Abstract
Drivers of large-scale variability in parasite prevalence are not well understood. For logistical reasons, explorations of spatial patterns in parasites are often performed as observational studies. However, to understand the mechanisms that underlie these spatial patterns, standardized and controlled comparisons are needed. Here, we examined spatial variability in infection of an important fishery species and ecosystem engineer, the oyster (Crassostrea virginica) by its pea crab parasite (Zaops ostreus) across 700 km of the southeastern USA coastline. To minimize the influence of host genetics on infection patterns, we obtained juvenile oysters from a homogeneous source stock and raised them in situ for 3 months at multiple sites with similar environmental characteristics. We found that prevalence of pea crab infection varied between 24 and 73 % across sites, but not systematically across latitude. Of all measured environmental variables, oyster recruitment correlated most strongly (and positively) with pea crab infection, explaining 92 % of the variability in infection across sites. Our data ostensibly suggest that regional processes driving variation in oyster recruitment similarly affect the recruitment of one of its common parasites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The abundance of parasites, like most free-living organisms, varies substantially through space, often at multiple scales (Fredensborg et al. 2006; Smith 2007; Byers et al. 2008, 2011; Blakeslee et al. 2012). Determining the controls of this variability is paramount to understanding larger ecological and evolutionary ramifications of parasites. For example, the controls of parasitic infection can underlie the strength and variability in host life-history evolution (Tschirren and Richner 2006; Crossan et al. 2007), host population regulation (Perrin et al. 1996; Hudson et al. 1998; Hatcher et al. 1999), and community-level ecological consequences of host infection (Wood et al. 2007). Additionally, understanding the controls on parasite infection may aid predictions of host population infection rates in the face of global change (Sutherst 2001; Wiedermann et al. 2007; Tinsley et al. 2011).
Spatial explorations of parasites at very large scales are almost exclusively observational because of the logistical challenges associated with conducting controlled host–parasite experiments. These explorations have provided insight into the potential effects of several environmental factors on parasite patterns at large scales (e.g., Poulin and Mouritsen 2003; Brown et al. 2008; Byers et al. 2008; Thieltges et al. 2009). However, the causal mechanisms controlling large-scale spatial heterogeneity in infection are not able to be directly explicated from observation alone. Therefore, it is often unknown whether an observed pattern is driven by spatial variation in the parasite, the host, the biotic and abiotic environment, or an interaction of these (Mouritsen et al. 2003; Hawley and Altizer 2011).
Throughout many coastal areas of the North Atlantic, the ecologically and economically important Eastern oyster (Crassostrea virginica) is infected by the parasitic oyster pea crab Zaops ostreus (=Pinnotheres ostreum). The small crab (<1.5 cm width) lives most of its life inside the gills of an oyster, and occasionally other bivalves, using the host for protection and food acquisition (Sandifer 1972). Pea crabs spawn in the summer and their larvae take about 1 month to develop in the water column before locating and infecting their oyster hosts (Sandoz and Hopkins 1947). Pea crab infection often causes gill damage (Stauber 1945) and affects the condition index (Sandoz and Hopkins 1947; Mercado-Silva 2005) and gonad development of oysters (O’Beirn and Walker 1999), but does not seem to increase mortality. While the natural history and ecology of Z. ostreus is well known (Stauber 1945; Sandoz and Hopkins 1947; Christensen and McDermott 1958), it has hitherto been studied primarily on a local scale (e.g., O’Beirn and Walker 1999), and reported prevalence in oysters varies tremendously from 1 to 100 % (e.g., Galtsoff 1964; O’Beirn and Walker 1999). However, because the host and parasite species coexist over a large latitudinal range, this system provides the opportunity to examine potential drivers of spatial structure of variability in infection.
To determine whether spatial differences in oyster infection stem from characteristics of the host, the parasite, trophic interactions, and/or the environment, we conducted a controlled comparison of parasite infection at a biogeographic scale. Along more than 700 km of coastline of the southeastern US, we deployed juvenile oysters to multiple sites in a standardized protocol that controlled the densities and identities of interacting species and as many characteristics of the host as possible (including a homogeneous genetic stock, age, and size). Also, by deploying host oysters concurrently for the same length of time at the same tidal height and similar salinities at every site, we controlled for several highly influential environmental aspects that collectively control exposure time to infective pea crab stages (Beach 1969). We tracked the major remaining abiotic and biotic variables (i.e., temperature, mean daily submergence, ambient oyster density, and recruitment) among sites to use as covariates in our analyses.
Materials and methods
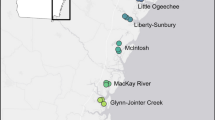
To examine the biogeographic variability in parasite infection rates, we exposed host oysters at six estuarine sites throughout the Southeastern Atlantic Bight to natural pea crab infection (Fig. 1). Because our emphasis was on large-scale patterns driving pea crab variability, we chose the estuary as our primary level of replication. To standardize our comparisons across this biogeographic scale and to help isolate the roles of larger scale processes, we tried to minimize variability in potentially influential microhabitat factors among sites as much as possible. To this end, each selected site was polyhaline (>18ppt), near the mouth of an estuary, and had dense oyster reefs nearby. We also matched sites for similar water flow and sediment properties. While maintaining these similar environmental characteristics, we chose sites systematically spaced throughout this range at least 100 km apart. The two sites in Florida, however, were spaced closer than desired because of the difficulty in finding similar matching microhabitat characteristics at more broadly dispersed sites in northern Florida. However, post hoc examination of the data verified the independence among our sites, including the two Florida sites, in regard to both predictor and response variables. We also considered grouping sites into three regions, but with only six total sites we felt that this approach stretched the limits of our data.
The southeastern US showing our six experimental sites. Inset in upper left corner denotes the location of the Southeastern Atlantic Bight which is shown in fuller detail in the larger map. PF Pellicer Flats, Marineland, Florida; SA Crescent Beach, St. Augustine, Florida; SKIO Skidway Island, Georgia; ACE Ace Basin, South Carolina; MB Masonboro Island, North Carolina; HH Hoop Hole Creek, near Morehead City, North Carolina
Because we wanted to isolate factors relating to the parasite and the environment that drive variability, we used a homogeneous source stock for our experimental oyster hosts. The host stock did not derive from any of our experimental sites to avoid possible preference (or avoidance) of local parasites for local hosts, or better resistance of a local host genotype in its home environment. The experimental oysters were spawned at a Florida hatchery in March 2011 from parent stock collected from Pine Island (Lee County), Florida, on the Gulf Coast. The parent stock was composed of ~25 males and 14 females. Eggs released by females were placed in separate containers; sperm released from males was mixed in a common container and then distributed to the eggs to fertilize them. The oysters were raised to small juvenile stage and then shipped to our field sites.
At each location, multiple teams created deployment units that were comprised of 12 juvenile oysters (also known as spat) (initial length 0.7–1.9 cm) equally spaced in a checkerboard pattern on an 11 × 11 cm ceramic tile. The spat were adhered to the tile in their natural upright positions with a small amount of marine epoxy (Z-spar A-788 Splash Zone Compound). To control the oyster abundance immediately surrounding our focal oyster hosts as well as the predators that had access to them, at each site we constructed replicate oyster reefs on intertidal mudflats that had no present oyster population. We chose these mudflats to match tidal height elevations of natural oysters in the area (~+0.75 m MLLW). At each site, we installed nine circular Vexar mesh cages (2.4 m diameter, 1 m height, 6.4 mm mesh size), which we positioned 3 m from each other. We dug the cages 30 cm into the ground, supported the sides with rebar poles, and enclosed the tops with 6.4-mm mesh bird netting. We constructed oyster reefs (1.5 m diameter) in the center of each cage by first laying down one bushel (35.2 L) of dead oyster shell for stability and then placing on top three bushels of locally-collected live oyster clusters which had been washed and soaked in freshwater to remove all epifaunal species. This technique was highly effective and oysters were held in flowing seawater tanks in the laboratory for a day following to ensure that the process had no adverse effects on their survival. To avoid the possibility of differential exposure to predators causing stress-induced susceptibility to parasites (Raffel et al. 2010), we stocked each cage with standardized combinations of common reef predators, including mud crabs (Panopeus herbstii), oyster drills (Urosalpinx cinerea), bluecrabs (Callinectes sapidus), and toadfish (Opsanus tau). These include some of the most common predators (i.e., mud crabs) and represent a range of predators types (secondary vs. tertiary; sit-and-wait vs. active; selective vs. generalist).
Within each of these cages, we deployed six experimental tiles to which the 12 oyster spat had been affixed. Each tile was glued with silicone to a brick (15 × 15 × 4.5 cm) for support and mounted vertically by securing the brick upright to a post. Tiles were placed 25 cm inward from the edge of the reef, facing outward, and were evenly spaced around the perimeter of the reef. To increase the number of focal oysters surviving to the end of experiment, we placed a subcage made of 6.4-mm PVC-coated wire mesh (43 cm × 18 cm × 16 cm high) over every other one of these six tiles to exclude any consumers. To quantify the similarity of our study systems, at each site we deployed Hobo gauges throughout the experiment that recorded temperature as well as tidal submergence. The experiment ran at all sites for 56–88 days from early June to late August 2011 when all tiles were recovered and frozen for subsequent analysis (Appendix A).
To ensure proper analytical consistency, all tiles were processed by a single investigator. We measured individual shell lengths of a random subsample of 20 spat per cage, or the maximum available if <20 spat survived. At one Florida site (PF), there were three cages in which we processed up to 31 spat to boost the overall sample size. Each oyster was dissected to determine the presence or absence of pea crabs.
To add to our list of tracked ecological variables, we enumerated the number of oyster spat that naturally recruited to each tile and support brick during the course of the experiment and averaged these values across the six replicates within each cage and across the nine cages. At the Georgia and South Carolina sites (SKIO and ACE), these recruits were also useful in a second manner. Due to a large number of spat that naturally recruited to the tiles during the course of the experiment, we separately quantified pea crab infection prevalence at these two sites in >150 naturally recruited spat for comparison with our deployed focal spat.
Finally, to quantify relative host availability and related settling/recruitment cues for pea crabs, we quantified the density of large adult oysters (≥7.5 cm) on oyster reefs at each site. Specifically, we placed a 0.25-m2 quadrat on five separate reefs separated by at least 100 m that were located in the vicinity of our experimental set-up. All living adult oysters within each quadrat were excavated and enumerated, and adult oyster densities for the five quadrats at each site were averaged. In this manner, we could use adult oyster density in our analyses to examine if it is related to pea crab prevalence.
Data analysis
To determine if host oysters varied in pea crab prevalence across sites, we performed a Chi square analysis on the overall effect of site (Proc logistic; SAS). Because site was significant in this analysis, we followed up with a test to probe whether any of our measured environmental variables might systematically explain the site-level variation. First, to ensure that experimental duration did not influence site-level infection prevalence, we regressed pea crab prevalence against experimental duration. Finding no effect of experimental duration on pea crab infection rates (F 1,5 = 0.074, P = 0.80, R 2 = 0.018), we proceeded to investigate other site-level variables. For this subsequent analysis, the site-level mean of pea crab prevalence was regressed against latitude, mean water temperature, mean hours of daily submergence, mean large adult oyster density, and mean oyster recruitment. The latter two variables were natural log-transformed. We created a series of nested generalized linear models, including an intercept-only null model and then all possible single- and two-factor additive models (the degrees of freedom limited including more terms simultaneously to create more complex candidate models). The most parsimonious model was identified according to Akaike’s information criterion corrected for small sample sizes AICc. Models were ranked according to their Akaike weight (w i ), which was calculated as the model likelihood normalized by the sum of all model likelihoods; values close to 1 indicate greater confidence in the selection of the best model.
To analyze the effect of oyster size on pea crab infection prevalence, we performed a logistic regression using individual oyster sizes, site, and their interaction as the independent variables. Because oyster size measurements were not conducted for the two Florida sites, they were not included in this formal analysis.
Results
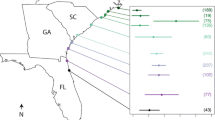
The prevalence of pea crab infection varied between 24 and 73 % and differed significantly across sites throughout the Southeastern Atlantic Bight (χ 2 = 61.7, P < 0.0001; Fig. 2; Appendix A). Oysters at three sites (ACE, SKIO, and HH) had pea crab prevalence greater than 60 %. The average size of oyster spat across our sites was 28.7 mm ± 9.2 (SD). Logistic regression indicated that the size of a focal oyster did not influence its pea crab infection probability (χ 2 = 0.12, df = 1, P = 0.73), nor did the interaction of size with site (χ 2 = 3.2, df = 3, P = 0.37). Within infected spat across all sites, the predominately small size of pea crabs (<2 mm carapace width) suggested that they were very recent infections. At ACE, due to high sedimentation smothering oyster spat, spat survival was very low and resulted in a small sample size. In contrast to the high infection rates for focal spat, the prevalence of pea crabs in naturally recruiting spat when measured was considerably lower (SKIO = 0.05; ACE = 0.09; Fig. 2). These oysters were smaller [21.4 mm ± 4.8 (mean ± SD)] and substantially younger hosts with far fewer days of environmental exposure.
Percentage of experimental oysters (Crassostrea virginica) from the same genetic stock infected with pea crabs (Zaops ostreus) at our six experimental sites. Sites are listed from south to north (with sample sizes): PF Pellicer Flats, Marineland, Florida (154); SA Crescent Beach, St. Augustine, Florida (95); SKIO Skidway Island, Georgia (62); ACE Ace Basin, South Carolina (21); MB Masonboro Island, North Carolina (129); HH Hoop Hole Creek, near Morehead City, North Carolina (127). The two bars on the far right show the pea crab prevalence in naturally recruiting oyster spat which not only differed in genetic composition from the experimental oysters, but were also much younger and thus less exposed. The total number of oysters sampled is printed above the bar for each site
The abundance of oyster spat recruitment (ln-transformed) across sites was highly and positively correlated with pea crab prevalence (R 2 = 0.92; pea crab prevalence = 11.8 [ln(oyster spat abundance)] + 10.4; Table 1; Fig. 3). With a model weight of 0.88, this single variable model was the strong favorite in our AIC model comparison. Although there were interesting spatial patterns of other predictor variables, none of them were considered strong explanations of pea crab infection (Table 1). For example, although mean water temperature only differed by 2 °C across our sites, it was strongly and positively correlated with the prevalence of pea crabs, explaining 80 % of the spatial variability (R 2 = 0.80; Table 1; Appendix B). However, the model with temperature had low Akaike weight (Table 1). Ambient large adult oyster density varied considerable among sites and was moderately correlated with pea crab prevalence; however, ultimately, it was not an influential variable in our model (Table 1). For the most part, submersion time was high and very similar at all sites, although site MB had considerably less submersion. Mean submersion time per tidal cycle at MB was 11.9 h compared to 17.5–19.4 h at the other five sites. However, this variable appeared to have little influence on pea crab infection rates (Table 1). Likewise, latitude did not explain a significant portion of the variability in pea crab prevalence across sites. Latitude also failed to explain site level differences in water temperature (R 2 = 0.013, F 1,5 = 0.05, P = 0.83). Models with two predictor variables were also explored but they showed substantially higher AICc values and thus had extremely low Akaike weights.
Discussion
Despite standardizing several important host and environmental characteristics, the prevalence of pea crab infection of eastern oysters still varied by a factor of three across the Southeastern Atlantic Bight. This large difference in infection rates suggests that spatial variability in the pea crab parasites themselves or their interactions with abiotic and biotic factors are driving the observed pattern across a biogeographic range of 700 km. This spatial pattern of infection does not appear to vary linearly with latitude, suggesting that infection levels differ based on site-level variables, perhaps driven by larger-scale, non-linear processes.
Because pea crab prevalence was positively correlated with oyster recruitment, our study suggests that one of the most likely mechanisms driving variation in pea crab infection at large scales is regional variation in the supply of pea crab propagules. The highest levels of infection occurred in the Georgia/South Carolina region, which also had the highest levels of oyster recruitment in our study. The high levels of both pea crab prevalence and recruitment of natural oyster spat suggest that this may be a region that aggregates larvae or is particularly hospitable for them. This region does have unusually high tidal amplitude, and the associated high water flux could readily influence larval delivery (Butman 1987). Regardless, the extremely high correlation of oyster recruitment and pea crab prevalence suggests that processes driving variation in oyster recruitment, such as hydrodynamics, or possibly food supply, similarly affect the recruitment of one of its common parasites. Oysters and pea crabs both have planktotrophic larvae that are long-lived, often residing in the water column for a month or more (Sandoz and Hopkins 1947; Dekshenieks et al. 1993). Both are spawned in the summer, with pea crabs typically trailing about 1 month after oysters, presumably so that they have sufficiently large, newly settled hosts to recruit to when they settle (Christensen and McDermott 1958). These findings suggest that recruitment limitation may affect pea crab populations, as has been suggested in some trematode parasite systems (Smith 2001; Fredensborg et al. 2006; Byers et al. 2008).
Several factors support the notion that the pea crabs were new recruits and not local crabs that moved hosts. First, the pea crabs we observed were very small, and thus very young and consistent with the age and size of new settlers. Second, female pea crabs typically do not move from their host once settled. Male pea crabs can move between hosts in order to copulate; however, they live for less than 1 year (Christensen and McDermott 1958), and only new recruits should have been present in the months we sampled.
Temperature correlated with infection rates despite relatively small mean temperature differences between the sites. The effect of temperature was not spatially systematic, in that it did not vary significantly with latitude. The lack of such a relationship is not surprising, especially in summer, since solar insolation is high and similar throughout this region, allowing microsite differences in depth, flushing rate, and tidal factors to have a greater influence on temperatures (e.g., Helmuth et al. 2006). Although the correlation between infection and temperature is not necessarily causal, there is a possible mechanistic expectation of such a linkage. Specifically, given the increased metabolism, population growth rates, and stress of most organisms at higher temperatures, both parasite exposure and the efficacy of infection often increase in a host with increasing environmental temperature (e.g., Mouritsen and Jensen 1997; Harkonen et al. 2010; Garamszegi 2011; Paull and Johnson 2011; Macnab and Barber 2012; Zamora-Vilchis et al. 2012). It remains to be seen, however, whether such small differences in mean summer temperature actually contribute to such influences in this system, or whether these temperatures are correlated with more influential variables such as hydrodynamics.
We were surprised that submersion time had no influence on pea crab infection across sites. This variable would seemingly control the exposure time of oyster hosts to pea crab propagules in the water. Pea crabs have been found to be more prevalent in subtidal compared to intertidal oysters (Galtsoff 1964; Linton 1968; Parks 1968; O’Beirn and Walker 1999). However, our data suggest that submersion time is not a limiting factor for pea crab infections in oyster spat, at least over the range of values captured here. Our oysters’ submersion period admittedly was large at >11.8 h per day, and may have already provided saturating levels of submersion. Furthermore, the sites (apart from the MB site) were not only all exceptionally high in submersion time but differed little between one another, likely limiting our ability to detect its significance on pea crab infection rates (Appendix A).
In our analyses, the duration of the experiment, or number of days of exposure of oysters to the environment, also did not influence pea crab prevalence. However, logically at some point, a positive relationship between exposure time and prevalence likely exists. But our results suggest that the effect of the number of exposure days on prevalence does not build at a universal or linear rate. The PF site had 30 fewer days of exposure than our maximally exposed site at SA that was only 10 km away, yet PF had 15 % more infection. Perhaps the main effect of increased exposure days is increasing the probability of oysters being exposed during an episodic pulse of pea crab recruits.
Prevalence of infection in our focal experimental spat was substantially higher than in naturally recruiting spat, which we were able to compare at two sites (Fig. 2). At least three factors could explain this difference. First, we examined natural spat that settled on the deployment tiles with our mounted focal oysters. These naturally setting spat were necessarily younger than our mounted focal oysters because they settled after the tiles were deployed, and thus their exposure time to contract infection was less than that of the focal oysters. The newly recruiting natural oyster spat possibly missed the main pulse of pea crab recruitment. Second, due to their Gulf Coast origin, the focal oysters likely differed from naturally recruiting oysters in their genetic composition, which was perhaps more susceptible to infection by local pea crabs. Finally, our focal oysters could have had higher susceptibility due to transport stress and acclimation to their new transplanted environments. All these factors were held constant among our focal oysters, and thus should not have influenced relative comparisons among sites, which was the primary intention of our study.
O’Beirn and Walker (1999) measured the prevalence of infection in adult intertidal oysters near our SKIO site in 1993–94 to be between 1 and 8 %, also sharply lower than values for our focal spat. In addition to the reasons for differences in natural versus experimental spat discussed above, there are two further reasons for differences between their findings and ours. First, our oysters were far younger, and younger oysters usually have higher prevalence of infection, perhaps because older oysters are more capable of shedding pea crab infection (Christensen and McDermott 1958). Also, based on several lines of evidence, Christensen and McDermott (1958) suggest that pea crabs may preferentially invade oyster spat. Second, our study also differed from O’Beirn and Walker (1999) in that the studies occurred nearly 20 years apart. Temporal variability in pea crab prevalence may be as strong as spatial variability. Stauber (1945), working at a site in two consecutive years in Delaware Bay, found that pea crab prevalence changed by 25 % from the first year to the next. Collectively, these results highlight the need for further investigations aimed at disentangling the factors that control spatial and temporal variation in host–parasite dynamics.
Small-scale studies of parasite heterogeneity (e.g., Hechinger and Lafferty 2005; Lopez et al. 2005; Torchin et al. 2005; de Montaudouin and Lanceleur 2011) have an advantage because the environment, and to some extent host genetics, are held constant. Differences in infection between patches of hosts can often be presumed to be due to aspects of the parasite. But at a large scale, clearly more factors are free to vary, thus rendering an experimental approach particularly useful for disentangling mechanisms that affect host–parasite dynamics more broadly. The large-scale variation in prevalence of the parasite in our study appears to be driven by the abundance and infection efficacy of the parasite and the characteristics of the environment, and less by aspects of the host directly. A likely explanation for the high spatial variability is that there are differences in the source pool of pea crab propagules. In fact, because pea crab prevalence and recruitment of natural oyster spat were highly correlated, it suggests that certain hotspots like the Georgia/South Carolina region are generally attractive areas for larvae. This could be due to regional level processes that aggregate larvae in the area, or more localized conditions such as high water column productivity and warm temperatures that foster pelagic life stages. Temperature may play a small contributing role on pea crab infection processes, though its effects do not covary with latitude as systematically as one might initially expect.
Ultimately, our work has suggested that these potential biotic and abiotic mechanisms can drive high spatial variability in parasite infection in our system across a large regional scale. Whether these results can be extrapolated to additional host–parasite systems will require the increased use of large-scale experimentation. This study reminds us that exposing the large-scale patterns of parasites and the mechanisms behind them may prove increasingly important to predicting responses of infection to global change, since effects are not always most strongly driven by temperature or along latitudinal gradients.
References
Beach NW (1969) The oyster crab, Pinnotheres ostreum Say, in the vicinity of Beaufort, North Carolina. Crustaceana 17:87–199
Blakeslee AMH, Altman I, Miller AW, Byers JE, Hamer CE, Ruiz GM (2012) Parasites and invasions: a biogeographic examination of parasites and hosts in native and introduced ranges. J Biogeogr 39:609–622
Brown HE, Childs JE, Diuk-Wasser MA, Fish D (2008) Ecological factors associated with West Nile virus transmission, northeastern United States. Emerg Infect Dis 14:1539–1545
Butman CA (1987) Larval settlement of soft-sediment invertebrates: the spatial scales of pattern explained by active habitat selection and the emerging role of hydrodynamical processes. Oceanogr Mar Biol 25:113–165
Byers JE, Blakeslee AMH, Linder E, Cooper AB, Maguire TJ (2008) Controls of spatial variation in the prevalence of trematode parasites infecting a marine snail. Ecology 89:439–451
Byers JE, Altman I, Grosse AM, Huspeni TC, Maerz JC (2011) Using parasitic trematode larvae to quantify an elusive vertebrate host. Conserv Biol 25:85–93
Christensen AM, McDermott JJ (1958) Life history and biology of the oyster crab, Pinnotheres ostreum Say. Biol Bull 114:146–179
Crossan J, Paterson S, Fenton A (2007) Host availability and the evolution of parasite life-history strategies. Evolution 61:675–684
de Montaudouin X, Lanceleur L (2011) Distribution of parasites in their second intermediate host, the cockle Cerastoderma edule: community heterogeneity and spatial scale. Mar Ecol Prog Ser 428:187–199
Dekshenieks MM, Hofmann EE, Powell EN (1993) Environmental effects on the growth and development of eastern oyster, Crassostrea virginica (Gmelin, 1791), larvae: a modeling study. J Shellfish Res 12:241–254
Fredensborg BL, Mouritsen KN, Poulin R (2006) Relating bird host distribution and spatial heterogeneity in trematode infections in an intertidal snail-from small to large scale. Mar Biol 149:275–283
Galtsoff PS (1964) The American oyster, Crassostrea virginica, Gmelin. United State Fish ad Wildlife Service, Fish Bull 64:1–480
Garamszegi LZ (2011) Climate change increases the risk of malaria in birds. Glob Change Biol 17:1751–1759
Harkonen L et al (2010) Predicting range expansion of an ectoparasite: the effect of spring and summer temperatures on deer ked Lipoptena cervi (Diptera: Hippoboscidae) performance along a latitudinal gradient. Ecography 33:906–912
Hatcher MJ, Taneyhill DE, Dunn AM, Tofts C (1999) Population dynamics under parasitic sex ratio distortion. Theor Popul Biol 56:11–28
Hawley DM, Altizer SM (2011) Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol 25:48–60
Hechinger RF, Lafferty KD (2005) Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc R Soc Lond B 272:1059–1066
Helmuth B et al (2006) Mosaic patterns of thermal stress in the rocky intertidal zone: implications for climate change. Ecol Monogr 76:461–479
Hudson PJ, Dobson AP, Newborn D (1998) Prevention of population cycles by parasite removal. Science 282:2256–2258
Linton TL (1968) Feasibility of raft culturing oysters in Georgia. In: Linton TL (ed) Proceedings of the Oyster Culture Workshop, July 11–13, 1967. University of Georgia and Georgia Game and Fisheries Commission, Marine Fisheries Division, Contribution Series Number 6, pp 69–73
Lopez JE, Gallinot LP, Wade MJ (2005) Spread of parasites in metapopulations: an experimental study of the effects of host migration rate and local host population size. Parasitology 130:323–332
Macnab V, Barber I (2012) Some (worms) like it hot: fish parasites grow faster in warmer water, and alter host thermal preferences. Glob Change Biol 18:1540–1548
Mercado-Silva N (2005) Condition index of the eastern oyster, Crassostrea virginica (Gmelin, 1791) in Sapelo Island Georgia: Effects of site, position on bed and pea crab parasitism. J Shellfish Res 24:121–126
Mouritsen KN, Jensen KT (1997) Parasite transmission between soft-bottom invertebrates: temperature mediated infection rates and mortality in Corophium volutator. Mar Ecol Prog Ser 151:123–134
Mouritsen KN, McKechnie S, Meenken E, Toynbee JL, Poulin R (2003) Spatial heterogeneity in parasite loads in the New Zealand cockle: the importance of host condition and density. J Marine Biol Assoc UK 83:307–310
O’Beirn FX, Walker RL (1999) Pea crab, Pinnotheres ostreum Say, 1817, in the eastern oyster, Crassostrea virginica (Gmelin, 1791): prevalence and apparent adverse effects on oyster gonad development. Veliger 42:17–20
Parks P (1968) A comparison of chemical composition of oysters (Crassostrea virginica) from different ecological habitats. In: Linton TL (ed) Proceedings of the Oyster Culture Workshop, July 11–13, 1967. University of Georgia and Georgia Game and Fisheries Commission, Marine Fisheries Division, Contribution Series Number 6, pp 147–173
Paull SH, Johnson PTJ (2011) High temperature enhances host pathology in a snail-trematode system: possible consequences of climate change for the emergence of disease. Freshw Biol 56:767–778
Perrin N, Christe P, Richner H (1996) On host life-history response to parasitism. Oikos 75:317–320
Poulin R, Mouritsen KN (2003) Large-scale determinants of trematode infections in intertidal gastropods. Mar Ecol Prog Ser 254:187–198
Raffel TR, Hoverman JT, Halstead NT, Michel PJ, Rohr JR (2010) Parasitism in a community context: trait-mediated interactions with competition and predation. Ecology 91:1900–1907
Sandifer PA (1972) Growth of young oyster crabs, Pinnotheres ostreum Say, reared in the laboratory. Chesapeake Sci 13:221–222
Sandoz M, Hopkins SH (1947) Early life history of the oyster crab, Pinnotheres ostreum (Say). Biol Bull 93:250–258
Smith NF (2001) Spatial heterogeneity in recruitment of larval trematodes to snail intermediate hosts. Oecologia 127:115–122
Smith NF (2007) Associations between shorebird abundance and parasites in the sand crab, Emerita analoga, along the California coast. J Parasitol 93:265–273
Stauber LA (1945) Pinnotheres ostreum, parasitic on the American oyster, Ostrea (Gryphaea) virginica. Biol Bull 88:269–291
Sutherst RW (2001) The vulnerability of animal and human health to parasites under global change. Int J Parasitol 31:933–948
Thieltges DW, Fredensborg BL, Poulin R (2009) Geographical variation in metacercarial infection levels in marine invertebrate hosts: parasite species character versus local factors. Mar Biol 156:983–990
Tinsley RC, York JE, Everard ALE, Stott LC, Chapple SJ, Tinsley MC (2011) Environmental constraints influencing survival of an African parasite in a north temperate habitat: effects of temperature on egg development. Parasitology 138:1029–1038
Torchin ME, Byers JE, Huspeni TC (2005) Differential parasitism of native and introduced snails: replacement of a parasite fauna. Biol Invasions 7:885–894
Tschirren B, Richner H (2006) Parasites shape the optimal investment in immunity. Proc R Soc Lond B 273:1773–1777
Wiedermann MM, Nordin A, Gunnarsson U, Nilsson MB, Ericson L (2007) Global change shifts vegetation and plant-parasite interactions in a boreal mire. Ecology 88:454–464
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AMH (2007) Parasites alter community structure. Proc Natl Acad Sci USA 104:9335–9339
Zamora-Vilchis I, Williams SE, Johnson CN (2012) Environmental temperature affects prevalence of blood parasites of birds on an elevation gradient: Implications for disease in a warming climate. Plos ONE 7:ARTN: e39208. doi:10.31371/journal.pone.0039208
Acknowledgments
We thank Luke Dodd, H. Garland, P. Langdon, Jenna Malek, E. Pettis, Walt Rogers, and Heidi Weiskel for help in the field and Tom McCrudden for spawning and growing our hatchery oyster spat. This work was financially supported by the National Science Foundation (NSF-OCE-0961853).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jeff Shima.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Byers, J.E., Rogers, T.L., Grabowski, J.H. et al. Host and parasite recruitment correlated at a regional scale. Oecologia 174, 731–738 (2014). https://doi.org/10.1007/s00442-013-2809-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2809-2