Abstract
Background and aims
Root niche partitioning among trees/shrubs and grasses facilitates their coexistence in savannas, but little is known regarding root distribution patterns of co-occurring woody plants, and how they might differ on contrasting soils.
Methods
We quantified root distributions of co-occurring shrubs to 2 m on argillic and non-argillic soils.
Results
Root biomass in the two shrub communities was 3- to 5- fold greater than that in the grassland community. Prosopis glandulosa, the dominant overstory species was deep-rooted, while the dominant understory shrub, Zanthoxylum fagara, was shallow-rooted (47% vs. 25% of root density at depths >0.4 m). Shrubs on argillic soils had less aboveground and greater belowground mass than those on non-argillic soils. Root biomass and density on argillic soils was elevated at shallow (< 0.4 m) depths, whereas root density of the same species on non-argillic soils were skewed to depths >0.4 m. Root density decreased exponentially with increasing distance from woody patch perimeters.
Conclusions
Belowground biomass (carbon) pools increased markedly with grassland-to-shrubland state change. The presence/absence of a restrictive barrier had substantial effects on root distributions and above- vs. belowground biomass allocation. Differences in root distribution patterns of co-occurring woody species would facilitate their co-existence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The proliferation of woody species in grass-dominated ecosystems has occurred in many arid and semi-arid regions around the world during the past century (van Auken 2000; Archer et al. 2001; Stevens et al. 2017). This geographically extensive land cover change appears to be driven largely by chronic livestock grazing, fire suppression, rising atmospheric CO2 concentrations, and their interactions (Wigley et al. 2010; Archer et al. 2017; Brunsell et al. 2017; Devine et al. 2017; Venter et al. 2018), and has the potential to alter grassland biodiversity, hydrology, biogeochemistry, and landscape evolution (Boutton et al. 1999; Huxman et al. 2005; Ratajczak et al. 2012; Zhou et al. 2017a, 2018a, b).
In the Rio Grande Plains of southern Texas, C3 trees and shrubs have formed multispecies patches within the original C4 grassland matrix, resulting in a subtropical savanna parkland landscape (Archer 1995; Boutton 1996; Archer et al. 2001). Upland soils of this area are sandy loams characterized by a laterally continuous argillic (Bt) horizon interspersed with patches where the argillic horizon is absent (Archer 1995; Zhou et al. 2017a). Previous studies in this region showed that the development of woody patches is initiated by the colonization of N2-fixing Prosopis glandulosa, which then facilitate the recruitment of a diverse assemblage of other shrub species under their canopies to form small discrete clusters (< 100 m2) (Archer et al. 1988, 2001; Archer 1995). Where non-argillic inclusions are present, discrete clusters expand laterally and coalesce to form large groves (> 100 m2) (Archer 1995; Bai et al. 2012; Zhou et al. 2017a). However, where the argillic horizon is present, woody clusters remain relatively small and isolated within the grassland matrix and do not fuse with other clusters. This suggests that the presence/absence of the argillic horizon is influencing vegetation dynamics and landscape evolution, perhaps by affecting root distribution patterns of the encroaching woody species.
Root architecture and distribution are, in part, genetically determined, but are also influenced by soil properties (e.g. texture and bulk density) and soil resources (e.g. water and nutrients) (Valverde-Barrantes et al. 2013; Wachsman et al. 2015; Morris et al. 2017; Fan et al. 2017). The impact of temporally and spatially heterogeneous soil resources on root distribution patterns of individual or coexisting species has long been studied (Walter 1971; Parrish and Bazzaz 1976) and reviewed recently (Hutchings et al. 2003; Hodge 2004; de Kroon et al. 2012; Ward et al. 2013). Site-specific soil physical attributes such as texture can also significantly influence root distribution (e.g., Schenk and Jackson 2005; Xu and Li 2009). Several studies have reported negative correlations between soil clay content and root density (Strong and La Roi 1985; Plante et al. 2014), likely a result of reduced soil porosity, hydraulic conductivity, shrink/swell shearing forces and/or increased soil resistance, all of which can inhibit root growth and elongation (Ludovici 2004). In addition, the formation of clay-rich subsurface soil horizons (e.g. argillic horizons) has been shown to affect vertical root distribution (Macinnis-Ng et al. 2010). For example, Sudmeyer et al. (2004) found that where argillic horizons were present, tree root densities were high in the coarse-textured surface soil, but decreased sharply below the subsurface clay-rich argillic horizon.
Root distribution patterns of coexisting species affect vegetation dynamics and landscape evolution by influencing interspecific competition for resources (Parrish and Bazzaz 1976; Tilman 1985; Schenk 2006; Ratajczak et al. 2011; Zhou et al. 2018c). During stand development, for example, early successional species often have a significantly greater proportion of roots occurring deeper in the soil profile than late successional species (Gale and Grigal 1987), ostensibly enabling them to exploit larger volumes of soil with limited water and nutrients. Shallow-rooted species appearing at later successional stages are better adapted to exploit resources that have accumulated over time in the surface soil, thereby facilitating their establishment and coexistence with earlier successional species via reduced interspecific competition. Numerous studies in savanna ecosystems have used differences in root distribution between trees and grasses to explain their coexistence via niche partitioning of water uptake (e.g. Walter 1971; Sankaran et al. 2004; February and Higgins 2010; Holdo 2013; Zhou et al. 2018c). However, few studies have quantified root distribution patterns of co-occurring trees and shrubs in multispecies woody patches in savanna ecosystems, especially in response to contrasting soils.
The purpose of this study was to quantify root distribution patterns of co-occurring woody plants in small, discrete clusters on soils with a well-developed argillic horizon (hereafter argillic soils), and in groves that occur on soils lacking an argillic horizon (hereafter non-argillic soils) in a subtropical savanna parkland where the abundance of woody species has increased substantially during the past century (Archer 1995; Boutton et al. 1998; Bai et al. 2009; Zhou et al. 2017a). Specific objectives were to quantify: (1) the influence of the presence or absence of an argillic horizon on root distribution; (2) the vertical root distributions of co-occurring woody plant species; (3) patterns of belowground vs. aboveground biomass allocation of woody plants on argillic and non-argillic soils; and (4) the extension of woody plant lateral roots beyond cluster perimeters. Results from these investigations were then used to explain observed vegetation dynamics and patterns of landscape development in this subtropical savanna parkland.
Methods and materials
Study site
This study was conducted at the Texas A&M AgriLife La Copita Research Area (27°40̍ N, 98°12̍ W) located in the Rio Grande Plains of southern Texas, approximately 65 km west of Corpus Christi. Climate of this region is subtropical, with mean annual temperature and precipitation of 22.4 °C and 680 mm, respectively. Annual rainfall is bimodal with peaks in May and September. Soil moisture to a depth of 1.2 m ranges from 2 to 10% (gravimetric soil water content) during the growing season (unpublished data). Elevation ranges from 75 to 90 m above sea level. Topography consists of uplands with slopes of 1–3% surrounded by lower-lying intermittent drainages. Upland portions of the landscape where this study was conducted classify as fine-loamy, siliceous, hyperthermic, Typic Argiustolls (Boutton et al. 1998) and are characterized by a well-developed, nearly continuous clay-rich argillic (Bt) horizon. However, on some portions of the upland landscape, there are patches where the argillic horizon is absent, and these soils classify as coarse-loamy, siliceous, hyperthermic, Pachic Haplustolls (Boutton et al. 1998). Soil pH was neutral at the surface, increased slightly (max = 7.8) with depth to 1.2 m, and did not differ between argillic and non-argillic soils (Zhou et al. 2017b). Prior to the colonization of woody plants, nitrogen and phosphorus content of argillic and on non-argillic soils to a depth of 1.2 m were comparable (Zhou et al. 2018b). The presence/absence of the argillic horizon in upland soils is a strong determinant of vegetation cover, as discussed below. The region, including the La Copita Research Area, has been grazed continuously by domestic livestock since the mid- to late-1800s. Grazing pressure at the La Copita has been light to moderate since it was designated as a research area in 1981, and no fires have been documented for at least the past 50 yrs.
Evidence from sequential aerial photography, tree ring analyses, vegetation dynamics modeling, and the isotopic composition of soils all indicate that this region was once relatively open grassland and that woody plant encroachment into those grasslands has occurred over the past 150 yrs., apparently in response to livestock grazing and fire suppression (Archer et al. 1988, 2001, 2004; Boutton et al. 1998, 1999). Our prior research at this site shows that woody encroachment into grassland is initiated by the establishment of Prosopis glandulosa (honey mesquite, hereafter Prosopis), a N2-fixing legume (Zitzer et al. 1996; Soper et al. 2015) with an arborescent growth form. P. glandulosa then serves as a nurse plant facilitating the recruitment of other fruticose shrub species beneath its canopy, resulting in the formation of small (< 100 m2) discrete woody clusters (Archer et al. 1988). Where the argillic horizon is absent, these shrub clusters expand laterally and fuse to form larger (> 100 m2) groves (Archer 1995; Bai et al. 2012; Zhou et al. 2017a. Therefore, upland portions of this landscape currently consist of discrete clusters and groves scattered within a matrix of remnant C4 grassland (Fig. 1), which is similar to the two-phase vegetation pattern described by Whittaker et al. (1979) for this region. Discrete clusters and groves have similar plant species composition, with an overstory dominated by Prosopis and an understory dominated by Zanthoxylum fagara (lime prickly ash, hereafter Zanthoxylum) (Liu et al. 2013). See Appendix S1 for a listing of other common understory woody species. Perennial grasses, including Paspalum setaceum, Setaria geniculata, Bouteloua rigidiseta, and Chloris cucullata, dominate the remnant grassland matrix. Species names follow Hatch et al. (1990).
Field sampling
Two discrete clusters and two groves located within a 260 × 200 m upland grazing exclosure established in 1984 were selected (Fig. 1). A vegetation inventory was conducted in May of 1991 prior to excavating trenches for quantifying root distribution patterns (Table S1). Location, species, height, and canopy diameter of each woody plant were recorded for each discrete cluster, and in a 4 m wide belt immediately overlying the planned trench locations in each grove. Aboveground biomass was quantified in plots (0.5 m × 0.5 m) spaced evenly along the center-line of the planned trenches in each discrete cluster (n = 3/cluster) and grove (n = 5/grove). Shrubs were cut at ground level and biomass sorted by species, oven-dried (60 °C) to constant weight, and weighed. Age of Prosopis occurring within the harvested plots was estimated by counting annual rings (Flinn et al. 1994).
Four main trenches (1 m wide × 2 m deep) were carefully excavated in June 1991, using a backhoe to quantify vertical and horizontal root distribution patterns. The trench through discrete cluster 1 was oriented north-south, while that for discrete cluster 2 was oriented east-west. Trenches for the two groves were oriented the same as those for clusters and extended from their canopy perimeters to their centers.
Short secondary trenches perpendicular to main trenches of each discrete cluster were excavated to determine the magnitude of lateral root extension beyond woody canopy boundaries (Fig. S1). One of the secondary trenches was placed with the inner face 1 m from the edge of the cluster canopy; the other was excavated at the opposite end of the main trench with the inner face 3 m from the edge of cluster canopy edge. Data on shrub root density at 1, 2, 3, and 4 m beyond the edge of cluster canopies were thus generated (Fig. S1).
Roots were exposed by removing 3–5 mm of soil from trench faces with a small-toothed scraper after wetting with a garden sprayer. This procedure ensured that roots obscured by backhoe excavation were accounted for. Root density was quantified during June through August 1991 by placing a gridded frame (0.2 × 0.2 m cells) against the trench face and counting the number of roots in three diameter classes (1–3 mm, 3–10 mm, and > 10 mm) in each grid cell. These root diameter classes were chosen to facilitate parameterization of the CENTURY model (Parton et al. 1992). Root density was quantified continuously from the soil surface to a depth of 2 m along the entirety of all trench faces in clusters and groves. Shrub roots were visually distinct from grass and herbaceous dicot roots and were identified to species using a key based on physical characteristics, such as color, texture and morphology (Appendix S1). Roots of some species had black or brown sheaths that distinguished them from other species, but no features that allowed us to distinguish them from each other. Accordingly, we lumped species with ‘black’ or ‘brown’ roots that could not identified to species. We made no attempt to distinguish between live and dead roots.
Root biomass was quantified within 0.2 m × 0.2 m × 0.2 m soil blocks arrayed continuously from the soil surface to a depth of 2 m (Fig. S2). This sampling was conducted at 1 m horizontal intervals along the trench faces in discrete clusters, and at 2 m intervals in groves. Coarse woody plant roots were removed by hand from each soil block, sorted by species and diameter class (1–3 mm, 3–10 mm and > 10 mm), and washed gently in water to remove adhering soil particles. Roots not identified to species were recorded as black or brown. Then, 300 cm3 subsamples from each soil block were used to isolate fine roots (< 1 mm) using a hydropneumatic elutriation system (Gillison’s Variety Fabrication, Inc., Benzonia, MI) with primary (760 μm) and secondary (410 μm) sieves (Smucker et al. 1982). Non-root organic matter was separated manually from fine roots following elutriation. Fine roots could not be identified to species. All root samples were oven-dried (60 °C) to a constant weight for biomass determination.
Subsamples from each soil block were analyzed to determine both wet and dry Munsel color (Pendleton and Nickerson 1951; Owens and Rutledge 2005). Munsel colors were used in conjunction with other soil attributes to map soil horizons (Table S2). In addition, subsamples of soil from selected blocks (Block C and E for both discrete clusters, Block A, I and Q for grove 1, and Block A, K, and U for grove 2, see Fig. S2) were analyzed for soil texture using the hydrometer method (Sheldrick and Wang 1993) (Table S3).
We were also interested in determining the extent to which root biomass in the shrub communities on argillic and non-argillic sites differed from that of the herbaceous matrix within which they were embedded. To quantify root biomass in the herbaceous area surrounding groves and clusters, we drove 5 cm diameter cores to a depth of 1.2 m using a pneumatic hammer. Cores were then partitioned into depth increments (0–20, 20–40, 40–60, 60–80, 80–100, and 100–120 cm). Root biomass within soil cores was determined as described above. There were no remnant grassland patches on non-argillic soils.
Data analysis
Root density and root biomass for cluster and grove data sets were standardized as the number of roots counted per m2 of trench face (roots m−2), and as grams per m2 (g m−2), respectively. Both root numbers and root biomass were summed over 1 m horizontal by 0.4 m vertical increments to a depth of 2 m to achieve a degree of spatial integration. Preliminary data analysis indicated correlations between means and standard deviations, so a natural logarithm transformation was applied to all data for subsequent statistical analyses (McDonald 2014).
Root density was analyzed using the General Linear Models procedure for multivariate analysis of variance (ANOVA). The dependent variable was root density, and the multivariate was depth (0.0–0.4, 0.4–0.8, 0.8–1.2, 1.2–1.6, and 1.6–2.0 m). Independent variables were soil type (argillic vs. non-argillic soil), root diameter class (1–3, 3–10 and > 10 mm), replication (1 or 2), trench face (left or right), relative horizontal position (exterior = outside cluster canopy perimeters, edge = within 1 m (discrete clusters) or 4 m (groves) of the canopy perimeter, and interior = all other data under canopy), and plant species. For lateral root extension analysis, independent variable distance (1, 2, 3, or 4 m from the canopy perimeter) and block (relative horizontal position along perpendicular trench (1–4)) were also included. Significance was tested at α = 0.05. The Bonferroni correction (α = 0.05/n) was used for each of n pairwise comparisons, to achieve an experiment wide error rate of α = 0.05. All analyses were performed on SAS 6.07 software (SAS Institute, Cary, NC, USA).
Results
Shrub cluster and grove vegetation and soil properties
A total of 403 plants representing 18 woody species were inventoried in the two discrete clusters and the two 4 m wide belt transects in groves (Table S1). Both discrete clusters and groves contained 10–15 woody species, and plant densities were greater in discrete clusters (5.4 m−2) than in groves (1.7 m−2) (Table 1). Prosopis was the largest plant with respect to aboveground biomass, height, and canopy area. Based on annual ring counts, the largest Prosopis in groves (74.0 years) were older than the largest Prosopis in discrete clusters (61.5 years). Prosopis and Zanthoxylum dominated the overstory-understory aboveground biomass, respectively, of both discrete clusters and groves, accounting for ~ 85% of the total.
Soils in discrete clusters and the perimeter of groves were similar and were characterized by the presence of an argillic horizon (Bt) and distinct structure and horizon boundaries (Fig. S2). Toward the interior of groves, the argillic horizon gradually disappeared, and horizon boundaries became less distinct. In both clusters and groves, sand content decreased from the surface to a depth of 2 m, while silt content remained relatively constant and clay content gradually increased (Table 1). However, clay content in the 0.8–1.2 m depth increment in the interior of both groves (20–28%) was sharply reduced compared to values near the canopy perimeter (34–38%), reflecting the absence of the argillic horizon beneath groves (Table S3).
Root distribution patterns in shrub clusters and groves
Results from multivariate ANOVA of root density showed a significant depth × soil type interaction, indicating that the presence or absence of the argillic horizon significantly influenced root densities in the vertical plane (Table S4). Compared to non-argillic soils, root densities on argillic soils were significantly greater above 0.4 m depths, but similar or significantly lower at depths >0.4 m (with the exception of 1–3 mm diameter roots at 0.4–0.8 m depth) (Fig. 2). There was also a significant depth × root diameter interaction, reflecting the fact that small roots (1–3 and 3–10 mm diam.) decreased exponentially with depth, whereas declines in large roots (> 10 mm diam.) were more monotonic with depth (Table S4, Fig. 2).
When plant species were included in the multivariate ANOVA, significant depth × species interactions were obtained for both argillic and non-argillic soils, indicating significant differences in root vertical distribution among species (Table S5). More specifically, Prosopis was the deepest-rooted species on each soil type, with root densities below 0.4 m accounting for >47% and 59% of the total root density on argillic vs. non-argillic soils, respectively (Fig. 3). Only Prosopis had roots >10 mm diameter at depths >0.4 m (Fig. 3). Compared to Prosopis plants on argillic soils, those on non-argillic soils had higher root densities in all size classes (Fig. 3). Zanthoxylum, a relatively shallow-rooted species with >75% of its total roots occurring above 0.4 m on both soil types, dominated the density of small-to-medium root diameter classes on argillic soils, and was second only to Prosopis on non-argillic soils (Fig. 3). In contrast to Prosopis, Zanthoxylum had higher root densities on argillic soils than on non-argillic soils (Fig. 3).
Relative root densities (mean percentage of root density in each depth interval to total root density from the ground surface to a depth of 2 m) by species and root diameter classes on argillic (a, b, and c) and non-argillic soils (d, e, and f). Values to right of bars are mean (± SE) total root densities (roots m−2) for each plant species
Excluding black and brown roots that we could not identify to species, Prosopis and Zanthoxylum together accounted for ~ 80% of total root density in non-argillic soils compared to 49% in argillic soils. This was especially true for 1–3 mm diameter roots on argillic soils where other woody genera (Lycium, Schaefferia, Celtis, and Opuntia) comprised ~ 60% of the total (Fig. 3). Differences between Prosopis and other understory species in root biomass distribution patterns were also evident (Fig. S3).
Root biomass and plant biomass allocation in shrub clusters and groves
Mean total root biomass to a depth of 2 m among shrubs on argillic soils (2712 g m−2) was higher than that of shrubs on non-argillic soils (1740 g m−2) (Fig. 4 and Table 2). Root biomass in the upper 0.4 m accounted for 69% of the total root biomass on argillic soils, compared to 38% on non-argillic soils (Fig. 4). Root biomass for all diameter classes on argillic soils was significantly higher than that on non-argillic soils at depths <0.4 m. However, at depths >1.2 m, root biomass on non-argillic soils was higher than that on argillic soils, especially for roots >3 mm diameter.
Biomass allocation above- and belowground was strongly affected by the presence/absence of an argillic horizon. The mean belowground to aboveground biomass ratio (root: shoot ratio) on argillic soils was ~3X greater than that on non-argillic soils (0.57 vs. 0.20) (Table 2). This was also true for the dominant overstory (Prosopis glandulosa) and understory (Zanthoxylum fagara) species (Table 2).
Root extension beyond discrete cluster perimeters
Contour maps of root density along trench faces within the discrete clusters showed an inverted cone-shape distribution (Fig. 5). Root densities were highest near the center and gradually decreased towards the cluster canopy/grassland interface. Root penetration was deepest near discrete cluster centers and decreased toward cluster perimeters. Multivariate ANOVA showed a significant interaction between horizontal position and soil depth (Tables S4 and S5), confirming the root distribution patterns visually apparent in Fig. 5.
Contour maps of root densities (roots m−2) along trench faces for discrete clusters 1 (a) and 2 (b). Values are the mean of both trench faces. Solid black horizonal line denotes top of argillic horizon (Fig. S2); dashed vertical lines denote location of shrub cluster canopy perimeters
Moving beyond the discrete cluster boundary into the surrounding grasslands, root density typically decreased exponentially with increasing distance from cluster canopy perimeters (Fig. 6). The amount of variation in root density explained by distance from discrete cluster perimeters generally declined with increasing soil depth and root diameter. Results from the multivariate ANOVA revealed a significant depth × distance interaction which was a consequence of decreasing rooting depth with increasing distance from the cluster perimeter (Table S6). The root diameter × distance interaction term was also significant, reflecting the fact that distance-from-cluster effects diminished with increases in root size (Fig. 6).
Mean (± SE) root densities (roots m−2) at 0, 1, 2, 3 and 4 m beyond discrete cluster canopy perimeters for root diameter classes 1–3 mm, 3–10 mm, and > 10 mm. Bars with different letters are significantly different (p < 0.05). Exponential decay functions were fitted to the means of root density vs. the distances from cluster canopy perimeters for each root diameter class. Panels without equations indicate failed fit with exponential decay functions. Results from depths >1.2 m in the soil profile are not presented, as there were no significant differences in root densities with distance from cluster canopy perimeters, but can be found in Table S8
Root biomass in grasslands, shrub clusters and groves
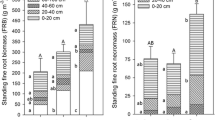
Total root biomass in each of these patch types was highest in the upper 40 cm and declined markedly with depth to 1.2 m (Fig. 7). The root biomass in cluster and grove communities at each depth was substantially greater than that in the grassland community. Summarized over all depths, root biomass in the two shrub communities was 3 - to 5- fold greater than that of the grassland community (ranging from 1.4 - to 7.3- fold greater, depending on depth).
Discussion
Edaphic influences on root distribution and biomass partitioning
The ability to identify roots to species for the predominant and co-occurring woody plants in this system afforded unique and novel perspectives on plant-plant and plant-soil relationships in this savanna parkland. Differences in root distribution with depth on soils with and without a well-developed argillic horizon (Figs. 2 and 4) ostensibly reflect the combined effect of physical restrictions to root penetration, resource availability and plant genetic potential. Soil clay content is often correlated negatively with root density and root growth (Strong and La Roi 1985; Plante et al. 2014). This may result from increased soil strength and reduction in soil aeration which inhibit root growth and metabolism (Gerard et al. 1982; Clark et al. 2003; Bengough et al. 2006; Haling et al. 2011). Bulk density > 1.5 g cm3 [similar to those of the argillic horizon on this site (Zhou et al. 2017b)], can generally reduce root growth through reduced pore spaces (Clark et al. 2003; Ludovici 2004; Xu and Li 2009; Plante et al. 2014). Accordingly, we observed an abrupt decrease in root density and biomass below 0.4 m in shrub clusters where the argillic horizon begins (Figs. 2 and 4). This is consistent with several other studies reporting that the presence of a concentrated clay hardpan in subsoils had strong influences on root vertical distribution patterns (Dracup et al. 1992; Sudmeyer et al. 2004; Macinnis-Ng et al. 2010).
Except where physical restrictions occur, roots are known to proliferate in zones of high moisture availability (Eissenstat and Caldwell 1988; Schenk and Jackson 2002; Metcalfe et al. 2008; Fan et al. 2017). Thus, perching of water above and retention of water within the argillic horizon, coupled with limited recharge of subsoil moisture (Archer 1995), likely contributed to enhanced root production in surficial soils while simultaneously inhibiting root penetration and retarding growth in deeper soil. However, it should be noted that the proportion of Zanthoxylum root biomass and density in the upper 0.4 m of soil was comparable on argillic and non-argillic soils (> 68% for both variables). Rooting patterns of Prosopis were also relatively consistent on both soils (> 47% and > 59% of roots below 0.4 m when the argillic horizon was present and absent, respectively). These data suggest that Zanthoxylum and Prosopis are intrinsically and genetically shallow- and deep-rooted, respectively, and that soil physical properties are of secondary importance in influencing their vertical root distribution patterns.
Root: shoot ratios are an indicator of photosynthate allocation priorities. The ratios observed at this site (Table 2) generally fell within global range for subtropical dry woodlands (0.26–0.72, Mokany et al. 2006). Root: shoot ratios are affected by a variety of factors related to stand development. As noted in Mokany et al. (2006), root: shoot ratios generally decrease with a variety of factors all of which are consistent with our data: increasing stand age (61.5 vs. 74.0 years for largest Prosopis in discrete clusters and groves), height (3.5 vs. 4.7 m), and mean basal diameter (11.1 vs. 15.9 cm), and decreasing plant density (5.4 vs 1.7 m−2). In addition, edaphic characterizations in this study compliment those of Loomis (1989), Stokes (1999), and Zhou et al. (2017b) on this site and further substantiate that patches of grassland and discrete clusters of woody plants occur where there is a well-developed argillic horizon and that large groves of woody plants have developed where soils are coarse-textured throughout the profile. Our data illustrating the larger size of Prosopis in groves relative to discrete clusters (Table 1) agree with more extensive surveys (Archer 1995; Boutton et al. 1998) and indicate that upland soil inclusions lacking an argillic horizon are more productive sites for woody plants. We hypothesize that a greater abundance of soil moisture, associated with receipt of surface runoff and subsurface interflow from portions of landscape with a continuous argillic horizon, has enabled woody plants in groves to support greater aboveground biomass with substantially less investment in root biomass.
Depth distribution of root density and biomass revealed that small roots (< 10 mm) on both argillic and non-argillic soils decreased exponentially with depth, whereas large roots (> 10 mm) on argillic soils presented different vertical patterns compared to those on non-argillic soils (Figs. 2 and 4). Accordingly, multivariate ANOVA of root density showed a significant soil type × root diameter interaction (Table S4) at depths >0.4 m. As discussed above, non-argillic soils are more productive for woody plants (Archer 1995; Boutton et al. 1998) and support larger woody plants compared to argillic soils (Table 1), potentially resulting in more large roots (> 10 mm) in non-argillic soils, especially at depths >0.4 m. More large roots in non-argillic soils then contributed to the lower root: shoot ratios in grove communities (Table 1).
Species rooting patterns and plant succession
Root systems of co-occurring plant species often overlap substantially, but differences in distribution, turnover rates, activity, phenology and size may be sufficient to affect competitive interactions and resource partitioning (Walter 1971; Seghieri 1995; Hipondoka et al. 2003; Sankaran et al. 2004; Schenk 2006; February and Higgins 2010; Stokes and Archer 2010; Zhou et al. 2018c). Extensive intermingling of species root systems was observed in this subtropical savanna ecosystem, but contrasting patterns were evident for key co-occurring species (Fig. 3). Prosopis, the pioneer species in discrete cluster development that facilitates the subsequent ingress and establishment of subordinate shrub species (Archer 1990 and 1995), was deep-rooted with 47% and 59% of roots present below 0.4 m in discrete clusters and groves, respectively. This deep rooting system not only enhances the survival of Prosopis during the critical seedling establishment phase by reducing competition with grasses for water (Brown and Archer 1999; Ansley et al. 2014), but also facilitates subsequent recruitment of subordinate shrub species by providing nutrient-enriched soil conditions (Hibbard et al. 2001; Zhou et al. 2018a, b). Prosopis is known to form symbiotic associations with N-fixing bacteria, and plants at this site do nodulate and fix N (Zitzer et al. 1996; Soper et al. 2015). As a result, soil N in the upper 30 cm of the profile is greater under Prosopis canopies than adjacent herbaceous zones (Archer 1995; Hibbard et al. 2001; Boutton and Liao 2010; Zhou et al. 2018a). In addition, deep-rooted Prosopis transfers phosphorus (P) from deeper soil layers and enlarges the P pool in the upper soil layers where it is more actively cycled (Zhou et al. 2018b).
Zanthoxylum is among the first shrub species to colonize beneath Prosopis canopies, and typically dominates the understory layer (Archer et al. 1988; Archer 1990). Although the precise mechanisms of this facilitation have not yet been fully addressed experimentally, the shallow root systems of these early arrivals (Fig. 3) would make them well-suited to take advantage of (1) N- and P-enriched surface soils resulting from the establishment and growth of Prosopis (Hibbard et al. 2001; Zhou et al. 2018a, b); and (2) redistribution of soil moisture from deep to surface horizons that might occur as the result of hydraulic redistribution, as has been shown on this site (Zou et al. 2005). In addition, the dense shallow root systems of these early arrivals underneath Prosopis canopies may contribute to the observed, significant declines in shallow-rooted grasses and forbs by intensifying competition for soil resources while simultaneously reducing light levels. This, in turn, may create opportunities for the establishment of shade-tolerant understory woody species appearing later during woody patch development.
Different root distribution patterns among shrub species would diffuse competition and enable the dynamic co-existence of woody species in discrete clusters and groves. For example, the natural abundance of 2H and 18O in stem water indicates that shallow-rooted understory species such as Zanthoxylum are closely coupled to availability of surface soil moisture, while deep-rooted Prosopis acquires water from deeper soil layers (Boutton et al. 1999). However, as the size and density of understory species increases over time, co-existence appears to give way to asymmetric competition (Archer 1989, 1995), as field observations indicate that the overstory Prosopis in about 25% of discrete clusters have died. One explanation supported by experimental evidence is that roots of Zanthoxylum and other shallow-rooted shrubs sequester water and nutrients that might otherwise percolate to depths where Prosopis roots dominate and adversely impact Prosopis growth (Barnes and Archer 1999). Since the demise of the central Prosopis is less common in groves on non-argillic soils, we hypothesize that these competitive effects would be most pronounced in discrete clusters on soils with a well-developed argillic horizon, where near-surface understory root densities and biomass are higher (Figs. 2 and 4) and where the clay-rich argillic horizon restricts percolation of water and nutrients to deeper soil layers (Archer et al. 1988 and Archer 1995).
Root extension and landscape development
The current two-phase landscape (woody clusters and groves interspaced within a grassland matrix, Fig. 1) on the upland portions of this study site has been hypothesized to represent an intermediate stage in landscape development from open grassland to monophasic woodland (Archer et al. 1988; Archer 1995; Boutton 1996; Boutton et al. 1998; Zhou et al. 2017b). Within this two-phase landscape, 95% of woody clusters are within 15 m of another, and seedlings of Prosopis occur in high densities in the grassland matrix (Archer et al. 1988), suggesting the potential for cluster formation through the aforementioned vegetation succession. This hypothesis presumes that interactions between woody clusters are minimal and that as new clusters form and grow, coalescence will occur.
However, cluster coalescence may not occur if intense competition from laterally extensive root systems of existing clusters significantly reduces the survival probability of Prosopis seedlings in the grassland matrix or limits subsequent cluster formation and development. Our data showed that root density decreased exponentially with increasing distance from cluster perimeters and that few roots extended more than 1 m beyond cluster perimeters, especially at soil depths below 0.4 m (Figs. 5 and 6, Table S7). These results indicate that Prosopis seedlings in the grassland matrix likely receive no or limited competition from existing woody plants and have the potential to grow and recruit understory species to form discrete clusters. Indeed, a chronological sequence of aerial photos at this site spanning the period 1930 to 2016 documents the active formation of discrete clusters and their expansion and coalescence (Archer et al. 2001; Bai et al. 2009; Zhou et al. 2017b), providing direct evidence to support this hypothesis. Therefore, our results suggest that succession to monophasic woodland may occur on upland portions of this study site, with the rate being mediated by edaphic heterogeneity and the nature of future climate and disturbance (e.g. grazing and fire) regimes.
Belowground consequences of woody plant proliferation in grassland
Shrub clusters and groves in this subtropical system have developed on former grasslands (Archer 1995; Boutton et al. 1998). The consequences for this change with respect to aboveground biomass, primary production and near-surface (upper 20 cm) soil carbon pools and fluxes have been summarized in earlier work (e.g., Archer et al. 2001; Hibbard et al. 2003; McCulley et al. 2004; Boutton and Liao 2010). Here, we show that the change in community structure from grass to woody plant dominance has profoundly increased the magnitude of the carbon pool associated with plant roots to depths of 1.2 m (3- to 5-fold, Fig. 7). Our ability to predict how root biomass might change with changes in growth form/life form composition presently relies on a ‘space-for-time substitution’ approach. For example, if a tropical savanna transitioned to a tropical evergreen forest or a tropical deciduous forest, we could use data such as that presented in Jackson et al. (1996) to predict the consequences for below ground biomass with depth. The veracity of this prediction would be predicated on the strength of the assumption that such a space-for-time substitution is accurate and robust. Although our data is also based on a space-for-time approach, it is at more local scale which might make the results more germane to developing accurate predictions. In any case, global budgets for root biomass, surface area and nutrient content (e.g. Jackson et al. 1997) will be challenged to take into account shifts from grass to woody plant dominance and how such shifts will play out on various soil types. To further complicate things, root longevity, turnover and decay likely vary with species, size (diameter) and depth to determine the dynamics of this substantial belowground carbon pool.
Conclusions and implications
Edaphic characteristics strongly influenced root distribution patterns and biomass allocation of co-occurring woody plants in this subtropical savanna parkland. Root density and biomass in soils with a well-developed argillic horizon were generally greater above a depth of 0.4 m and lower below 0.8 m of the profile compared to similar depths in soils where the argillic horizon was absent. Woody plants maintained greater aboveground biomass with substantially less root biomass on soils lacking an argillic horizon. The dominant overstory species Prosopis was deep-rooted with a high proportion of its total roots below 0.4 m. In contrast, the dominant understory shrub, Zanthoxylum, and other subordinate species were relatively shallow-rooted. This would facilitate their early co-existence with Prosopis -- by minimizing interspecies competition during the formation of woody clusters and groves -- and later form the basis for asymmetric competition that may hasten the demise of the Prosopis overstory. Root density decreased exponentially with increasing distance from cluster perimeters, and few woody plant roots extended >1 m beyond cluster canopies, thus minimizing the potential for density dependent constraints on future cluster development. The ability to identify roots to species for the predominant and co-occurring woody plants in this system afforded unique and novel perspectives on plant-plant and plant-soil relationships and provide valuable insights for explaining vegetation dynamics and landscape development of this subtropical savanna parkland.
Root distribution datasets of co-occurring woody species, such as those presented in this study, are comparatively rare and novel, as the savanna literature has focused primarily on rooting differences between woody plants and grasses. The patterns of species-specific root distribution revealed in this study have relevance to understanding plant succession and landscape development. Our data provide potential insights into the structure-function relationships of grass-woody, shrub-shrub and shrub-tree growth forms and life forms in savanna, parkland and woodland settings. Our results show the broad range of rooting patterns occurring in dryland woody plants and challenge prevailing generalization that they are deep rooted and/or that they have extensive shallow lateral root systems. We clearly demonstrate how edaphic factors affecting water regimes are critical in shaping biomass partitioning (Tomlinson et al. 2012) and hence global patterns of rooting depth distribution (Fan et al. 2017). Furthermore, root biomass in shrub cluster and grove communities to a depth of 1.2 m were 3- to 5-times greater than that of the grasslands they ostensibly replaced (Fig. 7), suggesting belowground carbon inputs can increase dramatically when woody plants proliferate in grasslands. This substantive change in the magnitude of belowground C pools would markedly influence assessments of C-sequestration potential and source-sink relationships (Barger et al. 2011). Accordingly, improvements in our understanding rooting patterns of dryland woody species is paramount to advancing our ability to predict species interactions, vegetation dynamics, and responses of mixed growth form/life form systems to future environmental conditions.
References
Ansley RJ, Boutton TW, Jacoby PW (2014) Root biomass and distribution patterns in a semi-arid mesquite savanna: responses to long-term rainfall manipulation. Rangel Ecol Manag 67:206–218
Archer S (1989) Have southern Texas savannas been converted to woodlands in recent history? Am Nat 134:545–561
Archer S (1990) Development and stability of grass/woody mosaics in a subtropical savanna parkland, Texas, USA. J Biogeogr 17:453–462
Archer S (1995) Tree-grass dynamics in a Prosopis-thornscrub savanna parkland: reconstructing the past and predicting the future. Ecoscience 2:83–99
Archer S, Scifres C, Bassham CR, Maggio R (1988) Autogenic succession in a subtropical savanna: conversion of grassland to thorn woodland. Ecol Monogr 58:111–127
Archer S, Boutton TW, Hibbard KA (2001) Trees in grasslands: biogeochemical consequences of woody plant expansion. In: Schulze E-D (ed) Global biogeochemical cycles in the climate system. Academic Press, San Diego, pp 115–137
Archer S, Boutton TW, McMurtry C (2004) Carbon and nitrogen accumulation in a savanna landscape: field and modeling perspectives. In: Shiomi M, Kawahata H, Koizumi H, Tsuda A, Awaya Y (eds) Global environmental change in the ocean and on land. Terrapub, Tokyo, pp 359–373
Archer SR, Andersen EM, Predick KI, Schwinning S, Steidl RJ, Woods SR (2017) Woody plant encroachment: causes and consequences. In: Briske DD (ed) Rangeland systems: processes, management and challenges. Springer, New York, pp 25–84
Bai E, Boutton TW, Wu XB, Liu F, Archer SR (2009) Landscape-scale vegetation dynamics inferred from spatial patterns of soil δ13C in a subtropical savanna parkland. J Geophys Res-Biogeosci 114:G01019. https://doi.org/10.1029/2008JG000839
Bai E, Boutton TW, Liu F, Wu XB, Archer SR (2012) Spatial patterns of soil δ13C reveal grassland-to-woodland successional processes. Org Geochem 42:1512–1518
Barger NN, Archer S, Campbell J, Huang C, Morton J, Knapp A (2011) Woody plant proliferation in north American drylands: a synthesis of impacts on ecosystem carbon balance. J Geophys Res Biogeosci 116:G00K07. https://doi.org/10.1029/2010JG001506
Barnes PW, Archer S (1999) Tree-shrub interactions in a subtropical savanna parkland: competition or facilitation? J Veg Sci 10:525–536
Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA (2006) Root responses to soil physical conditions; growth dynamics from field to cell. J Exp Bot 57:437–447
Boutton TW (1996) Stable carbon isotope ratios of soil organic matter and their use as indicators of vegetation and climate change. In: Boutton TW, Yamasaki SI (eds) Mass spectrometry of soils. Marcel Dekker, New York, pp 47–82
Boutton TW, Liao JD (2010) Changes in soil nitrogen storage and δ15N with woody plant encroachment in a subtropical savanna parkland landscape. J Geophys Res-Biogeosci 115:G03019. https://doi.org/10.1029/2009JG001184
Boutton TW, Archer SR, Midwood AJ, Zitzer SF, Bol R (1998) δ13C values of soil organic carbon and their use in documenting vegetation change in a subtropical savanna ecosystem. Geoderma 82:5–41
Boutton TW, Archer SR, Midwood AJ (1999) Stable isotopes in ecosystem science: structure, function and dynamics of a subtropical savanna. Rapid Commun Mass Spectrom 13:1263–1277
Brown JR, Archer S (1999) Shrub invasion of grassland: recruitment is continuous and not regulated by herbaceous biomass or density. Ecology 80:2385–2396
Brunsell NA, Van Vleck ES, Nosshi M, Ratajczak Z, Nippert JB (2017) Assessing the roles of fire frequency and precipitation in determining woody plant expansion in central U.S. grasslands. J Geophys Res-Biogeosci 122:2683–2698
Clark LJ, Whalley WR, Barraclough PB (2003) How do roots penetrate strong soil? In Roots: The Dynamic Interface Between Plants and the Earth. Springer Netherlands, pp:93–104
de Kroon H, Hendriks M, van Ruijven J, Ravenek J, Padilla FM, Jongejans E, Visser EJW, Mommer L (2012) Root responses to nutrients and soil biota: drivers of species coexistence and ecosystem productivity. J Ecol 100:6–15
Devine AP, McDonald RA, Quaife T, Maclean IMD (2017) Determinants of woody encroachment and cover in African savannas. Oecologia 183:939–951
Dracup M, Belford RK, Gregory PJ (1992) Constraints to root growth of wheat and lupin crops in duplex soils. Aust J Exp Agr 32:947–961
Eissenstat DM, Caldwell MM (1988) Seasonal timing of root growth in favorable microsites. Ecology 69:870–873
Fan Y, Miguez-Macho G, Jobbágy EG, Jackson RB, Otero-Casal C (2017) Hydrologic regulation of plant rooting depth. Proc Natl Acad Sci 114:10572–10577
February EC, Higgins SI (2010) The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. S Afr J Bot 76:517–523
Flinn RC, Archer SR, Boutton TW, Harlan T (1994) Identification of annual rings in an arid-land woody plant, Prosopis glandulosa. Ecology 75:850–853
Gale MR, Grigal DF (1987) Vertical root distributions of northern tree species in relation to successional status. Can J For Res 17:829–834
Gerard CJ, Sexton P, Shaw G (1982) Physical factors influencing soil strength and root growth. Agron J 74:875–879
Haling RE, Simpson RJ, Culvenor RA, Lambers H, Richardson AE (2011) Effect of soil acidity, soil strength and macropores on root growth and morphology of perennial grass species differing in acid-soil resistance. Plant Cell Environ 34:444–456
Hatch SL, Ghandi KN, Brown LE (1990) A checklist of the vascular plants of Texas. Texas Agricultural Experiment Station, College Station
Hibbard KA, Archer S, Schimel DS, Valentine DW (2001) Biogeochemical changes accompanying woody plant encroachment in a subtropical savanna. Ecology 82:1999–2011
Hibbard KA, Schimel D, Archer S, Ojima D, Parton W (2003) Grassland to woodland transitions: integrating changes in landscape structure and biogeochemistry. Ecol Appl 13:911–926
Hipondoka MHT, Aranibar JN, Chirara C, Lihavha M, Macko SA (2003) Vertical distribution of grass and tree roots in arid ecosystems of southern Africa: niche differentiation or competition? J Arid Environ 54:319–325
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Holdo RM (2013) Revisiting the two-layer hypothesis: coexistence of alternative functional rooting strategies in savannas. PLoS One 8(8):e69625. https://doi.org/10.1371/journal.pone.0069625
Hutchings MJ, John EA, Wijesinghe DK (2003) Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology 84:2322–2334
Huxman TE, Wilcox BP, Breshears DD, Scott RL, Snyder KA, Small EE, Hultine K, Pockman WT, Jackson RB (2005) Ecohydrological implications of woody plant encroachment. Ecology 86:308–319
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108:389–411
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci U S A 94:7362–7366
Liu F, Archer S, Gelwick F, Bai E, Boutton T, Wu X (2013) Woody plant encroachment into grasslands: spatial patterns of functional group distribution and community development. PLoS One 8:e84364. https://doi.org/10.1371/journal.pone.0084364
Loomis LE (1989) Influence of heterogeneous subsoil development on vegetation patterns in a subtropical savanna parkland, Texas. PhD thesis, Texas A&M University, College Station, TX, USA
Ludovici KH (2004) Tree roots and their interaction with soil. In: Burley J, Evans J, Youngquist JA (eds) Encyclopedia of forest sciences. Elsevier, Oxford, pp 1195–1201
Macinnis-Ng CMO, Fuentes S, O’Grady AP, Palmer AR, Taylor D, Whitley RJ et al (2010) Root biomass distribution and soil properties of an open woodland on a duplex soil. Plant Soil 327:377–388
McCulley RL, Archer SR, Boutton TW, Hons FM, Zuberer DA (2004) Soil respiration and nutrient cycling in wooded communities developing in grassland. Ecology 85:2804–2817
McDonald JH (2014) Handbook of biological statistics, 3rd edn. Sparky House Publishing, Baltimore
Metcalfe DB, Meir P, Aragão LE, da Costa AC, Braga AP, Gonçalves PH et al (2008) The effects of water availability on root growth and morphology in an Amazon rainforest. Plant Soil 311:189–199
Mokany K, Raison R, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Glob Chang Biol 12:84–96
Morris EC, Griffiths M, Golebiowska A, Mairhofer S, Burr-Hersey J, Goh T et al (2017) Shaping 3D root system architecture. Curr Biol 27:919–930
Owens PR, Rutledge EM (2005) Morphology. In: Hillel D, Hatfield JL (eds) Encyclopedia of soils in the environment. Elsevier, Amsterdam, pp 511–520
Parrish JAD, Bazzaz FA (1976) Underground niche separation in successional plants. Ecology 57:1281–1288
Parton WJ, McKeown B, Kirchner V, Ojima D (1992) CENTURY User’s Manual. Natural Resource Ecology Laboratory, Colorado State University, Fort Collins, CO.
Pendleton RL, Nickerson D (1951) Soil colors and special soil color charts. Soil Sci 71:35–44
Plante PM, Rivest D, Vézina A, Vanasse A (2014) Root distribution of different mature tree species growing on contrasting textured soils in temperate windbreaks. Plant Soil 380:429–439
Ratajczak Z, Nippert JB, Hartman JC, Ocheltree TW (2011) Positive feedbacks amplify rates of woody encroachment in Mesic tallgrass prairie. Ecosphere 2:1–14
Ratajczak Z, Nippert JB, Collins SL (2012) Woody encroachment decreases diversity across north American grasslands and savannas. Ecology 93:697–703
Sankaran M, Ratnam J, Hanan NP (2004) Tree–grass coexistence in savannas revisited–insights from an examination of assumptions and mechanisms invoked in existing models. Ecol Lett 7:480–490
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90:480–494
Schenk HJ, Jackson RB (2005) Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 126:129–140
Seghieri J (1995) The rooting patterns of woody and herbaceous plants in a savanna; are they complementary or in competition? Afr J Ecol 33:358–365
Sheldrick BH, Wang C (1993) Particle size distribution. In: Carter MR (eds) Soil sampling and methods of analysis. Canadian Society of Soil Science, Lewis Publishers, Ann Arbor, pp: 499–511
Smucker AJM, McBurney SL, Srivastava AK (1982) Quantitative separation of roots from compacted soil profiles by the hydropneumatic elutriation system. Agron J 74:500–503
Soper FM, Boutton TW, Sparks JP (2015) Investigating patterns of symbiotic nitrogen fixation during vegetation change from grassland to woodland using fine scale δ15N measurements. Plant Cell Environ 38:89–100
Stevens N, Lehmann CE, Murphy BP, Durigan G (2017) Savanna woody encroachment is widespread across three continents. Glob Change Biol 23:235–244
Stokes CJ (1999) Woody plant dynamics in a south Texas savanna: pattern and process. PhD Dissertation, Texas A&M University, College Station, TX USA
Stokes CJ, Archer SR (2010) Niche differentiation and neutral theory: an integrated perspective on shrub assemblages in a parkland savanna. Ecology 91:1152–1162
Strong WL, La Roi GH (1985) Root density-soil relationships in selected boreal forests of Central Alberta, Canada. Forest Ecol Manag 12:233–251
Sudmeyer RA, Speijers J, Nicholas BD (2004) Root distribution of Pinus pinaster, P. radiata, Eucalyptus globulus and E. kochii and associated soil chemistry in agricultural land adjacent to tree lines. Tree Physiol 24:1333–1346
Tilman D (1985) The resource ratio hypothesis of succession. Am Nat 125:827–852
Tomlinson KW, Sterck FJ, Bongers F, da Silva DA, Barbosa ER, Ward D, Bakker FT, van Kaauwen M, Prins HHT, de Bie S, van Langevelde F (2012) Biomass partitioning and root morphology of savanna trees across a water gradient. J Ecol 100:1113–1121
Valverde-Barrantes OJ, Smemo KA, Feinstein LM, Kershner MW, Blackwood CB (2013) The distribution of below-ground traits is explained by intrinsic species differences and intraspecific plasticity in response to root neighbours. J Ecol 101:933–942
van Auken OW (2000) Shrub invasions of north American semiarid grasslands. Annu Rev Ecol Evol Syst 31:197–215
Venter ZS, Cramer MD, Hawkins HJ (2018) Drivers of woody plant encroachment over Africa. Nat Commun 9:2272. https://doi.org/10.1038/s41467-018-04616-8
Wachsman G, Sparks EE, Benfey PN (2015) Genes and networks regulating root anatomy and architecture. New Phytol 208:26–38
Walter H (1971) Ecology of tropical and subtropical vegetation. Oliver and Boyd, Edinburgh, UK
Ward D, Wiegand K, Getzin S (2013) Walter's two-layer hypothesis revisited: back to the roots! Oecologia 172:617–630
Whittaker RH, Gilbert LE, Connell JH (1979) Analysis of two phase pattern in a mesquite grassland, Texas. J Ecol 67:935–952
Wigley BJ, Bond WJ, Hoffman MT (2010) Thicket expansion in a south African savanna under divergent land use: local vs. global drivers? Glob Chang Biol 16:964–976
Xu GQ, Li Y (2009) Rooting depth and leaf hydraulic conductance in the xeric tree Haloxyolon ammodendron growing at sites of contrasting soil texture. Funct Plant Biol 35:1234–1242
Zhou Y, Boutton TW, Wu XB, Yang C (2017a) Spatial heterogeneity of subsurface soil texture drives landscape-scale patterns of woody patches in a subtropical savanna. Landsc Ecol 32:915–929
Zhou Y, Boutton TW, Wu XB (2017b) Soil carbon response to woody plant encroachment: importance of spatial heterogeneity and deep soil storage. J Ecol 105:1738–1749
Zhou Y, Mushinski RM, Hyodo A, Wu XB, Boutton TW (2018a) Vegetation change alters soil profile δ15N values at the landscape scale. Soil Biol Biochem 119:110–120
Zhou Y, Boutton TW, Wu XB (2018b) Woody plant encroachment amplifies spatial heterogeneity of soil phosphorus to considerable depth. Ecology 99:136–147
Zhou Y, Boutton TW, Wu XB, Wright CL, Dion AL (2018c) Rooting strategies in a subtropical savanna: a landscape-scale three-dimensional assessment. Oecologia 186:1127–1135
Zitzer SF, Archer SR, Boutton TW (1996) Spatial variability in the potential for symbiotic N2-fixation by woody plants in a subtropical savanna ecosystem. J Appl Ecol 33:1125–1136
Zou CB, Barnes PW, Archer S, McMurtry CR (2005) Soil moisture redistribution as a mechanism of facilitation in savanna tree–shrub clusters. Oecologia 145:32–40
Acknowledgements
This research was supported by NSF grant BSR-9109240, NASA grant NAGW-2662, NSF Doctoral Dissertation Improvement Grant DEB/DDIG-1600790, USDA/NIFA Hatch Project 1003961. Yong Zhou was supported by a Sid Kyle Graduate Merit Assistantship from the Department of Ecosystem Science and Management and a Tom Slick Graduate Research Fellowship from the College of Agriculture and Life Sciences, Texas A&M University. Stephen Watts was supported by a Regents’ Fellowship from the Office of Graduate and Professional Studies at Texas A&M University. We thank Rob Flinn and Dr. Stephen Zitzer for identification of woody plants and assistance with fieldwork; Dr. Lynn Loomis of USDA/NRCS for help with soil descriptions; and, Dr. Mike Longnecker of Department of Statistics at Texas A&M for help with statistical analyses. Several undergraduate students also contributed to the project, including Keith Beatty, Brian Hays, James Loughlin, Patty Mentch, Wesley Roark, and Marcus Simpson. Assistance with on-site logistics at the La Copita Research Area was provided by David and Stacy McKown.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alexia Stokes.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1041 kb)
Rights and permissions
About this article
Cite this article
Zhou, Y., Watts, S.E., Boutton, T.W. et al. Root density distribution and biomass allocation of co-occurring woody plants on contrasting soils in a subtropical savanna parkland. Plant Soil 438, 263–279 (2019). https://doi.org/10.1007/s11104-019-04018-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04018-9