Abstract
The invasion by winter-annual grasses (AGs) such as Bromus tectorum into sagebrush steppe throughout the western USA is a classic example of a biological invasion with multiple, interacting climate, soil and biotic factors driving the invasion, although few studies have examined all components together. Across a 6000-km2 area of the northern Great Basin, we conducted a field assessment of 100 climate, soil, and biotic (functional group abundances, diversity) factors at each of 90 sites that spanned an invasion gradient ranging from 0 to 100 % AG cover. We first determined which biotic and abiotic factors had the strongest correlative relationships with AGs and each resident functional group. We then used regression and structural equation modeling to explore how multiple ecological factors interact to influence AG abundance. Among biotic interactions, we observed negative relationships between AGs and biodiversity, perennial grass cover, resident species richness, biological soil crust cover and shrub density, whereas perennial and annual forb cover, tree cover and soil microbial biomass had no direct linkage to AG. Among abiotic factors, AG cover was strongly related to climate (increasing cover with increasing temperature and aridity), but had weak relationships with soil factors. Our structural equation model showed negative effects of perennial grasses and biodiversity on AG cover while integrating the negative effects of warmer climate and positive influence of belowground processes on resident functional groups. Our findings illustrate the relative importance of biotic interactions and climate on invasive abundance, while soil properties appear to have stronger relationships with resident biota than with invasives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding plant community assembly and species distributions are desired, fundamental goals of community ecology. The abundance and distribution of individual species are constrained by a set of unique abiotic filters and biotic interactions that have developed over evolutionary time scales (Götzenberger et al. 2012). Traditionally, climate and soil factors are assumed to govern the distribution of species at macroscales (>10,000 km2), while biotic interactions are more important at finer scales (<100 m2) (Pearson and Dawson 2003). However, biotic interactions may also affect community assemblages and shape species distribution across larger spatial scales, and those biotic interactions may influence species-climate and species-soil relationships (Duffield 1956; Araújo and Rozenfeld 2013; Wisz et al. 2013; Jeffers et al. 2015). Realistically, there are suites of biotic, climate and soil factors that simultaneously influence species’ distributions and abundances through a chain of complex interactions across spatial and temporal scales. Major environmental shifts, such as climate change and biological invasions, have disrupted long-standing abiotic filters and biotic interactions, altering the abundances of natural populations, species and entire plant communities (McLane and Aitken 2011; Pyšek et al. 2012).
When biological invasions occur, new biotic agents are introduced into already complex systems of abiotic and biotic interactions. Invasive species have not evolved under the same abiotic and biotic conditions as those present in their new environment, although numerous examples have demonstrated a considerable ability of invasive plants to rapidly acclimate and adapt to novel conditions (Sakai et al. 2001). Invasive species abundances have repeatedly been linked to climate conditions (Benowicz et al. 2001; Bradford and Lauenroth 2006), soil factors (Peeler and DeBell 1987; Blank et al. 2007; Vasquez et al. 2008) and biotic interaction (e.g., competition, predation) (Mangla et al. 2011; Eskelinen and Harrison 2013), indicating that distributions of invasive species are also subject to abiotic and biotic constraints when they colonize new habitats. Knowing which constraints are strongest on invasive and resident species distributions and abundances within an ecosystem will provide critical information for modeling and managing the spread of invasives (Benowicz et al. 2001; McLane and Aitken 2011).
The invasion by winter-annual grasses (AGs) such as Bromus tectorum into the semi-arid sagebrush steppe ecosystem throughout the western USA is one of most ecologically devastating biological invasions in recent human history (Pyšek et al. 2012), and has justifiably received vast amounts of scientific resources to better understand which environmental factors promote or limit the success of AGs (Mack 1996; Chambers et al. 2007; Mangla et al. 2011). The scientific literature identifies numerous biotic interactions (competitive abilities, life history), climate factors (temperature, precipitation) and soil properties (texture, nutrients) that are associated with the distribution and abundance of invasive AGs (Melgoza et al. 1990; Anderson and Inouye 2001; Gelbard and Belnap 2003; Blank et al. 2007; Chambers et al. 2007; Maestre et al. 2010; Rau et al. 2011; Bansal et al. 2014a, b). Other factors such as cattle grazing are also associated with the expansion of AG into sagebrush steppe (Bower and Aitken 2006; Reisner et al. 2013; Rodhouse et al. 2014; Jones et al. 2015; Piper et al. 2015). Implementing changes to many of these factors (e.g., grazing) have important implications for social, economic and ecological values (Sheley et al. 2010; McLane and Aitken 2011; Rodhouse et al. 2014).
There is a well-established approach in community ecology to use naturally occurring ecological gradients to model changes in species abundances in response to environmental factors (Whittaker 1967). When addressing the roles of multiple factors, it becomes necessary to assess several intersecting gradients, and then integrate relationships among correlated factors using path analysis or structural equation modeling (SEM) (Grace 2006; Grace et al. 2010). Key requirements for these types of analyses include (1) a sufficiently large environmental space to infer conclusions regarding species-environment relationships, (2) fine-scale measurements conducted over large spatial areas, and (3) appropriate indicator factors that represent each major (relevant) component of a system (Wisz et al. 2013). Relatively few studies have applied this technique in invaded ecosystems (Seabloom et al. 2006; Chambers et al. 2007; Reisner et al. 2013), despite its considerable potential to identify key factors and processes governing the spread of invasive species.
We conducted a field assessment of nearly 100 climate, soil, and biotic (functional group abundances, diversity) factors at each of 90 sites that spanned an invasion gradient from 0 to 100 % AG cover. The large topographic range and dynamic geologic history in the study area (Morrison 1991) provided large gradients in climate and soil properties across the study area, despite the relatively small geographic area sampled (6000-km2) compared to the entire distribution of AGs in the western USA (>100,000 km2) (Menakis et al. 2003). Using this data set, we followed an iterative, exploratory process in which we used (1) correlative relationships and a priori knowledge to infer the relative importance of biotic and abiotic factors, and (2) regression and SEM to explore how multiple abiotic and biotic factors can be integrated to describe above- and belowground processes associated with AG abundance.
Materials and methods
Study system
The study was conducted over a 6000-km2 area across a sagebrush steppe ecosystem in the northern Great Basin, USA (43°24–44°11′N, 117°10–119°43′W). Bromus tectorum (L.) was the dominant AG species in the area. Other AG species found in the study region included Taeniatherum caput-medusae (L.) Nevski and B. japonicus Thunb, and trace amounts of Eremopyrum triticeum (Gaertn.) Nevski. The dominant resident perennial grass species were Pseudoroegneria spicata (Pursh) A. Love, Poa secunda J. Presl and Elymus elymoides (Raf.) Swezey (see Online Resource 1 for histogram of cover distribution of AG and perennial grasses across sites). The primary shrub species were Artemisia tridentata Nutt, ssp. wyomingensis Beetle and Young, Artemisia tridentata Nutt ssp. tridentata, and Artemisia tridentata Nutt ssp. vaseyana (Rydb.) Beetle, Chrysothamnus nauseosus (Pall. ex Pursh) G.L. Nesom & Baird, C. viscidiflorus (Hook.) Nutt., and Sarcobatus vermiculatus (Hook.) Torr.; the only tree species was Juniperus occidentalis Hook. The eastern edge of the study area was located at the lower transition zone of sagebrush steppe into high-elevation desert, while the western edge was located at the upper transition zone of sagebrush steppe into Pinus ponderosa forest, thus capturing a range of sagebrush steppe community types. Across these sagebrush steppe communities, there was a complete gradient of AG abundance from 0 to 100 % AG cover (i.e., completely non-invaded native communities to complete monocultures of AG).
We used ArcGIS 10.1 (ESRI, Redlands, CA) to randomly generate the locations of 90 sites within the study area (Fig. 1). The elevation, mean annual temperature and mean annual precipitation ranged from 700 to 1600 m, 10.9–6.3 °C, and 212–415 mm, respectively. The climate of the area is characterized by warm/dry summers and cool/snowy winters. Sites were constrained to publicly accessible land (Bureau of Land Management) and within 500 m of an undeveloped road to facilitate access. Each site was ~30 × 30 m in size with similar vegetation structure and topography across the site.
Sampling approach
We selected several abiotic and biotic factors to sample under natural, field conditions that represent each of ten general categories associated with biotic and abiotic factors including the plant community, ground layer, plant traits, reproductive output, climate, soil chemical, physical and biotic properties, and grazing (see Online Resource 1 for means and SDs of each abiotic and biotic factor). Our goal was to capture as much variation as possible in each of the measured factors. The majority of our measured factors could be (and have been) examined in much greater detail, but the primary scientific objective of this study was to broadly identify which factors were most associated with each of the functional groups’ abundances. Factors were sampled over the course of 1 calendar year (September 2011–September 2012). Many of the measured factors are seasonally dynamic, and therefore we selected sampling times for each factor that corresponded with a stage of phenological development that would provide consistent and relevant information regarding each factor’s relationship to AGs.
Plant community
We measured species-level abundances of all herbaceous vegetation at each site in June/July 2012, which corresponded with the maximum biomass phase during phenological development for the majority of herbaceous species. At each site, herbaceous vegetation canopy cover (percent), densities (individuals per square meter) and heights (centimeter) were measured by species on 15 randomly located 0.20-m2 quadrats (40 × 50-cm frame). Mean cover, density and height values for each functional group were calculated from individual species measurements. On at least three randomly chosen quadrats per site, we collected aboveground biomass of herbaceous vegetation and litter to develop regression equations using cover and height data to estimate site-level biomass for each functional group (see Online Resource 2 for equations). Shrub height and diameter were estimated using 15 representative shrubs per species per site. Shrub heights and diameters were used to determine shrub volume and canopy cover per individual. Shrub densities were determined by counting total number of shrubs by species within the study site. Site-level shrub cover was estimated by multiplying the average shrub cover per individual by shrub density. Tree crown cover was estimated using a modified angle gauge method. Species richness (total number of different species found across all 15 quadrats) was separated into resident and invasive groups. Biodiversity was calculated as the total number of species (per square meter) divided by the number of individuals (per square meter).
Ground layer
Litter cover and height (millimeters), moss and crust lichen cover (collectively biological soil crusts), woody debris (>1-cm diameter), and exposed rock cover (percent) were measured within each quadrat in which plant community variables were measured.
Plant traits and reproductive output
We used specific leaf area (SLA) as an indicator of plant relative growth rates for annual and perennial species (Garnier 1992). At each site, we collected fresh leaf tissue from 20 individuals of each of the annual and perennial grass, shrub and tree species in late spring 2013, which corresponded with a period of rapid growth. Plants were selected arbitrarily by tossing a pin on the ground and collecting the closest individual to the pin. Fresh leaves were laid flat and photographed with a scale to determine leaf area (square centimeter) using image processing software (Image J; National Institutes of Health, Bethesda, MD). The leaves were then dried at 60 °C and weighed (±0.1 mg). Values of SLA were calculated as fresh leaf area divided by dry leaf mass.
In September 2012, after seeds were fully developed on the plants, we collected seeds from ten individuals of the most abundant AG species at each site. Plants for seed collection were selected arbitrarily by tossing a pin on the ground and collecting from the closest individual to the pin. Seeds were counted and weighed to determine mean values for the number of seeds per individual and seed mass (milligrams) at each site. The average number of seeds per individual was multiplied by AG density to determine AG seeds per square meter.
Climate factors
We recorded soil temperature (5-cm depth) every 4 h at each site using temperature probes (iButton Thermochron; Maxim Integrated Products, TX). Two probes were buried at each site in winter 2011 and extracted in summer 2012. Daily minimum, mean and maximum temperatures were used to calculate mean values for each site during the measurement period. We additionally acquired historical average temperatures and precipitation data for each site using ClimateWNA (Wang et al. 2012). Other factors measured at each site included slope, aspect, elevation, latitude and longitude. Slopes were converted to radians and aspects were folded (so higher values indicated more southerly slopes) and were used to calculate heat load and solar insolation (McCune and Keon 2002).
Soil factors
Soil physical and chemical properties were determined by collecting five randomly placed soil cores (10-cm diameter, 0- to 10-cm depth) in late spring (April/May 2012), during a period of rapid growth for herbaceous species. The soil cores were bulked, mixed and weighed to determine soil bulk density (grams per cubic meter). Fresh soils were sieved (2 mm) and remaining roots and rocks were separated, dried (roots only) and weighed to determine the relative fraction of roots and rocks in the soils at each site (grams per cubic meter). Ammonium and nitrate (micrograms per gram) were extracted from fresh soils using a 2N potassium chloride solution; concentrations of the soil extract solutions were determined spectrophotometrically (UVmini-1240; Shimadzu, Colombia, MD) (Miranda et al. 2001). Phosphate was measured on air-dried soils using the Bray method (Bray and Kurtz 1945). Soil moisture content was determined gravimetrically by weighing fresh and dried soils (110 °C for 48 h). Field gravimetric water content (GWC) was calculated as (fresh weight–dry weight)/dry weight. The dried soils were then used to determine soil texture using the hydrometer method (Gee and Bauder 1986). The pH of the soils was measured in a 1:2 soil:distilled water solution using a pH probe after 60 min of shaking (pHI 295; Beckman Coulter, Pasadena, CA). Total C and N, and soil organic matter were determined using dry combustion analysis (LECO CN 1000; LECO, St. Joseph, MI). Water holding capacity (WHC) was determined by hydrating a subsample of dry soil (10 g) via capillary action [funnel method (Cheng 2009)]; WHC was calculated as (wet weight–dry weight)/dry weight.
Microbial biomass
Soil microbial biomass was estimated using the substrate-induced respiration (SIR) method (Anderson and Domsch 1978). SIR was not measured on 12 sites due to relatively high pH (>7.0). A 6-g sample of fresh sieved soil was separated into two 3-g subsamples, placed into 125-ml Erlenmeyer flasks, and adjusted to 120 % WHC. One subsample was amended with distilled water for measurements of basal respiration and the other received a glucose solution (20 mg ml−1). The flasks were then sealed with a rubber septum and kept at 22 °C. Concentrations of CO2 in the flask headspace were measured 1 and 4 h after sealing using an infrared gas analyzer (modified LI-COR 6400; LI-COR, Lincoln, NE). The increase in CO2 in the headspace was used to calculate basal respiration and SIR (micrograms carbon dioxide per gram soil per hour). The relative fraction of soil C as microbial biomass was estimated from SIR per milligram soil C. The respiratory quotient was calculated as the ratio of basal respiration to SIR.
Grazing
Current grazing utilization was determined using cattle exclosures. Cattle exclosures were placed in the field over representative vegetation at each site. Percent utilization was calculated as the average difference in heights between forage species inside and outside the exclosures during vegetation measurements. Bureau of Land Management records were used to determine animal unit months (AUM) designated for each site.
Data analyses
We performed correlation, multiple regression and SEM analyses to infer the functional relationships among abiotic and biotic factors associated with AG cover. For the correlations, Pearson correlation coefficients between factors were determined using mean values for each factor for each site (R version 3.0.1; R Core Development Team, Vienna). To account for multiple comparisons, we used the Bonferroni correction and reported significant relationships at α = 0.0005, although our interpretations were primarily based on coefficients (r-values) and a priori knowledge. Due to non-independence of related factors (e.g., perennial grass cover, density, and biomass), we reported the factor that had the highest r-value. For example, AG cover correlated with perennial grass cover (r = −0.63), density (r = −0.57) and biomass (r = −0.55), therefore we only report perennial grass cover in Table 1.
For the multiple regression analyses to predict AG cover, AG cover was constrained between 0 and 100 % and modeled using generalized linear models with a β error distribution and a logit link function (betareg function in the betareg R package) (Grün et al. 2012). Separate models with AG cover as the response variable were developed for each general category (plant community, ground layer, plant traits, reproductive output, climate, soil chemical, physical and biotic properties and grazing) using the measured factors within each category (e.g., perennial grass cover, biodiversity, shrub density, etc.) as predictor variables; interactions among factors or second-order terms were not included in the models. Regression models were selected based on corrected Akaike information criterion (AICc) score (Burnham and Anderson 2002). For each category-specific model, all possible combinations of explanatory factors within each category were evaluated and ranked in order of AICc scores to determine the most parsimonious model with the lowest AICc score (dredge function in MuMIn R package) (Bartoń 2013). If the most parsimonious model contained multiple factors, then the relative explanatory power of each factor was assessed by dropping factors one at a time and observing the increase in model AICc (i.e., calculating the ΔAICc values of the simplified models), with larger increases in AICc indicating greater explanatory power of the removed factor. If removal of a factor resulted in an increase in AICc by 10, 2, or <2, then the factor was considered to have relatively high, moderate, or low, respectively, explanatory power. After separate models for each category were developed, then an additional multiple regression analysis across all categories was conducted to predict AG cover using those factors that were included in each of the final, category-specific models.
We used SEM (AMOS version 19; IBM, Chicago, IL) to integrate aboveground and belowground factors to explore possible direct and indirect influences of climate, soil and biotic factors on AG cover. Given the large number of measured factors in this study, we developed one (of potentially many) plausible model in which the selection of factors and their relationships was based on results from our correlation/multiple regression analyses and strongly supported by the scientific literature. Our correlations and regressions suggested the overwhelming influence of biotic interactions on AG cover compared to other factors, and therefore we had direct paths from perennial grass cover and resident species richness to AG cover, both of which have been experimentally shown to negatively impact AGs in the study area (James et al. 2008; Sheley and James 2010; Davies et al. 2014). Climate variables (temperature, precipitation) were related to both AGs and resident functional groups, but had relatively strong relationships (based on r-values) with resident groups, which are well known to be affected by climate (Anderson and Inouye 2001). Based on the strong climate relationships to resident functional groups, we included direct paths from climate factors to resident species richness, perennial grasses cover and perennial forbs cover. The direct relationship of climate to resident functional groups, combined with the direct path from resident groups to AGs, created indirect paths from climate to AGs. The belowground factors we used were based on many studies that show strong linkages between native vegetation to soil properties in semi-arid ecosystems. Specifically, studies have shown that perennial vegetation allocates up to eight times more biomass to roots compared to annuals (Monaco et al. 2003), thus sites with greater perennial grass cover were expected to have greater total amount of root biomass in soils, which in turn increases soil WHC, soil C and N (Pregitzer et al. 1995; Norton et al. 2004; Rasse et al. 2005). The increases in soil C and N are known to increase microbial biomass and activity in the rhizosphere (Smith and Paul 1990; Cheng 2009), which correspondingly increase the long-term transformation and storage of mineral N in soils (Williams and Sparling 1988; Wardle 1992). A relatively stable supply of mineral N produced through natural belowground processes (as opposed to fertilization or deposition) generally favors native perennial forb vegetation over invasive annuals (McLendon and Redente 1992; Bansal et al. 2014b). Therefore, we suggest that an increase in perennial grass cover can mechanistically improve soil conditions to favor perennial forbs through belowground linkages. Given that perennial forbs was the functional group with the greatest species diversity in our study, an increase in perennial forbs contributed most to increased resident species richness. Thus, relationships between perennial grasses, soil properties and resident species richness create an indirect link between perennial grass cover and AG cover via belowground factors. Cover values for each functional group could also be influenced by their respective relative growth rates, which were indicated by SLA values. Indirect paths coefficients were calculated by taking the products of standardized coefficients of the direct paths (Grace et al. 2010). Model fit was determined using the χ2 goodness-of-fit statistic; maximum likelihood was used for parameter estimations. For SEM, a non-significant P-value indicates that the hypothesized model does not diverge significantly from the observed data. All factors in the model were manifest (directly measured or observed) variables and all relationships were linear with homoscedastic error variance.
For all statistical analyses, site was the unit of replication, cover data (percent) were arcsine square root transformed, and density data and number of seeds (per square meter) were log transformed. Seventy-five of 90 sites were dominated by B. tectorum or a mix of B. tectorum with other AG species; 29 of 90 sites had recorded disturbance history (e.g., fire) in the previous 80 years. An initial analysis (not shown here) revealed that relationships among factors generally did not change among sites dominated by B. tectorum compared with other AG species or mixtures of species, or when disturbed sites were included or excluded from the models.
Results
Annual grasses
AG had many strong relationships with biotic factors in the plant community and functional group categories (Table 1; Fig. 2). Biodiversity (Fig. 2a) and resident species richness (Fig. 2d) decreased, and invasive species richness increased with increasing AG cover. AG cover decreased with increasing perennial grass cover (Fig. 2b), shrub density (Fig. 2e) and biological soil crust cover (Fig. 2f). AG cover also had many strong relationships with climate variables (Table 1; Fig. 3). AG cover increased with increasing minimum soil temperatures, mean warm month temperature and aridity (annual heat-to-moisture index), and decreased with increasing elevation, precipitation as snow and mean annual precipitation. There were no significant relationships between AG cover and soil factors (Table 1; Fig. 3). AG cover had relatively strong relationships with the ground layer and reproductive output. Litter cover, litter height and AG seeds (per square meter) increased with increasing AG cover. There was a decrease in AG cover with increasing grazing utilization.
Relationships between annual grass cover (%) and a, d plant community traits and b, c, e, f individual functional groups along an invasion gradient in sagebrush steppe. Each point represents the mean values for a site. Solid lines indicate significant relationships (P < 0.0005). Symbols represent different dominant annual grass species or mixtures of species including Bromus tectorum (BRTE), Bromus japonicas (BRJA) and Taeniatherum caput-medusae (TACA). y-Axes are backtransformed values from arcsine square root transformation of percentage data
Positive (black, solid lines) and negative (gray, dashed lines) correlative relationships (P < 0.0005) between abiotic and biotic factors observed and a annual grass (AG) and b perennial grass cover across a sagebrush steppe landscape. Thicker lines correspond with higher r-values following Table 1. PG perennial grass, BSC biological soil crust, Precip. precipitation, T temperature, MCMT mean cold month temperature, SIR substrate-induced respiration, SLA specific leaf area, C carbon, GWC gravimetric water content, N nitrogen, MAP mean annual precipitation
In the multiple regression analyses for each category separately, the factors with the highest predictive power generally matched those with the highest correlation coefficients (Table 2). Models generated using factors from the plant community, climate, ground layer and functional group categories had relatively high predictive power (i.e., lowest AICc values), while models using factors from the plant traits, reproductive output, soil physical, soil biotic, and soil chemical categories had weaker predictive power. The best-fit, all-category model explained a large fraction of the variance in AG cover, with a pseudo-R 2 of 0.89.
Perennial grasses
Perennial grasses had relatively strong negative correlations with AG cover and AG seeds (per square meter) and positive correlations with biodiversity and resident richness (Table 1; Fig. 3). Perennial grass cover increased with increasing elevation and precipitation as snow, and decreased with increasing mean soil temperature and aridity. Compared to AGs, perennial grasses had stronger relationships with soil variables (Table 1; Fig. 3). Root mass in soils, soil C, soil N and GWC increased with increasing perennial grass cover.
Annual and perennial forbs
Annual forbs had no significant relationships with any measured factors (Table 1; Online Resource 3). Perennial forb cover had positive relationships with resident species richness (Table 1; Online Resource 3). Perennial forb cover increased with increasing elevation, precipitation as snow and mean annual precipitation, and decreased with increasing aridity, extreme maximum temperature and mean soil temperature. Perennial forbs also had relatively strong positive relationships with soil chemical and physical properties, including soil N, soil C, GWC, WHC and mineral N.
Shrubs and trees
Shrub cover had relatively weak relationships with climate, soil or biotic factors (Table 3; Online Resource 3). Coarse woody debris, microbial biomass, and mineral N increased with increasing shrub cover. Tree cover had positive relationships with biological soil crust cover, maximum soil temperature, AG seeds (per square meter), litter biomass and rock cover, and negative relationships with perennial forb height, AG seeds (per individial) and GWC (Table 3; Online Resource 3).
Microbial biomass and biological soil crusts
Microbial biomass had relatively strong positive relationships to soil properties including soil N, soil C, WHC, mineral N and clay content (Table 3; Online Resource 3). Microbial biomass increased with increasing shrub cover, and decreased with increasing maximum soil temperature and aridity. Biological soil crust cover decreased with increasing litter cover, litter height and AG biomass, and increased with increasing tree cover (Table 3; Online Resource 3).
Plant community
Resident species richness had its strongest relationships with climate factors (Table 4; Online Resource 3). Resident richness increased with increasing elevation and precipitation and decreased with aridity and warm temperature climate variables. Resident species richness also had relatively strong relationships with soil properties. Resident richness increased with increasing GWC, soil C, WHC and soil N. Values of pH, SIR per gram soil C, respiratory quotient and basal respiration decreased with increasing resident richness. Invasive species richness had weaker relationships with climate variables compared to resident species richness (Table 4; Online Resource 3). Invasive richness decreased with increasing elevation and increased with increasing latitude and longitude.
Structural equation model
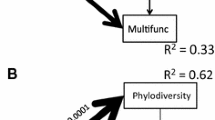
Our SEM model linking aboveground and belowground factors showed a close fit with our observed data (χ 2 = 26.65, P = 0.23; df = 22), and explained 51 % of the variance in AG cover, 63 % of resident species richness, 31 % of perennial grass cover, 29 % of perennial forb cover, and 36 % of microbial biomass (Fig. 4). When temperature and precipitation were both used as exogenous climate factors, estimates between precipitation and perennial grass cover or resident species richness were not significant, and therefore precipitation was not included in the final model. Among the measured and derived estimates of temperature that we tested, the mean warm month temperatures (from ClimateWNA) explained the most variance in perennial grass cover and resident species richness. Mean warm month temperatures had direct negative effects on perennial grass cover and resident richness, leading to an indirect positive effect of temperature on AG cover (standardized indirect coefficient = 0.45). Adding SLA of perennial grasses into the model increased the R 2 of perennial grass from 0.17 to 0.31. The standardized estimate between AG and perennial grass was more than double the estimate between AG and resident species richness (Fig. 4). The influence of perennial grasses on AG via belowground processes was relatively low (standardized indirect coefficient = −0.01), although belowground factors were tightly associated with each other.
Structural equation model integrating climate, vegetation and soil factors in a sagebrush steppe ecosystem invaded by annual grasses. Values adjacent to lines are standardized coefficients for positive (black, solid lines) and negative (gray, dashed lines) relationships. Values of R 2 are squared multiple correlations for each functional group, which represent the amount of variance of each functional group accounted for by the explanatory factors. For abbreviations, see Fig. 3
Discussion
After analyses of nearly 100 factors associated with climate, soils and biota across an AG invasion gradient in sagebrush steppe, we found that the abundance of invasive AGs was most tightly associated with biotic factors and with climate, while soil properties appeared to have more direct relationships with resident biota than with invasives.
There is a general paradigm that species abundances and distributions are affected by biotic interactions at fine scales, but the relative influence of climate and soils increase at larger scales (Pearson and Dawson 2003; Maestre et al. 2010). Our study was conducted over a relatively small geographic area (compared to the entire range that B. tectorum and other AGs are distributed), but still captured relatively large gradients in biotic, climate and soil properties. Our observations across an ecologically diverse (but geographically small) area revealed that strong linkages between AGs and their environment were through negative plant–plant associations, indicating the likely importance of biotic interactions during a biological invasion at local (and possibly larger) scales (Davies 2011; Souza et al. 2011; Rodhouse et al. 2014). Given the correlative nature of our study, we cannot explicitly conclude if native plants were limiting AG cover, or if AG cover was driving a decline in the native plants [i.e., AGs as “passengers” or “drivers” of the plant community (Didham et al. 2005; MacDougall and Turkington 2005)]. A number of manipulative, experimental studies have demonstrated that increasing native species abundances and diversity reduces establishment of invasives in sagebrush steppe (Chambers et al. 2007; e.g., Davies et al. 2010; Sheley and James 2010; Mazzola et al. 2011; Leger et al. 2014; Blank et al. 2015; Liao et al. 2015) and in other ecosystems (reviewed in Levine et al. 2004), suggesting AGs are passengers. Alternatively, the rapid establishment of invasive AGs through priority effects and propagule pressure following disturbances is known to inhibit subsequent growth and establishment of native species (Melgoza et al. 1990; Humphrey and Schupp 2004; Bradford and Lauenroth 2006; Simberloff 2009), suggesting AGs as drivers. Based on these previous studies, it is likely that AGs fall along a continuum between passengers and drivers of plant community composition, mediated by external factors such as climate conditions, soil properties and disturbances that favor (or hinder) the competitive abilities of native species (Melgoza et al. 1990; Belnap and Sherrod 2009; McGlone et al. 2011; Bauer 2012; Alba et al. 2015). Clearly biotic interactions play an key role for both native and invasive species in sagebrush steppe, and disentangling the mechanisms of competition under a range of conditions should be a continued area of research.
Our study not only assessed the overall importance of biotic vs. abiotic factors on AG invasion, but also examined specific factors within each general category. Among biotic interactions, we observed negative relationships between AGs and (in rank order) biodiversity, perennial grass cover, resident species richness, biological soil crust cover and shrub density, whereas annual forb cover, tree cover and soil microbial biomass had no direct linkage to AGs. Studies that examine biotic interactions have established the importance of the perennial grasses over other functional groups in controlling weed populations (Sheley and James 2010; Rodhouse et al. 2014; Stark and Norton 2015). Direct exploitative competition between AGs and perennial grasses for soil moisture, nutrients, light and space has been demonstrated at neighborhood and site scales (Melgoza et al. 1990; Corbin and D’Antonio 2004; Chambers et al. 2007; Vasquez et al. 2008). Our data confirm the relatively strong negative relationship between AGs and perennial grasses compared to other functional groups, and show that the AG-perennial grass relationship is one of the strongest negative associations for AGs compared to any biotic or abiotic factor. Biological soil crusts and shrub densities were also negatively related to AG cover primarily as a function of decreasing variation in AG cover with increasing cover of crusts or shrubs (Fig. 3). This trend of decreasing variation is consistent with biological soil crusts and shrubs playing a primary role in limiting AG cover only when their abundances are relatively high. In contrast, the higher variation in AG cover at lower cover of crusts and shrubs indicates that other factors could be more important in constraining AG cover when biological soil crust or shrub abundances were relatively low.
There are several studies, meta-analyses and reviews that assert climate change will favor the spread of invasive species (Benowicz et al. 2001; Bradford and Lauenroth 2006; McLane and Aitken 2011; Bellard et al. 2013). Indeed, we found that AG abundance was positively related to warmer temperatures, particularly during their establishment phase in winter. Manipulative studies have shown that warm temperatures can increase AG germination, establishment, biomass production and reproductive output when coupled with adequate soil moisture (Bradford and Lauenroth 2006; Chambers et al. 2007; Zelikova et al. 2013; Stark and Norton 2015). At the same time, there were strong, negative relationships between resident functional group cover and temperature, which could have influenced (or been influenced by) AG-temperature relationship, bringing into question cause and effect. Our study did not experimentally confirm causation, but instead described the functional relationship between factors. In reality, the relationships among AGs, resident plants and climate characterize an aboveground set of factors that are tightly integrated. Independent of the causal mechanisms, all direct and indirect relationships indicate that warmer temperatures will co-vary with increased AG abundance and decreased resident diversity and abundance in sagebrush steppe.
The role of the microbial community is often not measured in many ecological studies on biological invasion, despite its fundamental ecological role in belowground processes (Peeler and DeBell 1987). AG invasion has been linked to reduced soil organic C, increased C:N ratios and lower rates of mineralization (Koteen et al. 2011; Rau et al. 2011; but see Stark and Norton 2015) due to the low quality litter of AGs, all of which are associated with decreased microbial activity (Schaeffer et al. 2012). We observed a strong positive relationship between AG abundance and the litter layer, but no direct relationships with microbial biomass. Similarly, there were very weak relationships between AGs and soil nutrients, which have also been mechanistically linked to AG invasion in many manipulative studies (Gundale et al. 2008; James et al. 2008, 2010; Vasquez et al. 2008; Bansal et al. 2014b), albeit not in all (e.g., Bradford and Lauenroth 2006). Some evidence suggests that AG invasion alters microbial community composition and structure (Belnap and Phillips 2001; Piper et al. 2015), rather than affecting total microbial biomass. Even though there were limited direct relationships between AGs and the microbial community or other belowground factors, native functional groups did have many strong and positive relationships to soil factors, which may indirectly affect AG invasion.
We used SEM to describe linkages among aboveground and belowground factors, and how they directly and indirectly influence AG invasion. One of the benefits of using SEM is the ability to explore potential management scenarios. Our data supported a passenger-based model indicating that manipulating perennial grass cover and resident diversity could have a direct negative impact on AGs, although the relative influence of perennial grasses was nearly double that of species diversity (Fig. 4). Land managers are frequently faced with a decision during restoration efforts to either seed with a few dominant species or with a mixture of diverse species. Our model, and other empirical studies, suggests that increasing perennial grass abundance could be more effective at controlling AGs compared to increasing species diversity (Davies et al. 2014). The model also demonstrates how an increase in perennial grass abundance could potentially yield indirect benefits to other native functional groups such as perennial forbs and resident diversity via improved belowground conditions (Fig. 4). Consequently, increasing perennial grass cover coupled with supplementary amendments to belowground soil factors such as organic C and mineral N may provide maximum effectiveness for controlling AG abundance and for restoring native sagebrush steppe communities.
In sagebrush steppe, grazing has been linked to AG invasion (D’Antonio and Vitousek 1992; Knapp 1996), although the magnitude and direction of grazing’s effect is inconsistent in the literature (Bower and Aitken 2006; Reisner et al. 2013; Jones et al. 2015; Stark and Norton 2015). We found relatively weak relationships between grazing and AG abundance, particularly compared to biotic or climate relationships. Other studies that have incorporated abiotic and biotic factors typically show indirect or context-dependent effects of grazing on the abundance of AGs such as B. tectorum (Gelbard and Belnap 2003; Bower and Aitken 2006; Chambers et al. 2007; Jones et al. 2015; Stark and Norton 2015). One of the reasons for the inconsistencies between studies may be due to difference in indicator variables for grazing. Our study only examined current-year grazing, whereas the long-term effects of grazing likely have greater impact on the plant community. Also, even though cattle are a biotic component in this ecosystem, their abundance, distribution and impacts are highly regulated. Thus, grazing is not subject to natural environmental constraints as other functional groups, and is therefore less likely to develop consistent relationships with abiotic factors such as climate or soils. Instead, grazing may have more potential to disrupt historic plant-soil-climate relationships among resident biota, indirectly favoring AGs (Reisner et al. 2013). This indirect effect on resident biota may be particularly strong on the biological soil crust community, which was linked to AG cover in our study (Fig. 3) and others (Belnap et al. 2006; Maestre et al. 2010; Serpe et al. 2013).
In the Great Basin of the western USA, AGs are widespread, and the abundance of AGs can vary from 0 to 100 % cover within a local region. After a broad-spectrum assessment of nearly 100 environmental factors, we found that biotic and climate relationships were relatively strong for AGs and many resident functional groups. Drier summers and warmer winters in the Pacific Northwest (Abatzoglou et al. 2013) will likely exacerbate the negative biotic relationship between invasive and resident functional groups (Benowicz et al. 2001). This information highlights the necessity to incorporate biotic interactions into climate-based species distribution and abundance models for both invasive and resident species. Also, soil factors had relatively strong relationships with resident functional groups, but had limited relationships with AGs. Clearly, there are many factors that control ecosystem structure that are highly interconnected, and predictions and management of invasive species in sagebrush steppe will require a multi-factor approach for maximum effectiveness.
References
Abatzoglou JT, Rupp DE, Mote PW (2013) Seasonal climate variability and change in the Pacific Northwest of the United States. J Clim 27:2125–2142. doi:10.1175/JCLI-D-13-00218.1
Alba C, Skálová H, McGregor KF, D’Antonio C, Pyšek P (2015) Native and exotic plant species respond differently to wildfire and prescribed fire as revealed by meta-analysis. J Veg Sci 26:102–113. doi:10.1111/jvs.12212
Anderson J, Domsch K (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Anderson JE, Inouye RS (2001) Landscape-scale changes in plant species abundance and biodiversity of a sagebrush steppe over 45 years. Ecol Monogr 71:531–556. doi:10.1890/0012-9615(2001)071[0531:lscips]2.0.co;2
Araújo MB, Rozenfeld A (2013) The geographic scaling of biotic interactions. Ecography 37:406–415
Bansal S, James JJ, Sheley RL (2014a) The effects of precipitation and soil type on three invasive annual grasses in the western United States. J Arid Environ 104:38–42
Bansal S, Sheley RL, Blank B, Vasquez EA (2014b) Plant litter effects on soil nutrient availability and vegetation dynamics: changes that occur when annual grasses invade shrub-steppe communities. Plant Ecol 215:367–378
Bartoń K (2013) MuMIn: multi-model inference. R package version 1
Bauer J (2012) Invasive species: “back-seat drivers” of ecosystem change? Biol Invasions 14:1295–1304. doi:10.1007/s10530-011-0165-x
Bellard C, Thuiller W, Leroy B, Genovesi P, Bakkenes M, Courchamp F (2013) Will climate change promote future invasions? Glob Change Biol 19:3740–3748
Belnap J, Phillips SL (2001) Soil biota in an ungrazed grassland: response to annual grass (Bromus tectorum) invasion. Ecol Appl 11:1261–1275. doi:10.1890/1051-0761(2001)011[1261:sbiaug]2.0.co;2
Belnap J, Sherrod SK (2009) Soil amendment effects on the exotic annual grass Bromus tectorum L. and facilitation of its growth by the native perennial grass Hilaria jamesii (Torr.) Benth. In: Van der Valk AG (ed) Herbaceous plant ecology. Springer, the Netherlands, pp 345–357
Belnap J, Phillips SL, Troxler T (2006) Soil lichen and moss cover and species richness can be highly dynamic: the effects of invasion by the annual exotic grass Bromus tectorum, precipitation, and temperature on biological soil crusts in SE Utah. Appl Soil Ecol 32:63–76. doi:10.1016/j.apsoil.2004.12.010
Benowicz A, L’Hirondelle S, El-Kassaby YA (2001) Patterns of genetic variation in mountain hemlock (Tsuga mertensiana (Bong.) Carr.) with respect to height growth and frost hardiness. For Ecol Manage 154:23–33. doi:10.1016/S0378-1127(00)00607-1
Blank RR, Chambers J, Roundy B, Whittaker A (2007) Nutrient availability in rangeland soils: influence of prescribed burning, herbaceous vegetation removal, overseeding with Bromus tectorum, season, and elevation. Rangel Ecol Manage 60:644–655. doi:10.2111/06-120r2.1
Blank RR, Morgan T, Allen F (2015) Suppression of annual Bromus tectorum by perennial Agropyron cristatum: roles of soil nitrogen availability and biological soil space. AoB Plants 7:plv006. doi:10.1093/aobpla/plv006
Bower AD, Aitken SN (2006) Geographic and seasonal variation in cold hardiness of whitebark pine. Can J For Res 36:1842–1850. doi:10.1139/x06-067
Bradford JB, Lauenroth WK (2006) Controls over invasion of Bromus tectorum: the importance of climate, soil, disturbance and seed availability. J Veg Sci 17:693–704. doi:10.1111/j.1654-1103.2006.tb02493.x
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer Science & Business Media, New York
Chambers JC, Roundy BA, Blank RR, Meyer SE, Whittaker A (2007) What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecol Monogr 77:117–145. doi:10.1890/05-1991
Cheng W (2009) Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C–N budgets. Soil Biol Biochem 41:1795–1801
Corbin JD, D’Antonio CM (2004) Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology 85:1273–1283
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu Rev Ecol Syst 23:63–87
Davies KW (2011) Plant community diversity and native plant abundance decline with increasing abundance of an exotic annual grass. Oecologia 167:481–491
Davies KW, Nafus AM, Sheley RL (2010) Non-native competitive perennial grass impedes the spread of an invasive annual grass. Biol Invasions 12:3187–3194. doi:10.1007/s10530-010-9710-2
Davies K, Johnson D, Nafus A (2014) Restoration of exotic annual grass-invaded rangelands: importance of seed mix composition. Invasive Plant Sci Manage 7:247–256
Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20:470–474. doi:10.1016/j.tree.2005.07.006
Duffield JW (1956) Damage to western Washington forests from November 1955 cold wave. Research Notes. Pacific Northwest Forest and Range Experiment Station 5
Eskelinen A, Harrison S (2013) Exotic plant invasions under enhanced rainfall are constrained by soil nutrients and competition. Ecology 95:682–692. doi:10.1890/13-0288.1
Garnier E (1992) Growth analysis of congeneric annual and perennial grass species. J Ecol 80:665–675
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis. Part 1. Physical and mineralogical methods. American Society of Agronomy, Madison, pp 383–411
Gelbard JL, Belnap J (2003) Roads as conduits for exotic plant invasions in a semiarid landscape. Conserv Biol 17:420–432. doi:10.1046/j.1523-1739.2003.01408.x
Götzenberger L et al (2012) Ecological assembly rules in plant communities—approaches, patterns and prospects. Biol Rev 87:111–127
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Grace JB, Anderson TM, Olff H, Scheiner SM (2010) On the specification of structural equation models for ecological systems. Ecol Monogr 80:67–87
Grün B, Kosmidis I, Zeileis A (2012) Extended beta regression in R: shaken, stirred, mixed, and partitioned. J Stat Softw 48:1–25
Gundale MJ, Sutherland S, DeLuca TH (2008) Fire, native species, and soil resource interactions influence the spatio-temporal invasion pattern of Bromus tectorum. Ecography 31:201–210. doi:10.1111/j.0906-7590.2008.5303.x
Humphrey LD, Schupp EW (2004) Competition as a barrier to establishment of a native perennial grass (Elymus elymoides) in alien annual grass (Bromus tectorum) communities. J Arid Environ 58:405–422. doi:10.1016/j.jaridenv.2003.11.008
James JJ, Davies KW, Sheley RL, Aanderud ZT (2008) Linking nitrogen partitioning and species abundance to invasion resistance in the Great Basin. Oecologia 156:637–648
James JJ, Drenovsky RE, Monaco TA, Rinella MJ (2010) Managing soil nitrogen to restore annual grass-infested plant communities: effective strategy or incomplete framework? Ecol Appl 21:490–502. doi:10.1890/10-0280.1
Jeffers ES, Bonsall MB, Froyd CA, Brooks SJ, Willis KJ (2015) The relative importance of biotic and abiotic processes for structuring plant communities through time. J Ecol 103:459–472. doi:10.1111/1365-2745.12365
Jones R, Chambers J, Johnson D, Blank R, Board D (2015) Effect of repeated burning on plant and soil carbon and nitrogen in cheatgrass (Bromus tectorum) dominated ecosystems. Plant Soil 386:47–64. doi:10.1007/s11104-014-2242-2
Knapp PA (1996) Cheatgrass (Bromus tectorum L) dominance in the Great Basin Desert: history, persistence, and influences to human activities. Glob Environ Change 6:37–52. doi:10.1016/0959-3780(95)00112-3
Koteen LE, Baldocchi DD, Harte J (2011) Invasion of non-native grasses causes a drop in soil carbon storage in California grasslands. Environ Res Lett 6:1–10. doi:10.1088/1748-9326/6/4/044001
Leger EA, Goergen EM, Forbis de Queiroz T (2014) Can native annual forbs reduce Bromus tectorum biomass and indirectly facilitate establishment of a native perennial grass? J Arid Environ 102:9–16. doi:10.1016/j.jaridenv.2013.10.015
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Liao H, Luo W, Peng S, Callaway RM (2015) Plant diversity, soil biota and resistance to exotic invasion. Divers Distrib 21:826–835. doi:10.1111/ddi.12319
MacDougall AS, Turkington R (2005) Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86:42–55. doi:10.1890/04-0669
Mack RN (1996) Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biol Conserv 78:107–121
Maestre FT et al (2010) Do biotic interactions modulate ecosystem functioning along stress gradients? Insights from semi-arid plant and biological soil crust communities. Philos Trans R Soc B Biol Sci 365:2057–2070. doi:10.1098/rstb.2010.0016
Mangla S, Sheley RL, James JJ, Radosevich SR (2011) Intra and interspecific competition among invasive and native species during early stages of plant growth. Plant Ecol 212:531–542
Mazzola M et al (2011) Effects of resource availability and propagule supply on native species recruitment in sagebrush ecosystems invaded by Bromus tectorum. Biol Invasions 13:513–526. doi:10.1007/s10530-010-9846-0
McCune B, Keon D (2002) Equations for potential annual direct incident radiation and heat load. J Veg Sci 13:603–606. doi:10.1111/j.1654-1103.2002.tb02087.x
McGlone CM, Sieg CH, Kolb TE (2011) Invasion resistance and persistence: established plants win, even with disturbance and high propagule pressure. Biol Invasions 13:291–304. doi:10.1007/s10530-010-9806-8
McLane SC, Aitken SN (2011) Whitebark pine (Pinus albicaulis) assisted migration potential: testing establishment north of the species range. Ecol Appl 22:142–153. doi:10.1890/11-0329.1
McLendon T, Redente E (1992) Effects of nitrogen limitation on species replacement dynamics during early secondary succession on a semiarid sagebrush site. Oecologia 91:312–317. doi:10.1007/bf00317618
Melgoza G, Nowak R, Tausch R (1990) Soil water exploitation after fire: competition between Bromus tectorum (cheatgrass) and two native species. Oecologia 83:7–13. doi:10.1007/bf00324626
Menakis J, Osborne D, Miller M (2003) Mapping the cheatgrass-caused departure from historical natural fire regimes in the Great Basin, USA. USDA For Serv Proc RMRS-P-29
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71. doi:10.1006/niox.2000.0319
Monaco TA et al (2003) Contrasting responses of Intermountain West grasses to soil nitrogen. J Range Manage 56:282–290
Morrison RB (1991) Quaternary stratigraphic, hydrologic, and climatic history of the Great Basin, with emphasis on Lakes Lahontan, Bonneville, and Tecopa. Quat Nonglac Geol Conterm US 2:283–320
Norton JB, Monaco TA, Norton JM, Johnson DA, Jones TA (2004) Soil morphology and organic matter dynamics under cheatgrass and sagebrush steppe plant communities. J Arid Environ 57:445–466. doi:10.1016/s0140-1963(03)00104-6
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr 12:361–371. doi:10.1046/j.1466-822X.2003.00042.x
Peeler KC, DeBell DS (1987) Variation in damage from growing-season frosts among open-pollinated families of red alder. USDA, Forest Service, Pacific Northwest Research Station
Piper CL, Siciliano SD, Winsley T, Lamb EG (2015) Smooth brome invasion increases rare soil bacterial species prevalence, bacterial species richness and evenness. J Ecol 103:386–396. doi:10.1111/1365-2745.12356
Pregitzer KS, Zak DR, Curtis PS, Kubiske ME, Teeri JA, Vogel CS (1995) Atmospheric CO2, soil nitrogen and turnover of fine roots. New Phytol 129:579–585
Pyšek P et al (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Change Biol 18:1725–1737. doi:10.1111/j.1365-2486.2011.02636.x
Rasse D, Rumpel C, Dignac M-F (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356. doi:10.1007/s11104-004-0907-y
Rau BM, Johnson DW, Blank RR, Lucchesi A, Caldwell TG, Schupp EW (2011) Transition from sagebrush steppe to annual grass (Bromus tectorum): influence on belowground carbon and nitrogen. Rangel Ecol Manage 64:139–147. doi:10.2111/rem-d-10-00063.1
Reisner MD, Grace JB, Pyke DA, Doescher PS (2013) Conditions favouring Bromus tectorum dominance of endangered sagebrush steppe ecosystems. J Appl Ecol 50:1039–1049. doi:10.1111/1365-2664.12097
Rodhouse TJ et al (2014) Predicting foundation bunchgrass species abundances: model-assisted decision-making in protected-area sagebrush steppe. Ecosphere 5:1–16. doi:10.1890/ES14-00169.1
Sakai AK et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Schaeffer S, Ziegler S, Belnap J, Evans RD (2012) Effects of Bromus tectorum invasion on microbial carbon and nitrogen cycling in two adjacent undisturbed arid grassland communities. Biogeochemistry 111:427–441. doi:10.1007/s10533-011-9668-x
Seabloom EW, Williams JW, Slayback D, Stoms DM, Viers JH, Dobson AP (2006) Human impacts, plant invasion, and imperiled, plant species in California. Ecol Appl 16:1338–1350. doi:10.1890/1051-0761(2006)016[1338:hipiai]2.0.co;2
Serpe MD, Roberts E, Eldridge DJ, Rosentreter R (2013) Bromus tectorum litter alters photosynthetic characteristics of biological soil crusts from a semiarid shrubland. Soil Biol Biochem 60:220–230. doi:10.1016/j.soilbio.2013.01.030
Sheley RL, James JJ (2010) Resistance of native plant functional groups to invasion by medusahead (Taeniatherum caput-medusae). Invasive Plant Sci Manage 3:294–300. doi:10.1614/ipsm-d-09-00056.1
Sheley RL, James JJ, Smith B, Vasquez E (2010) Applying ecologically based invasive-plant management. Rangel Ecol Manage 63:605–613. doi:10.2111/rem-d-09-00187.1
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Smith J, Paul E (1990) The significance of soil microbial biomass estimations. In: Megusar E, Gantar M (eds) Soil biochemistry, vol 6. Dekker, New York, pp 460–466
Souza L, Bunn WA, Simberloff D, Lawton RM, Sanders NJ (2011) Biotic and abiotic influences on native and exotic richness relationship across spatial scales: favourable environments for native species are highly invasible. Funct Ecol 25:1106–1112
Stark J, Norton J (2015) The invasive annual cheatgrass increases nitrogen availability in 24-year-old replicated field plots. Oecologia 177:799–809. doi:10.1007/s00442-014-3093-5
Vasquez E, Sheley R, Svejcar T (2008) Nitrogen enhances the competitive ability of cheatgrass (Bromus tectorum) relative to native grasses. Invasive Plant Sci Manage 1:287–295
Wang T, Hamann A, Spittlehouse DL, Murdock TQ (2012) ClimateWNA-high-resolution spatial climate data for western North America. J Appl Meteorol Climatol 51:16–29
Wardle D (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Whittaker RH (1967) Gradient analysis of vegetation. Biol Rev 42:207–264
Williams BL, Sparling GP (1988) Microbial biomass carbon and readily mineralized nitrogen in peat and forest humus. Soil Biol Biochem 20:579–581. doi:10.1016/0038-0717(88)90078-8
Wisz MS et al (2013) The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev 88:15–30. doi:10.1111/j.1469-185X.2012.00235.x
Zelikova TJ, Hufbauer RA, Reed SC, Wertin T, Fettig C, Belnap J (2013) Eco-evolutionary responses of Bromus tectorum to climate change: implications for biological invasions. Ecol Evol 3:1374–1387
Acknowledgments
We thank the many technicians who assisted us with field data collection and laboratory analyses, especially C. Park, C. Gearhart and B. Bingham. We are also indebted to numerous scientists that helped with the development of various protocols used in this study, with special thanks to Drs. W. Cheng for help with the SIR protocol, M. Reisner, K. Ford and B. Cade who provided statistical advice, and M. Jonsson and two anonymous reviewers for editing. This research was funded through the USDA-Agricultural Research Service Areawide Project for Ecologically-based Invasive Plant Management of Annual Grasses in the Great Basin Ecosystem. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Author contribution statement
S. B. and R. L. S. conceived and designed the study and wrote the manuscript. S. B collected and analyzed the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Tim Seastedt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bansal, S., Sheley, R.L. Annual grass invasion in sagebrush steppe: the relative importance of climate, soil properties and biotic interactions. Oecologia 181, 543–557 (2016). https://doi.org/10.1007/s00442-016-3583-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3583-8