Abstract
Ectomycorrhizal fungal (EMF) communities vary among microhabitats, supporting a dominant role for deterministic processes in EMF community assemblage. EMF communities also differ between forest and clearcut environments, responding to this disturbance in a directional manner over time by returning to the species composition of the original forest. Accordingly, we examined EMF community composition on roots of spruce seedlings planted in three different microhabitats in forest and clearcut plots: decayed wood, mineral soil adjacent to downed wood, or control mineral soil, to determine the effect of retained downed wood on EMF communities over the medium and long term. If downed and decayed wood provide refuge habitat distinct from that of mineral soil, we would expect EMF communities on seedlings in woody habitats in clearcuts to be similar to those on seedlings planted in the adjacent forest. As expected, we found EMF species richness to be higher in forests than clearcuts (P ≤ 0.01), even though soil nutrient status did not differ greatly between the two plot types (P ≥ 0.05). Communities on forest seedlings were dominated by Tylospora spp., whereas those in clearcuts were dominated by Amphinema byssoides and Thelephora terrestris. Surprisingly, while substrate conditions varied among microsites (P ≤ 0.03), especially between decayed wood and mineral soil, EMF communities were not distinctly different among microhabitats. Our data suggest that niche partitioning by substrate does not occur among EMF species on very young seedlings in high elevation spruce-fir forests. Further, dispersal limitations shape EMF community assembly in clearcuts in these forests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different ectomycorrhizal fungal (EMF) species colonize seedlings in forests and clearcuts (Jones et al. 2003; Dickie et al. 2009; Ding et al. 2011). These differences may result from limited inoculum and/or dispersal (Hagerman et al. 1999; Taylor and Bruns 1999; Dickie and Reich 2005; Peay et al. 2010), and altered abiotic properties (Kranabetter and Friesen 2002; Dickie et al. 2009; Peay et al. 2010; Ding et al. 2011) in clearcuts. Some EMF species propagate effectively by vegetative growth from root system to root system (Simard et al. 1997; Nara 2005), while others rely more on dispersal from spores (Deacon and Fleming 1992; Izzo et al. 2006; Blaalid et al. 2012). By removing all large trees, clearcutting greatly reduces hyphal extension from colonized roots as a source of inoculum. Consequently, seedlings planted within the rooting zone of forest edges are colonized by a greater range of EMF species than those planted further into the clearcut (Hagerman et al. 1999; Dickie and Reich 2005). The loss of these important inoculum sources leaves only ‘resistant propagules’ such as spore banks and sclerotia (Taylor and Bruns 1999; Jones et al. 2003; Izzo et al. 2006). Dispersal limitation resulting from this loss of inoculum, in addition to species-specific colonization mechanisms (i.e., from living hyphae versus from spore) (e.g., Kennedy et al. 2011; Peay et al. 2010) will influence the composition of the EMF community in young stands developing after clearcutting.
Clearcuts differ significantly from forests in their abiotic properties, exhibiting greater diurnal fluctuations in temperature and moisture than forest soils (Bunnell and Houde 2010). In addition, nitrate levels often increase for several years after clearcut logging (Wall 2008), while the thickness of the forest floor decreases (Laiho and Prescott 2004). One of the major differences between clearcuts and old-growth stands is the amount of downed wood on the soil surface (Berg et al. 1994; Smith et al. 2000). In some subalpine forests in British Columbia, coarse woody debris (CWD), defined as downed wood larger than 8–10 cm in diameter, covers 14 % of the ground surface, and is estimated to remain on the ground for an average of 320–350 years (Hollstedt and Vyse 1997). In various jurisdictions, 30–50 % of natural CWD levels represent a threshold below which species are lost (Berg et al. 1994; BC Ministry of Forests and Range Chief Forester. personal communication, 2010). Downed logs influence the soil environment for soil microbes, including EMF (Harvey et al. 1979; Elliott et al. 2007), and are considered to be legacy material from the previous stand (Elliott et al. 2007; Molina et al. 2010). In mature forests, decayed wood often supports an EMF community distinct from those in organic or mineral soils (Goodman and Trofymow 1998; Tedersoo et al. 2003). Consequently, decayed wood remaining from the previous stand may be important for preserving forest EMF species in young disturbed stands, by providing habitats with abiotic characteristics more similar to mature forests than the rest of the clearcut (Smith et al. 2000).
The creation or maintenance of microsites with specific abiotic environments due to the retention of downed wood would be expected to be most influential if deterministic processes are more important than stochastic processes as drivers of community assembly by EMF. Deterministic processes are those where differences in heritable traits among species favor establishment of different species in different environments (Chase and Myers 2011). Stochastic processes are ecologically neutral processes such as random dispersal or disturbance events. Within an EMF community, species with specialized niches should be most affected by deterministic processes (Bruns 1995). Interpretation of mycorrhizal fungal community data in the light of community assembly processes is quite recent (e.g., Lekberg et al. 2007; Peay et al. 2010), but several biogeographic and successional studies have concluded that stochastic effects dominate the assembly of root-associated fungi (Jumpponen 2003; Queloz et al. 2011). Specifically, the reduction in species richness of EMF on tree islands at increasing distances from intact forests indicated the importance of stochastic immigration and extinction events to Peay et al. (2010). Likewise, Blaalid et al. (2012) concluded that the patchy distribution of EMF communities among Bistorta vivipara plants at a glacial forefront was evidence for the dominance of stochastic dispersal events. However, environmental properties such as host species and soil chemistry also strongly and directionally structure mycorrhizal assemblages (Bruns 1995; Tedersoo et al. 2008; Koide et al. 2011). The relative importance of these stochastic and deterministic processes in EMF communities on clearcuts is unknown.
In the current study, we examined the composition of the EMF community on roots of spruce seedlings planted in three different microsites in both forest and clearcut plots in a high elevation forest. The microsites were decayed wood, mineral soil adjacent to a large intact piece of downed wood, or control mineral soil microsites. Our objective was to determine whether the community composition of EMF on young seedlings differed among these microsites, especially in clearcuts where downed and decayed wood are expected to provide refuge habitat distinct from that of mineral soil. We predicted that EMF communities would vary among microsites in both forest and clearcut plots. Furthermore, we expected EMF communities on seedlings in decayed wood or adjacent to downed wood in clearcuts to be similar to communities on seedlings planted in the adjacent forest. Taken together, these results would be consistent with a dominant role for deterministic processes in EMF communities in this forest type.
Materials and methods
Field site description and experimental design
The Sicamous Creek Silvicultural Systems Trial is located in the Engelmann spruce–subalpine fir wet–cold biogeoclimatic zone in the southern interior of British Columbia, Canada (http://www.for.gov.bc.ca/hre/becweb/resources/). Soils are Humo-Ferric Podzols, and the current forest began to re-establish after a fire event more than 350 years ago (Hollstedt and Vyse 1997). These unlogged areas are dominated by subalpine fir (Abies lasiocarpa Hook.), with a smaller proportion of Engelmann spruce (Picea engelmannii Parry ex. Engelm.), and a white rhododendron (Rhododendron albiflorum) and huckleberry/blueberry (Vaccinium spp.) understory (Craig et al. 2006). The 150-ha experimental area includes three replicate 10-ha clearcut blocks, which were harvested in the winter of 1994/95, and range in elevation from 1,580 m (Block A), through 1,675 m (Block B), to 1,770 m (Block C) (Hollstedt and Vyse 1997). In 1996, two 1-ha treatment plots were established inside each cutblock: one where CWD generated during the harvest was retained and one where CWD was removed. Engelmann spruce was then operationally planted. Subalpine fir is now regenerating naturally in the clearcuts, which are primarily occupied by Vaccinium spp., and a number of herbaceous plants including Valeriana sitchensis and Arnica latifolia (Hagerman et al. 1999). The small size and low density of the trees (<2 m tall, and >3 m apart) allows for exposed conditions at the soil surface in the absence of herbaceous cover. In 2007, we established a 1-ha control plot in the undisturbed forest, 30 m inside the southern margin of each cutblock.
In early May 2007, hybrid P. engelmannii × Picea glauca (Moench) Voss (native interior hybrid spruce) were grown from surface-sterilized seed (seedlot number 26212; B.C. Ministry of Forests Seed Center, ID DWD 20070064A) in sterile 1:1 peat:vermiculite potting mix. After 2 weeks of hardening off outdoors, 75 8-week old seedlings were gently shaken free of the potting mix and planted in each 1-ha plot, with 25 planted in each of three different microsites at randomly-selected locations. Control microsites comprised primarily mineral soil located at least 50 cm from any visible downed wood. Downed wood microsites were mineral soil within 5 cm, and- on the northern and downhill side, of a piece of downed wood at least 10 cm in diameter and of the Vegetation Resource Inventory decay class 1–3 (Government of BC Vegetation Resource Inventory, 2004). Decay classes 1–3 range from hard, intact logs with bark and twigs attached, to sagging, partly decayed logs with roots invading the sapwood. The decayed wood microsites were within wood of Vegetation Resource Inventory decay classes 4, 5, or beyond: sunken logs that are no longer round and which have roots invading the heartwood, as well as small, soft portions of wood on the ground. Because removal plots contained no logs, downed wood microsites in those plots were located adjacent to major above-ground horizontal roots associated with stumps. Twelve seedlings were randomly selected before and at planting, and were found to be non-mycorrhizal. Any seedlings that died over the first 8 weeks were replaced in early September.
Seedling harvesting and root tip sampling procedures
In late September 2008, we harvested five seedlings per microsite per plot by cutting a 5 × 5 × 20-cm-deep block of soil surrounding the root system. Samples were transported to the lab in plastic bags, on ice, then stored at 4 °C. Morphotyping of all live ectomycorrhizal root tips per seedling was based on Agerer’s (1987–2002) descriptions and the instructions of Goodman et al. (1996). Following examination at ×50 or ×100 and ×1,000, mycorrhizal morphotypes were distinguished by the type of branching, color, texture, abundance of hyphae, presence of rhizomorphs, and other microscopic features of the mantle (e.g., mantle pattern) and emanating hyphae (e.g., presence of septa). Two representative tips of each morphotype per seedling were frozen at −80 °C for DNA extraction and molecular identification.
Soil abiotic properties
In the center of each plot, one Decagon ‘Em5b’ datalogger with three ‘ECH2O’ soil moisture sensors attached (Decagon Devices, Pullman, WA, USA) was installed, and three Onset ‘Stowaway Tidbit’ temperature loggers (Onset Computer, Pocasset, MA, USA) were buried. The three pairs of sensors were buried 5 cm deep immediately adjacent to a seedling planted at each of three microsites in each retention (clearcut) and forest plot. Minimum daily moisture and maximum daily temperature were recorded throughout the snow-free growing season (July–September 2008).
Air-dried subsamples (approx. 10 g) of the soils surrounding the root system of three seedlings per microsite type per plot (3 seedlings × 3 microsite types × 3 plot treatments = 27 samples) were ground with a mortar and pestle, and these were individually tested for exchangeable cations and effective cation exchange capacity (Al, Ca, Fe, K, Mg, Mn, Na, and CEC) (0.1 M barium chloride, ICP spectrometer), mineralizeable N (expressed as ammonium-N, 1 N KCl anaerobic incubation) and extractable nitrate-N and ammonium-N (2 N KCl, colorimetric-autoanalyzer), and total C and N (combustion elemental analyzer). These tests were performed at the British Columbia Ministry of Forests and Range, Analytical Chemistry Services Laboratory in Victoria, British Columbia.
Molecular identification of fungi from ectomycorrhizae
Fungal DNA was extracted from frozen root tips by following the protocols of the Sigma Extract-N-Amp Plant PCR Kit (Sigma-Aldrich, St. Louis MO, USA), and the Internal Transcribed Spacer (ITS) region of nuclear rDNA was amplified with GoTaq (Promega, Madison WI, USA) using the forward primer ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) (Gardes and Bruns 1993) and the reverse primer ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. 1990). The 50-μl GoTaq reaction mixture included 2.5 μl of each 10 μM primer, 25 μl of GoTaq master mix 2×, 18.5 μl of sterile water, and 1.5 μl of template DNA. Thermal cycler conditions were: a 3-min initial denaturation at 94 °C, followed by 35 cycles of 94 °C for 1 min, 50 °C for 1 min, 72 °C for 1 min, and a final 10-min extension at 72 °C. Amplicons were visualized on a 1 % agarose gel, and single bands were cleaned with Agencourt AMPure XP magnetic beads according to the 96-well plate procedure (Beckman Coulter, Beverly, MA, USA), then quantified with a NanoDrop micro-volume spectrophotometer (Thermo Scientific, Wilmington, DE, USA) prior to in-house Sanger sequencing. Amplicons were sequenced with forward primer ITS1F and reverse primer ITS4 using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA).
Sequence contigs of fungal DNA from ectomycorrhizae were aligned, and base-pair calls corrected manually as necessary after examining chromatograms, using Sequencher 4.2 (Gene Codes, Ann Arbor, MI, USA). Primers were subsequently removed with MOTHUR v.1.16.0 (Schloss et al. 2009), and sequences of at least 450 bp were submitted for further analysis. The entire ITS region was isolated using the Fungal ITS Extractor (Nilsson et al. 2010) and compared against the GenBank nucleotide database (BLASTn; Altschul et al. 1997) via the ITS Pipeline (Nilsson et al. 2009), which groups sequences taxonomically. Sequences were also aligned with MAFFT v.5 (Katoh et al. 2002), then assembled into distance matrixes and clustered in MOTHUR (countends = F, cutoff = 0.10). By plotting the range of accumulation curves for operational taxonomic units (OTUs) from this site, we determined that 95 % molecular sequence similarity, although somewhat more inclusive than commonly used (Buée et al. 2009; Tedersoo et al. 2010), gave the best estimate of the number of fungal taxonomic groups for this dataset, as it has for previous studies (Jumpponen and Jones 2009; Walker et al. 2012). The OTUs, once defined, were assigned a species name if the representative sequence had ≥97 % similarity over at least 450 bp to a vouchered BLASTn database match. Remaining OTUs were subsequently given a genus name if there was 94–96 % similarity, a family name for a 91–93 % match, and an order, class, or division name if <90 % similarity.
Statistical analyses
The experimental design was a balanced hierarchical model with three factors: block (cutblocks A, B, C, and adjacent forest), plot treatment (control forest, CWD retention, CWD removal), and microsite (control mineral soil, decayed wood, and downed wood). Block was designated as a random factor; plot treatment and microsite were fixed factors, with microsite nested in plot.
After assessing assumptions of normality by plotting histograms and by performing Shapiro–Wilk’s tests, soil chemical data were cube-root transformed. Multivariate analyses were performed on two groups of soil chemical properties once it was determined that they covaried: (1) mineralizeable N, extractable ammonium, extractable nitrate, total C, and total N, and (2) exchangeable cations and CEC. These data were tested using hierarchical multivariate and univariate ANOVA (when multivariate results warranted subsequent analysis) (R v.2.15.0; R Development Core Team 2012). Post-hoc Tukey HSD tests were used to compare differences between all pairs of means when ANOVA P < 0.05, with Bonferroni correction. Daily minimum soil moisture and daily maximum soil temperature data were analyzed by permutational multivariate analysis; this was performed using ADONIS in the Vegan package of R (Oksanen et al. 2012).
Rarefied observed (Mau Tau) and estimated (Jackknife 1) fungal taxon richness, Shannon diversity, and evenness per microsite and per plot were calculated in EstimateS (v.8.2) (Colwell 2009). Sample-based rarefaction with replacement was applied to correct richness estimates for the unequal number of tips per sample. Indicator Species Analysis (Dufrêne and Legendre 1997) was used (PC-ORD; McCune and Mefford 1999) to explore the importance of individual fungal taxa to decayed wood, downed wood, and control microsite communities or to those in forest and clearcut plots. Analyses of community composition were based on relative abundance of each fungal taxon per seedling (i.e., number of mycorrhizae formed by each taxon divided by the total number of mycorrhizae on that seedling). These data were visualized using Nonmetric Multidimensional Scaling (NMDS) in R v.2.15.0, and compared using a mixed-effect hierarchical multivariate permutational ANOVA (Permanova; Anderson 2005). Pairwise post hoc tests were used to compare differences between all pairs of means when P < 0.05.
Results
Abiotic properties of microsites and plots
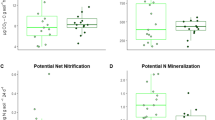
Soils collected with the seedlings differed in their chemical properties among plot and microsite treatments. When analyzed as a group, mineralizeable N, extractable ammonium and nitrate, total C and N, and C:N differed between forest and clearcut samples (Table S1). Although none of soil properties differed by plot type when analyzed individually, a combination of slightly higher mineralizeable N and available ammonium in forest soils (Fig. S1) seems to be responsible for the multivariate result. By contrast, whether analyzed as a group or individually, concentrations of exchangeable Al, Ca, Na, K, Fe, Mg, and Mn, plus CEC, did not differ between forest and clearcut plots. None of the soil chemical properties differed between the two types of clearcut plots and nor did we detect a block effect (Table S1). As expected, total C was much greater (eight-fold when untransformed values were considered) in decayed wood than in mineral soil microsites, but proximity to downed wood also resulted in a doubling of soil C as compared to mineral soil (Fig. 1). C:N ratios were almost four times higher in decayed wood than in either mineral soil microsites. Although mineralizeable N, available ammonium, and total N appeared to be highest in decayed wood and lowest in control mineral soil microsites, differences were not significant after Bonferroni correction (P = 0.05/6 variables = 0.008). Of the base cations analyzed, exchangeable K and Mg were higher in decayed wood than control microsites (almost three-fold and four-fold, respectively), but with a detectable increase also associated with the presence of downed wood (Fig. 2). As expected, the only cation that tended to be lower in decayed wood was the sole acid cation, Al.
a–c Mineralizeable and extractable nitrogen and d–f nitrogen and carbon status of substrate sampled from three microsite types within both forest and clearcut plots. Decayed decayed wood, downed mineral soil beside hard, downed wood, and Mineral control mineral soil >50 cm from downed wood. Raw data are presented in the figure, but all data were cube-root transformed for statistical analyses. P values are from univariate ANOVAs; lower case letters designate differences detected by Tukey HSD tests. Because of the non-independent nature of these variables, only P values <0.008 (Bonferroni correction = 0.05/6) represent significant differences among microsites. The boxes surrounding median values represent the first and third quartiles, while the whiskers show the smaller (and larger) of either the maximum (and minimum) vales or 1.5× the interquartile range (approximately ±2SD), n = 3. In the case of the latter, the outliers are plotted individually
a–e Concentration of exchangeable cations and f cation exchange capacity in substrates sampled from three microsite types within both forest and clearcut plots. Decayed decayed wood, Downed beside hard, downed wood, and mineral control mineral soil >50 cm from downed wood. Raw data is presented in the figure, but all data was cube root transformed for statistical analyses. P values are from univariate ANOVAs; lower case letters designate differences detected by Tukey HSD tests. Because of the non-independent nature of these variables, only P values <0.006 (Bonferroni correction = 0.05/8) represent significant differences among microsites. The boxes surrounding median values represent the first and third quartiles, while the whiskers show the smaller (and larger) of either the maximum (and minimum) vales or 1.5× the interquartile range (approximately ±2SD), n = 3. In the case of the latter, the outliers are plotted individually
Soil microclimate also varied between forest and clearcut and by microsite type over the 3-month growing season. Maximum daily soil temperature was higher in the clearcut plots than in the forest (P = 0.001, permutation multivariate ANOVA; Fig. S2a). In the forest, temperature was higher in decayed wood than in soil (P = 0.01). Within clearcut plots where wood had been retained, temperatures were lowest beside pieces of downed wood (P = 0.04), making these temperatures closest to those of forest soils.
Minimum daily soil moisture varied considerably among blocks because of elevation differences across the site (block P = 0.05, permutation multivariate ANOVA). When analyzed over the entire growing season, minimum daily soil moisture did not differ between forest and clearcut plots (P = 0.21) or among microsites across plot types (P = 0.66). Nevertheless, through much of the growing season, soil moisture appeared to be higher in decayed and downed wood microsites in the forest plots, compared to other microsites in forest or clearcut (Fig. S2b). By contrast, mineral soils in the forest remained drier and similar in moisture status to clearcut soils until late in the growing season. When the fall rains began (moderately on 20 August 2008, and then heavily on 24 September 2008), the relationship among microsites changed such that soil moisture in downed wood microsites in the clearcuts approached and exceeded that of forest soils.
Fungal community identification
A total of 121 spruce seedlings with active ectomycorrhizae on their root systems were collected; the remaining 14 were dead, dying, or not found upon sampling. Of 3,518 root tips morphotyped (mean number of active EMF root tips per seedling = 27.2; SD = 21.0), 321 were submitted for molecular analysis, and 86.9 % of these were identified. The sequences clustered into 63 OTUs. Prior to rarifying the taxon richness, the overall mean number of taxa per seedling was 1.83; SD = 0.91.
Fungal DNA amplified from the ectomycorrhizae was primarily from EMF taxa; 44 EMF OTUs, 15 OTUs from fungi known or suspected of forming ectomycorrhizae or other mycorrhizal associations, or representing fungal groups known to contain mycorrhizal species, and only 4 saprotrophic OTUs. The four known saprotrophs were not included in subsequent analyses. Thelephora terrestris, Amphinema byssoides, and Tylospora sp. 1 (likely T. asterophora) made up the largest proportion of identified EMF colonizing spruce roots (Table S2). Subdominant EMF taxa included Tylospora sp. 2 (possibly T. fibrillosa), Russula sp. 2 (possibly R. aeruginea) and a member of the Pyronemataceae (perhaps a Wilcoxina sp.).
Fungal community richness and evenness
A Coleman rarefaction curve of all EMF taxa successfully identified on seedlings sampled from all microsites at all plots approached an asymptote (Fig. S3). Estimated overall taxon richness was 66.7; SD = 4.6 (Jacknife1) across the site. Approximately twice as many EMF species were observed or estimated to occur in forest than clearcut plots, whereas microsite type did not affect EMF richness or evenness (Table 1). A significant block effect was detected for observed richness (data not shown).
Fungal community composition
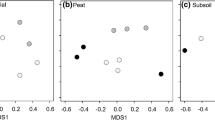
Ectomycorrhizal fungal communities on root tips of spruce seedlings were structured primarily by plot type (Fig. 3), but with a significant interaction with block (Table 2). Different communities of fungi colonized seedlings planted in forest and clearcut plots, whereas communities in the two types of clearcut plots did not differ. Several fungal taxa were responsible for the differences between forest and clearcut communities. Tylospora sp. 1 occurred more often in forest plots than in CWD removal plots (P = 0.05), while Tylospora sp. 2 (P = 0.04) and Tylospora fibrillosa (P = 0.02) occurred only in forest plots, and were both indicator species for this plot type (P ≤ 0.05). By contrast, Thelephora terrestris was more abundant in clearcut than forest plots (P < 0.001) and was an indicator species for CWD retention plots (P = 0.003). Although multivariate analyses did not detect differences in communities on seedlings between retention and removal clearcut plots (Table 2), univariate analyses on the relative abundances of individual taxa indicated that Amphinema byssoides was more abundant on seedlings in removal than retention plots (P = 0.02), and was a weak indicator species for the removal treatment (P = 0.06).
Two-dimensional NMDS ordination of EMF species relative abundance in decayed wood, downed wood, and control mineral soil microsites within forest, CWD removal, and CWD retention plots, sampled at three blocks: A (circled), B (labeled), and C (circled). Stress for this Euclidean solution was 0.16. Each icon within a block represents the EMF community in one of the three microsite types from one plot type in that block, n = 3–5. Dashed line surrounds EMF communities from all microsite types in all three forest plots
The EMF community did not differ among microsites across the site (Table 2), and, while block by microsite interactions were detected, EMF communities within a block did not cluster by microsite (Fig. 3). The block × microsite (plot) interaction is strongly driven by the nesting of microsite in plot, but post hoc pairwise analyses of Permanova results did not statistically support the patterns seen among microsites in some plot types in some blocks (Fig. 3). Indeed, examination of the relative abundance of EMF species among microsites within plots and blocks reveals that these apparent patterns are not driven by the consistent detection of distinct EMF species in certain microhabitats, but by the many zeroes common in a community dataset (Table S3). In addition, and contrary to our expectations, decayed wood microsites in the clearcut plots were no more aligned with forest samples than they were to control microsites in their respective plots.
Discussion
We initiated this study to investigate the factors driving assembly of EMF communities in clearcuts. By planting seedlings in various microsites in both forests and clearcuts, we hoped to be able to distinguish the relative importance of stochastic versus deterministic processes. Our results support a role for dispersal limitation in structuring EMF communities on young seedlings. The dispersal limitation arose both from differences in dispersal strategies among species and differences in the distributions of species across the site. There was less evidence for niche partitioning by microsite than we expected, but this may have been because of restricted colonization potential by such young seedlings.
Differences in the EMF community between forest and clearcuts
We had expected the clear distinction observed between EMF communities in both forest and clearcut plots. Our results agree with a well-known pattern wherein EMF species composition of the two communities always differs, even though EMF diversity may be higher or lower in forests than clearcuts (Jones et al. 2003 and references therein; Ding et al. 2011). In our case, EMF species richness was higher on seedlings planted in forests, with Amphinema byssoides and Thelephora terrestris emerging as the most abundant EMF species in clearcut plots, and Tylospora spp. occurring more often in forest plots. These differences in abundance seem to reflect differences in dispersal strategies among the three EMF taxa. A. byssoides and T. terrestris are dominant pioneer species on a range of hosts in these subalpine (Hagerman et al. 1999; Jones et al. 2002, 2009) and other temperate forests (Kranabetter and Friesen 2002; Hagerman and Durall 2004). Their abundance on nursery seedlings grown in sterile substrates (Kernaghan and Khasa 2003; Rudawska et al. 2006) indicates that they can disperse and colonize effectively from air-borne spores. Thelephora terrestris is also able to colonize from a persistent spore bank (Kranabetter and Friesen 2002). With these dispersal strategies, which do not rely on vegetative spread of hyphae from mycorrhizal roots of neighboring hosts, it is not surprising that A. byssoides and T. terrestris were dominant colonizers in the clearcut plots.
Tylospora spp. are common mycorrhizal symbionts in mature spruce forests in both North America and Europe (e.g., Flynn et al. 1998; Wallander et al. 2010), occurring on both seedlings and mature trees (Palfner et al. 2005) and in a range of substrates (Tedersoo et al. 2008). Less is known about their dispersal strategies; however, to our knowledge, they have not been observed on spruce seedlings in nurseries or disturbed environments such as very recent clearcuts, suggesting that they are not especially effective at colonizing from air-borne spores. Furthermore, the presence of T. asterophora only on spruce seedlings that had access to roots of mature trees (Tedersoo et al. 2008) indicates that root-to-root spread was the dominant form of dispersal for this species in Estonian forests. Although Tylospora spp. have been able to colonize the roots of 12-year-old saplings in the clearcuts at Sicamous Creek (Walker et al. 2012), our data indicate that Tylospora spp. are ineffective competitors with T. terrestris and A. byssoides for colonization of roots of young seedlings when dispersing from spores or resistant propagules. These differences in dispersal strategy likely explain differences in the EMF communities observed in the spruce seedlings in clearcuts and forest plots.
Although recent clearcuts can differ in microclimate and soil chemistry from established forests (Hannam and Prescott 2003; Wall 2008), such differences did not appear to play a role in partitioning species between clearcut and forest communities in this study. Most studies that report an effect of increased inorganic N, especially nitrate, are performed on very recent clearcuts. Approximately 15 years after harvest, differences in soil chemistry between clearcut and forest soils at Sicamous Creek were almost undetectable (this study). Microclimate did differ, however. Soils were warmer through the growing season and tended to be drier, depending on microsite. Nevertheless, the high relative abundance of Tylospora spp. on roots of 12-year-old spruce saplings growing in the clearcuts (Walker et al. 2012) indicates that soil microclimate did not restrict Tylospora spp. to forest environments, and further supports our conclusion that poorly effective dispersal from spores limits colonization by Tylospora spp. in clearcuts over the short term.
Lack of niche partitioning by ectomycorrhizal fungi among microsites
The decayed wood, downed wood, and control microsites at Sicamous Creek differed considerably in nutrient content and microclimate (this paper. and J.M.K. Walker, unpublished). If, as concluded by Bruns (1995) and others (Parrent et al. 2010; Koide et al. 2011), niche partitioning among substrates shapes EMF communities, these microsites would be expected to support different EMF communities. Nevertheless, in this high elevation forest, the EMF colonizing young planted spruce seedlings did not vary with microsite. We were surprised by this result, as others have found clear differences among EMF communities sampled from microsites, including decayed wood, in mature forests (Goodman and Trofymow 1998; Tedersoo et al. 2003; Iwánski and Rudawska 2007). Specifically, several studies (Goodman and Trofymow 1998; Tedersoo et al. 2003, 2008; Elliott et al. 2007; Iwánski and Rudawska 2007) found an abundance of Thelephoroid and Atheliod mycorrhizae associated with decomposed logs. While these groups represented the most dominant EMF detected in our studies, they were no more abundant in decayed wood than in mineral soil.
Limited dispersal of decayed-wood specialists from the forest, combined with a lack of niche specialization in ruderal EMF species, could explain the lack of microsite-specific EMF communities in the clearcuts. As discussed above, one group of EMF commonly found in decayed wood, Tylospora spp. (Tedersoo et al. 2003, 2008), seems to migrate only slowly from mature forests into clearcuts. Furthermore, the powerful influence of experimental block on EMF communities across the site suggests that dispersal limitation may well have prevented some fungi from colonizing seedlings in other parts of the site. Clearcut dominants, such as T. terrestris and A. byssoides, can be labeled as ruderal species because of their facile dispersal and establishment from spores in disturbed environments. In general, ruderal species are considered to be more homogeneous in their functional traits compared to competitive species (Grime 1977). Certainly, Barker et al. (2013) found remarkably similar EMF communities on 1- to 2-year-old Douglas-fir seedlings regenerating after stand-destroying wildfire and clearcut timber harvest, even though the nutrient status of the two soils differed considerably. Clearcut logging may have a homogenizing effect on EMF communities, as it does on plant communities (Brewer et al. 2012).
The same arguments cannot explain the results in forest plots, however. Dispersal was unlikely to limit colonization of seedlings by substrate specialists in forest plots because roots of mature trees, together with their fungal symbionts, were common in decayed logs. Over two growing seasons, even in the low temperature soils of this subalpine forest, we would expect hyphae of decayed-wood specialists to be able to reach and colonize seedling root tips. In addition, given the possibility for vegetative spread of hyphae from existing hosts to the seedlings (Tedersoo et al. 2008), we would not classify the fungal community on seedlings in the forest as ruderal (Deacon and Fleming 1992). Overall then, we reject the idea that any restriction in dispersal of substrate specialists contributed to our results. Instead, we propose that the young age and size of the bioassay seedlings acted as a filter to restrict colonization to a subset of the available pool of EMF, specifically those fungi with lower carbon demands. In a classic study, Gibson and Deacon (1988) found that EMF species stratified their colonization of birch root tips depending on the age of the root region, with some species colonizing only young roots. Furthermore, species colonizing younger parts of the root system, and/or those able to colonize from spores, appeared to require less carbon to grow and colonize than did other species (Gibson and Deacon 1990). Druebert et al. (2009) confirmed that restricting the carbon supply to roots restricted the number of EMF species able to colonize beech roots. This may be especially relevant for P. engelmannii because, unlike many other tree species, P. engelmannii seedlings from Sicamous Creek did not increase their photosynthetic activity under increased irradiance, instead retaining fixed carbon above ground (McKinnon and Mitchell 2003). Although seedlings in forests can become part of mycorrhizal networks with older trees (Beiler et al. 2010), thereby having some of the carbon needs of the fungus met by those other hosts (Wu et al. 2001), forest seedlings are still only colonized by a subset of the EMF found on the mature trees that surround them (Jonsson et al. 1999). This reduced species pool may be less likely to contain substrate specialists. In agreement with this hypothesis, Christy et al. (1982) and Jones et al. (2009) found no differences in the EMF communities on the roots of 1-year-old seedlings of Tsuga heterophylla or P. engelmannii seedlings growing in decayed wood versus mineral soil. Although differences in EMF communities have been detected on roots of young seedlings growing in decayed wood versus mineral soil substrates, these have sometimes been confounded by extreme differences between substrates (e.g., >3 pH units in Baier et al. 2006) or continual disturbance (e.g., erosion and flooding of mineral soil microsites in Tedersoo et al. 2008). EMF communities in forest types that commonly experience frequent and drastic perturbations (e.g., boreal forests, where most seedlings establish naturally after wildfire) have been shown to vary among microsites (Iwánski and Rudawska 2007), but in general, EMF communities on young seedlings growing in long-lived wet temperate forests, appear to be more uniform (e.g., Christy et al. 1982; Jones et al. 2009; this study).
Role of coarse woody debris in structuring EMF communities
In addition to the expectation that forest microsites would contain specialized EMF communities, we also expected that decayed wood in the clearcuts would retain species of EMF already colonizing it prior to clearcutting. This is the ‘legacy’ property attributed to downed and decayed wood by Molina et al. (2010). Fourteen years post-harvest, we found that nutrient availability was generally lower in clearcut plots than forest plots, but that downed and decayed wood microsites within the clearcuts had nutrient levels similar to those of forest soils. Furthermore, soils adjacent to downed wood on clearcuts were cooler than mineral soils, making them more similar to microclimates in forests. Nevertheless, even though microclimates created by the woody microsites in clearcuts were similar to conditions in the forest, EMF communities in decayed wood microsites in clearcuts were no more similar to forest communities than they were to the other microsite types in clearcuts.
Although we did not observe unique EMF communities in the decayed wood in this study, we suggest that over the medium and long term, retaining coarse woody debris in clearcuts will influence the development of EMF communities in or near the logs. Earlier studies have found that more unique communities of EMF develop in decayed wood in older seedlings than the ones studied here (Christy et al. 1982; Tedersoo et al. 2008). Furthermore, coarse woody debris can retain (Wall 2008) and accumulate (Preston et al. 2012) N as well as mineral elements such as calcium (Shortle et al. 2012). Our findings indicate that changes in edaphic conditions due to retention of post-harvest woody debris are detectable after almost 15 years, and that retention of large downed wood and its subsequent decay provide microsites that create forest-like habitat inside drier, hotter, and nutrient-depleted clearcut plots.
Conclusion
We found evidence for both stochastic and deterministic processes influencing the composition of EMF communities on young, planted spruce seedlings, but with some surprising aspects. Specifically, fine-scale differences in substrate type did not affect community assembly. Instead, the geographic location on the site significantly affected the EMF communities, suggesting that the species pool available to colonize seedlings varied across the site and that, at scales of tens of hectares, stochastic processes were influential. Within each 30-ha treatment unit, a difference in dispersal strategy among fungal species, which is deterministic because it favours differential colonization in sites with or without a high density of EMF hosts, appeared responsible for distinct forest and clearcut communities. We hypothesize that colonization by generalized ruderal species in the clearcuts, combined with a restricted pool of EMF able to colonize these very small seedlings in both forest and clearcut plots, contributed to the surprising lack of niche partitioning among microsites influenced by downed wood over the short or long term. Nevertheless, we were able to detect significant differences in soil nutrient status and microclimate among microsites and expect that niche-specific communities will develop there in older hosts. Consequently, we expect retention of coarse woody debris after clearcut logging to increase the diversity of the EMF community at the hectare scale as this forest re-establishes.
References
Agerer R (1987–2002) Colour atlas of ectomycorrhizae. Einhorn Eduard Dietenberger, Schwäbisch Gmünd
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST—a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Anderson MJ (2005) PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. University of Auckland, Department of Statistics, New Zealand
Baier R, Ingenhaag J, Blaschke H, Göttlein A, Agerer R (2006) Vertical distribution of an ectomycorrhizal community in upper soil horizons of a young Norway spruce (Piceaabies [L.] Karst.) stand of the Bavarian limestone Alps. Mycorrhiza1 6:197–206
Barker JS, Simard SW, Jones MD, Durall DM (2013) Ectomycorrhizal fungal community assembly on regenerating Douglas-fir after wildfire and clearcut harvesting. Oecologia. doi: 10.1007/s00442-012-2562-y
Beiler KJ, Durall DM, Simard SW, Maxwell SA, Kretzer AM (2010) Architecture of the wood-wide web: rhizopogon species genets link multiple Douglas-fir cohorts. New Phytol 185:543–553. doi:10.1111/j.1469-8137.2009.03069.x
Berg Å, Ehnström B, Gustafsson L, Hallingbäck T, Jonsell M, Weslien J (1994) Threatened plant, animal, and fungus species in Swedish forests: distribution and habitat associations. Conserv Biol 8:718–731. doi:10.1046/j.1523-1739.1994.08030718.x
Blaalid R, Carlsen T, Kumar S, Halvorsen R, Ugland KI, Fontana G, Kauserud H (2012) Changes in the root-associated fungal communities along a primary succession gradient analysed by 454 pyrosequencing. Mol Ecol 21:1897–1908. doi:10.1111/j.1365-294X.2011.05214.x
Brewer JS, Bertz CA, Cannon JB, Chesser JD, Maynard EE (2012) Do natural disturbances or the forestry practices that follow them convert forests to early- successional communities? Ecol Appl 22:442–458. doi:10.1890/11-0386.1
Bruns TD (1995) Thoughts on the processes that maintain local species diversity of ectomycorrhizal fungi. Plant Soil 170:63–73. doi:10.1007/BF02183055
Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, Martin F (2009) 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol 184:449–456. doi:10.1111/j.1469-8137.2009.03003.x
Bunnell F, Houde I (2010) Down wood and biodiversity-implications to forest practices. Environ Rev 18:397–421. doi:10.1139/A10-019
Chase JM, Myers JA (2011) Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B 366:2351–2363. doi:10.1098/rstb.2011.0063
Christy EJ, Sollins P, Trappe JM (1982) First-year survival of Tsuga heterophylla without mycorrhizae and subsequent ectomycorrhizal development on decaying logs and mineral soil. Can J Bot 60:1601–1605. doi:10.1139/b82-206
Colwell RK (2009) EstimateS: Version 8.2. Statistical estimation of species richness and shared species from samples. http://purl.oclc.org/estimates
Craig VJ, Klenner W, Feller MC, Sullivan TP (2006) Relationships between deer mice and downed wood in managed forests of southern British Columbia. Can J For Res 36:2189–2203. doi:10.1139/x06-118
Deacon JW, Fleming LV (1992) Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning: an integrative plant-fungal process. Chapman & Hall, New York, pp 249–300
Dickie IA, Reich PB (2005) Ectomycorrhizal fungal communities at forest edges. J Ecol 93:244–255. doi:10.1111/j.1365-2745.2005.00977.x
Dickie IA, Richardson SJ, Wiser SK (2009) Ectomycorrhizal fungal communities and soil chemistry in harvested and unharvested temperate Nothofagus rainforests. Can J For Res 39:1069–1079. doi:10.1139/X09-036
Ding Q, Liang Y, Legendre P, X-h He, Pei K-q Du, X-j Ma K-p (2011) Diversity and composition of ectomycorrhizal community on seedling roots: the role of host preference and soil origin. Mycorrhiza 12:669–680. doi:10.1007/s00572-011-0374-2
Druebert C, Lang C, Valtanen K, Polle A (2009) Beech carbon productivity as driver of ectomycorrhizal abundance and diversity. Plant Cell Env 32:992–1003. doi:10.1111/j.1365-3040.2009.01983.x
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Elliott JC, Smith JE, Cromack K Jr, Chen H, McKay D (2007) Chemistry and ectomycorrhizal communities of coarse wood in young and old-growth forests in the Cascade Range of Oregon. Can J For Res 37:2041–2051. doi:10.1139/X07-014
Flynn D, Newton AC, Ingleby K (1998) Ectomycorrhizal colonization of Sitka spruce (Picea sitchensis (Bong.) Carr) seedlings in a Scottish plantation forest. Mycorrhiza 7:313–317. doi:10.1007/s005720050198
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for Basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. doi:10.1111/j.1365-294X.1993.tb00005.x
Gibson F, Deacon JW (1988) Experimental study of establishment of ectomycorrhizas in different regions of birch root systems. Trans Br Mycol Soc 91:239–251
Gibson F, Deacon JW (1990) Establishment of ectomycorrhizas in aseptic culture: effects of glucose, nitrogen and phosphorus in relation to successions. Mycol Res 94:166–172. doi:10.1016/S0953-7562(09)80608-4
Goodman DM, Trofymow JA (1998) Distribution of ectomycorrhizas in microhabitats in mature and old-growth stands of Douglas-fir on southeastern Vancouver Island. Soil Biol Biochem 30:2127–2138. doi:10.1016/S0038-0717(98)00094-7
Goodman DM, Durall DM, Trofymow JA, Berch SM (1996) A manual of concise descriptions of North American Ectomycorrhizae. Mycologue, Sydney, pp 3A1–3A5
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Hagerman SH, Durall DM (2004) Ectomycorrhizal colonization of greenhouse-grown Douglas-fir (Pseudotsuga menziesii) seedling by inoculum associated with the roots of refuge plants sampled from a Douglas-fir forest in the southern interior of British Columbia. Can J Bot 82:742–751. doi:10.1139/b04-047
Hagerman SH, Jones MD, Bradfield GE, Gillespie M, Durall DM (1999) Effects of clear- cut logging on the diversity and persistence of ectomycorrhizas at a subalpine forest. Can J For Res 29:124–134. doi:10.1139/x98-186
Hannam KD, Prescott CE (2003) Soluble organic nitrogen in forests and adjacent clearcuts in British Columbia, Canada. Can J For Res 33:1709–1718. doi:10.1139/x03-091
Harvey AE, Larsen MJ, Jurgensen MF (1979) Comparative distribution of ectomycorrhizae in soils of three western Montana forest habitat types. For Sci 25:350–358
Hollstedt C, Vyse A. (1997) Sicamous Creek Silvicultural Systems Project: Workshop Proceedings April 24–25, 1996. Work Pap 24/1997. Res Br BC Min For, Victoria, B.C., Canada
Iwánski M, Rudawska M (2007) Ectomycorrhizal colonization of naturally regenerating Pinus sylvestris L. seedlings growing in micro-habitats in boreal forests. Mycorrhiza 17:461–467. doi:10.1007/s00572-007-0132-7
Izzo A, Nguyen DT, Bruns TD (2006) Spatial structure and richness of ectomycorrhizal fungi colonizing bioassay seedlings from resistant propagules in a Sierra Nevada forest: comparisons using two hosts that exhibit different seedling establishment patterns. Mycologia 98:374–383. doi:10.3852/mycologia.98.3.374
Jones MD, Hagerman SH, Gillespie M (2002) Ectomycorrhizal colonization and richness of previously colonized, containerized Picea engelmannii does not vary across clearcuts when planted in mechanically site-prepared mounds. Can J For Res 32:1425–1433. doi:10.1139/x02-069
Jones MD, Durall DM, Cairney JGW (2003) Ectomycorrhizal fungal communities in young stands regenerating after clearcut logging. New Phytol 157:399–422. doi:10.1046/j.1469-8137.2003.00698.x
Jones MD, Grenon F, Peat H, Fitzgerald M, Holt L, Philip LJ, Bradley R (2009) Differences in 15 N uptake among spruce seedlings colonized by three pioneer ectomycorrhizal fungi in the field. Fungal Ecol 2:110–120. doi:10.1016/j.funeco.2009.02.002
Jonsson L, Dahlberg A, Nilsson M-C, Kårén O, Zackrisson O (1999) Continuity of ectomycorrhizal fungi in self-regenerating boreal Pinus sylvestris forests studied by comparing mycobiont diversity on seedlings and mature trees. New Phytol 142:151–162. doi:10.1046/j.1469-8137.1999.00383.x
Jumpponen A (2003) Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytol 158:569–578. doi:10.1046/j.1469-8137.2003.00767.x
Jumpponen A, Jones KL (2009) Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184:438–448. doi:10.1111/j.1469-8137.2009.02990.x
Katoh K, Misawa K, Kuma K, Miyata T (2002) Mafft: a novel method for rapid multiple sequence alignment based on fast fourier transform. Nucleic Acids Res 30:3059–3066. doi:10.1093/nar/gkf436
Kennedy P, Higgins LM, Rogers RH, Weber MG (2011) Colonization-competition tradeoffs as a mechanism driving successional dynamics in ectomycorrhizal fungal communities. PLoS ONE 6:1–10. doi:10.1371/journal.pone.0025126
Kernaghan G, Khasa D (2003) Mycorrhizal and root endophytic fungi of containerized Picea glauca seedlings assessed by DNA sequence analysis. Microb Ecol 45:128–136. doi:10.1007/s00248-002-1024-1
Koide RT, Fernandez C, Petprakob K (2011) General principles in the community ecology of ectomycorrhizal fungi. Ann For Sci 68:45–55. doi:10.1007/s13595-010-0006-6
Kranabetter JM, Friesen J (2002) Ectomycorrhizal community structure on western hemlock (Tsuga heterophylla) seedlings transplanted from forests into openings. Can J Bot 80:861–868. doi:10.1139/b02-071
Laiho R, Prescott CE (2004) Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: a synthesis. Can J For Res 34:763–777. doi:10.1139/x03-241
Lekberg Y, Koide RT, Rohr JR, Aldrich-Wolfe L, Morton JB (2007) Role of niche restrictions and dispersal in the composition of arbuscular mycorrhizal fungal communities. J Ecol 95:95–105. doi:10.1111/j.1365-2745.2006.01193.x
McCune B, Mefford MJ (1999) PC-Ord. Multivariate analysis of ecological data, Version 5.0. MJM Software Design, Gleneden Beach
McKinnon LM, Mitchell AK (2003) Photoprotection, not increased growth, characterizes the response of Engelmann spruce (Picea engelmannii) seedlings to high light, even when resources are plentiful. New Phytol 160:69–79. doi:10.1046/j.1469-8137.2003.00854.x
Molina R, Horton TR, Trappe JM, Marcot BG (2010) Addressing uncertainty: how to conserve and manage rare or little-known fungi. Fungal Ecol 4:134–146. doi:10.1016/j.funeco.2010.06.003
Nara K (2005) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169:169–178. doi:10.1111/j.1469-8137.2005.01545.x
Nilsson RH, Bok G, Ryberg M, Kristiansson E, Hallenberg N (2009) A software pipeline for processing and identification of fungal ITS sequences. Source Code Biol Med 4:1. doi:10.1186/1751-0473-4-1
Nilsson RH, Veldre V, Hartmann M, Unterseher M, Amend A, Bergsten J, Kristiansson E, Ryberg M, Jumpponen A, Abarenkov K (2010) An open source software package for automated extraction of ITS1 and ITS2 from fungal ITS sequences for use in high-throughput community assays and molecular ecology. Fungal Ecol 3:284–287. doi:10.1016/j.funeco.2010.05.002
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2012) vegan: community ecology package. R package version 2.1 http://R-Forge.R-project.org/projects/vegan/
Palfner G, Cassanova-Katny MA, Read DJ (2005) The mycorrhizal community in a forest chronosequence of Sitka spruce (Picea sitchensis (Bong. (Carr) in Northern England. Mycorrhiza 15:571–579. doi:10.1007/s00572-005-0364-3
Parrent JL, Peay K, Arnold AE, Comas LH, Avis P, Tuininga A (2010) Moving from pattern to process in fungal symbioses: linking functional traits, community ecology and phylogenetics. New Phytol 185:882–886. doi:10.1111/j.1469-8137.2010.03190.x
Peay KG, Garbelotto M, Bruns TD (2010) Evidence of dispersal limitation in soil microorganisms: isolation reduces species richness on mycorrhizal tree islands. Ecology 91:3631–3640. doi:10.1890/09-2237.1
Preston CM, Trofymow JA, Nault JR (2012) Decomposition and change in N and organic composition of small-diameter Douglas-fir woody debris over 23 years. Can J For Res 42:1153–1167. doi:10.1139/x2012-076
Queloz V, Sieber TN, Holenrieder O, McDonald BA, Grünig CR (2011) No biogeographical pattern for a root-associated fungal species complex. Glob Ecol Biogeogr 20:160–169. doi:10.1111/j.1466-8238.2010.00589.x
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/
Rudawska M, Leski T, Trocha LK, Gornowicz R (2006) Ectomycorrhizal status of Norway spruce seedlings from bare-root forest nurseries. For Ecol Manag 236:375–384. doi:10.1016/j.foreco.2006.09.066
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing MOTHUR: open-source, platform- independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi:10.1128/AEM.01541-09
Shortle WC, Smith KT, Jellison J, Schilling JS (2012) Potential of decaying wood to restore root- available base cations in depleted forest soils. Can J For Res 42:1015–1024. doi:10.1139/x2012-056
Simard SW, Perry DA, Smith JE, Molina R (1997) Effects of soil trenching on occurrence of ectomycorrhizae on Pseudotsuga menziesii seedlings grown in mature forests of Betula papyrifera and Pseudotsuga menziesii. New Phytol 136:327–340. doi:10.1046/j.1469-8137.1997.00731.x
Smith JE, Molina R, Huso MMP, Larsen MJ (2000) Occurrence of Piloderma fallax in young, rotation-age, and old-growth stands of Douglas-fir (Pseudotsuga menziesii) in the Cascade Range of Oregon, U.S.A. Can J Bot 78:995–1001. doi:10.1139/b00-085
Taylor DL, Bruns TD (1999) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol Ecol 8:1837–1850. doi:10.1046/j.1365-294x.1999.00773.x
Tedersoo L, Kõljalg U, Hallenberg N, Larsson K-H (2003) Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol 159:153–165. doi:10.1046/j.1469-8137.2003.00792.x
Tedersoo L, Suvi T, Jairus T, Kõljalg U (2008) Forest microsite effects on community composition of ectomycorrhizal fungi on seedling of Picea abies and Betula pendula. Environ Microbiol 10:1189–1201. doi:10.1111/j.1462-2920.2007.01535.x
Tedersoo L, Nilsson RH, Abarenkov K, Jairus T, Sadam A, Saar I, Bahram M, Bechem E, Chuyong G, Kõljalg U (2010) 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological bias. New Phytol 188:291–301. doi:10.1111/j.1469-8137.2010.03373.x
Walker JKM, Ward V, Paterson C, Jones MD (2012) Coarse woody debris retention in subalpine clearcuts affects ectomycorrhizal root tip community structure within fifteen years of harvest. J Appl Soil Ecol 60:5–15. doi:10.1016/j.apsoil.2012.02.017
Wall A (2008) Effect of removal of logging residue on nutrient leach and nutrient pools in the soil after clearcutting in a Norway spruce stand. For Ecol Manag 3256:1372–1383
Wallander H, Johansson U, Sterkenburg E, Durling MB, Lindahl BD (2010) Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests. New Phytol 187:1124–1134. doi:10.1111/j.1469-8137.2010.03324.x
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Wu BY, Nara K, Hogetsu T (2001) Can C-14 labeled photosynthetic products move between Pinus densiflora seedlings linked by ectomycorrhizal mycelia? New Phytol 149:137–146. doi:10.1046/j.1469-8137.2001.00010.x
Acknowledgments
Funding for this project was provided by a Discovery Grant from the Natural Sciences and Engineering Research Council, and by the Forest Science Program of the British Columbia Forest Investment Account to M.J. J.W. acknowledges support from UBC Okanagan and the Province of BC for scholarships. We thank Alan Vyse for safe access to the field site, MaryAnn Olson and Fawn Ross for extensive fieldwork, and Valerie Ward for unsurpassed assistance in the field and laboratory. This manuscript was greatly improved with the help of constructive comments from two anonymous reviewers.
Conflict of interest
The authors declare that no financial conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hakan Wallander.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Walker, J.K.M., Jones, M.D. Little evidence for niche partitioning among ectomycorrhizal fungi on spruce seedlings planted in decayed wood versus mineral soil microsites. Oecologia 173, 1499–1511 (2013). https://doi.org/10.1007/s00442-013-2713-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2713-9