Abstract
Different growth rates of grasses from South American natural grasslands are adaptations to soils of low fertility. Grasses with fast growth rate are species with an accumulation of nutrients in soluble forms, with a high metabolic rate. This work aimed to study whether grasses with different growth rates have different phosphorus (P) uptake and efficiency of P use with high and low P availability in soil, as well as whether phosphatase activity is related to the species growth rate and variations in P biochemical forms in the tissues. Three native grasses (Axonopus affinis, Paspalum notatum, and Andropogon lateralis) were grown in pots with soil. Along plant growth, biomass production and its structural components were measured, as well as leaf acid phosphatase activity and leaf P chemical fractions. At 40 days of growth, leaf acid phosphatase activity declined by about 20–30% with an increase of P availability in soil for A. affinis and P. notatum, respectively. Under both soil P levels, P. notatum showed the highest plant total biomass, leaf dry weight and highest P use efficiency. A. affinis presented the higher P uptake efficiency and soluble organic P concentration in the leaf tissues. A. lateralis showed P-Lipid concentration 1.6 and 1.3 times higher than A. affinis and P. notatum, respectively. In conclusion, acid phosphatase activity in grass of higher growth rate is related to higher remobilization of P due to higher demand, as in A. affinis, and higher growth rates are associated with higher P uptake efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient availability frequently limits plant growth in natural communities (Wang et al. 2010). Plant adaptations that could be important under low fertility soils include: (1) low relative growth rate, preventing exhaustion of internal nutrient reserves (Chapin 1980); (2) high capacity to scavenge nutrients from soil (Vance et al. 2003); and (3) differences in nutrient use efficiency (Veneklaas et al. 2012). Slow growth rate of plants under low fertility soils is related to their longer leaf life span, which appears to be an adaptation to low translocation and remobilization capacity (Aerts and Chapin 1999); therefore, these plants may have a limited response to the increase of nutrient availability in soil.
In general, a longer life span of leaves is related to mineral nutrients and photoassimilates being allocated into complex leaf structures, i.e., with a large amount of structural tissues. These species have a higher content of dry matter in leaves (LDMC), which results in a reduction in leaf area per carbon unit, evaluated as specific leaf area (SLA) (Wright and Westoby 2003). Considering these leaf traits, plants with lower LDMC and higher SLA show a higher growth rate, a faster and intense use of mineral nutrients, and great efficiency of nutrient remobilization (Garnier et al. 2007).
A higher efficiency of phosphorus (P) remobilization may be related to the activity of enzymes such as acid phosphatases. The activity of these enzymes is related to the alteration of P tissue concentration (Duff et al. 1994), leading to an activity regulation through P demands as a feedback regulation effect (McLachlan and March 1982). When inorganic phosphorus (Pi) supply by soil is lower than the plant demand, a higher remobilization of P stored in old leaves can occur translocating it to young leaves, as well as an increase in root growth, especially for species with a higher growth rate (Doerner 2008; Shen et al. 2011).
The remobilization of P is not just a matter of senescence, but it occurs even in young tissues, supporting the idea that a specific fraction of P is cycled in the plant (Lambers et al. 2006; Shen et al. 2011). Remobilization is an important source of P for growth related to P use efficiency (Wieneke 1990). However, other concepts such as P allocation capacity in different organs must be considered. Low quantities of P allocated in complex structural forms (phospholipids, for example) preserves P in easier transported forms and may be an alternative for increased nutrient use efficiency. In this regard, Elliott and Läuchli (1986) suggested that the P partitioning between inorganic and organic forms is the major determinant of P use efficiency.
In southwestern Australian plant species, both high nutrient remobilization efficiency (Wright and Westoby 2003; Denton et al. 2007) and high leaf longevity (Wright et al. 2004; Lambers et al. 2012) are common as expected by the status of the P impoverished environment (Hopper 2009). However, concerning the South American natural grasslands species, there is little scientific information regarding the interrelationships among phosphate adaptation strategies, phosphorus metabolism, and plant growth. Therefore, we performed an experiment with three South American native grasses (Axonopus affinis, Paspalum notatum, and Andropogon lateralis) which have a range of leaf life span (Machado et al. 2013) and different growth rates under low and high P availability.
These grass species grow in Brazilian natural grasslands that are in a region with subtropical climate and acidic soils (pH ranging from 4.4 to 5.1) which have low P availability (ranging from 0.1 to 7.6 mg kg−1) (Oliveira et al. 2014; Tiecher et al. 2014). Such grassland areas occupy 2.07% of the Brazilian territory (176,496 km2), but are described as the main forage source for approximately 13 million cattle and 5 million sheep (de Carvalho PC and Batello 2009).
We intended to focus on the following questions: (1) Do grass species with different leaf traits and growth rates have a consistent pattern for the efficiency of P uptake and use in both high and low P soil availability? (2) Is the phosphatase activity related to the plant growth rate of the grasses and biochemical P form in the grasses? (3) Do grasses with low growth rate allocate more P in structural fraction in their tissues? To answer these questions, we grew young plants in soil under two levels of P availability in a greenhouse during successive re-growth and measured the distribution and quantity of biomass, leaf acid phosphatase activity, and concentrations of the major P chemical fractions in leaves.
Materials and methods
Soil and plant species
Soil and plant species for the pot experiment were collected from a natural grassland community of Campos (Allen et al. 2011) in Santa Maria, southern Brazil. This grassland community occupies large areas (location at 29°45′S, 53°42′W; 95 m a. s. l.) where the predominant soil type is Ultisol, which was utilized in this work. In the top horizon (0–20 cm depth), the clay content was 180 g kg−1, total organic carbon 14.5 g kg−1, soil pH in water (1:1 v/v) 4.6; available P and K (extracted by Mehlich 1) were 3.0 and 76.0 mg kg−1, respectively, and there were 1.9, 2.8 and 1.4 cmolc kg−1 of exchangeable Al, Ca, and Mg extracted by 1.0 M KCl, respectively.
Three South American native grasses (Axonopus affinis Chase, Paspalum notatum Flüggé, and Andropogon lateralis Nees) were selected for our work because they are the most frequent grasses in the natural grassland community. Axonopus affinis is a grass that grows by stolons and is most commonly found in lowland areas with high soil moisture. Paspalum notatum is the species that occurs the most in natural grasslands of Southern Brazil and its growth form is based on rhizomes. Axonopus affinis and Paspalum notatum are species with high relative growth rate. Andropogon lateralis is a potential tussock grass, but changes in its growth structure with high grazing pressure, is well distributed on the landscape, and is a species with low relative growth rate.
Treatment and experimental conditions
The experiment was carried out in a greenhouse with an average temperature of 23.5 °C, light intensity of 1100 µmol m−2 s−1 PAR, and relative humidity of 76.4% in the spring period of 2012 (September to November). Plant evaluation occurred during two periods for each species after soil fertilization. During the last week of May 2012, five juvenile individuals (tillers) of similar size of each species were transplanted into pots with soil. Pots with 23 cm diameter and 20 cm height were filled with 5 kg of homogenized dry soil from the top horizon (0–20 cm depth). After an acclimation period (since May 2012), on September 13, all plants were clipped to a height of 5 cm and randomly assigned to fertilizer treatments.
The treatments consisted of: (1) one application of 50 mg P kg−1 of soil (named P+) and (2) natural availability of P (no application) (named P−). The amount of P added was previously determined by an incubation test of doses of P in the soil. The selected dose was determined by reaching the critical level of P availability for plant growth proposed by the Southern Brazilian Soil Fertility and Chemical Committee (CQFS-RS/SC 2016). Phosphorus was added as potassium phosphate (KH2PO4). Potassium (K) was added in P− in the same amount as for P+ as potassium chloride (KCl) to avoid K limitation for growth. Pots were maintained at around 70–80% of field capacity by daily weighing and watering. For each grass species, the P treatments were arranged in a randomized block design with four replications. Blocking criterion was the position of the plots on the testing bench, nearest or farthest from the refrigeration system.
Sample collection
The period for sampling was estimated through the accumulated mean temperature (thermal sum) needed for expansion of 2.5 leaves (leaf expansion duration, a period for the leaf to obtain its maximum elongation). The thermal sum used was 437° days (DD) for the three species [phyllochron utilized is found in Machado et al. (2013)]. The mean temperature utilized was the average between the maximum and minimum temperature of each day in the greenhouse (Fig. 1). On October 27, 2012, corresponding to 40 days after treatment application, the plants of the three species were harvested.
Measurements
Leaf traits
Following the recommendations of the protocols proposed by Cornelissen et al. (2003), ten individuals (tillers) per replicate were selected for evaluation. Two traits were measured: specific leaf area (SLA) and leaf dry matter content (LDMC). For this purpose, ten newly expanded leaves from tillers were scanned (HP Scanjet g 4050) and leaf areas were measured using the ImageJ software. After that, the same ten leaves were used to calculate LDMC (leaf DM per unit fresh mass of water-saturated leaves) (Garnier et al. 2001; Cornelissen et al. 2003).
Plant biomass
For each replicate, plant biomass was separated into leaves (blades), stems (sheath, stems, stolons, and rhizomes), and roots, which were weighed after drying for 72 h at 60 °C.
Phosphorus determination
Soil P availability was estimated after 40 days of growth by extraction with Mehlich-1 extractor (HCl 0.05 N + H2SO4 0.025 N) (Tedesco et al. 1995). P concentration in the extracts were determined by the Murphy and Riley (1962) method (Table 1).
Total P concentration was determined in leaves (Pleaf), stems, and roots by the Murphy and Riley (1962) method after digestion with concentrated sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) (Tedesco et al. 1995). The average P plant concentration (Pplant) was calculated through weighting the average P concentration at each organ and its dry matter mass. Phosphorus uptake was estimated by multiplying the total dry mass by plant P concentration.
Phosphorus use efficiency and allocation
Phosphorus use efficiency (PUE) was calculated as follows (Siddiqi and Glass 2008):
Phosphorus uptake efficiency (PUtE) was evaluated according to Swiader et al. (1994) as follows:
Phosphorus allocation in plants was analyzed using the index proposed by Fitter and Setters (1988) and Dale and Causton (1992). The P allocation index (PAI) in an organ was expressed as follows:
where Po and Pp are phosphorus concentration in the referred organ and in the whole plant, respectively. The Mo is the biomass of the organ, and Mp is the biomass of the whole plant. The phosphorus concentration of the organ is:
A PAI > 1 for a particular organ indicates a greater proportional allocation of P (Dale and Causton 1992).
Acid phosphatase activity
The acid phosphatase activity (APA) was determined for each replicate on the three youngest and fully expanded leaves (Tabaldi et al. 2007). The leaves were frozen in liquid nitrogen and stored at − 20 °C. Subsequently, the leaves were manually ground in liquid nitrogen and put in a reaction medium consisting of 3.5 mM NaN, 2.5 mM NaCl, and 100 mM citrate buffer (pH 5.5) to a final volume of 200 µL. The inorganic phosphate was measured at 630 nm in a spectrophotometer SF325NM (Bel Engineering, Italy) using malachite green as a reagent and KH2PO4 colorimetric standard calibration for the curve.
Biochemical fractionation
The leaves were subjected to sequential P chemical fractionation according to the methodology proposed by Miyachi and Tamiya (1961), with adaptations proposed by Casali et al. (2011) and Schmidt and Thannhauser (1945). Phosphorus fractions obtained were total P soluble in acid (P-Totalsol), inorganic P soluble in acid (P-Inor), organic P soluble in acid (P-Org—obtained by the difference between P-Totalsol and P-Inor), lipid P (P-Lipid), P associated with RNA (P-RNA), P associated with DNA (P-DNA), and residual P fraction (P-residual).
Statistical analyses
For the analysis of variance (ANOVA) of soil and grass species characterization, mass production and P parameters, the following model was used:
where µ is the overall experimental average; B is the blocks (i = 1, 2, 3, 4); F is the fertilizing treatments (j = 1, 2); S is the grass species (k = 1, 2, 3); and error is the experimental error. When treatment effects were significant at 5% probability of error by F test, the differences between the means of fertilized treatments and grass species were compared by Tukey’s honestly significant difference (HSD) (P < 0.05).
For the analysis of variance (ANOVA) of P chemical fractions, the grass species were compared within the treatments (P− and P+) by the following model:
where µ is the overall experimental average; B is the blocks (i = 1, 2, 3, 4); S is the grass species (j = 1, 2, 3) and error is the experimental error. When treatment effects were significant at the 5% probability level by the F test, the differences between means of fertilized treatments and grass species were compared by Tukey’s honestly significant difference (HSD) (P < 0.05).
A multivariate analysis was performed to explore the relations between P chemical fractions and other parameters of plants and soil. The variables were chosen for their correlations between them and were submitted to principal coordinates ordination analyses (PCoA), with the data previously processed by the amplitude and Euclidean distance as a similarity measure, using the software MULTIV (Pillar 2001). The decision to choose PCoA was made because our focus was on the grass species (sampling units) growing in different treatments, and not on the variables (P fractions).
Results
Soil phosphorus availability
Regardless of the grass species tested, the soil P availability extracted by Mehlich-1 was 16 times higher on addition of 50 mg P kg−1 of soil (P+) when compared with the control (P−) (Table 1). In the P+ treatment, the soil showed a higher availability of P (28.2 mg kg−1) when grown with A. lateralis plants as compared to the two other grass species (22.5 mg kg−1). However, for all three grasses, soil P availability was higher than 21 mg kg−1, which represents the critical level of P recommended by the South Brazilian Soil Fertility and Chemical Committee (CQFS-RS/SC 2016). Moreover, P availability (1.5 mg kg−1) in P− soil was considered to be very low (CQFS-RS/SC 2016).
Plant functional traits and growth
Specific leaf area (SLA) and leaf dry matter content (LDMC) were not affected by changes in soil P availability (Table 1). However, there were significant differences among species, in which P. notatum plants showed higher SLA (23 m2 kg−1) than A. lateralis (19 m2 kg−1), but both species had lower values when compared with A. affinis (33 m2 kg−1). Conversely, the LDMC followed in the opposite order of SLA, in which these species showed the following decreasing order: A. lateralis (311 g kg−1), P. notatum (273 g kg−1), A. affinis (209 g kg−1).
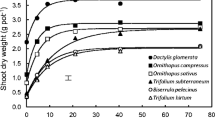
Plant total dry mass was 15, 48, and 75% higher in the P+ treatment for A. affinis, P. notatum, and A. lateralis, respectively, when compared with the P− treatment (Table 2). Leaf and stem dry masses of A. affinis were 19 and 26% higher in P+, respectively, when compared with the P− plants. Plant total dry mass of P. notatum was 66 and 113% higher than that of A. affinis in both P− and P+ (Table 2). Biomass partitioning to leaves and stems was higher for A. affinis and P. notatum than for A. lateralis (Table 2).
Biomass partitioning to roots was higher for A. lateralis than for A. affinis and P. notatum. Therefore, the root/shoot ratio (R/S) was higher for A. lateralis when compared with the other species either grown in P− or P+ (Table 2). With the exception of A. lateralis, there was no fertilization treatment effect on R/S. The total leaf area (TLA) was 33, 68 and 19% higher in P+ treatment when compared with the P− treatment for A. affinis, P. notatum, and A. lateralis, respectively (Table 2). TLA was similar between A. affinis and P. notatum and lower for A. lateralis.
Phosphorus concentration and acid phosphatase activity in plant
The P concentration in the whole plant (Pplant) was 119 and 59% higher in P+ for A. affinis and P. notatum than for A. lateralis. The P concentration in the leaves (Pleaf) was, respectively, 155% and 100% higher in P+ for A. affinis and P. notatum when compared with the P− treatment (Table 2). However, for A. lateralis, the levels of Pplant (0.5 mg g −1) and Pleaf (0.7 mg g−1) were similar between P+ and P− treatments.
Leaf acid phosphatase activity (APA) decreased 20 and 25% with increasing soil P availability for A. affinis and P. notatum, respectively (Table 2). A. lateralis showed a different pattern of APA response, in which APA was similar between P+ and P−. In low soil P availability, both A. affinis and P. notatum had higher leaf APA as compared to A. lateralis, which had the highest APA under high soil P availability (Table 2). Furthermore, leaf APA was higher in A. affinis than in P. notatum under high P soil availability.
For P use efficiency (PUE), P. notatum presented the highest values in low and high P availability (Table 2). However in P+, the PUE was lower for A. affinis and P. notatum compared to P−. Phosphorus uptake efficiency (PUtE) was higher with increasing soil P availability for A. affinis and P. notatum. Independently of soil P availability, PUtE showed a tendency to be higher for A. affinis as compared to the other species.
Phosphorus allocation index
The P allocation index (PAI) between organs of the three grasses showed a similar response under P− and P+ (Fig. 2). The three grasses showed higher P allocation to leaf and stem tissues (PAI > 1) as compared to the roots (PAI < 1).
Phosphorus chemical fractions in leaves
At low soil P availability (P−), the soluble inorganic P concentration (P-inor) in leaves of A. lateralis and P. notatum was 26% higher as compared to the values found in A. affinis (Table 3). However, the soluble organic P (P-org) concentration was 43 and 138% higher in A. affinis as compared to that of P. notatum and A. lateralis plants. In addition, under low soil P availability, the P-Lipid concentration was 59 and 32% higher in A. lateralis when compared to A. affinis and P. notatum, respectively. The P-DNA concentration and the sum of all P fractions (total phosphorus) were 29 and 16% higher in A. lateralis as compared to that found in the other species. However, under low soil P availability, the P-residual and P-RNA concentrations were similar among all evaluated species.
With high soil P availability (P+), the P-Inor concentration was 26% higher in A. affinis and P. notatum, when compared with that in A. lateralis (Table 3). Although not significant, the P-Org concentration was 75% higher in A. affinis as compared to that in P. notatum and A. lateralis. Independently of soil P availability, the P-Lipid concentration was 62 and 23% higher in A. lateralis than that in P. notatum and A. affinis. In P+ treatment, the P-DNA and total P concentrations were similar between species.
Considering all the evaluated fractions of P in the plant, P-Inor and P-RNA were the most abundant (Table 3). Under low soil P availability (P−), the P-Inor fraction was higher for P. notatum and A. lateralis as compared to A. affinis. Conversely, under high P availability (P+), P-Inor was higher for A. affinis and P. notatum as compared to A. lateralis. For the three grasses, both P-DNA and P-Resid fractions were the least affected by soil P availability. In high soil P availability (P+), P-Lipid represented 14% of total P in tissues for A. lateralis, higher than in A. affinis (8%) and P. notatum (11%).
Ordination analysis
The first two axes of the PCoA explained 69% of the variability when grass species were grown under P− treatment (Fig. 3a). Three P chemical fractions and two P parameters had a strong contribution to the X axis with the absolute correlation coefficients being higher than 0.70: P-Inor, P-Org, P-DNA, P uptake (Pup), and APA (Fig. 3a).
Ordination diagram of species Axonopus affinis (triangles), Paspalum notatum (squares), and Andropogon lateralis (circles) growing under low (a) and high (b) soil P availability as a function of acid phosphatase activity (APA), P use efficiency (PUE), P-uptake efficiency (PUtE), P uptake (Pup), leaf P concentration (Pleaf), plant P concentration (Pplant), soil available P by Mehlich-1 (Mehlich I), and leaf P chemical fractions: soluble inorganic (Inor), soluble organic (Org), lipid, RNA, DNA, and residual (Resid)
The ordination analyses of variables showed that when grass species were grown under low soil P availability (P−), the P chemical fractions in leaves such as P-Inor, P-Lipid, P-RNA, and P-Resid tend to be higher for A. lateralis, and depended on higher Pplant and Pleaf concentration (Fig. 3a). However, it was inversely correlated with APA in leaves. APA in leaves was positively related in P. notatum and A. affinis. A. affinis also showed a positive correlation with PUtE and P-Org. Conversely, P. notatum showed a positive correlation with P uptake (Pup) and PUE. Thus, APA was the variable related with PUE, PUtE, Pup, and P-Org and inversely related to P-Inor, Pplant, and Pleaf.
The first two axes of the PCoA explained 74% of the variability when the species were grown in P+ treatment (Fig. 3b). The chemical fractions P-Inor and the five P parameters had a strong contribution to the X axis: leaf P concentration (Pleaf), plant P concentration (Pplant), phosphorus uptake efficiency (PUtE), acid phosphatase activity (APA), soil P available (Mehlich-I), whereas P chemical fractions P-DNA and P-RNA and P use efficiency (PUE) had a strong contribution to the Y axis; their absolute correlation coefficients were higher than 0.70 (Fig. 3b).
When ordination analysis was done with variables of plants grown in high P soil availability, the P chemical fractions in leaves (P-Inor, P-Org, P-Resid) were related to A. affinis as well as Pplant and Pleaf concentrations (Fig. 3b). Thus, P-Inor, P-Org, P-Resid were inversely correlated with APA in leaves. On the other hand, the APA in leaves was positively correlated with P-Lipid fraction and, consequently, positively related in A. lateralis. As before, PUtE and P-Org variables were positively related to A. affinis and inversely to APA. PUE was related positively with P. notatum.
Discussion
Biomass production and efficiency of phosphorus uptake and use
Under higher soil P availability, all tested grasses showed an increase in the dry mass of leaves, stems, and roots, and consequently in the total dry mass (Table 2). This increase in plant dry mass may be associated with higher total leaf area (TLA) (Table 2), which has been shown to be related to the increase in CO2 assimilation rate (Fujita et al. 2004). This relationship becomes clear when we analyze the concentration of P in the soil. In the soil cultivated with grass species of higher dry matter production, P concentration was lower (Table 1), indicating a higher absorption rate.
However, the total plant dry matter and TLA response to soil P availability was higher for P. notatum as compared to A. affinis. It is possible to relate the higher P response capacity with leaf traits and physiological tissue construction strategy, which has a lower density per unit of leaf area and highest P use efficiency. This characterizes a fast growth rate species (Machado et al. 2013), and it has been shown that fast growth rate species have a higher capacity of response to nutrient availability (Chapin et al. 1982). A high growth rate is advantageous mainly in periods with higher nutrient availability or in a natural fertile environment that allows a rapid production of leaf and root biomass, which enables plants to absorb water and nutrients.
Under both low and high soil P availability, the total dry matter was approximately two times higher in P. notatum than in A. affinis. This result shows that from the same number of transplanted units (tillers), P. notatum was a grass with higher dry matter accumulation. The reasons for its higher biomass production capacity are not yet clear. P. notatum higher dry matter accumulation can be related to its ability to allocate more than 45% of the biomass in the rhizomes (measured as stems) and 28% in roots (Table 2), and leaves with relatively intermediate dry matter content (Table 1). Moreover, the P allocation index (PAI) for P. notatum shows that stems and roots are not the target for P allocation, but leaves show higher PAI (Fig. 2). In this way, its capacity to have a higher biomass production in stems and roots with low PAI can, consequently, be a result of a higher PUE in P− as well as in P+.
A. affinis had a higher PUtE as compared to P. notatum in high soil P availability, and as compared to A. lateralis under both soil P availabilities (Table 2). As described in other studies, a large difference in P uptake rate among species is not expected (absorption rate per unit mass of root measured under standardized conditions) (Aerts and Chapin 1999). In general, P uptake rate is slightly higher or very similar in fast growing species than in slow growing species (Chapin 1980; Chapin et al. 1982). In addition, this is not the most important adaptive mechanism for low soil P availability, because P diffusion at the root surface might decrease P uptake. Therefore, even a low nutrient uptake capacity is adequate to absorb those nutrients reaching the roots (Aerts and Chapin 1999). These results indicate that there are important root morphological differences among these three tested species.
In one study correlating traits of leaf and root, Fort et al. (2013) showed similar tissue construction strategies between shoot and root biomass. However, according to these authors, for the same leaf trait strategy, drought-tolerant grass species displayed more conservative root strategies than drought-sensitive species. Thus, we suggest that a difference in PUtE between P. notatum, mostly abundant in drier sites, and A. affinis, more frequently found in moist sites, could be attributed to different conservative root strategy traits. P. notatum uses higher amounts of carbon for more root production, and thus it explores more soil volume and reaches P with less P allocation in roots under P− treatment (Fig. 2). On the other hand, A. affinis has lower root biomass (Table 2) and can have higher specific root length and fine root percentage (Fort et al. 2013), and, consequently, is more efficient in capturing P than P. notatum in both treatments (P+ and P−) (Table 2).
Acid phosphatase activity and phosphorus chemical fractions
The acid phosphatase activity (APA) in leaves of native grasses proved to be a parameter inversely correlated with plant and/or leaf P status for all three species (Table 2 and Fig. 3). With this parameter, it was possible to show that when plants were grown under low soil P availability there was a significant increase in APA. This trend was less evident for A. lateralis compared to the other grasses. The lower APA under P deficiency in A. lateralis can be related to its lower P remobilization and this may be related to its higher leaf life span (Machado et al. 2013) and, consequently, its slow growth rate. In addition, it shows a reasonable relationship between slow growth rates and less instantaneous P demand, as well as a lower need for P remobilization (Aerts and Chapin 1999).
The high APA of P. notatum also indicates the hypothesis that Campos native grasses with leaf traits for resources capture (Table 1) probably have higher P remobilization and use than species with conservation strategies. Moreover, we showed that when species were grown under high soil P availability, APA decreased for A. affinis and P. notatum, but not for A. lateralis (Table 2). In the same way, the ordination analyses showed that the APA was related to A. affinis and P. notatum in P− (Fig. 3a) and with A. lateralis in P+ (Fig. 3b) and inversely correlated with the concentration of Pplant and Pleaf (Fig. 3). The explanation for the decrease in APA was related to a feedback regulation effect (McLachlan and March 1982) and confirms that APA is a parameter that can be useful to study differences in plant resource acquisition strategies based on the tissue P concentration (Duff et al. 1994).
Phosphorus chemical functions and relationship with the growth rate
With the increase of soil P availability, the concentration of all chemical P fractions in the plant tissues increased in a similar way regardless of the tested species, except P-Inor for A. affinis that increased 46% more than for the other tested species (Table 3). The increase in concentration of all major P fractions with increasing P supply has also been documented in other studies. For example, Bieleski (1973) and Chapin et al. (1982) showed that P ester is less responsive to alteration of the P supply and P-Inor is the most responsive. P-Inor is the major form in which P is stored in non-meristematic cells of higher plants (Bieleski 1973).
The higher level of P-Inor, P-Lipid, and P-DNA from A. lateralis compared to other grasses (Table 3), when grown under low soil P availability, can be explained by the higher total P concentration in leaves (Pleaf) in A. lateralis. For a given nutrient supply, slow growing species of typical low nutrient availability habitats have greater tissue concentration of nutrients than rapidly growing species (Clarkson 1967; White 1972).
The data of P chemical fractionation in leaves of native species grown under both low and high soil P availability showed higher P-Org concentration in leaves of A. affinis compared to A. lateralis (Table 3). A. lateralis tends to accumulate P bound to lipids, or even linked to a structural form such as the membranes. Chisholm and Blair (1988) have emphasized and showed that P remobilization of phospholipids is possible when reserves of other P fractions are sufficiently reduced. However, the results of Adugyamfi et al. (1990) do not support the phospholipid remobilization hypothesis, because the P-Lipid content in pigeon pea (Cajanus cajan (L) Millsp.; species tolerant to low P supply) and soybean (Glycine max (L) Merr cv. Tamahomare; less tolerant species to low P supply) showed a similar reduction of 65% when compared with low and intermediate P supply.
The lower P-Lipid concentration in A. affinis compared to other species under low soil P availability can indicate higher substitution of phospholipids in membranes by sulfolipids and/or galactolipids in response to P deficiency (Veneklaas et al. 2012). In southwestern Australia, species belonging to Proteaceae which are adapted to P-impoverished soils clearly show that a low investment in phospholipids, relative to non-phospholipids, offers a partial explanation for a high photosynthetic rate per unit leaf P (Lambers et al. 2012).
The higher P-Org concentration in leaves of A. affinis compared to the other grasses may be related with a higher APA (Fig. 3a). Thus, A. affinis’ strategy to maintain growth could be due to its capacity to remobilize the soluble organic P fractions, increasing P use efficiency (Table 2) which allows this grass to grow also in sites with low soil P availability.
Conclusions
This work demonstrates that natural grasslands have species of grasses that show different leaf and physiological traits in response to P availability. High SLA and low LDMC were associated with higher growth rate, generating a greater total leaf area. Higher capacity of remobilization of soluble organic fractions of P and higher uptake efficiency, especially for the species with the highest growth rate, allowed higher P use efficiency under low P availability in soil, especially for the species with higher growth rates, A. affinis and P. notatum. The fractions P-Inor and P-RNA were higher under high availability of P in the soil, and high levels of these forms were associated negatively to phosphatase activity. APA was also related to the highest growth rates, independent of soil P availability. The high APA in grass species with higher growth rate is related to higher remobilization of P due to higher demand, as in A. affinis. In addition, higher growth rates for these species were associated with higher P uptake efficiency.
References
Adugyamfi JJ, Fujita K, Ogata S (1990) Phosphorus fractions in relation to growth in pigeon pea [Cajanus cajan (L) Millsp.] at various levels of P supply. Soil Sci Plant Nutr 36:531–543. https://doi.org/10.1080/00380768.1990.10416790
Aerts R, Chapin FS III (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67. https://doi.org/10.1016/S0065-2504(08)60016-1
Allen VG, Batello C, Berretta EJ et al (2011) An international terminology for grazing lands and grazing animals. Grass Forage Sci 66:2–28. https://doi.org/10.1111/j.1365-2494.2010.00780.x
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252. https://doi.org/10.1146/annurev.pp.24.060173.001301
Casali CA, Kaminski J, Arbugeri FE et al (2011) Mineralização das formas de fósforo do tecido de plantas de cobertura. Informações Agronômicas 135:21–24
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260. https://doi.org/10.1146/annurev.es.11.110180.001313
Chapin FS III, Follett JM, O’Connor KF (1982) Growth, phosphate absorption, and phosphorus chemical fractions in two Chionochloa species. J Ecol 70:305–321. https://doi.org/10.2307/2259881
Chisholm RH, Blair GJ (1988) Phosphorus efficiency in pasture species. II. Differences in the utilization of P between major chemical fractions. Aust J Agric Res 39:817–826
Clarkson DT (1967) Phosphorus supply and growth rate in species of Agrostis L. J Ecol 55:111–118. https://doi.org/10.2307/2257720
Cornelissen JHC, Lavorel S, Garnier E et al (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. https://doi.org/10.1071/BT02124
CQFS-RS/SC (2016) Manual de calagem e adubação para os estados do Rio Grande do Sul e Santa Catarina. Sociedade Brasileira de Ciência do Solo, Porto Alegre
Dale MP, Causton DR (1992) The ecophysiology of Veronica chamaedrys, V. montana and V. officinalis. IV. Effects of shading on nutrient allocations—a field experiment. J Ecol 80:517–526. https://doi.org/10.2307/2260695
de Carvalho PC F, Batello C (2009) Access to land, livestock production and ecosystem conservation in the Brazilian Campos biome: the natural grasslands dilemma. Livest Sci 120:158–162. https://doi.org/10.1016/j.livsci.2008.04.012
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant, Cell Environ 30:1557–1565. https://doi.org/10.1111/j.1365-3040.2007.01733.x
Doerner P (2008) Phosphate starvation signaling: a threesome controls systemic Pi homeostasis. Curr Opin Plant Biol 11:536–540. https://doi.org/10.1016/j.pbi.2008.05.006
Duff SMG, Sarath G, Plaxton WC (1994) The role of acid phosphatases in plant phosphorus metabolism. Physiol Plant 90:791–800. https://doi.org/10.1111/j.1399-3054.1994.tb02539.x
Elliott GC, Läuchli A (1986) Evaluation of an acid phosphatase assay for detection of phosphorus deficiency in leaves of maize (Zea mays L.). J Plant Nutr 9:1469–1477. https://doi.org/10.1080/01904168609363542
Fitter AH, Setters NL (1988) Vegetative and reproductive allocation of phosphorus and potassium in relation to biomass in six species of viola. J Ecol 76:617–636. https://doi.org/10.2307/2260563
Fort F, Jouany C, Cruz P (2013) Root and leaf functional trait relations in Poaceae species: implications of differing resource-acquisition strategies. J Plant Ecol 6:211–219. https://doi.org/10.1093/jpe/rts034
Fujita K, Kai Y, Takayanagi M et al (2004) Genotypic variability of pigeon pea in distribution of photosynthetic carbon at low phosphorus level. Plant Sci 166:641–649. https://doi.org/10.1016/j.plantsci.2003.10.032
Garnier E, Shipley B, Roumet C, Laurent G (2001) A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct Ecol 15:688–695. https://doi.org/10.1046/j.0269-8463.2001.00563.x
Garnier E, Lavorel S, Ansquer P et al (2007) Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 European sites. Ann Bot 99:967–985. https://doi.org/10.1093/aob/mcl215
Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322:49–86. https://doi.org/10.1007/s11104-009-0068-0
Lambers H, Shane MW, Cramer MD et al (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Lambers H, Bishop JG, Hopper SD et al (2012) Phosphorus-mobilization ecosystem engineering: the roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann Bot 110:329–348. https://doi.org/10.1093/aob/mcs130
Machado JM, Rocha MG, Quadros FLF et al (2013) Morphogenesis of native grasses of Pampa biome under nitrogen fertilization. Rev Bras Zootec 42:22–29. https://doi.org/10.1590/S1516-35982013000100004
McLachlan K, March D (1982) Acid phosphatase activity of intact roots and phosphorus nutrition in plants. III. Its relation to phosphorus garnering by wheat and a comparison with leaf activity as a measure of phosphorus status. Aust J Agric Res 33:1–11. https://doi.org/10.1071/AR9820001
Miyachi S, Tamiya H (1961) Distribution and turnover of phosphate compounds in growing Chlorella cells. Plant Cell Physiol 2:405–414
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Oliveira LB, Tiecher T, Quadros FLF et al (2014) Formas de fósforo no solo sob pastagens naturais submetidas à adição de fosfatos. Rev Bras Cienc do Solo 38:867–878. https://doi.org/10.1590/S0100-06832014000300018
Pillar VD (2001) Multivariate exploratory analysis, randomization testing and bootstrap resampling. User’s Guid. 1–41
Schmidt G, Thannhauser SJ (1945) A method for the determination of deoxyribonucleic acid, ribonucleic acid and phosphoproteins in animal tissues. J Biol Chem 161:83–89
Shen J, Lixing Y, Junling Z et al (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005. https://doi.org/10.1104/pp.111.175232
Siddiqi MY, Glass ADM (2008) Utilization index: a modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J Plant Nutr 4:289–302. https://doi.org/10.1080/01904168109362919
Swiader JM, Chyan Y, Freiji FG (1994) Genotypic differences in nitrate uptake and utilization efficiency in pumpkin hybrids. J Plant Nutr 17:1687–1699. https://doi.org/10.1080/01904169409364840
Tabaldi LA, Ruppenthal R, Cargnelutti D et al (2007) Effects of metal elements on acid phosphatase activity in cucumber (Cucumis sativus L.) seedlings. Environ Exp Bot 59:43–48. https://doi.org/10.1016/j.envexpbot.2005.10.009
Tedesco MJ, Gianello C, Bissani CA et al (1995) Análise de solo, plantas e outros materiais, 2nd edn. Departamento de Solos da Universidade Federal do Rio Grande do Sul, Porto Alegre
Tiecher T, Oliveira LB, Rheinheimer DS et al (2014) Phosphorus application and liming effects on forage production, floristic composition and soil chemical properties in the Campos biome, southern Brazil. Grass Forage Sci 69:567–579. https://doi.org/10.1111/gfs.12079
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Veneklaas EJ, Lambers H, Bragg J et al (2012) Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol 195:306–320. https://doi.org/10.1111/j.1469-8137.2012.04190.x
Wang X, Shen J, Liao H (2010) Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci 179:302–306. https://doi.org/10.1016/j.plantsci.2010.06.007
White RE (1972) Studies on mineral ion absorption by plants. Plant Soil 36:427–447. https://doi.org/10.1007/BF01373496
Wieneke J (1990) Phosphorus efficiency and phosphorus remobilization in two sorghum [Sorghum bicolor (L.) Moench] cultivars. Plant Soil 123:139–145. https://doi.org/10.1007/BF00011259
Wright IJ, Westoby M (2003) Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Funct Ecol 17:10–19. https://doi.org/10.1046/j.1365-2435.2003.00694.x
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Acknowledgements
We thank Dr. Pablo Cruz (INRA, France), Dr. Tales Tiecher (UFRGS, Brazil), and Giselle Lee (Arizona State University–EUA) for the review with helpful suggestions for improving the manuscript.
Author information
Authors and Affiliations
Contributions
LBO, ACRM, RP, and JGF performed the experiment, laboratory analyses, and statistical analyses. FLFQ, FTN, and GB organized the experiment and helped to write the manuscript.
Corresponding author
Additional information
Communicated by Russell K. Monson.
Rights and permissions
About this article
Cite this article
de Oliveira, L.B., Marques, A.C.R., de Quadros, F.L.F. et al. Phosphorus allocation and phosphatase activity in grasses with different growth rates. Oecologia 186, 633–643 (2018). https://doi.org/10.1007/s00442-018-4059-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4059-9