Abstract
The functional response is a key element of predator–prey interactions. Basic functional response theory explains foraging behavior of individual predators, but many empirical studies of free-ranging predators have estimated functional responses by using population-averaged data. We used a novel approach to investigate functional responses of an avian predator (the rough legged-buzzard Buteo lagopus Pontoppidan, 1763) to intra-annual spatial variation in rodent density in subarctic Sweden, using breeding pairs as the sampling unit. The rough-legged buzzards responded functionally to Norwegian lemmings (Lemmus lemmus L. 1758), grey-sided voles (Myodes rufocanus Sundevall, 1846) and field voles (Microtus agrestis L. 1761), but different rodent prey were not utilised according to relative abundance. The functional response to Norwegian lemmings was a steep type II curve and a more shallow type III response to grey-sided voles. The different shapes of these two functional responses were likely due to combined effects of differences between lemmings and grey-sided voles in habitat utilisation, anti-predator behaviour and size-dependent vulnerability to predation. Diet composition changed less than changes in relative prey abundance, indicating negative switching, with high disproportional use of especially lemmings at low relative densities. Our results suggest that lemmings and voles should be treated separately in future empirical and theoretical studies in order to better understand the role of predation in this study system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Theoretical and empirical work suggest that predation can explain why certain prey populations fluctuate in time and space (Murdoch et al. 2003; Turchin 2003). In order to fully understand how predator–prey systems are structured, it is necessary to quantify two interactive processes—the functional and numerical responses of predators (Solomon 1949).
The functional response, defined as the predator consumption rate in relation to prey density, is the basic predatory response to changes in prey numbers, as energy for predator reproduction and growth is derived from consumed prey. The functional response involves the full range of foraging behaviours and occurs on a fast time scale. Holling (1959a, b) described three functional response types that are regarded as the theoretical basis for predator–prey interactions. The type I functional response illustrates a linear relationship between kill rate and prey density, whereas the type II response has a concave shape, and the type III functional response is sigmoid in shape. Given their dissimilar contributions to stability in a predator–prey system (Kuno 1987; Murdoch and Oaten 1975), it is of central importance to distinguish between different functional response curves.
However, despite functional response being an important concept in theoretical ecology, the number of empirical field estimates is relatively low compared to theoretical work (Abrams and Ginzburg 2000). This can partly be explained by difficulties in measuring functional responses under field conditions. For instance, it is not clear which temporal, spatial and population/individual scales that are most relevant in order to test predator–prey theory. According to Holling’s first principles (Holling 1959a), the functional response is the consumption rate of an individual predator measured over a short period of time and has an immediate effect on prey mortality. This is in contrast to the numerical response, which describe changes in natality and mortality rates—demographic processes that cause long-term effects in the predator and prey populations. The total effect of predation can be derived by multiplying the functional and numerical responses (Sinclair and Pech 1996), but the logical inconsistency of combining processes operating at different time scales has been pointed out (Inchausti and Ballesteros 2008; Oksanen et al. 1992). As a result, functional responses are often studied on the same temporal scale as predator population dynamics. An alternative to population-level functional responses is to study how individual predators or packs react to changes in prey density in their territories or home ranges. This approach is a closer approximation to underlying foraging theories and is likely to offer better mechanistic explanations and understanding of foraging decisions, but has only rarely been adopted for field studies (e.g. Jost et al. 2005; Koivunen et al. 1996; Moleón et al. 2012).
Further, the focus of many empirical studies has been on a single resource species, or similar resource species treated collectively as a functional group. But predators are often selective in their prey choice and kill some prey species disproportionately more often in relation to their abundance. Such prey selection can have effects on functional responses in multi-prey systems (Fryxell and Lundberg 1998; Messier 1995) and may also contribute to community dynamics. In multi-prey communities, predation patterns are therefore not only shaped by absolute prey densities, but also by relative densities of different prey, which may lead to switching behaviour (Murdoch 1969). The classic case is “positive switching” in which the predator directs disproportionately more attention to the more abundant prey—a behaviour that will have a stabilising impact on community dynamics (Oaten and Murdoch 1975). The opposite pattern, termed “negative switching” (Chesson 1984), has received less attention but is not uncommon (Rindorf et al. 2006), and increases the probability of local extinctions of rare prey. But although the concepts of different functional response types and switching are related, there is no generally applicable link or theoretical framework between them (Asseburg 2006).
A prerequisite for estimation of functional responses is that prey density is highly variable. This is a characteristic feature of Arctic and boreal communities where many herbivores show fluctuations in population size with amplitudes ranging over several orders of magnitude (e.g. Krebs and Myers 1974; Stenseth and Ims 1993). These herbivores and their predators are therefore promising study systems (Boutin 1995). In northern Europe, interactions between predators and Microtus and Myodes voles have received particular attention (reviewed by Hanski et al. 2001; Henttonen and Hanski 2000). Population dynamics of voles in this region are primarily influenced by interactions between Microtus voles and mustelids Mustela spp. (Henttonen et al. 1987; Turchin and Hanski 1997), but there is also an important multi-species component involving Myodes voles and a guild of various rodent predators, both avian and mammalian (Korpimäki et al. 2002). Indeed, modelling suggests that differential prey vulnerability and species-specific functional responses are necessary to explain the complex community dynamics of boreal voles (Hanski and Henttonen 1996). These results can be extended to the Fennoscandian mountain region, where the rodent community is similar to the well-studied community in the boreal zone, but with different species involved. The key species in the mountain birch forest is the grey-sided vole Myodes rufocanus (Sundevall, 1846), whereas the Norwegian lemming (Lemmus lemmus L., 1758) is the most important species in mountain tundra (Henttonen and Wallgren 2001; Ims and Fuglei 2005). Population dynamics of the grey-sided vole are more likely to be caused by predator–prey interactions (Hansen et al. 1999; Turchin et al. 2000), whereas lemming dynamics are more consistent with a lemming–vegetation interaction in northernmost Fennoscandia (Oksanen et al. 2008). It has been proposed that the Norwegian lemming is a less suitable prey than voles (Hagen 1952; Taitt 1993), a suggestion that has received some experimental support (Andersson 1976; Barth et al. 2000). However, empirical studies describing functional responses and prey preference regarding grey-sided voles and Norwegian lemmings are, to our knowledge, lacking.
In this study, we studied interactions between a migratory avian predator, the rough-legged buzzard (Buteo lagopus Pontoppidan, 1763), and the key rodents in the Fennoscandian subarctic region, Norwegian lemmings, grey-sided voles, and field voles (Microtus agrestis L., 1761). We investigated prey density, diet choice and prey selection in separate territories of rough-legged buzzards within a single breeding season characterised by peak rodent densities, using a breeding pair as our focal unit. Our objectives are to investigate predation patterns of buzzards by (1) modelling prey-dependent functional responses, (2) analyse whether the buzzards exhibited different functional responses to each prey species, and (3) whether relative prey densities was related to prey selection, or whether non-random prey selection causes prey switching.
Materials and methods
Study area
This study was conducted in Stora Sjöfallet National Park with surroundings in NW Sweden (67°45′N, 17°30′E). The study area encompassed 150 km2 of subarctic environment. The northern part of the study area comprised the alpine heaths surrounding the lakes Autajaure and Sitasjaure (altitudes ranging from 600 to 1,000 m a.s.l.). The vegetation was dominated by fresh and dry heaths, with mostly dwarf birch (Betula nana L., 1753), crowberry (Empetrum hermaphroditum Hagerup, 1927) and bilberry (Vaccinium myrtillus L., 1753). The southern part represented lower altitudes (450–700 m a.s.l.) along the northern shore of lake Akkajaure, where the main vegetation was moderately productive mountain birch (Betula pubescens ssp. czerepanovii Orlova, 1791) woodland.
Rough-legged buzzard monitoring
The rough-legged buzzard has a circumpolar distribution in the northern hemisphere (Hagemeijer and Blair 1997), and breeds in mountain regions, boreal taiga and on arctic tundra (Cramp and Simmons 1980). In peak rodent years, it is the most abundant avian predator in the Fennoscandian mountains, particularly in the transition zone between mountain birch forests and alpine tundra, where the nest usually is built on a cliff ledge. The rough-legged buzzard is widely considered as a specialist predator and shows strong aggregative and reproductive responses to fluctuations in small mammal populations (e.g. Hagen 1969; Potapov 1997; Sundell et al. 2004). Our study was part of a long-term study on raptors and owls in Stora Sjöfallet National Park, where the rough-legged buzzard population closely tracks the rodent cycle. The numerical response of the rough-legged buzzard at our study site is illustrated in Fig. 1, showing that density of breeding pairs was almost perfectly correlated with rodent density during 2001–2006.
Fluctuations in small mammal abundance (open circles, dashed lines, right y-axis) and the numerical response of rough-legged buzzards (Buteo lagopus) (filled circles, solid lines, left y-axis) to small mammals at Stora Sjöfallet National Park in northern Sweden 2001–2006. Rodent abundance was estimated by snap trapping, index values are presented as number of captures per 100 units of effort (trap days). Number of pairs that laid at least one egg were included in the rough-legged buzzard variable, density of breeding pairs expressed as the number of breeding pairs/10 km2
The present study was conducted in a single year, 2001. Field surveys started in May 2001 shortly after the rough-legged buzzards had arrived in the study area. Of 32 occupied nesting territories, 9 were included in this study, representing breeders both on alpine heath (n = 4) and in the adjacent birch forest (n = 5). Occupied territories were visited 1–3 times during the summer to obtain data on brood size. Sampling from two habitat types was necessary for covering an adequate range of densities for functional response estimation. Territories included in this study were selected because adult hunting areas and roosting sites were observable (and not overlapping between territories) and partly also by topographic constraints (territories in boulder fields, steep slopes and on the border between the birch forest and heath habitats were not included). The minimum distance between two nest sites included in this study was 1.3 km (mean ± S.E. 4.6 ± 1.8).
Rodent monitoring
We used the trapping protocol described by Krebs et al. (2002) to estimate density of rodent populations within buzzard territories. Two parallel snap-trap lines separated by 100 m were set out 300–500 m from the nest during the late nestling period of the buzzards in the period 14–27 July 2001. Each trap line had 20 stations placed at 15-m intervals. A small flag was placed at the centre of each station, and three traps were evenly distributed at a distance of 1–2 m from the flag. Traps were set for 48 h per territory and checked four times at 12-h intervals. We used peanut butter and raisins as bait. The trapping effort was 240 trap-nights per territory, and we used the number of captured individuals of each species per 100 trap-nights as an index of rodent density. The species-specific indices were calculated as 100 × number of captures/corrected effort. We took non-availability of sprung traps into account by calculating the corrected effort as (modified from Nelson and Clark 1973):
which in our case meant that we subtracted 0.25 from the total effort for each trap sprung (sprung traps included all captures of all species as well as trap errors).
The rough-legged buzzards perched in top of trees or hovered while hunting, which allowed us to observe where each individual hunted. Consequently, the trap lines were placed in the most frequently used hunting areas of each territory. Our rodent monitoring methods have been evaluated by an ethical commitee and are licensed by the Swedish Board of Agriculture.
We have no information from our study are regarding rodent abundance prior to 2001. But according to other monitoring studies in northern Fennoscandia, 2001 was a peak year for lemmings and voles at several sites (Kilpisjärvi, Cornulier et al. 2013; Ammarnäs and Stora Sjöfallet, Ecke et al. 2010; Sarek/Padjelanta, Nyström et al. 2006; Abisko and Vassijaure, Olofsson et al. 2009).
Diet composition
Diet composition was studied by analysis of regurgitated pellets and prey remains collected at nests and roost sites. Pellets of adult buzzards were collected at roosts and perches. Pellets from nestlings accumulated in and below the nest and were collected after the brood had fledged. Small mammals (voles, lemmings and shrews) were identified to species by molar teeth patterns or characteristic fur (Niethammer and Krapp 1982). The minimum number of ingested mammalian prey was estimated by counting the number of unique molar teeth in each pellet. Bird remains were identified to taxonomic order by using the keys of Brom (1986) and Day (1966). If a pellet contained only fur or feathers, it was assumed that one prey specimen had been ingested. Totals of each prey type were converted to biomass using mean values from all trapping sessions for rodents (grey-sided vole: 31.5 g; Northern-red backed vole, Myodes rutilus Pallas, 1779: 30.0 g; field vole: 31.3 g; Norwegian lemming: 48.6 g) and literature values for other mammals (Siivonen 1976) and birds (Huhtala et al. 1996; Pasanen and Sulkava 1971). Adult buzzards do not process small rodent prey before delivery at the nest (Hellström, unpublished data), a behaviour that is common in some raptor species (Korpimäki et al. 1994 and references therein). Therefore, our comparisons of adult and juvenile diets are not confounded by pre-delivery prey processing by adults.
Estimation of consumption rates
We estimated kill rates based on the combined adult and nestling diets. The number (NP) of grey-sided voles, field voles and lemmings killed by each rough-legged buzzard pair per day during the nestling period was calculated in accordance with Lindén and Wikman (1983), a formula that is widely used in the raptor literature:
where DER is the daily energy requirements (average food intake in grams per day) of adults and nestlings, respectively, and brood size is the number of fledged nestlings. PP is the proportion of the prey type (by biomass) in the diet of each buzzard pair and MMP is the mean mass (g) of a single prey item of species i. Daily energy requirements (DER) of rough-legged buzzards have been estimated in previous studies (Pasanen and Sulkava 1971; Potapov 1993; Reid et al. 1997). These three studies have found DER to be in the range 100–140 g/day under conditions with no food stress, with high agreement across studies. For adults, we therefore used DER = 110 g/day (average of estimates for males and females in the detailed study on energy budget in Potapov 1993, sect. 10.2), and for nestlings DER = 140 g/day (averaged from Pasanen and Sulkava 1971, sect. VII; Potapov 1993, fig. 9.25).
Prey selection and switching
We calculated two measures of prey selection or preference at both population and pair level—selection ratios (Manly et al. 2002) and standardised selection ratios. Selection indices are often referred to as preference indices. For our purposes, preference means any deviation from random use of prey, and thus not necessarily an active choice made by the predator.
Selection ratios are calculated as r i /n i , where r i = proportion of species i in the diet and n i = proportion of prey i available in the environment. Selection ratios from 0 to 1 reflect avoidance, and ratios from 1 to infinity relative over-representation or preference. Selection ratios can be standardised so that values range from 0 to 1 (and sum to 1) according to:
Standardised selection ratios are equivalent to Manly’s alpha index (Chesson 1978; Manly et al. 1972), and estimates the probability that the next prey item will be of class i if it were possible to make all resources equally available (Manly et al. 2002, p. 51). In the null case of no selective feeding, α i = 1/m, where m is the number of possible prey types. α i > 1/m indicates preference.
Switching behaviour was evaluated with a graphical approach (Murdoch 1969; O’Donoghue et al. 1998) by comparing the percent of a given prey species in the diet with its relative availability. The null hypothesis is that the relative availability should fall on a line with unit slope, but at strong preference the null hypothesis is a curve (convex for preferred prey and concave for non-preferred prey; see Murdoch and Oaten 1975). If points fall below the null-hypothesis curve at low relative densities, and above the curve at high relative densities, this is taken as evidence for positive switching (Oksanen et al. 2001), while the opposite pattern indicates negative switching.
Statistical analyses
Diet composition was modelled with multinomial logit models (Venables and Ripley 2002), where the response matrix comprised counts of grey-sided voles, lemmings, field voles and birds in pellets. Habitat (birch forest or tundra heath) and age (nestling or adult) were included as predictor variables. Multinomial models were fitted with the multinom function in the nnet R-package (v.7.3-7; Venables and Ripley 2002). Territory A was excluded from this analysis, since diet data for adults could not be obtained as pellets were deposited on inaccessible cliff roosts.
Analyses of prey selection ratios and standardised selection ratios were calculated in accordance with Manly et al. (2002). Since prey availability and consumption was measured separately for each rough-legged buzzard pair, our study was of the design III sampling protocol (see chap. 4 in Manly et al. 2002). Selection ratios and associated standard errors were calculated with the widesIII-function in the R-package adehabitatHS (v.0.3.8; Calenge 2006), by assuming that proportions of each prey type available were estimated (and not accurately known). The null-hypothesis curve for prey switching was calculated following the approach by O’Donoghue et al. (1998).
Prey-dependent functional responses were modelled as Holling’s functional responses (hyperbolic type II and two versions of the sigmoid type III), Lotka-Volterra (linear type I without intercept) and as constant (null model, type 0). We investigated three different sets of hypotheses: (1) no difference in the functional response to different prey species, (2) the functional response differs between species, but the type is similar for all prey species, and (3) the functional response differs between species and the predator shows different (non-linear) types of responses to different prey species.
Nonlinear functional responses were modelled as:
where subscripts denote observation i of prey species j (in our analysis, j had two levels, 1 = lemmings and 2 = grey-sided voles) and x is prey density. We used calculated kill rates and % of prey biomass as the response variable y in separate analyses. Equation 4 belongs to the family of Michaelis–Menten equations, and can be re-parameterised to Holling’s original notation (Real 1977). Parameter a represents the asymptotic kill rate, and b (the half-saturation constant) is the prey density at half the maximum kill rate a. θ controls the shape of the curve (concave down or sigmoid). The type II response was obtained by setting θ = 1. If θ = 2, we obtain the most commonly used (sigmoid) type III response (hereafter referred to as type IIIa). The phenomenological form of the type III response (hereafter type IIIb), where θ is an estimated parameter was also modelled. Hypothesis 1 (a single curve describes the functional response to both species) was evaluated by dropping the subscripts j in Eq. 4. Hypothesis 2 corresponded to fixing the parameter θ to the same value for both prey species for type II (θ j = 1) and IIIa (θ j = 2) responses, while having no constraints on θ j for type IIIb. Finally, different response types (hypothesis 3) to different prey species was evaluated by fixing θ j at a particular value (1 or 2) for at least one prey species and not allowing θ 1 = θ 2 . The linear type I functional response was described as \(y_{ij} = N\left( {a_{j} \times x_{ij} ,\sigma_{j}^{2} } \right)\) where a equals search rate, and the constant null model as \(y_{ij} = N\left( {\mu_{j} ,\sigma_{j}^{2} } \right)\).
From our set of hypotheses, we constructed 14 candidate models (Table 1). The functional response models were fitted to the data with (nonlinear) generalised least squares (Pinheiro and Bates 2000) by using the gnls-function in the R-package nmle (Pinheiro et al. 2013). In all models, the variance was estimated separately for each prey species.
Territory I was excluded from analyses of prey selection ratios and the functional response to lemmings, as we could not estimate lemming density in this territory. The hunting territory of the male in this pair was divided between a high ridge, where lemmings were abundant, and the adjacent birch forest (grey-sided vole habitat). Trap lines could only be placed in the birch forest due to the impassable terrain, and lemming density was consequently underestimated. This territory was included in the analyses of functional responses to grey-sided voles, as both adults were observed to frequently catch this species in the area where our traps were set.
The information-theoretic approach (Burnham and Anderson 2002) was used to select the best model among candidate models, defined as the model with the lowest information criteria, in this case AICC (Akaike’s Information Criterion corrected for small sample size). All statistical analyses were performed in R 3.0.2 (R Development Core Team 2013).
Results
Rodent density
The population estimates of the four arvicoline rodent species in the study area (grey-sided vole, Northern red-backed vole, field vole and Norwegian lemming) indicated a high degree of both intra- and interspecific spatial variation in density within the same summer season. The most abundant species, the grey-sided vole, was trapped in all territories, but density estimates decreased along the productivity gradient from birch forest to heath vegetation. There was a 41-fold difference in density between highest and lowest density estimates. Lemmings were trapped in all territories except one, but showed less intraspecific variation in density (a 14-fold difference) than grey-sided voles. Highest lemming indices were obtained above the tree line, particularly in high-altitude territories dominated by fresh heath vegetation. Field voles and Northern red-backed voles showed a patchy distribution pattern. High densities of field voles were recorded in two territories in a mosaic landscape with a high proportion of dry fens and low herb meadows. The Northern red-backed vole was restricted to low altitudes in the birch forest, and occurred at very low densities.
Diet composition
A total of 959 prey items were identified from 505 pellets and occasional larger prey remains. The dietary analysis confirmed that the rough-legged buzzard relied heavily on small mammalian prey, as rodents constituted 79–98 % of all prey items and 69–98 % of prey in terms of biomass. Overall, lemmings (41 %) and grey-sided voles (36 %) constituted three-quarters of biomass intake on a population level. An additional 11 % of the diet was made up by field voles. The remaining proportion of the diet was shared between other small mammals (0.2 %) and birds (13 %). Diet composition (in terms of proportion of all prey items) was best explained by an interaction between habitat (birch forest or tundra heath) and age of bird (nestling or adult) (Fig. 2), as this model (AICC = 1,814.55, K = 12, weight = 0.65) was (weakly) supported over the model with additive effects of habitat and age (AICC = 1,815.55, K = 9, weight = 0.35). Remaining models including only age or habitat as predictors had very low AICc-weights (<0.0001). As expected, based on the distribution pattern of prey species, grey-sided voles were more common in the diet in the birch forest than in tundra heath, while the opposite was observed for lemmings, field voles and birds. Further, in both habitats, adults had a higher proportion of lemmings and field voles in the diet than nestlings, while nestling diet comprised a larger share of grey-sided voles and birds (Fig. 2). The interaction effect between habitat and age was likely caused by the larger difference between the proportion of birds in nestling and adult diets in the heath habitat than in the birch forest habitat (Fig. 2).
Diet composition of rough-legged buzzards in relation to habitat (birch forest, tundra heath) and age class (nestling, adult). Pooled data from 8 territories (4 territories in each habitat type). Proportions refer to proportions of prey items per habitat and age class. Number of identified prey items: birch forest n nestlings = 346, n adults = 248, and in tundra heath n nestlings = 133 and n adults = 174
Functional response
The rough-legged buzzards exhibited strong functional responses to both grey-sided voles and lemmings (Fig. 3). They also responded functionally to field voles (not shown), but since we only have two estimates of field vole density >0, the shape of this response could not be analysed. We therefore focused on the functional response to grey-sided voles and lemmings.
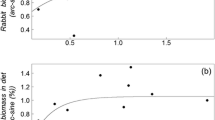
Prey-dependent functional responses of rough-legged buzzards to grey-sided voles (Myodes rufocanus) and Norwegian lemmings (Lemmus lemmus). a Percentage of ingested prey biomass in relation to prey density index, compositional data that was converted to: b the estimated number of killed prey individuals by a single breeding pair (including prey items fed to their nestlings) per day. Density estimates of respective prey species were obtained by snap-trapping. The functional response to grey-sided voles (filled circles, solid line) was best described by a type IIIa response, and the response to lemmings (open circles, dashed line) was of type II (open crossed circle data point not included in the analysis; see “Materials and methods” for explanation)
Models with species-specific parameters were strongly supported over global models in which grey-sided voles and lemmings shared parameters. All five global models had Akaike-weights <0.001 (Set 1 in Table 1) and the species-specific models thus received almost all support. Analyses with different response variables (kill rates and % of prey biomass) gave very similar results regarding the overall shapes of the functional responses (Table 1). The highest ranked model contained a type IIIa response to grey-sided voles and a type II response to lemmings. However, the model with type IIIa responses to both species received similar support (Table 1). The functional response to grey-sided voles was weak at low densities and clearly sigmoid in shape. The inflection point of the type IIIa response to lemmings was at very low density. Such a functional response is density-dependent only over a narrow spectrum of low densities, and probably of limited importance in reality. The type II response was therefore the more likely predation pattern on lemmings.
For the analysis with kill rates as the response variable, both asymptotic kill rates and half-saturation constants differed between responses to grey-sided voles and lemmings. Asymptotic kill rate of grey-sided voles was 2.17 times greater than the corresponding asymptote for lemmings, whereas the half-saturation constant for lemmings was 13.94 times lower than the half-saturation constant in the response to grey-sided voles. The difference in the asymptote of the functional response was not evident when % of prey biomass was analysed as the response variable (as the functional response leveled out at ~65 % for both species), but half-saturation constants differed in the same direction as for kill rates (ratio grey-sided voles/lemmings: 5.19). The two functional responses thus differed markedly in the rate of approach to the asymptote, which was expressed as a substantially steeper functional response to lemmings than to grey-sided voles (Fig. 3; Table 2).
Prey selection and switching
According to selection ratios, different rodent prey categories were not utilised in accordance with relative availability, neither on the population (Table 3) nor territory (Fig. 4) levels. On the population level, grey-sided voles occurred half as often in pellets compared to alternative prey (i.e. lemmings and field voles) as would be predicted from their relative abundance. Selection ratios for lemmings versus alternative prey (all vole species combined) showed that lemmings were taken 1.7 times more often than suggested by relative availability. However, this pattern was not consistent at the individual (or pair) level. Analyses of data obtained from each pair showed that grey-sided voles were taken in proportion to relative abundance at low relative densities (Fig. 3a), but that standardised selection ratios declined with relative abundance (GLM, Gaussian error structure with logit-link: \({\text{logit}}\left( {\text{selection ratio}} \right) = - 0. 2 6 - 1. 5 8x\), analysis of deviance χ 21 = 0.08, p = 0.0009). At low relative densities, lemmings were apparently more vulnerable to predation than grey-sided voles (Fig. 3b), but as for grey-sided voles, standardised selection ratios for lemmings declined as relative density increased [GLM, Gaussian error structure with logit-link: logit(selection ratio) = 1.55 − 2.90 x, analysis of deviance χ 21 = 0.28, p < 0.0001]. The graphical analysis presented in Fig. 4 indicated that the predation patterns on grey-sided voles and lemmings were not consistent with positive switching, but rather with negative switching.
Selection of prey by each rough-legged buzzard pair in relation to relative prey abundance. a Grey-sided voles versus alternative rodent prey (lemmings + field voles), b lemmings versus alternative prey (grey-sided voles + field voles). The dashed line indicates equal representation in diet and environment, the solid line represents the null hypothesis calculated from population-level selection ratios. Proportions are proportions of prey items
Discussion
In this study, we demonstrated that an avian predator, the rough-legged buzzard, showed different functional responses to its two most important prey species, Norwegian lemmings and grey-sided voles. The response curve to lemmings was noticeably steeper at low-to-intermediate prey densities than the response to grey-sided voles. The rough-legged buzzard can thus be categorised as a rodent specialist with an opportunistic foraging strategy, which has also been observed in Siberian rough-legged buzzards (Wiklund et al. 1998). But we also observed that grey-sided voles and lemmings were not preyed upon according to relative abundance, and further that diet composition differed between adults and nestlings.
We analysed functional responses to spatial heterogeneity in prey densities, rather than to temporal variation. Spatial within-year heterogeneity of rodents is common in taiga and tundra landscapes of Fennoscandia, and is related to productivity patterns and habitat preferences of different rodent species (Oksanen and Henttonen 1996; Oksanen et al. 1999). Thus, this high degree of within-year variation was not a consequence of our sampling protocol and selection of specific predator territories, and long-term studies in our study area have shown that the habitat-related pattern of vole and lemming abundance observed here was consistent over three cycles from 2001 to 2011 (Taylor 2009; Hellström, unpublished data). We assumed that density indices obtained by snap-trapping was highly correlated and linearly related to true densities, a suggestion supported by previous studies on Myodes and Lemmus species (Gruyer et al. 2008; Hanski et al. 1994). However, the relationship between snap-trap indices and density estimates might differ depending on species and habitats (e.g. Øvrejorde 2007), which can potentially bias calculations of particularly prey preference. But various removal estimators (White et al. 1982) applied to snap-trapping data for lemmings and grey-sided voles in our study area yield abundance estimates that are linearly related to density indices and further do not support a species-dependent relation between snap-trap indices and abundance estimates (Hellström and Angerbjörn, unpublished data). Current data therefore indicate that removal trapping using snap-traps reflects true abundance (both absolute and relative) without major biases and differences between species.
Scaling issues and functional responses
It is necessary to test predictions from foraging theory using various observational scales. Arditi and Ginzburg (1989) suggested that functional responses should be studied on the same temporal scale as population dynamics (i.e. numerical responses), otherwise predator–prey models are contradictory due to the combination of fast and slow processes. This is in contrast to mechanistic explanations for the functional response, which was derived from behaviours of individual predators (Holling 1959a, b). Population level responses are indeed the cumulative effects of individual responses occurring on a behavioural time scale, and both the population and individual level approaches are thus necessary for finding scale-independent features of predation. Researchers concerned with regulation of prey populations have primarily adopted the population scale approach, usually by averaging kill rates and prey density within a breeding interval and then repeating the sampling over several seasons (Abrams 1994). But the variation in both predator and prey behaviour would be lost by such averaging of important parameters, and can lead to unexpected results (Chesson 1984). Instead, the “individual scale approach” allows for a detailed study of how individuals vary in their response to prey density.
Patterns detected on longer time scales may further be obscured by changes in densities of other species other than the focal predator–prey unit. For instance, the rough-legged buzzard responds numerically mainly to vole fluctuations, and lemmings are not a pre-requisite for successful breeding (Hellström 2007). Although vole and lemming cycles are generally tightly coupled, lemmings repeatedly fail to reach peak densities when voles do (Henttonen and Kaikusalo 1993). It is possible that the functional response to grey-sided voles would shift from a type III curve in simultaneous lemming and vole peaks to a steep type II function in years when only voles peak. Such context-dependent responses could have interesting consequences on population dynamics, but have so far largely been over-looked.
Time-scale averaging of functional responses further excludes the possibility to take spatial variation in predation pressure into account, a factor that is often ignored in investigations of predator–prey systems, but might be an important component of vole and lemming dynamics (Ekerholm et al. 2001). Oksanen et al. (1999) found that the dynamics of tundra-living grey-sided voles were characterised by dynamics with more truncated peaks than grey-sided vole dynamics in more productive patches. The functional response of buzzards to grey-sided vole is one plausible explanation to this pattern, as the type III response indicates that buzzard functional response can be density-dependent at low densities (i.e. on tundra heath vegetation), and hence could dampen population peak densities in less preferred habitats of grey-sided voles. In birch forests, where grey-sided voles are highly abundant, per capita rates of predation pressure from buzzards on grey-sided voles were likely less important as the functional response reached saturation level at intermediate densities. Predation on lemmings followed a different pattern, and was characterised by negative switching where the proportion of lemmings in the diet of buzzards were higher than expected at low relative abundance and lower than expected in mountain tundra heaths where lemmings are the dominant prey. Low absolute and relative densities of lemmings largely coincided with productive patches such as birch forest or grasslands. Oksanen (1993) suggested that lemmings could not establish populations in forested areas due to apparent competition mediated by least weasel (Mustela nivalis) predation, a hypothesis that can be refined to also include avian predators given the negative switching by buzzards presented here (but see Ims et al. 2011 for an alternative hypothesis). Negative switching is likely to have a destabilising impact on community dynamics (Chesson 1984) and has been observed in other raptors (e.g. Palma et al. 2006). There are several proposed explanations for negative switching, including confusion of predator’s search by more abundant (and less preferred) prey (Kean-Howie et al. 1988), changes in behaviour under high predation risk (Abrams and Matsuda 1993), differences in nutritional status between prey types (Abrams 1987), and a need for information-sampling of the availability of different prey types. In relation to foraging theory, negative switching does not support the classic energy-maximising model (i.e. optimal diet or contingency models; Fryxell and Lundberg 1998; Stephens and Krebs 1986), but the observed dietary conservatism instead supports the balanced diet hypothesis. In terms of raw energy content, the larger lemmings are more profitable prey, and energy-maximising models would predict that buzzards should ignore other prey types at high lemming densities, but we instead found that voles comprised a large share of the diet even at the highest lemming densities. For a full evaluation of contrasting hypotheses, we would however need to obtain direct estimates of handling times of lemmings and voles, as measures of profitability should also take species-specific handling time into account. Balanced diets are typically attributed to herbivores as plants often differ in chemical composition. Carnivores, on the other hand, mostly feed on prey species with similar nutrient and chemical composition, but a difference between voles and lemmings in this aspect has long been discussed (see below).
Prey selection and provisioning
Our study contrasts with the common view that lemmings are inferior prey, a viewpoint largely based on Hagen’s large collection of prey items (Hagen 1952) that has been repeatedly cited in the literature (e.g. Framstad et al. 1997). On a population level, lemmings were captured more often than expected based on relative availability, whereas voles were underrepresented in buzzard diet. Hagen (1952, pp. 539–540) reported that lemmings were a surprisingly rare prey in his studies of the diets of raptors and owls in Norway, and further that lemmings were frequently regurgitated by raptors only partially digested. Taitt (1993) suggested that lemmings might be distasteful because of substances emitted by their dorsal skin glands (Wallin 1967). There are, however, other alternate explanations for why lemmings may be more vulnerable to predation than voles.
First, encounter rates with different prey depend on prey density and overlap in predator/prey habitat selection. Rough-legged buzzards mainly hunt in open areas or in patches with sparse vegetative cover (Sonerud 1986; Sylvén 1978), which is the typical habitat for lemmings during summer (Heske and Steen 1993). Grey-sided and field voles on the other hand mainly occupy habitats with a high proportion of shrub cover or boulder fields (Hambäck et al. 1998; Johannesen and Mauritzen 1999; Magnusson et al. 2013), where the risk of encounters with avian predators supposedly is lower. Several studies of selective predation by birds of prey have demonstrated that a preference for a prey type can be explained by coincident habitat choice, both in space and time, of the predator and its preferred prey (e.g. Dickman 1992; Dickman et al. 1991; Norrdahl and Korpimäki 1993; Rohner and Krebs 1996). It is also possible that predators detect lemmings more easily than voles in most habitats. Lemmings, in their “bright yellow, reddish brown, white and contrasting jet black hues” (Andersson 1976) are certainly more conspicuous than both grey-sided and field voles, whose pelage is reddish brown.
Second, rodent-eating avian predators seem to attack different prey species in proportion to their relative densities (Hakkarainen et al. 1992; Nishimura and Abe 1988; Temple 1987), rather than actively select to attack or ignore certain prey types. Prey utilisation patterns therefore seem to be also governed by differences in capture success between prey types. Lemmings are larger and much slower than voles (Oksanen 1993), which suggests that lemmings are easier to catch. Lemmings are also known for their aggressive anti-predator behaviour (Koponen et al. 1961) and often try to ward off predators (Myllymäki et al. 1962), a tactic that can be successful at least against long-tailed skuas (Stercorarius longicaudus Viellot, 1819) (Andersson 1976). Voles have adopted another behaviour by outright fleeing when approached by a predator (Sundell and Ylönen 2008), a strategy that is less likely to result in being captured (Shifferman and Eilam 2004).
The overall higher vulnerability of lemmings is thus probably a result of a high encounter rate due to similar habitat choice and high capture success due to their slower mobility and behaviour. The cause of selective predation on lemmings needs further investigation, but seems to lie with the prey rather than with the predator. Prey vulnerability is also likely related to habitat, as we found that selection ratios were variable at the predator-pair level, with both grey-sided voles and lemmings having lower selection ratios in their respective favoured habitat. The cause for this shift in vulnerability warrants further study, but can be caused by intra-specific processes and unfamiliarity with a non-preferred habitat, but also by competitive interactions between lemmings and voles where the inferior competitor is forced the occupy patches with high predation risk.
It is important to note that the functional responses and prey selection ratios would have been different if we had analysed pellets and prey remains collected only at nests, since there was a consistent difference between adult and nestling diets. For instance, lemmings occurred more frequently in the diet of adults, and predation rates on lemmings would have been biased low if the functional response analysis had been based only on nestling diet. Grey-sided voles were found more often in nestling diet, and this pattern does not support a load-size effect (Orians and Pearson 1979; Sonerud 1992), since the expected outcome under such a scenario is that lemmings should be more frequent in nestling pellets than voles. This difference in prey selection can be a consequence of differential patch use (Markman et al. 2004) or foraging behaviour (e.g. Davoren and Burger 1999) during self-feeding and nestling provisioning, tactics that can be explained by adaptive foraging (Ydenberg 2007). An alternate hypothesis not invoking adaptive behaviour is that nestling size and development determine provisioning decisions and delivery of prey of different sizes to the nest. This is partly supported by direct observations of nestling feeding behaviour (Hellström, unpublished data), as lemmings are frequently difficult to handle, process and swallow for nestlings until the very latest stage of the nestling period.
To conclude, there is a need for further theoretical and empirical work on community interactions with species-specific or multi-species functional responses to different prey (Hanski and Henttonen 1996; Koen-Alonso 2007; Matthiopoulos et al. 2007; Sinclair et al. 1998). We advocate that, in studies and analyses of the rodent community in subarctic Fennoscandia, species should be treated separately and not as a collective unit, which has been a debated issue (see discussions in Falck et al. 1995; Turchin 2003). The novel approach adopted in this study also revealed that functional responses can vary depending on within-year variation in the density of different prey species. An alternative individual-based approach in which processes occurring on different spatio-temporal scales are both coupled and compared (e.g. Englund and Leonardsson 2008) therefore seems to be necessary to bridge the gap between theoretical and empirical studies of functional responses.
References
Abrams P (1987) The functional responses of adaptive consumers of two resources. Theor Popul Biol 32:262–288. doi:10.1016/0040-5809(87)90050-5
Abrams PA (1994) The fallacies of ‘ratio-dependent’ predation. Ecology 75:1842–1850. doi:10.2307/1939644
Abrams PA, Ginzburg LR (2000) The nature of predation: prey dependent, ratio dependent or neither? Trends Ecol Evol 15:337–341. doi:10.1016/S0169-5347(00)01908-X
Abrams P, Matsuda H (1993) Effects of adaptive predatory and anti-predator behaviour in a two-prey-one-predator system. Evol Ecol 7:312–326. doi:10.1007/BF01237749
Andersson M (1976) Lemmus lemmus: a possible case of aposematic coloration and behavior. J Mammal 57:461–469. doi:10.2307/1379296
Arditi R, Ginzburg LR (1989) Coupling in predator-prey dynamics: ratio-dependence. J Theor Biol 139:311–326. doi:10.1016/S0022-5193(89)80211-5
Asseburg C (2006) A Bayesian approach to modelling field data on multi-species predator-prey interactions. PhD thesis, University of St. Andrews, St. Andrews
Barth L, Angerbjörn A, Tannerfeldt M (2000) Are Norwegian lemmings Lemmus lemmus avoided by arctic Alopex lagopus or red foxes Vulpes vulpes? A feeding experiment. Wildl Biol 6:101–109
Boutin S (1995) Testing predator-prey theory by studying fluctuating populations of small mammals. Wildl Res 22:89–100. doi:10.1071/WR9950089
Brom TG (1986) Microscopic identification of feathers and feather fragments of palearctic birds. Bijdr Dierkunde 56:181–204
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. In: A practical information-theoretic approach, 2nd edn. Springer, New York
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. doi:10.1016/j.ecolmodel.2006.03.017
Chesson J (1978) Measuring preference in selective predation. Ecology 59:211–215. doi:10.2307/1936364
Chesson PL (1984) Variable predators and switching behavior. Theor Popul Biol 26:1–26. doi:10.1016/0040-5809(84)90021-2
Cornulier T, Yoccoz NG, Bretagnolle V, Brommer JE, Butet A, Ecke F, Elston DA, Framstad E, Henttonen H, Hörnfeldt B, Huitu O, Imholt C, Ims RA, Jacob J, Jedrzejewska B, Millon A, Petty S, Pietiäinen H, Tkadlec E, Zub K, Lambin X (2013) Europe-wide dampening of population cycles in keystone herbivores. Science 340:63–66. doi:10.1126/science.1228992
Cramp S, Simmons KEL (eds) (1980) The birds of the Western Palearctic, vol 2. Oxford University Press, Oxford
Davoren GK, Burger AE (1999) Differences in prey selection and behaviour during self-feeding and chick provisioning in rhinoceros auklets. Anim Behav 58:853–863. doi:10.1006/anbe.1999.1209
Day MG (1966) Identification of hair and feather remains in the gut and faeces of stoats and weasels. J Zool 148:201–217. doi:10.1111/j.1469-7998.1966.tb02948.x
Development Core Team R (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Dickman CR (1992) Predation and habitat shift in the house mouse, Mus domesticus. Ecology 73:313–322. doi:10.2307/1938742
Dickman CR, Predavec M, Lynam AJ (1991) Differential predation of size and sex classes of mice by the barn owl, Tyto alba. Oikos 62:67–76. doi:10.2307/3545447
Ecke F, Christensen P, Rentz R, Nilsson M, Sandström P, Hörnfeldt B (2010) Landscape structure and the long-term decline of cyclic grey-sided voles in Fennoscandia. Landsc Ecol 25:551–560. doi:10.1007/s10980-009-9441-x
Ekerholm P, Oksanen L, Oksanen T (2001) Long-term dynamics of voles and lemmings at the timberline and above the willow limit as a test of hypotheses on trophic interactions. Ecography 24:555–568. doi:10.1034/j.1600-0587.2001.d01-211.x
Englund G, Leonardsson K (2008) Scaling up the functional response for spatially heterogeneous systems. Ecol Lett 11:440–449. doi:10.1111/j.1461-0248.2008.01159.x
Falck W, Bjørnstad ON, Stenseth NC (1995) Voles and lemmings: chaos and uncertainty in fluctuating populations. Proc R Soc Lond B 262:363–370. doi:10.1098/rspb.1995.0218
Framstad E, Stenseth NC, Bjørnstad ON, Falck W (1997) Limit cycles in Norwegian lemmings: tensions between phase-dependence and density-dependence. Proc R Soc Lond B 264:31–38. doi:10.1098/rspb.1997.0005
Fryxell JM, Lundberg P (1998) Individual behaviour and community dynamics. Chapman & Hall, London
Gruyer N, Gauthier G, Berteaux D (2008) Cyclic dynamics of sympatric lemming populations on Bylot Island, Nunavut, Canada. Can J Zool 86:910–917. doi:10.1139/Z08-059
Hagemeijer WJM, Blair MJ (1997) The EBCC atlas of European breeding birds. Poyser, London
Hagen Y (1952) Rovfuglene og viltpleien. Gyldendal Norsk, Oslo
Hagen Y (1969) Norske undersøkelser over avkomproduksjonen hos rovfugler og ugler sett i relasjon til smågnagerbestandens vekslinger. Fauna 22:73–126
Hakkarainen H, Korpimäki E, Mappes T, Palokangas P (1992) Kestrel hunting behaviour towards solitary and grouped Microtus agrestis and M. epiroticus—a laboratory experiment. Ann Zool Fenn 29:279–284
Hambäck PA, Schneider M, Oksanen T (1998) Winter herbivory by voles during a population peak: the relative importance of local factors and landscape pattern. J Anim Ecol 67:544–553. doi:10.1046/j.1365-2656.1998.00231.x
Hansen TF, Stenseth NC, Henttonen H (1999) Multiannual vole cycles and population regulation during long winters: an analysis of seasonal density dependence. Am Nat 154:129–139. doi:10.1086/303229
Hanski I, Henttonen H (1996) Predation on competing rodent species: a simple explanation of complex patterns. J Anim Ecol 65:220–232. doi:10.2307/5725
Hanski I, Henttonen H, Hansson L (1994) Temporal variability and geographical patterns in the population density of microtine rodents: a reply to Xia and Boonstra. Am Nat 144:329–342. doi:10.1086/285678
Hanski I, Henttonen H, Korpimäki E, Oksanen L, Turchin P (2001) Small-rodent dynamics and predation. Ecology 82:1505–1520. doi:10.2307/2679796
Hellström P (2007) Interactions between rodents and rough-legged buzzards (Buteo lagopus) in northern Sweden. Phil. lic. thesis, Stockholm University, Stockholm
Henttonen H, Hanski I (2000) Population dynamics of small rodents in northern Fennoscandia. In: Perry JN, Smith RH, Woiwood IP (eds) Chaos in Real Data. Kluwer, Dordrecht, pp 73–96
Henttonen H, Kaikusalo A (1993) Lemming movements. In: Stenseth NC, Ims RA (eds) The biology of lemmings. Academic Press, London, pp 157–186
Henttonen H, Wallgren H (2001) Rodent dynamics and communities in the birch forest zone of northern Fennoscandia. In: Wielgolaski FE (ed) Nordic Mountain Birch Ecosystems. UNESCO, Carnforth
Henttonen H, Oksanen T, Jortikka A, Haukisalmi V (1987) How much do weasels shape microtine cycles in the northern Fennoscandian taiga? Oikos 50:353–365. doi:10.2307/3565496
Heske EJ, Steen H (1993) Interspecific interactions and microhabitat use in a Norwegian low alpine rodent assemblage. In: Stenseth NC, Ims RA (eds) The biology of lemmings. Academic, London, pp 397–409
Holling CS (1959a) The components of predation as revealed by a study of small mammal predation of the European pine sawfly. Can Entomol 91:293–320
Holling CS (1959b) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Huhtala K, Pulliainen E, Jussila P, Tunkkari PS (1996) Food niche of the gyrfalcon Falco rusticolus nesting in the far north of Finland compared with other choices of the species. Ornis Fenn 73:78–87
Ims RA, Fuglei E (2005) Trophic interaction cycles in tundra ecosystems and the impact of climate change. Bioscience 55:311–322. doi:10.1641/0006-3568(2005)055[0311:TICITE]2.0.CO;2
Ims RA, Yoccoz NG, Killengreen ST (2011) Determinants of lemming outbreaks. Proc Natl Acad Sci USA 108:1970–1974. doi:10.1073/pnas.1012714108
Inchausti P, Ballesteros S (2008) Intuition, functional responses and the formulation of predator–prey models when there is a large disparity in the spatial domains of the interacting species. J Anim Ecol 77:891–897. doi:10.1111/j.1365-2656.2008.01419.x
Johannesen E, Mauritzen M (1999) Habitat selection of grey-sided voles and bank voles in two subalpine populations in southern Norway. Ann Zool Fenn 36:215–222
Jost C, Devulder G, Vucetich JA, Peterson RO, Arditi R (2005) The wolves of Isle Royale display scale-invariant satiation and ratio-dependent predation on moose. J Anim Ecol 74:809–816. doi:10.1111/j.1365-2656.2005.00977.x
Kean-Howie JC, Pearre S Jr, Dickie LM (1988) Experimental predation by sticklebacks on larval mackerel and protection of fish larvae by zooplankton alternative prey. J Exp Mar Biol Ecol 124:239–259. doi:10.1016/0022-0981(88)90174-8
Koen-Alonso M (2007) A process-oriented approach to the multispecies functional response. In: Rooney N, McCann KS, Noakes DLG (eds) From energetics to ecosystems: the dynamics and structure of ecological systems. Springer, Dordrecht
Koivunen V, Korpimäki E, Hakkarainen H, Norrdahl K (1996) Prey choice of Tengmalm’s owls (Aegolius funereus): preference for substandard individuals? Can J Zool 74:816–823. doi:10.1139/z96-094
Koponen T, Kokkonen A, Kalela O (1961) On a case of spring migration in the Norwegian lemming. Ann Acad Sci Fenn Ser A 52:1–30
Korpimäki E, Tolonen P, Valkama J (1994) Functional responses and load-size effect in central place foragers: data from the kestrel and some general comments. Oikos 69:504–510. doi:10.2307/3545862
Korpimäki E, Norrdahl K, Klemola T, Pettersen T, Stenseth NC (2002) Dynamic effects of predators on cyclic voles: field experimentation and model extrapolation. Proc R Soc Lond B 269:991–997. doi:10.1098/rspb.2002.1972
Krebs CJ, Myers JH (1974) Population cycles in small mammals. Adv Ecol Res 8:267–399. doi:10.1016/S0065-2504(08)60280-9
Krebs CJ, Kenney AJ, Gilbert S, Danell K, Angerbjörn A, Erlinge S, Bromley RG, Shank C, Carriere S (2002) Synchrony in lemming and vole populations in the Canadian arctic. Can J Zool 80:1323–1333. doi:10.1139/z02-120
Kuno E (1987) Principles of predator-prey interaction in theoretical, experimental, and natural population systems. Adv Ecol Res 16:249–337. doi:10.1016/S0065-2504(08)60090-2
Lindén H, Wikman M (1983) Goshawk predation on tetraonids: availability of prey and diet of the predator in the breeding season. J Anim Ecol 52:953–968. doi:10.2307/4466
Magnusson M, Bergsten A, Ecke F, Bodin Ö, Bodin L, Hörnfeldt B (2013) Predicting grey-sided vole occurrence in northern Sweden at multiple spatial scales. Ecol Evol 3:4365–4376. doi:10.1002/ece3.827
Manly BFJ, Miller P, Cook LM (1972) Analysis of a selective predation experiment. Am Nat 106:719–736. doi:10.1086/282808
Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP (2002) Resource selection by animals—statistical design and analysis for field studies, 2nd edn. Kluwer, Dordrecht
Markman S, Pinshow B, Wright J, Kotler BP (2004) Food patch use by parent birds: to gather food for themselves or for their chicks? J Anim Ecol 73:747–755. doi:10.1111/j.0021-8790.2004.00847.x
Matthiopoulos J, Graham K, Smout S, Asseburg C, Redpath S, Thirgood S, Hudson P, Harwood J (2007) Sensitivity to assumptions in models of generalist predation on a cyclic prey. Ecology 88:2576–2586. doi:10.1890/06-0483.1
Messier F (1995) On the functional and numerical responses of wolves to changing prey density. In: Carbyn LN, Fritts SH, Seip DR (eds) Ecology and conservation of wolves in a changing world. Canadian Circumpolar Institute, Edmonton, pp 187–197
Moleón M, Sánchez-Zapata JA, Gil-Sánchez JM, Ballesteros-Duperón E, Barea-Azcón JM, Virgós E (2012) Predator–prey relationships in a Mediterranean vertebrate system: Bonelli’s eagles, rabbits and partridges. Oecologia (Berl) 168:679–689. doi:10.1007/s00442-011-2134-6
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol Monogr 39:335–354. doi:10.2307/1942352
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131. doi:10.1016/S0065-2504(08)60288-3
Murdoch WW, Briggs CJ, Nisbet RM (2003) Consumer-resource dynamics. Princeton University Press, Princeton
Myllymäki A, Aho J, Lind EA, Tast J (1962) Behaviour and daily activity of the Norwegian lemming, Lemmus lemmus (L.) during autumn migration. Ann Zool Soc Zool Bot Fenn Vanamo 24:1–31
Nelson L Jr, Clark FW (1973) Correction for sprung traps in catch/effort calculations of trapping results. J Mammal 54:295–298. doi:10.2307/1378903
Niethammer J, Krapp F (eds) (1982) Handbuch der Säugetiere Europas. Band 2/I, Nagetiere II (Cricetidae, Arvicolidae, Zapodidae, Spalacidae, Hystricidae, Capromyidae). Academische, Wiesbaden
Nishimura K, Abe MT (1988) Prey susceptibilities, prey utilization and variable attack efficiences of ural owls. Oecologia (Berl) 77:414–422. doi:10.1007/BF00378053
Norrdahl K, Korpimäki E (1993) Predation and interspecific competition in two Microtus voles. Oikos 67:149–158. doi:10.2307/3545105
Nyström J, Ekenstedt J, Angerbjörn A, Thulin L, Hellström P, Dalén L (2006) Golden Eagles on the Swedish mountain tundra—diet and breeding success in relation to prey fluctuations. Ornis Fenn 83:145–152
Oaten A, Murdoch WW (1975) Switching, functional response, and stability in predator-prey systems. Am Nat 109:299–318. doi:10.1086/282999
O’Donoghue M, Boutin S, Krebs CJ, Murray DL, Hofer EJ (1998) Behavioural responses of coyotes and lynx to the snowshoe hare cycle. Oikos 82:169–183. doi:10.2307/3546927
Oksanen T (1993) Does predation prevent Norwegian lemmings from establishing permanent populations in lowland forests? In: Stenseth NC, Ims RA (eds) The biology of lemmings. Academic, London, pp 425–437
Oksanen T, Henttonen H (1996) Dynamics of voles and small mustelids in the taiga landscape of northern Fennoscandia in relation to habitat quality. Ecography 19:432–443. doi:10.1111/j.1600-0587.1996.tb00254.x
Oksanen L, Moen J, Lundberg PA (1992) The time-scale problem in exploiter-victim models: does the solution lie in ratio-dependent exploitation? Am Nat 140:938–960. doi:10.1086/285449
Oksanen T, Schneider M, Rammul Ü, Hambäck P, Aunapuu M (1999) Population fluctuations of voles in north Fennocandian tundra: contrasting dynamics in adjacent areas with different habitat composition. Oikos 86:463–478. doi:10.2307/3546651
Oksanen T, Oksanen L, Schneider M, Aunapuu M (2001) Regulation, cycles and stability in northern carnivore-herbivore systems: back to first principles. Oikos 94:101–117. doi:10.1034/j.1600-0706.2001.11315.x
Oksanen T, Oksanen L, Dahlgren J, Olofsson J (2008) Arctic lemmings, Lemmus spp. and Dicrostonyx spp.: integrating ecological and evolutionary perspectives. Evol Ecol 10:415–434
Olofsson J, Oksanen L, Callaghan T, Hulme PE, Oksanen T, Suominen O (2009) Herbivores inhibit climate-driven shrub expansion on the tundra. Glob Change Biol 15:2681–2693. doi:10.1111/j.1365-2486.2009.01935.x
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 155–177
Øvrejorde A (2007) Calibrating abundance indices of small rodents in subarctic tundra. MSc thesis, University of Tromsø, Tromsø
Palma L, Beja P, Pais M, Cancela da Fonseca L (2006) Why do raptors take domestic prey? The case of Bonelli’s eagles and pigeons. J Appl Ecol 43:1075–1086. doi:10.1111/j.1365-2664.2006.01213.x
Pasanen S, Sulkava S (1971) On the nutritional biology of the rough-legged buzzard, Buteo lagopus Brünn., in Finnish Lapland. Aquilo Ser Zool 12:53–63
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer Science, New York
Pinheiro J, Bates D, DebRoy S, Sarkar D, The R Development Core Team (2013) nlme: Linear and monlinear mixed effects models. R package version 3.1-113
Potapov ER (1993) Ecology and energetics of rough-legged buzzard in the Kolyma river Lowlands. PhD thesis, University of Oxford, Oxford
Potapov ER (1997) What determines the population density and reproductive success of rough-legged buzzards, Buteo lagopus, in the Siberian tundra? Oikos 78:362–376
Real LA (1977) The kinetics of functional response. Am Nat 111:289–300. doi:10.1086/283161
Reid DG, Krebs CJ, Kenney AJ (1997) Patterns of predation on noncyclic lemmings. Ecol Monogr 67:89–108. doi:10.2307/2963506
Rindorf A, Gislason H, Lewy P (2006) Prey switching of cod and whiting in the North Sea. Mar Ecol Prog Ser 325:243–253. doi:10.3354/meps325243
Rohner C, Krebs CJ (1996) Owl predation on snowshoe hares: consequences of antipredator behaviour. Oecologia (Berl) 108:303–310. doi:10.1007/BF00334655
Shifferman E, Eilam D (2004) Movement and direction of movement of a simulated prey affect the success rate in barn owl Tyto alba attack. J Avian Biol 35:111–116. doi:10.1111/j.0908-8857.2004.03257.x
Siivonen L (1976) Nordeuropas däggdjur, 2nd edn. Norstedt, Stockholm
Sinclair ARE, Pech RP (1996) Density dependence, stochasticity, compensation and predator regulation. Oikos 75:164–173. doi:10.2307/3546240
Sinclair ARE, Pech RP, Dickman CR, Hik D, Mahon P, Newsome AE (1998) Predicting effects of predation on conservation of endangered prey. Conserv Biol 12:564–575. doi:10.1046/j.1523-1739.1998.97030.x
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–35. doi:10.2307/1578
Sonerud GA (1986) Effect of snow cover on seasonal changes in diet, habitat, and regional distribution of raptors that prey on small mammals in boreal zones of Fennoscandia. Holarct Ecol 9:33–47. doi:10.1111/j.1600-0587.1986.tb01189.x
Sonerud GA (1992) Functional responses of birds of prey: biases due to the load-size effect in central place foragers. Oikos 63:223–232. doi:10.2307/3545382
Stenseth NC, Ims RA (1993) The biology of lemmings. Academic, London
Stephens DW, Krebs CJ (1986) Foraging theory. Princeton University Press, Princeton
Sundell J, Ylönen H (2008) Specialist predator in a multi-species prey community: boreal voles and weasels. Integr Zool 3:51–63. doi:10.1111/j.1749-4877.2008.00077.x
Sundell J, Huitu O, Henttonen H, Kaikusalo A, Korpimäki E, Pietiäinen H, Saurola P, Hanski I (2004) Large-scale spatial dynamics of vole populations in Finland revealed by the breeding success of vole-eating avian predators. J Anim Ecol 73:167–178. doi:10.1111/j.1365-2656.2004.00795.x
Sylvén M (1978) Interspecific relations between sympatrically wintering common buzzards Buteo buteo and rough-legged buzzards Buteo lagopus. Ornis Scand 9:197–206. doi:10.2307/3675882
Taitt MJ (1993) Adaptive coloration in Lemmus lemmus: why aren’t Norwegian lemmings brown? In: Stenseth NC, Ims RA (eds) The biology of lemmings. Academic, London, pp 439–445
Taylor A (2009) Grey-sided vole dynamics in mountainous northern Sweden. MSc thesis, Stockholm University, Stockholm
Temple SA (1987) Do predators always capture substandard individuals disproportionately from prey populations? Ecology 68:669–674. doi:10.2307/1938472
Turchin P (2003) Complex population dynamics. Princeton University Press, Princeton
Turchin P, Hanski I (1997) An empirically based model for latitudinal gradient in vole population dynamics. Am Nat 149:842–874. doi:10.1086/286027
Turchin P, Oksanen L, Ekerholm P, Oksanen T, Henttonen H (2000) Are lemmings prey or predators? Nature 405:562–565. doi:10.1038/35014595
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Wallin L (1967) The dorsal skin gland of the Norwegian lemming Lemmus l. lemmus (L.). Z Morph Ökol Tiere 59:83–90. doi:10.1007/BF02427737
White GC, Anderson DR, Burnham KP, Otis DL (1982) Capture-recapture and removal methods for sampling closed populations. Los Alamos National Laboratory, Los Alamos
Wiklund CG, Kjellén N, Isakson E (1998) Mechanisms determining the spatial distribution of microtine predators on the Arctic tundra. J Anim Ecol 67:91–98. doi:10.1046/j.1365-2656.1998.00177.x
Ydenberg R (2007) Provisioning. In: Stephens DW, Brown JS, Ydenberg RC (eds) Foraging. Behavior and ecology. University of Chicago Press, Chicago, pp 273–303
Acknowledgments
We are grateful to Johan S. Eklöf for field work, and to Peter Lindberg who conducted surveys in our study area during 1966–1978. Charles J. Krebs gave instructive comments on rodent trapping methods. Geir A. Sonerud, Peter Abrams, Bertil Borg, Love Dalén, Hannu Ylönen, Janne Sundell and anonymous referees provided constructive comments that improved the manuscript. Vattenfall AB, Porjus, supported us with lodging facilities. This project was financed by grants to A. Angerbjörn from The Strategic Foundation for Environmental Research (to the Mountain-MISTRA project), Formas, and Swedish Polar Research Secreteriat/Swedish Research Council. P. Hellström received financial support from Alvin’s foundation for bird protection, the CLUB 300 foundation for bird protection and the Göran Gustafsson foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Janne Sundell.
Rights and permissions
About this article
Cite this article
Hellström, P., Nyström, J. & Angerbjörn, A. Functional responses of the rough-legged buzzard in a multi-prey system. Oecologia 174, 1241–1254 (2014). https://doi.org/10.1007/s00442-013-2866-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2866-6