Abstract
Foraging theory predicts that predators should prefer foraging in habitat patches with higher prey densities. However, density depends on the spatial scale at which a “patch” is defined by an observer. Ecologists strive to measure prey densities at the same scale that predators do, but many natural landscapes lack obvious, well-defined prey patches. Thus one must determine the scale at which predators define patches of prey. We estimated the scale at which guppies, Poecilia reticulata, selected patches of zooplankton prey using a behavioral assay. Guppies could choose between two prey arrays, each manipulated to have a density that depended on the spatial scale at which density was calculated. We estimated the scale of guppy foraging by comparing guppy preferences across a series of trials in which we systematically varied the scale associated with “high” prey density. This approach enables the application of foraging theory to non-discrete habitats and prey landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal foraging theory (OFT) is a commonly accepted framework for understanding predatory foraging behavior (Pyke et al. 1977; Krebs and Davies 1986; Stephens and Krebs 1986; Wellenreuther and Connell 2002). It predicts that, all else being equal, a predator choosing among patches of prey will select the patch that provides the highest net rate of energy acquisition, which is typically the patch with the highest prey density (Emlen 1966; MacArthur and Pianka 1966; Stephens and Krebs 1986). The predictions of OFT have been empirically supported in a variety of taxa including mammals (Bergman et al. 2001; Johnson et al. 2001), birds (Krebs et al. 1974; Smith and Sweatman 1974; Zach and Falls 1976; Cowie 1977), fishes (Townsend and Winfield 1985), insects (Pyke 1978; Schellhorn and Andow 2005), and parasitoids (Waage 1979). Understanding how predators choose when and where to forage is an important aspect of understanding life history patterns and community ecology (Pyke et al. 1977; Mangel and Clark 1986; Bax 1998; Osborne et al. 2008).
The predictions of OFT are straightforward to apply when predators choose between obviously distinct patches of prey, such as chickadees choosing between artificial cones hanging in groups (Krebs et al. 1974) or parasitoids choosing between moth hosts (Waage 1979). It is more difficult to determine how best to apply OFT predictions in more continuous prey landscapes such as on coral reefs, forests, or grasslands (Bond 1980). In those landscapes, the environment does not consist of obvious patches (obvious to a human observer, at least). Does a roving reef fish choose “patches” corresponding to a single coral colony, or a cluster of several colonies, or a larger expanse of reef? The answer to that question is important, because in any landscape the spatial scale at which one measures prey density (i.e., the denominator in the calculation of prey per unit area) strongly affects the estimate of density, particularly if prey are clumped (under-dispersed) in space. Moreover, it is clear that predators estimate and respond to prey density at particular spatial scales. For example, Wellenreuther and Connell (2002) found that magpie perch (Cheilodactylus nigripes) had longer feeding bouts on large (>160 cm2) than small (<40 cm2) prey patches even though prey density per patch were equal for both patch sizes. Ecologists are frequently exhorted to ensure that the scale of their measurements corresponds to the scale of the relevant ecological processes (e.g., Levin 1992), but in this case it is difficult to know the scale at which predators define patches.
The most common approach for determining the scale of a predator’s foraging decisions is to examine the spatial scale at which predation produces density-dependent mortality in their prey. In general, predators’ aggregative and functional responses should produce directly density-dependent mortality in their prey, but this will only be detectable if the observer is measuring prey density on the same spatial scale at which the predator is foraging (Ray and Hastings 1996). At smaller spatial scales, predators are likely foraging randomly with respect to prey density, so mortality may be inversely density dependent when measured at that scale (White et al. 2010). This approach has been used to estimate the scale of predator foraging decisions in several species of insects (Turchin and Kareiva 1989; Stiling et al. 1991; Mohd Norowi et al. 2000; Schellhorn and Andow 2005) and coral reef fishes (White and Warner 2007; White et al. 2010). Similarly, observations of seabird foraging patterns have been used to estimate the spatial scale at which they respond to the density of their pelagic fish prey (Burger et al. 2004). However, there has not yet been a direct experimental determination of the spatial scale at which predators respond to prey density.
Determining the spatial scale at which predators define prey patches and make foraging decisions is essential to applying OFT to continuous habitats, and has strong implications for prey population dynamics because of the relationship between foraging scale and density-dependent mortality (Ray and Hastings 1996; White et al. 2010; White 2011). In this study, we used guppies, Poecilia reticulata, as a model species to estimate the spatial scale of predation directly using a behavioral assay. Guppies were given systematic choices between configurations of their prey (the cladoceran Daphnia magna) arranged at different scale-dependent densities, and their preferences revealed the spatial scale at which they defined prey patches.
Materials and methods
Study species
We used the guppy, Poecilia reticulata Peters 1859, as our model predator because it is a convenient representative of visual-searching predatory fishes for use in laboratory experimentation (Abrahams 1989; Day et al. 2001; Swaney et al. 2001). Male individuals were purchased from Carolina Biological Supply Company (Burlington, NC), divided haphazardly into three experimental groups of approximately six individuals (due to natural mortality and reproduction there were three to nine individuals per group by the end of the experiment), and housed in separate 38-l aquaria at 25 °C with a 12-h light/dark cycle. Guppies were acclimatized for at least 2 weeks before being used in behavioral trials. When not used in trials, guppies were fed commercial brine shrimp flakes ad libitum daily. All experimental procedures were approved by the University of North Carolina Wilmington (UNCW) Institutional Animal Care and Use Committee, protocol number A1112-010.
Cladocerans, Daphnia magna (hereafter “Daphnia”), were utilized as our model prey species because they are easily cultured and have been used in prior studies on optimal foraging behavior in teleost fishes (Werner and Hall 1974; Ioannou and Krause 2008; Ioannou et al. 2009). Multiple Daphnia were fed to guppies daily for at least 1 week prior to performing trials to familiarize the guppies with Daphnia as a food source.
Behavioral assay
Our assay consisted of numerous trials which each gave guppies a choice between two prey groups which were arranged in groups of differing compaction. The guppies’ preferences between prey arrangements were observed to determine their foraging spatial scale. In order to maintain constant prey abundance and density throughout each trial, each prey group was composed of 24 Daphnia distributed into 12 test tubes (7.5 cm height, 1 cm diameter) which were arranged in a linear array. Daphnia were distributed in six arrangements with increasingly compact densities from two Daphnia per test tube (distributed across all 12 test tubes; least compact) to 24 Daphnia per test tube (only one of the 12 test tubes contained Daphnia; most compact). Thus all six arrangements had the same density when measured at the spatial scale of the entire 12-tube array, but more compact arrangements had higher densities when measured at smaller spatial scales (Fig. 1). Hereafter we refer to arrangements with higher densities at smaller scales to be more “compact.” If guppies define a patch of prey at some characteristic scale, we assumed that the guppies would prefer a prey array that had high density at that scale (or smaller scales) over a prey array that was less compact and had a lower density at that scale, even if the two arrays had the same density at the largest scale of 12 tubes. We systematically compared each possible combination of prey group arrangements in pairwise trials to determine the spatial scale at which the guppies defined a patch of prey.
Diagram of all six prey group arrangements (A2, A3, A4, A6, A12, A24). Circles represent test tubes, with numbers inside indicating number of Daphnia individuals. All arrangements had 24 Daphnia total and thus, equal prey density at a spatial scale ≥12 test tubes. At smaller spatial scales, prey density differs between arrangements
To illustrate how these comparisons indicated patch size, consider the most extreme prey group comparison: arrangement 2 (A2) and arrangement 24 (A24) (Fig. 2). If the perceived patch size is smaller than 12 tubes, then A24 concentrates the prey into a space smaller than the patch size, inflating the effective within-patch prey density perceived by the guppies, and we would expect the guppies to prefer that arrangement over A2. Similar logic applied to all other comparisons; if the more compact prey arrangement concentrated prey into a space smaller than the perceived patch size, we expected the guppies would prefer that arrangement due to the higher perceived prey density.
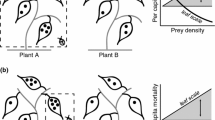
Comparison of two prey group arrangements with two hypothetical patch sizes indicated by ovals. a If guppies, Poecilia reticulata, defined a patch of prey at a spatial scale 12 test tubes wide, then the prey density in both perceived patches would have been equal and guppies would have exhibited no preference for either prey group. b If, however, guppies defined a patch of prey at a spatial scale <12 test tubes wide (i.e. one test tube wide), then the guppies would have perceived arrangement 2 as 12 separate patches each with prey density = 2 prey items, and arrangement 24 as one patch with prey density = 24 prey items, and exhibited a preference for the more compacted arrangement 24
Experimental apparatus
In each behavioral trial, one of the three groups of adult male guppies (1.5–2 cm standard length [SL]) was transferred with a dip net from its “home” aquarium to an experimental aquarium (0.9 m × 0.6 m × 0.3 m) (Fig. 3). The experimental aquarium was lined with opaque corrugated plastic and divided into three sections: a holding area where guppies were initially released and acclimatized for 10 min, the foraging arena, and a section with a light source and heaters. An 18-cm-wide gate separated the holding area from the foraging arena and plastic mesh netting separated the arena from the light and heaters. Water was 25 °C and ~12 cm deep in the experimental aquarium. This was deep enough to cover the test tube arrays, but constrained guppy behavior to primarily two dimensions. We withheld food from guppies for 24 h prior to each trial to ensure interest in prey during the trial. Two prey arrays were randomly assigned to both sides of the arena.
Experimental aquarium divided into three sections. The dashed line represents an 18-cm-wide gate that was raised for guppies, P. reticulata, to enter the foraging arena and lowered when the trials began. The dotted line represents plastic mesh netting. Guppies were considered “near” the prey arrays if they were inside the thin quarter circle arcs in the arena (radius = 27 cm). Note that guppies are not drawn to scale
Trials began when the gate was opened and at least three guppies swam into the foraging arena. The gate was immediately closed and the trial was conducted for 10 min. Multiple individuals were used because guppies forage naturally in shoals (Day et al. 2001), which led to more normal behavior than in isolated individuals, which behaved erratically. Guppies were defined as “near” a prey group when they were observed within 27 cm of the prey. This distance was half the width of the foraging arena and within the distance that visually foraging fish have been observed to detect Daphnia (Werner and Hall 1974). When guppies were ≥27 cm from either prey group (~25 % of the arena area), they were defined as choosing neither group. Each guppy group was used one to two times for each prey comparison treatment with four to six replications per treatment in total.
Analysis
Trials were video recorded from above the arena with a Canon VIXIA HF G10 high-definition video camera and frames were extracted from the videos in 30-s intervals using Matlab 7.13 (Mathworks, Natick, MA). Frames in which no guppies were observed near either prey group were excluded from analysis because they provided no information on prey preference (the guppies were not exhibiting foraging behavior); for two trials in which at least 75 % of the frames were excluded, the entire trials were discarded. In each remaining trial frame, the number of guppies near each prey group was noted and the difference in the number of guppies between the more compact prey group and the less compact prey group was calculated. The mean difference for all 20 frames in each trial was recorded. In each prey comparison treatment, we tested the one-tailed hypothesis that the mean difference would be positive (i.e., guppies spent more time, and thus, preferred the more compact prey group). We predicted that this would occur when the guppies defined a patch scale smaller than the size of the less compact prey group.
We tested for a positive difference between more and less compact arrays using a linear mixed-effects model with the intercept as the only fixed effect (i.e., the model would estimate the mean difference in the number of guppies) and guppy population (home aquarium) as the random effect. Fish from each aquarium were used in more than one replicate in each trial, so this random effect allowed us to account for the non-independence of those replicates. Statistical significance for a particular prey comparison treatment was determined by examining the posterior distribution of the fixed effect. All analyses were conducted in R version 2.15.0 (R Development Core Team 2012); mixed models were performed with the function lmer in the lme4 package (Bates et al. 2012). To estimate the mean and precision of the fixed effect, we used the function sim in the arm package (Gelman and Su 2013) to simulate the posterior distribution (Gelman and Hill 2007). We chose the simulation approach rather than the typical parametric approach to estimating confidence intervals on parameters because there is uncertainty regarding how best to estimate df in a mixed-effects model (Bolker et al. 2009).
Although our individual experiments were designed to test narrow hypotheses (e.g., that guppies prefer prey arrangement A24 to arrangement A2), our ultimate goal was to test for an overall preference for prey aggregated at or below a particular spatial scale. Specifically, we intended to test whether the perceived patch spatial scale was <12, <8, <6, <4, or <2 test tubes wide. For example, if the scale is <12 test tubes, we would expect that guppies would show a preference for any arrangement more compact than A2 (two Daphnia in each tube). To test for that preference, we used a meta-analysis approach. We used Fisher’s combined probability test (Fisher 1932) to combine the p-values from the individual comparisons A2 vs. A24, A2 vs. A12, A2 vs. A6, A2 vs. A4, and A2 vs. A3 (each testing the one-tailed hypothesis that the more compact arrangement is preferred), producing a p-value for the aggregate metahypothesis that the spatial scale is <12 test tubes. The α-value for each metahypothesis was adjusted to lessen the likelihood of a type-I error due to combined probability values from multiple hypotheses by the equation: \(\alpha = 0.05\left( {k + 1} \right)/\left( {2 k} \right)\) where k denotes the number of treatment hypotheses pooled.
There is controversy regarding the use of one-tailed tests, as they often appear to be a ploy to increase power in the face of an arbitrary α-value (Hurlbert and Lombardi 2009). However we used one-tailed tests in order to obtain p-values relevant to our metahypotheses (that there was a preference for more compact prey arrangements at a particular spatial scale); evaluating a two-tailed null hypothesis (there was a preference for either the more compact or less compact arrangement) would not allow the same metahypothesis test.
Results
We conducted a total of 139 trials, testing 19 prey comparison treatments. During the trials, guppies were often observed inspecting and biting at the test tubes that contained Daphnia, clearly showing interest in the prey items.
For our first metahypothesis (perceived patch spatial scale <12 test tubes), we predicted that guppies would have preferred the more compact prey arrangement in the five treatments comparing arrangement 2 to a more compact arrangement. Guppies spent a greater average time near the more compact prey arrangement in all five of these treatments (positive fixed-effect coefficient; Table 1; Table A1). This overall preference was statistically significant (Fisher’s combined probability test; p = 3.767 × 10−4, α = 0.030), leading us to accept our first metahypothesis.
We predicted given our second metahypothesis (perceived patch spatial scale less than eight test tubes) that guppies would prefer the more compact prey arrangement in the four treatments comparing arrangement 3 to a more compact arrangement. Unlike the first metahypothesis, guppies did not show a significant preference for the more compacted prey arrangements in any of the four treatments (fixed-effect coefficients near zero and p > 0.3 in all comparisons; Table 1; Table A1). We therefore rejected our second metahypothesis that the perceived patch spatial scale was less than eight test tubes wide (Fisher’s combined probability test; p = 0.560, α = 0.03125).
Similarly, we rejected all remaining metahypotheses since guppies did not prefer any prey arrangements in any treatments predicted by these metahypotheses (note near-zero or negative fixed-effect coefficients in Table 1; Fisher’s combined probability test, less than six test tubes: p = 0.831, α = 0.0333; less than four test tubes, p = 0.696, α = 0.0375; less than two test tubes, p = 0.401, α = 0.05; Table 1; Table A1).
Discussion
In our behavioral assay, predatory guppies defined a patch of prey at a spatial scale of eight or more, but <12, test tubes wide (10 cm ≤ patch scale width <15.2 cm). This is the first study, to our knowledge, that has determined the spatial scale of a prey patch as defined by a predator in a continuous landscape using a direct experimental trial rather than relying on indirect observations of density-dependent prey mortality (e.g., Stiling et al. 1991; Schellhorn and Andow 2005) or predator behavior [e.g., Schneider and Piatt 1986; Elliott and Kieckhefer 2000; Burger et al. 2004; but see Cummings et al. (1997) for an earlier demonstration that shorebirds did not respond to very small-scale differences in prey density]. Direct observation of predatory choices is a more powerful approach for determining the spatial scale of foraging than extrapolations from indirect observations of prey mortality, because the detection of density-dependent mortality in the field is fraught with other potentially confounding factors (e.g., Osenberg et al. 2002; White et al. 2010). As such we hope that our experimental approach for defining predators’ scale-dependent choices may lead to a better understanding of spatial foraging behavior in continuous prey landscapes.
Applying our results to foraging theory requires translating them into a prediction for how predators would behave in a real continuous landscape. One approach to do this would be to use our results to predict the spatial scale at which predators would produce density-dependent prey mortality. To test this prediction, one could allow predators to forage freely in an experimentally manipulated landscape similar to the one used here. Then one should calculate both density and mortality at a range of spatial scales to determine if density-dependent mortality is detected at the spatial scale predicted by our results. This approach would parallel the methodology of observational studies with insect predators such as Stiling et al. (1991) and Mohd Norowi et al. (2000) but with the goal of testing an a priori prediction rather than deducing a pattern from observational data. For fish predators, a similar type of experiment has already been performed in the field by Overholtzer-McLeod (2006), who compared the mortality of juvenile reef fishes (yellowhead wrasse, Halichoeres garnoti) on individual artificial coral reef heads that were either spaced closely or far apart. She found that on the closely spaced reef array, predatory snappers (Ocyurus chrysurus) foraged in home ranges that encompassed the entire array, and prey mortality was density independent at the scale of individual reefs. When reefs were widely spaced, prey mortality at the reef scale was density dependent, likely because the predators’ foraging scale encompassed only a single reef (Overholtzer-McLeod 2006; White et al. 2010). This suggests that the predators’ foraging scale is somewhat larger than the closely spaced reef array, but no independent test has confirmed this. Our approach provides a framework for performing that type of test and advancing the predictions of OFT in continuous prey landscapes where prey patches are most difficult to define as a human observer.
Although our experiment was conducted with only one species of predator, our findings are potentially applicable to other visually foraging benthic fishes. The prey patch width of our 1.5- to 2-cm SL guppies was 10–15.2 cm, or ~5–10 times a fish’s SL. A predator length:prey patch width ratio such as this may be a useful tool for estimating a predator’s patch scale in other fishes with similar hunting strategies to guppies. In fishes with dissimilar hunting strategies (i.e., ambush predators, highly mobile pelagic predators) this methodology may still reveal the spatial scale of a prey patch. These suggestions require further investigation.
Proper scaling of prey patches not only improves predictions of a predator’s optimal foraging behavior; it also improves predictions of prey’s optimal predator-avoidance behavior. If a predator defines a prey patch at a specific scale, it would be advantageous for prey within the patch (prey group size < patch size) to aggregate in order to achieve numerical risk dilution (Wrona and Dixon 1991), confusion effects (Landeau and Terborgh 1986), or information sharing (Lachmann et al. 2000). These behaviors would not increase overall prey density in the patch, but may reduce predation risk for individual prey, all else being equal (Pitcher and Parrish 1993; White et al. 2010). Prey aggregation at a scale equal to or larger than the predator’s patch scale, however, should increase prey density within the patch and increase predation risk, thus selecting for prey dispersion at this larger scale. Optimal prey behavior, therefore, is dependent on the behavioral decisions of the predators (Lima 2002). A better understanding of predators’ spatial decision-making thus provides new insight on the selective pressures affecting prey behavior.
References
Abrahams MV (1989) Foraging guppies and the ideal free distribution: the influence of information on patch choice. Ethol 82:116–126
Bates D, Maechler M, Bolker B (2012) lme4: linear mixed-effects models using S4 classes. R package version 0.999999-0. http://CRAN.R-project.org/package=lme4
Bax NJ (1998) The significance and prediction of predation in marine fisheries. ICES J Mar Sci 55:997–1030
Bergman CM, Fryxell JM, Gates CC, Fortin D (2001) Ungulate foraging strategies: energy maximizing or time minimizing? J Anim Ecol 70:289–300
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bond AB (1980) Optimal foraging in a uniform habitat: the search mechanism of the green lacewing. Anim Behav 28:10–19
Burger AE, Hitchcock CL, Davoren GK (2004) Spatial aggregations of seabirds and their prey on the continental shelf off SW Vancouver Island. Mar Ecol Prog Ser 283:279–292
Cowie RJ (1977) Optimal foraging in great tits (Parus major). Nature 268:137–139
Cummings VJ, Schneider DC, Wilkinson MR (1997) Multiscale experimental analysis of aggregative responses of mobile predators to infaunal prey. J Exp Mar Biol Ecol 216:211–227. doi:10.1016/S0022-0981(97)00097-X
Day RL, MacDonald T, Brown C et al (2001) Interactions between shoal size and conformity in guppy social foraging. Anim Behav 62:917–925
Elliott NC, Kieckhefer RW (2000) Response by coccinellids to spatial variation in cereal aphid density. Popul Ecol 42:81–90
Emlen JM (1966) The role of time and energy in food preference. Am Nat 100:611–617
Fisher RA (1932) Statistical methods for research workers, 4th edn. Oliver and Boyd, Edinburgh
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, Cambridge
Gelman A, Su Y-S (2013) arm: data analysis using regression and multilevel/hierarchical models. R package version 1.6-04. http://CRAN.R-project.org/package=arm
Hurlbert SH, Lombardi CM (2009) Final collapse of the Neyman-Pearson decision theoretic framework and rise of the neoFisherian. Ann Zool Fenn 46:311–349
Ioannou CC, Krause J (2008) Searching for prey: the effects of group size and number. Anim Behav 75:1383–1388
Ioannou CC, Morrell LJ, Ruxton GD, Krause J (2009) The effect of prey density on predators: conspicuousness and attack success are sensitive to spatial scale. Am Nat 173:499–506
Johnson CJ, Parker KL, Heard DC (2001) Foraging across a variable landscape: behavioral decisions made by woodland caribou at multiple spatial scales. Oecologia 127:590–602
Krebs JR, Davies NB (1986) Behavioural ecology. Blackwell Science, Sussex
Krebs JR, Ryan JC, Charnov EL (1974) Hunting by expectation or optimal foraging? A study of patch use by chickadees. Anim Behav 22:953–964
Lachmann M, Sell G, Jablonka E (2000) On the advantages of information sharing. Proc R Soc Lond B 267:1287–1293
Landeau L, Terborgh J (1986) Oddity and the ‘confusion effect’ in predation. Anim Behav 34:1372–1380
Levin SA (1992) The problem of pattern and scale in ecology: the Robert H. MacArthur Award Lecture. Ecology 73:1943–1967
Lima SL (2002) Putting predators back into behavioral predator–prey interactions. Trends in Ecol Evol 17:70–75
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Mangel M, Clark CW (1986) Towards a unified foraging theory. Ecology 67:1127–1138
Mohd Norowi H, Perry JN, Powell W, Rennolls K (2000) The effect of spatial scale on interactions between two weevils and their parasitoid. Ecol Entomol 25:188–196
Osborne JL, Martin AP, Carreck NL et al (2008) Bumblebee flight distances in relation to the forage landscape. J Anim Ecol 77:406–415
Osenberg CW, St. Mary CM, Schmitt RJ et al (2002) Rethinking ecological inference: density dependence in reef fishes. Ecol Lett 5:715–721
Overholtzer-McLeod KL (2006) Consequences of patch reef spacing for density-dependent mortality of coral-reef fishes. Ecology 87:1017–1026
Pitcher TJ, Parrish JK (1993) Functions of shoaling behavior in teleosts. In: Pitcher TJ (ed) Behaviour of teleost fishes. Chapman and Hall, London, pp 363–439
Pyke GH (1978) Optimal foraging: movement patterns of bumblebees between inflorescences. Theor Popul Biol 13:72–98
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
Ray C, Hastings A (1996) Density dependence: are we searching at the wrong spatial scale? J Anim Ecol 65:556–566
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Schellhorn NA, Andow DA (2005) Response of coccinellids to their aphid prey at different spatial scales. Popul Ecol 47:71–76
Schneider DC, Piatt JF (1986) Scale-dependent correlation of seabirds with schooling fish in a coastal ecosystem. Mar Ecol Prog Ser 32:237–246
Smith JNM, Sweatman HPA (1974) Food-searching behavior of titmice in patchy environments. Ecology 55:1216–1232
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, New Jersey
Stiling P, Throckmorton A, Silvanima J, Strong DR (1991) Does spatial scale affect the incidence of density dependence? A field test with insect parasitoids. Ecology 72:2143–2154
Swaney W, Kendal J, Capon H et al (2001) Familiarity facilitates social learning of foraging behaviour in the guppy. Anim Behav 62:591–598
Townsend CR, Winfield IJ (1985) The application of optimal foraging theory to feeding behavior in fish. In: Fish energetics. Springer, the Netherlands, pp 67–98
Turchin P, Kareiva P (1989) Aggregation in Aphis varians: an effective strategy for reducing predation risk. Ecology 70:1008–1016
Waage JK (1979) Foraging for patchily-distributed hosts by the parasitoid, Nemeritis canescens. J Anim Ecol 48:353–371
Wellenreuther M, Connell SD (2002) Response of predators to prey abundance: separating the effects of prey density and patch size. J Exp Mar Biol Ecol 273:61–71
Werner EE, Hall DJ (1974) Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus). Ecology 55:1042–1052
White JW (2011) Can inverse density dependence at small spatial scales produce dynamic instability in animal populations? Theor Ecol 4:357–370
White JW, Warner RR (2007) Safety in numbers and the spatial scaling of density-dependent mortality in a coral reef fish. Ecology 88:3044–3054
White JW, Samhouri JF, Stier AC, Wormald CL, Hamilton SL, Sandin SA (2010) Synthesizing mechanisms of density dependence in reef fishes: behavior, habitat configuration, and observational scale. Ecology 91:1949–1961
Wrona FJ, Dixon RWJ (1991) Group size and predation risk: a field analysis of encounter and dilution effects. Am Nat 137:186–201
Zach RJ, Falls JB (1976) Ovenbird (Aves: Parulidae) hunting behavior in a patchy environment: an experimental study. Can J Zool 54:1863–1879
Acknowledgments
The authors would like to thank Matt Kon and Joanna Lewis for assistance with animal care. M. A. Birk would also like to thank Dr. Fredrick Scharf and Dr. Mark Galizio for being committee members of this thesis and Jesus Christ for this opportunity. This study was funded by the UNCW Department of Biology and Marine Biology and a UNCW Center for the Support of Undergraduate Research and Fellowships Research Supplies Grant. All research was conducted in accordance with all applicable laws and rules set forth by the USA and the UNCW Institutional Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Birk, M.A., White, J.W. Experimental determination of the spatial scale of a prey patch from the predator’s perspective. Oecologia 174, 723–729 (2014). https://doi.org/10.1007/s00442-013-2818-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2818-1