified as grassland, cluster, or grove. Clusters were distinguished from groves based on their canopy area, as described above. At each sampling point, two adjacent soil cores (2.8 cm in diameter × 120 cm in length) were collected using a PN150 JMC Environmentalist’s Subsoil Probe (Clements Associates Inc., Newton, IA, USA).
Immediately after sampling, each soil core was subdivided into six depth increments (0–5, 5–15, 5–30, 30–50, 50–80, and 80–120 cm). One soil core was oven-dried (105 °C for 48 h) to determine soil bulk density, and the other was air-dried and passed through a 2 mm sieve prior to subsequent analysis of soil texture by the hydrometer method. To estimate root biomass, the oven-dried soil samples were soaked in water and gently washed via low-pressure spray through two sieves: a 0.5 mm mesh followed by a 0.25 mm mesh. Roots extracted on the 0.5 mm mesh were picked out manually and sorted into fine roots (< 2 mm) and coarse roots (> 2 mm). Soils and roots that passed the 0.5 mm mesh but were retained on the 0.25 mm mesh were transferred to a shallow tray, and water was run cautiously to separate the remaining roots from soils by flotation. Roots retrieved by flotation were added to fine root category. No attempt was made to distinguish between live or dead roots. All roots samples were oven-dried (65 °C) to constant weight for biomass determination.
Data analysis
To facilitate comparisons between the unequal sampling depth intervals, fine root density was expressed in units of kg m−3. The Normalized Difference Vegetation Index (NDVI) was calculated as (NIR − RED)/(NIR + RED), in which NIR and RED represent the spectral reflectance measurements from the near-infrared and the red regions, respectively, of the aerial photograph. Areas covered by woody plants have significantly higher NDVI values compared to grassland/bare soil areas (Fig. S3). Pearson correlation coefficients between fine root density, NDVI, soil bulk density, soil sand, silt and clay content within each depth increment were determined using R statistical software (R Development Core Team 2014). A modified t test (Dutilleul et al. 1993) which accounts for the effects of spatial autocorrelation was used to determine the significance levels of these correlation coefficients. For all these statistical analyses, p value < 0.05 was used as a cutoff for statistical significance.
Ordinary kriging was used for spatial interpolation based on values of 320 random sampling points within each depth increment and their spatial structure determined by fitting variogram models (Table S1). Kriged maps of fine root density for each depth increment with a 0.5 m × 0.5 m resolution were generated in ArcGIS 10.2.2 (ESRI, Redlands, CA, USA). Lacunarity analysis, a scale-dependent measurement of spatial heterogeneity or the “gappiness” of a landscape structure (Plotnick et al. 1996), was used to assess changes in spatial heterogeneity of fine root density along the soil profile. Briefly, a gliding box algorithm at seven different spatial scales with corresponding box sizes (side length of the gliding box, r) of 0.5, 1, 2, 4, 8, 16, and 32 m was used to determine the lacunarity value of each kriged map. The gliding box of a given size (r) was first placed at one corner of the kriged map, and the “box mass” S(r), the sum of fine root density of the pixels within the box, was determined. Then the box was systematically moved through the map one pixel at a time and the box mass was recorded at each location. The lacunarity was calculated according to:
where E (S(r)) is the mean and Var (S(r)) is the variance of the box mass S(r) for given box size r. All calculations were performed using R statistical software (R Development Core Team 2014). The lacunarity curve, natural log-transformed lacunarity Λ(r) against box size (r), was plotted to quantify the spatial heterogeneity of fine root distribution at different scales along the soil prolife, with a higher value of lacunarity indicating a more heterogeneous distribution pattern.
To more clearly evaluate and describe variation in root densities within woody patches vs. grasslands, edges of woody patches were delineated in the aerial photograph using ArcGIS 10.2.2. The distance from each sampling point to the nearest woody patch edge was calculated. Sampling points located within woody patches were assigned positive values; hence, large values indicated that sampling points were away from woody patch edges and near the centers of woody patches. In contrast, sampling points within the grassland matrix were assigned negative values. Thus, more negative values indicate that sampling points were farther away from woody patch edges.
Results
Since woody patches (both clusters and groves) had significantly higher fine root densities than grasslands throughout the entire soil profile (Table S2), spatial patterns of fine root density for each depth increment displayed a strong resemblance to that of vegetation pattern, especially the distribution of grove vegetation (Fig. 1). Fine root density was highest at the centers of woody patches, decreased towards the canopy edges, and reached lowest values in the grassland matrix (Fig. 1). This spatial trend was statistically supported by the significantly positive correlations between fine root density and distance from each sampling point to the nearest woody patch edge throughout the soil profile (Fig. 2).
Relationships between fine root density (kg m−3) and distance of each sampling point to the nearest woody patch edge (m) for a 0–5, b 5–15, c 15–30, d 30–50, e 50–80, and f 80–120 cm throughout the soil profile. Positive distances indicate that sampling points are within woody patches, whereas negative distances indicate that sampling points are within the grassland matrix. Please note that the scale on y-axis is different throughout the soil profile. Number of samples: grassland = 200 (triangles), grove = 79 (circles), and cluster = 41 (squares). (Color figure can be viewed in the online issue)
Quantitatively, lacunarity analysis indicated that spatial heterogeneities of fine root density were significantly higher in the upper and lower soil depth increments (i.e. 0–5, 5–15, and 80–120 cm) than in the intermediate depth increments (i.e. 15–30, 30–50, and 50–80 cm) (Fig. 3). The variabilities of fine root density based on coefficient of variation (CV) across this landscape decreased with soil depth and then increased in the two deepest increments (CVs from top to bottom along the soil profile = 71.34, 62.8, 53.27, 48.48, 54.26, and 68.26%) (Table S3). The ratios of fine root densities between woody patch and grassland were also higher in the upper and lower soil depth increments than in the intermediate depth increments (ratios along the soil profile from top to bottom = 2.93, 256, 2.09, 1.81, 1.89, and 2.23) (Fig. 4 and Table S2).
Fine root density was significantly and positively correlated with NDVI throughout the entire soil profile (Table 1). In addition, fine root density was significantly and positively correlated with soil sand content and negatively correlated with clay content, but only at depths > 30 cm below the soil surface where the argillic horizon starts to form across this landscape (Table 1).
Discussion
The three-dimensional spatial patterns of fine root distribution and the factors that determine them have the potential to influence plant water and nutrient acquisition, pool sizes and turnover rates of key limiting nutrients (e.g., N, P), and, ultimately, the spatial structure of aboveground vegetation. As expected, fine root densities were significantly higher beneath woody patches than beneath grasslands throughout the entire profile (Table S2, Fig. 1). This is consistent with prior studies at this site (e.g. Boutton et al. 1999), and with comprehensive literature reviews on root distribution patterns (Jackson et al. 1996; Schenk 2006). The resemblance between spatial patterns of fine root density and the spatial distribution of woody patches, and positive correlations between fine root density and NDVI throughout the entire soil profile, suggest that vegetation patterns were the primary factor driving fine root distribution across this landscape (Fig. 1 and Table 1). Fine root densities within each depth increment throughout the profile were highest near the centers of woody patches, especially for grove vegetation, decreased towards canopy edges, and reached the lowest in the grassland matrix (Figs. 1, 2). In a California oak savanna, Koteen et al. (2015) also reported this center-to-edge spatial variability in fine root biomass, and such variability was more pronounced with large trees or woody patches.
Although fine root density was significantly higher beneath small woody clusters compared to grasslands throughout the 1.2 m profile (Table S2), these differences were generally not evident in the kriged maps. The size of woody clusters across this landscape ranged from 1.3 to 78.7 m2, with an average of 13.4 m2 (n = 121) (Zhou et al. 2017b). In contrast, two randomly sampling points were selected in each 10 m × 10 m grid cell. Therefore, this inability to clearly resolve cluster vs. grassland differences in the kriged maps is attributable to our sampling intensity that could not detect this finer-scale spatial variability in this complex landscape (Liu et al. 2011).
Lacunarity analyses indicated that landscape-scale patterns of spatial heterogeneity in fine root density was relatively high in the surface soil (0–5 and 5–15 cm), decreased in the middle of the profile (15–80 cm), but then increased again in deeper portions (80–120 cm) of the soil profile (Fig. 3). These depth distribution patterns may be related to: (1) inherent differences in rooting depth between trees/shrubs and herbaceous species, as trees/shrubs in arid and semiarid ecosystems generally have deeper rooting systems than grasses/forbs (Jackson et al. 1996; Schenk 2006); and (2) the presence of non-argillic inclusions across this landscape. Several previous studies have reported a negative correlation between root density and soil clay content, and suggested that higher clay content would reduce soil porosity and hydraulic conductivity and increase soil resistance, thereby inhibiting root growth and elongation (Strong and La Roi 1985; Ludovici 2004; Macinnis-Ng et al. 2010; Plante et al. 2014). For this reason, the presence of a clay-accumulated subsurface soil horizon (i.e. argillic horizon) has been shown to affect vertical root distribution (Sudmeyer et al. 2004; Macinnis-Ng et al. 2010). For example, Sudmeyer et al. (2004) found that, in duplex soils, tree root densities were highest in the upper sandy portion of the profile, but then decreased sharply in subsurface clayey soils. Across this landscape, grove vegetation exclusively occupies those portions of the landscape with coarse-textured non-argillic inclusions, while grasslands occur on portions of the landscape where the argillic horizon is present at approximately 30–50 cm in the profile (Fig. S2, Archer 1995; Zhou et al. 2017b). This subsurface clay-rich argillic horizon may significantly impede the vertical distribution of fine roots beneath grasslands, while subsurface coarse-textured non-argillic inclusions beneath groves offer less resistance to fine root penetration. Therefore, we have observed significant negative correlations between fine root density and soil clay content at the 30–50, 50–80, and 80–120 cm depth increments (Table 1).
Size differences between plants (both above- and belowground) may potentially confer a competitive advantage for a larger individual to acquire disproportionately more resources over a smaller individual (Schwinning and Weiner 1998; Rajaniemi 2003; Schenk 2006; DeMalach et al. 2016). Fine roots are the primary functional components of root systems that enable plants to acquire water and nutrients from the highly heterogeneous soil environment (Pregitzer 2002). Although Walter’s two-layered niche differentiation hypothesis has long been proposed as an explanation for the coexistence of grasses and trees in savannas (Walter 1971), emerging studies have shown that trees and grasses actually occupy overlapping rooting niches (Hipondoka and Versfeld 2006; February and Higgins 2010; Holdo 2013; Kambatuku et al. 2013). Our results show that fine root densities beneath woody patches were always 2–3 times more abundant than grasslands in both upper and lower soil depth increments (Fig. 4, Table S2), consistent with other studies at this site (Midwood et al. 1998; Boutton et al. 1998). Greater abundance of fine root density in upper soil depth increments where soils generally have higher nutrient availability (Schlesinger et al. 1996; Jobbágy and Jackson 2001), and in lower soil depth increments where soils typically have higher water availability (Fig. S4, Boutton et al. 1999), suggest that woody species may be able to acquire disproportionately more resources than herbaceous species (Fig. 4), thereby facilitating their development, persistence, and encroachment across this landscape.
Overall, landscape scale spatial patterns of fine root density throughout the soil profile in this subtropical savanna were primarily controlled by the spatial distribution of woody patches in the horizontal plane, and by edaphic factors (presence/absence of argillic horizon, soil clay content) in the vertical plane, supporting both of our original hypotheses. These results have important implications for understanding belowground biogeochemical cycles and representing them in modeling studies. Characterized by fast turnover rates (Gill and Jackson 2000) and high decomposability (Silver and Miya 2001), fine roots represent a major pathway transferring carbon, nutrients, and energy from plants to soils (Matamala et al. 2003), especially to the subsurface soils (Rasse et al. 2005; Schmidt et al. 2011). At the individual tree or woody patch scale, spatial patterns of soil carbon storage closely mirror those of fine root biomass (Koteen et al. 2015), and the total amount of sub-canopy soil carbon storage generally increases linearly with the size of tree or woody patch (Throop and Archer 2008; Boutton et al. 2009; Koteen et al. 2015). Thus, we may expect that landscape scale spatial patterns of soil carbon storage would be tightly linked to the size and spatial distribution of root systems. However, soil characteristics that can affect the stabilization of organic carbon derived from fine root turnover (e.g., soil texture, organomineral interactions) may vary with depth and contribute to landscape scale heterogeneity in soil carbon storage in the vertical dimension. Therefore, it remains uncertain how this alteration of root distribution at the landscape scale following a shift from grass to woody plant dominance will affect the spatial patterns of soil biogeochemical properties and processes. Current models linking root traits and morphology to ecosystem function likely oversimplify the true complexity of belowground interactions (Nippert and Holdo 2015). Our results indicate that landscape-scale spatial heterogeneity of fine root distribution changes not only in response to vegetation cover, but also in response to soil textural variation with depth in the profile. The characterization of root biomass by soil depth, however, is absent in most terrestrial biosphere models and the ability to account for horizontal and vertical root spatial patterns could improve the predictive capabilities of these models (Warren et al. 2015). We suggest that root distribution patterns and soil processes in deeper portions of the soil profile, especially at the landscape scale, deserve greater attention in savanna ecology.
References
Archer S (1995) Tree-grass dynamics in a Prosopis-thornscrub savanna parkland: reconstructing the past and predicting the future. Ecoscience 2:83–99
Archer S, Scifres C, Bassham CR, Maggio R (1988) Autogenic succession in a subtropical savanna: conversion of grassland to thorn woodland. Ecol Monogr 58:111–127
Bai E, Boutton TW, Liu F, Wu XB, Archer SR (2009) Landscape-scale vegetation dynamics inferred from spatial patterns of soil δ13C in a subtropical savanna parkland. J Geophys Res 114:G01019. https://doi.org/10.1029/2008JG000839
Bai E, Boutton TW, Liu F, Wu XB, Archer SR (2012) Spatial patterns of soil δ13C reveal grassland-to-woodland successional processes. Org Geochem 42:512–1518
Boutton TW, Archer SR, Midwood AJ, Zitzer SF, Bol R (1998) δ13C values of soil organic carbon and their use in documenting vegetation change in a subtropical savanna ecosystem. Geoderma 82:5–41
Boutton TW, Archer SR, Midwood AJ (1999) Stable isotopes in ecosystem science: structure, function and dynamics of a subtropical savanna. Rapid Commun Mass Spectrom 13:1263–1277
Boutton TW, Liao JD, Filley TR, Archer SR (2009) Belowground carbon storage and dynamics accompanying woody plant encroachment in a subtropical savanna. In: Lal R, Follett R (eds) Soil carbon sequestration and the greenhouse effect, 2nd edn. Soil Science Society of America, Madison, pp 181–205
Caldwell MM, Manwaring JH, Durham SL (1996) Species interactions at the level of fine roots in the field: influence of soil nutrient heterogeneity and plant size. Oecologia 106:440–447
DeMalach N, Zaady E, Weiner J, Kadmon R (2016) Size asymmetry of resource competition and the structure of plant communities. J Ecol 104:899–910
Dutilleul P, Clifford P, Richardson S, Hemon D (1993) Modifying the t test for assessing the correlation between two spatial processes. Biometrics 49:305–314
February EC, Higgins SI (2010) The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. S Afr J Bot 76:517–523
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Hipondoka MHT, Versfeld WD (2006) Root system of Terminalia sericea shrubs across rainfall gradient in a semi-arid environment of Etosha National Park, Namibia. Ecol Indic 6:516–524
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Holdo RM (2013) Revisiting the two-layer hypothesis: coexistence of alternative functional rooting strategies in savannas. PLoS One 8(8):e69625. https://doi.org/10.1371/journal.pone.0069625
Jackson RB, Caldwell MM (1993) Geostatistical patterns of soil heterogeneity around individual perennial plants. J Ecol 81:683–692
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411
Jobbágy EG, Jackson RB (2001) The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry 53:51–77
Kambatuku JR, Cramer MD, Ward D (2013) Overlap in soil water sources of savanna woody seedlings and grasses. Ecohydrology 6:464–473
Koteen LE, Raz-Yaseef N, Baldocchi DD (2015) Spatial heterogeneity of fine root biomass and soil carbon in a California oak savanna illuminates plant functional strategy across periods of high and low resource supply. Ecohydrology 8:294–308
Kulmatiski A, Beard KH (2013) Woody plant encroachment facilitated by increased precipitation intensity. Nat Clim Change 3:833–837
Liu F, Wu XB, Bai E, Boutton TW, Archer SR (2011) Quantifying soil organic carbon in complex landscapes: an example of grassland undergoing encroachment of woody plants. Glob Change Biol 17:1119–1129
Loiola PP, Carvalho GH, Batalha MA (2016) Disentangling the roles of resource availability and disturbance in fine and coarse root biomass in savanna. Aust Ecol 41:255–262
Ludovici KH (2004) Tree roots and their interaction with soil. In: Burley J, Evans J, Youngquist JA (eds) Encyclopedia of forest sciences. Elsevier, Oxford, pp 1195–1201
Macinnis-Ng CMO, Fuentes S, O’Grady AP, Palmer AR, Taylor D, Whitley RJ, Yunusa I, Zeppel MJB, Eamus D (2010) Root biomass distribution and soil properties of an open woodland on a duplex soil. Plant Soil 327:377–388
Matamala R, Gonzalez-Meler MA, Jastrow JD, Norby RJ, Schlesinger WH (2003) Impacts of fine root turnover on forest NPP and soil C sequestration potential. Science 302:1385–1387
Midwood AJ, Boutton TW, Archer SR, Watts SR (1998) Water use by woody plants on contrasting soils in a savanna parkland: assessment with δ2H and δ18O. Plant Soil 205:13–24
Mudrak EL, Schafer JL, Fuentes-Ramirez A, Holzapfel C, Moloney KA (2014) Predictive modeling of spatial patterns of soil nutrients related to fertility islands. Landsc Ecol 29:491–505
Nippert JB, Holdo RM (2015) Challenging the maximum rooting depth paradigm in grasslands and savannas. Funct Ecol 29:739–745
NRCS (1979) Soil survey of Jim Wells County, Texas. Natural Resource Conservation Service, United States Department of Agriculture, Washington, DC
O’Donnell FC, Caylor KK, Bhattachan A, Dintwe K, D’Odorico P, Okin GS (2015) A quantitative description of the interspecies diversity of belowground structure in savanna woody plants. Ecosphere 6:1–15
Oliveira RS, Bezerra L, Davidson EA, Pinto F, Klink CA, Nepstad DC, Moreira A (2005) Deep root function in soil water dynamics in cerrado savannas of central Brazil. Funct Ecol 19:574–581
Plante PM, Rivest D, Vézina A, Vanasse A (2014) Root distribution of different mature tree species growing on contrasting textured soils in temperate windbreaks. Plant Soil 380:429–439
Plotnick RE, Gardner RH, Hargrove WW, Prestegaard K, Perlmutter M (1996) Lacunarity analysis: a general technique for the analysis of spatial patterns. Phys Rev E 53:5461–5468
Pregitzer KS (2002) Fine roots of trees–a new perspective. New Phytol 154:267–270
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. (http://www.r-project.org/)
Rajaniemi TK (2003) Evidence for size asymmetry of belowground competition. Basic Appl Ecol 4:239–247
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739
Schlesinger WH, Raikes JA, Hartley AE, Cross AF (1996) On the spatial pattern of soil nutrients in desert ecosystems. Ecology 77:364–374
Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Schwinning S, Weiner J (1998) Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 113:447–455
Scifres CJ, Koerth BH (1987) Climate, soils, and vegetation of the La Copita Research Area. Texas Agricultural Experiment Station MP-1626. Texas A&M University System, College Station
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Strong WL, La Roi GH (1985) Root density-soil relationships in selected boreal forests of central Alberta, Canada. For Ecol Manag 12:233–251
Sudmeyer RA, Speijers J, Nicholas BD (2004) Root distribution of Pinus pinaster, P. radiata, Eucalyptus globulus and E. kochii and associated soil chemistry in agricultural land adjacent to tree lines. Tree Physiol 24:1333–1346
Throop HL, Archer SR (2008) Shrub (Prosopis velutina) encroachment in a semidesert grassland: spatial–temporal changes in soil organic carbon and nitrogen pools. Glob Change Biol 14:2420–2431
Walter H (1971) Ecology of tropical and subtropical vegetation. Oliver and Boyd, Edinburgh
Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD (2015) Root structural and functional dynamics in terrestrial biosphere models–evaluation and recommendations. New Phytol 205:59–78
Whittaker RH, Gilbert LE, Connell JH (1979) Analysis of two-phase pattern in a mesquite grassland, Texas. J Ecol 67:935–952
Zhou Y, Boutton TW, Wu XB (2017a) Soil carbon response to woody plant encroachment: importance of spatial heterogeneity and deep soil storage. J Ecol 105:1738–1749
Zhou Y, Boutton TW, Wu XB, Yang C (2017b) Spatial heterogeneity of subsurface soil texture drives landscape-scale patterns of woody patches in a subtropical savanna. Landsc Ecol 32:915–929
Zhou Y, Boutton TW, Wu XB (2018) Woody plant encroachment amplifies spatial heterogeneity of soil phosphorus to considerable depth. Ecology 99:136–147
Acknowledgements
This research was supported by a Doctoral Dissertation Improvement Grant from the US National Science Foundation (DEB/DDIG1600790), USDA/NIFA Hatch Project (1003961), an Exploration Fund Grant from the Explorers Club, and the Howard McCarley Student Research Award from the Southwestern Association of Naturalists. Yong Zhou was supported by a Sid Kyle Graduate Merit Assistantship from Department of Ecosystem Science and Management and a Tom Slick Graduate Research Fellowship from the College of Agriculture and Life Sciences, Texas A&M University. We thank Dr. Chenghai Yang of USDA/ARS for acquiring the color infrared aerial photograph, and David and Stacy McKown for on-site logistics at the La Copita Research Area, and two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Contributions
YZ, TWB and XBW conceived and designed the experiments. YZ performed the experiments. YZ, CLW, ALD analyzed the data. YZ and TWB wrote the manuscript; other authors provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Data accessibility
We agree to archive the data associated with this manuscript should the manuscript be accepted.
Additional information
Communicated by Susanne Schwinning.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Y., Boutton, T.W., Wu, X.B. et al. Rooting strategies in a subtropical savanna: a landscape-scale three-dimensional assessment. Oecologia 186, 1127–1135 (2018). https://doi.org/10.1007/s00442-018-4083-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4083-9