Abstract
Climatic and land use changes have significant consequences for the distribution of tree species, both through natural dispersal processes and following management prescriptions. Responses to these changes will be expressed most strongly in seedlings near current species range boundaries. In northern temperate forest ecosystems, where changes are already being observed, ectomycorrhizal fungi contribute significantly to successful tree establishment. We hypothesised that communities of fungal symbionts might therefore play a role in facilitating, or limiting, host seedling range expansion. To test this hypothesis, ectomycorrhizal communities of interior Douglas-fir and interior lodgepole pine seedlings were analysed in a common greenhouse environment following growth in five soils collected along an ecosystem gradient. Currently, Douglas-fir’s natural distribution encompasses three of the five soils, whereas lodgepole pine’s extends much further north. Host filtering was evident amongst the 29 fungal species encountered: 7 were shared, 9 exclusive to Douglas-fir and 13 exclusive to lodgepole pine. Seedlings of both host species formed symbioses with each soil fungal community, thus Douglas-fir did so even where those soils came from outside its current distribution. However, these latter communities displayed significant taxonomic and functional differences to those found within the host distribution, indicative of habitat filtering. In contrast, lodgepole pine fungal communities displayed high functional similarity across the soil gradient. Taxonomic and/or functional shifts in Douglas-fir fungal communities may prove ecologically significant during the predicted northward migration of this species; especially in combination with changes in climate and management operations, such as seed transfer across geographical regions for forestry purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is expected to significantly influence the future ranges of plant species (IPCC 2013), with individual species experiencing expansion and/or contraction of regions that are suitable for establishment and growth (Iverson et al. 2004; Aitken et al. 2008; Coops and Waring 2011; Wang et al. 2012). Future distribution models are primarily based upon on temperature and moisture changes, and in northern hemisphere forests, rapid northward expansion of climatic suitability zones are predicted (Hamann and Wang 2006; Rehfeldt et al. 2014). Changes in climate are expected to alter interactions between plants and their fungal symbionts (Pickles et al. 2012), yet symbioses are rarely considered when modelling future species distributions (Dormann 2007). Recent paleoecological studies have indicated that historical range expansions did not simply follow climatic shifts, leading Elias (2013) to speculate that incompatibility with symbiont communities may have played a role in slowing host plant migrations in the Pacific Northwest. Thus, the contemporary distribution boundaries of host plants provide a useful ecological demarcation across which future range expansion can be examined with respect to host-symbiont interactions. Here, we assessed seedling interactions with symbionts across one such boundary to explore our hypothesis that mycorrhizal fungi could play a role in either limiting or facilitating host range expansion in response to climatic changes.
In western North America, Pseudotsuga menziesii var. glauca (interior Douglas-fir) is an ecologically and economically important constituent of forests, with a distribution that extends from isolated patches in Mexico around 19.0° N to approximately 54.5° N in western Canada. In British Columbia, interior lodgepole pine (Pinus contorta var. latifolia) and interior Douglas-fir co-occur within the same landscapes and across the same gradient of ecosystems (Pojar et al. 1987). Douglas-fir is thought to disappear from these ecosystems at its northern boundary because of high sensitivity to microsite conditions that favour the formation of frost pockets (Jull 1999; Griesbauer and Green 2010), though it is unlikely that this single factor controls the presence or absence of Douglas-fir. Thus, these two host species may display a degree of commonality in their regional and local symbiont species pools (for a discussion of the ‘species pool’ concept see Zobel 1997; Zobel et al. 1998, 2011) due to overlapping distributions, and the presence of lodgepole pine may facilitate the natural northward expansion of Douglas-fir through maintenance of symbiont reservoirs if environmental limitations to host distribution are removed through climatic changes (e.g. Reithmeier and Kernaghan 2013). Here, we refer to observed differences in the fungal communities of different hosts grown in the same soil as ‘host filtering’.
Formation of symbioses with ectomycorrhizal fungi (EMF) are important for seedling establishment in North American Douglas-fir forests (Simard 2009), with community differences observed between different life stages of Douglas-fir (Twieg et al. 2007). In our examination of the literature, we have found that, where multi-host EMF systems have been examined, it appears common for approximately one third of EMF species to be shared (with the exception of Alnus and its more specialised/selective community; Bogar and Kennedy 2013; Roy et al. 2013), thus a reservoir of suitable fungal symbionts may be available outside a host’s natural distribution through persistence on an alternate co-occurring host species (Ishida et al. 2007; Twieg et al. 2007; Timling et al. 2012; Lim and Berbee 2013). Indeed, the observed distribution of some EMF species covers temperate and boreal regions throughout the Northern hemisphere (e.g. Cortinarius spp.; Liimatainen et al. 2014), which suggests that many fungal symbionts are likely to be capable of utilising multiple hosts. Spore dispersal is also likely to provide hosts access to compatible symbionts, although this only appears relevant over short spatial scales (Peay et al. 2012), and the accumulation of potentially long-lived spores in soils (Bruns et al. 2009; Nara 2009; Nguyen et al. 2012) may increase the availability of symbionts in a manner analogous to the soil seed bank (Thompson 2000).
Alongside EMF, Douglas-fir and lodgepole pine are colonised by a variety of non-mycorrhizal root-associated fungi. Whereas the benefits of EMF are well established (Smith and Read 2008), the consequences of root colonisation by non-mycorrhizal fungi are less clear. In a meta-analysis of root endophytes, Mayerhofer et al. (2013) found that although beneficial effects of endophytes on plant biomass occurred, interactions were mostly neutral to negative; however, they also noted that plant responses are highly variable and specific to the environmental context. A meta-analysis of dark septate endophyte (DSE) fungi by Newsham (2011) reported increases in host root and shoot biomass when nitrogen was predominantly available in organic form and attributed this benefit to nutrient mineralisation. This latter hypothesis is especially compelling since beneficial effects of Phialocephala fortinii have been noted even in the absence of direct colonisation of the roots by the fungus (Ruotsalainen and Kytöviita 2004). Clearly, fungal root endophytes display a fundamentally different mode of action from EMF: whereas EMF generally aid the host via the direct uptake of nutrients by extramatrical hyphae, fungal endophytes are more variable in their effects upon growth but may improve nutrient uptake via mineralisation processes. For the purposes of this study, we considered the following to be fungal endophytes: (i) root endophytes, including DSE such as Phialocephala spp. (Addy et al. 2005), (ii) species for which there is limited evidence for definitive mycorrhizal status with the host e.g. Helotiales spp. (Tedersoo et al. 2009) and Meliniomyces sp. (Hambleton and Sigler 2005) and (iii) putative pathogenic fungi residing in roots (e.g. Nilsson et al. 2014).
Where a slowly dispersing host such as Douglas-fir is introduced into new regions, such as through management intervention (e.g. assisted migration; Pedlar et al. 2012), it may exhibit novel responses to the available symbiont communities in that region. As an extreme example, members of the Pinaceae have historically failed to establish in exotic forestry operations due to an absence of co-evolved symbionts (Mikola 1970; Nuñez et al. 2009). However, with range expansion or limited seed transfer, the relevant question is whether the formation of suboptimal symbioses outside of a tree’s range could lead to reduced resilience during colonisation of a new habitat (Elmqvist et al. 2003), perhaps resulting from a loss of functional redundancy (Rineau and Courty 2011). Possible mechanisms for compromised resilience are as follows: (a) a legacy of host absence may lead to fewer compatible fungal species being available (especially those considered to be ‘specialists’), (b) changes in the filters acting on the actual pool (environmental and biological) may lead to shifts in symbiont function and/or (c) symbionts may readily interact with the host but provide less benefit. If available symbiont diversity is lowered, or key symbionts are absent, this could contribute to the observed range limits of Douglas-fir. Kranabetter et al. (2012) suggests this as a factor for consideration in seed transfer operations involving coastal Douglas-fir, although further examination is required. Here, we use the term ‘habitat filtering’ from the assembly rules literature (e.g. Diamond 1975; Weiher and Keddy 1999) to represent the combination of environmental variables influencing successful symbiont colonisation of a host at a site, such that only those species with suitable ecological or physiological adaptations will form symbioses.
We hypothesised that the degree of compatibility with fungal symbionts could facilitate or limit interior Douglas-fir migration northwards in response to climate change. To test this, we examined seedling root colonisation by EMF and fungal endophytes across a northern distribution boundary, using interior Douglas-fir as an experimental species and interior lodgepole pine as a background control due to its extensive natural distribution throughout British Columbia (Little 1978). Host genotypes show heritability for several EMF traits (Rosado et al. 1994; Peterson and Bradbury 1999; Karst et al. 2009). Plants also display evidence of local adaptation to mycorrhizal symbionts (Johnson et al. 2010; Hoeksema et al. 2012; Ji et al. 2013). Given these observations, testing the responses of multiple seed provenances (local populations of known origin) of each host species was warranted. Thus, seeds from eight provenances of each host were grown in forest soil taken from five sites through British Columbia selected along an ecosystem gradient of biogeoclimatic zones (BEC zones; Pojar et al. 1987). This gradient crossed the northern distribution boundary of Douglas-fir but was well within the distribution of lodgepole pine. Functional differences were approximated using the ‘exploration type’ concept, in which mycorrhizal morphology (e.g. extent of extramatrical hyphae, rhizomorphs, etc.) reflects differences in nutrient acquisition strategies (Agerer 2001). Fungal endophytes were added as an additional functional type in recognition of the fact that, while we cannot yet quantify their effect on host plants with certainty, the evidence points to differences in function from EMF. The contributions of host and/or habitat filtering to seedling root-associated fungal communities were assessed.
Materials and methods
Seed selection and site descriptions
Seed of interior Douglas-fir and interior lodgepole pine from eight provenances per species (five class A, selectively bred ‘improved’, provenances (A1–A5) typically used in forestry operations and three class B, seed orchard or ‘wild’, provenances (B1–B3)) was provided by the British Columbia Ministry of Forests, Lands and Natural Resource Operations (BC MoFLNRO). Class B seed lots were selected from the BC MoFLNRO seed bank based on their close geographic proximity and climatic similarity (Hamann and Wang 2005; Wang et al. 2006) to that of class A seed orchards (Online Resource 1).
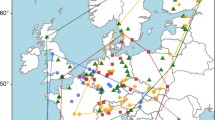
Study sites were established at five 1-ha areas within British Columbia (BC) where interior Douglas-fir is either (a) currently the dominant tree species or (b) not currently present but projected to become climatically suitable within the next 45 years (Fig. 1). Each site was continental in climate (mean annual temperature ranged from 0.8 to 3.4 °C), experienced frost over more than half of the year and was located within an undisturbed forest with an estimated stand age of approximately 80 years. These sites are referred to in the text using a four-letter code based on location and host species; climatic and soil nutrient data are provided for each site (Online Resource 2). The mono-dominant host species at each site (>95 % of the stand) was either interior Douglas-fir (VN-DF, PG-DF, JP-DF) or interior lodgepole pine (MZ-LP, FS-LP), with no mixing of these host species within each site.
Geographical distribution of study sites for soil collection and comparison with current natural range of interior Douglas-fir (green shading). White symbols were naturally regenerating interior Douglas-fir stands of harvestable age, with scattered Betula papyrifera (paper birch) also present. Black symbols were interior lodgepole pine stands of similar age structure, and scattered paper birch. Small numbers of Picea glauca (white spruce) were present in the FS-LP site. Each site had a ground layer of mosses and ericaceous plants, mostly Vaccinium myrtilloides (velvet leaf blueberry)
Greenhouse growth conditions
From mid-September to mid-October 2008, approximately 120 l of O-horizon soil (upper 15 cm, including small volumes of mineral horizon) was collected from five sub-locations per site, sieved to remove large debris (2 cm × 2 cm mesh) and transported for homogenisation in clean conditions. Seed was pretreated for germination using cold stratification and surface sterilisation (with hydrogen peroxide) techniques (Kolotelo et al. 2001). Twenty seedlings per provenance per soil (800 seedlings per host species) were established (two seeds planted with subsequent thinning if both emerged) in individual 650 ml Deepot™ seedling containers (Stewe and Sons, Oregon, USA). Containers were filled to within 1 cm of the rim with soil then covered with a layer of fine gravel to reduce surface evaporation and ‘damping off’. To stimulate mycorrhizal colonisation, no fertilisation was applied, but otherwise ‘non-limiting’ conditions (moisture, light and temperature) were maintained for approximately one growing season (8 months) to give time for mycorrhizal formation (initial examination at 3 months revealed little to no mantle formation). Soil moisture was maintained at a minimum of 80 % of saturated mass through irrigation only. Day length and temperature were standardised over the experimental period (16 h of daylight at 25 °C, 8 h of night at 20 °C, 15 min transition intervals of 22 °C) using 400-W high-pressure sodium lights (P.L. Light Systems, Beamsville, ON, Canada.).
Fungal identification
Following 8–9 months of growth, fungal community data was recorded for five randomly selected seedlings per provenance per soil (200 seedlings per host species; reduced to 195 Douglas-fir and 167 lodgepole pine due to seedling mortality). For each seedling individually, all fine roots were extracted and placed on a numbered grid in a container filled with de-ionised water. To avoid selection bias, 50 root tips were assessed from each root system using a random number generator to select grid squares. Excised fine roots were examined at ×40 magnification under a stereomicroscope and sorted into uncolonised (no discolouration or signs of hyphae or mantle formation) or individual fungal morphotypes. Five uncolonised tips and five tips of each putative morphotype were subsampled from each seedling and stored for later DNA analysis to ensure reliability of morphotyping. Where available, a minimum of 10 sequenced root tips per morphotype were used to confirm species identities. Root tips were homogenised using a micropestle, and fungal DNA was extracted using the DNeasy Plant Mini Kit according to the manufacturer’s instructions (Qiagen, ON, Canada). The internal transcribed spacer region (ITS) was amplified for each DNA sample using ITS1 (White et al. 1990) and NLB4 primers (Martin and Rygiewicz 2005). Amplifications were performed on a PTC Dyad Thermal Cycler (MJ Research Inc., MA, USA) in 50 μl volumes containing: nuclease-free H2O, 2 mM MgCl2, 5.0 μl buffer (16 mM (NH4)2SO4, 67 mM Tris-HCl (pH 8.8 at 25 °C), 0.01 % Tween-20)), 2 mM dNTP, 20 pmol each primer, 2.5 μl BSA, 1.15 units Platinum® Taq DNA polymerase (Invitrogen, ON, Canada) and 100 ng DNA. Thermocycling conditions: 3 min denaturation (95 °C), 35 cycles of denaturing, annealing and extension (95 °C for 30 s, 50 °C for 30 s and 72 °C for 1 min, respectively) and 5 min final extension (72 °C). PCR products were purified using the Agencourt AMPure system (Invitrogen) then sequenced using ABI BigDye v3.1 Terminator chemistry and an ABI 3130xl Genetic Analyser (Applied Biosystems, ON, Canada). Raw sequence data were analysed with SEQUENCHER Version 4.7 (Gene Codes Corp., MI, USA) before comparison with NCBI and UNITE (Kõljalg et al. 2013) databases using the BLAST algorithm. Names were assigned to morphotypes based on the combination of morphological characteristics and minimum 97 % sequence matches corresponding to the indicated species. These sequence data have been submitted to the GenBank database under accession numbers JF792502–JF792516 (Douglas-fir sequences) and KM008614-KM008632 (lodgepole pine sequences).
Based on the morphotyping-sequencing technique described above, root tip communities were divided into EMF, fungal endophytes and uncolonised categories then into the different exploration types. ‘Uncolonised’ root tips displayed no discernible mantle and an insignificant quantity of fungal DNA following extraction with the DNEasy kit. Further examination of subsampled tips with microscopy found no evidence of Hartig net formation. Occasionally, a root tip contained an EMF sequence and a ‘fungal endophyte’ sequence. In these cases, the EMF was considered to take functional priority due to the presence of hyphae and/or a mantle indicating structural changes to the root tip. Tips that only contained fungal endophyte DNA and showed no evidence of EMF colonisation were assigned to the ‘fungal endophyte’ category. Finally, to confirm that fungal species encountered in the greenhouse were present in the field, rather than ‘weedy’ species (e.g. Marx et al. 1982; Smith and Read 2008), fungal sequences were compared to those detected on mature host roots (see Online Resource 3) at each of the five field sites in a separate study (Pickles et al. unpublished data).
Statistical analyses
We note that for all analyses in which multiple testing took place, the Benjamini and Hochberg false discovery rate (FDR) correction was applied to P values (Verhoeven et al. 2005). Normality and variance of data was examined prior to selection of an appropriate parametric (GLM ANOVA) or nonparametric (K-W) statistical test using Minitab® 16.
Fungal community interactions were assessed using C-score analysis (Stone and Roberts 1990) in EcoSim v 7.72 (Gotelli and Entsminger 2009), which was used here to determine the extent to which species co-occurred between hosts, as compared to randomised data. A negative C-score indicates that species occur together on a host more often than would be expected by chance (interpreted as ‘facilitation’), whereas a positive C-score indicates species are less likely to occupy the same host than would be expected if they were randomly distributed (interpreted as ‘competition’, although in the original conception of co-occurrence analysis (Stone and Roberts 1990) competition was between sites or habitats, whereas here, we ask whether available fungal species will colonise one host species preferentially over the other). Each seedling root system was considered to be a ‘site’ which fungi could colonise. Null model constraints were set to ‘fixed-equiprobable’, meaning that the occurrence of each fungal species in any null community was the same as in the observed data, and each seedling was considered equally suitable for each fungal species; this is the most stringent option for analysis (Gotelli and Entsminger 2009). Species present on fewer than three seedlings were removed from the data set due to a lack of interpretive value. Expected C-scores were obtained from 10,000 Monte Carlo randomisations of the data using a sequential swap algorithm. Observed C-scores were compared to the null distribution of expected values and considered ecologically relevant if statistically significant at P < 0.05 and exhibiting a standardised effect size (SES) >2 or <−2 (a difference of two or more standard deviations from the expected mean). Null model assumptions were primarily applied to examine evidence for host filtering, which was expressed when competition was significant across all seedlings of both hosts and simultaneously nonsignificant when either host was considered separately. The overall presence or absence of habitat filtering could also be examined based on whether competition was significant across all sites for a single host and simultaneously nonsignificant within sites for that host (i.e. all site ‘competitive’ effects are purely due to differences in communities between sites, not actual competition between fungal species within sites). Where ecological relevance was detected, values in the upper (or lower) 97.5 % tail of the distribution of pairwise C-scores were considered to represent significant species interactions.
Patterns in fungal community data and exploration type data were assessed with nonparametric multi-dimensional scaling (NMS) using the Sørensen (Bray-Curtis) distance measure in PC-Ord 5 (MjM Software, OR, USA). Mean community data for each provenance was used in these analyses to account for variation in EMF communities within a site, resulting in eight data points per host per soil site. Each ordination began with a stress-minimising, resolution optimising step: 6-dimensional solution stepping down to 1-dimensional, instability criterion 0.0005, 200 iterations, 50 real data runs, 100 Monte Carlo simulations. Final ordinations used: 3-dimensional (fungal taxa) or 2-dimensional (exploration type) solution, stress-minimising starting configuration, no step-down in dimensionality, one real run, no randomised runs. Pearson’s (r) linear and Kendall’s (τ) rank correlations with ordination axes were assessed for fungal species data, soil chemistry and site-specific environmental factors. For factors correlated with each other at r 2 values ≥ 0.90, only the factor producing the strongest correlations to ordination axes was presented.
Differences in community composition were examined for both fungal species and exploration types using permutational multivariate analysis of variance (PERMANOVA; Anderson, 2001) in PC-Ord 5 (MjM Software, OR, USA). Provenance mean community or exploration type data was converted into Sørensen (Bray-Curtis) distances and observed values from the model ‘community dissimilarity ∼ Host*Site’ were compared to the expected values obtained from 5000 randomisations of the data. Pairwise site comparisons were determined by stratifying community permutations within each host species and significance was established after correcting for multiple testing. Since heterogeneity in group dispersion can lead to spurious PERMANOVA results, multivariate dispersion was examined in the R software environment (R Core Team 2014) using the Betadisper function in R-package ‘vegan’ (Oksanen et al. 2013).
Results
Seedling fungal communities and root colonisation
A total of 29 fungal species were encountered across both hosts and all soils, seven of which were shared between hosts (24.1 %), with nine exclusive to Douglas-fir (31.0 %) and 13 exclusive to lodgepole pine (44.8 %). Interior Douglas-fir seedlings were colonised by 14 EMF and three fungal endophyte species (Table 1) compared to 12 EMF and eight fungal endophytes on interior lodgepole pine seedlings (Table 2). The most frequently encountered fungal species on Douglas-fir seedlings were Pyronemataceae sp. (57.1 %) and Rhizopogon sp. 1 (48.2 %), which were the two most abundant ECM fungi on interior Douglas-fir at sites inside its distribution, yet had a negligible presence elsewhere (Fig. 2). Ectomycorrhizas of Rhizopogon spp. accounted for approximately 30–50 % of all Douglas-fir seedling root tips in soils from inside its range and only 5–10 % of root tips outside its range. The Douglas-fir associate Suillus lakei, considered a specialist symbiont of Douglas-fir, was only encountered on seedling roots in soils from outside the contemporary host distribution. For lodgepole pine, the fungal endophyte Meliniomyces sp. 1 was the most frequently encountered fungus overall (88.0 %), with Rhizopogon sp. 5 (49.7 %) and Cenococcum sp. (44.9 %) as the most frequent EMF (Fig. 2). Of these three fungi, the Meliniomyces sp. was abundant on the host at all five sites, the Rhizopogon sp. was only abundant at the three sites inside the distribution of Douglas-fir, and the Cenococcum sp. was present at low abundance across all five sites. Each fungal species observed on Douglas-fir seedlings grown in a given soil was also detected at the corresponding field sites, as were 13 of the fungal species encountered on lodgepole pine (Online Resource 3). Species detected in the greenhouse expressed similar frequency and abundance profiles to those detected in the field (data not shown) with the exception of Piloderma sp. (common in the field, rare in the greenhouse) and Cenococcum sp. (rare in the field, common in the greenhouse).
Examination of overall seedling colonisation patterns using three categories (uncolonised roots, EMF and fungal endophytes) indicated that each host species interacted differently with these groups (Table 3). Douglas-fir roots were significantly less colonised in the boreal soil (FS-LP) and significantly more colonised in the Douglas-fir BEC zone soil (VN-DF). One class A seed provenance showed consistently low root colonisation, and a further three of the ‘improved’ seed provenances had lower colonisation than the ‘wild’ seed provenances across all soils. No effect of seed provenance on root colonisation was noted for lodgepole pine seedlings, which were significantly less colonised in the boreal and Douglas-fir BEC zone soils. EMF colonisation of Douglas-fir was greatest in the soils inside its contemporary distribution. Lodgepole pine EMF colonisation was highest in the three sub-boreal soils and lowest in the boreal soil, where colonised root tips were primarily associated with a Leptodontidium sp. (considered a root endophyte of the DSE group and classified as a fungal endophyte; Fernando and Currah 1996) and in the interior Douglas-fir BEC zone soil where lodgepole pine is less common. Fungal endophyte species showed no significant differences in distribution across sites or provenances for lodgepole pine seedlings. Fungi of this category were not detected on Douglas-fir seedlings in the VN-DF or PG-DF soils but were abundant in soils outside its distribution (present on only 10.5 % of JP-DF seedlings, compared to 89.6 % of seedlings grown in MZ-LP and FS-LP soils).
Co-occurrence analysis
C-score analyses were performed across all seedlings of each species, and for each species separately, across all soils taken together and within each soil separately (Table 4). Removal of species with <3 occurrences (Helotiales sp., Meliniomyces sp. 3, Tuber sp. 3, Unknown 1, Unknown 2, Unknown 3) resulted in a maximum dataset of 362 samples (seedlings) in which 23 species occurred across both species and all soils. Large standardised effect sizes were primarily driven by significant fungal community differences between hosts (i.e. host filtering), as evidenced by their presence when seedlings of both species were considered together (all seedlings column, Table 4) and their absence when only a single host was considered (host only columns, Table 4). These tended to decrease in strength moving northwards along the BEC zone gradient, with only one soil indicating no significant difference between the fungal communities of the two host species (MZ-LP). The strong standardised effect size (SES) between all Douglas-fir fungal communities indicated significant ‘competition’ between sites coupled with nonsignificant SES ‘random assembly’ within sites; taken together, these observations were suggestive of habitat filtering. Conversely, lodgepole pine fungal communities showed no evidence of habitat filtering between sites (nonsignificant all-site SES) and two cases of strong within-site SES. These latter data points indicated facilitative interactions between fungal species in the JP-DF soil (negative SES) and competitive interactions in the MZ-LP soil (positive SES). Inspection of pairwise species combinations revealed that the JP-DF pattern was driven by the presence of Meliniomyces sp.1 on all but one seedling, and the MZ-LP pattern was caused by minimal co-occurrence of Suillus sp. 3 and Tuber sp. 2.
Multivariate community analysis
Ordination of seedling provenance fungal community data illustrated striking clustering and separation patterns in three dimensions (Fig. 3 and Online Resource 4), with two axes representing 75.2 % of the variance. For interior Douglas-fir, communities within the contemporary host distribution clustered closely together and were separated from communities outside the host distribution along the main axis of the ordination (axis 1, 61.9 % of the variance). Simultaneously, the Douglas-fir fungal communities formed in soils from outside of the host distribution were clustered together with all of the lodgepole pine communities, indicating that host filtering was taking place within the natural range of Douglas-fir but not outside of it. For interior lodgepole pine seedlings, there was no distinct separation of fungal communities between sites along axis 1, indicating that lodgepole pine fungal communities were not affected by the transition zone demarcating the range of Douglas-fir. None of the BEC zone climatic or edaphic factors were strongly correlated with this ordination axis. The second axis of the ordination (axis 2, 13.3 % of the variance) was strongly correlated with BEC zone temperature, length of frost-free period, soil cation exchange capacity and with biologically available soil nitrogen (mineral N; soluble nitrogen). Symbiont species and abiotic factors that were strongly correlated with ordination axes are provided for each host (Online Resource 5). Use of exploration types as functional proxies to categorise fungal species yielded significant separation of Douglas-fir provenance communities, but not lodgepole pine communities (Fig. 4a). Long-range and contact exploration types were the primary root colonists of Douglas-fir provenances inside its contemporary range, with short-range exploration types, uncolonised roots and fungal endophytes defining all lodgepole pine provenances and the Douglas-fir provenances grown in the more northerly BEC zone soils (Fig. 4b). Temperature-related factors were strongly correlated with axis 2 of the exploration type ordination (Online Resource 5).
NMS ordination of seedling root tip fungal species data illustrating community differences between hosts and sites, with bi-plots of selected environmental and soil properties correlated with axes at r 2 > 0.40 (dotted lines indicate direction of increase). Each data point is the mean community composition of a seedling provenance. Full solution is 3-dimensional (Stress = 16.49, P < 0.05): a Axes 1 and 2; b axes 1 and 3
NMS ordination of seedling root tip exploration type data illustrating: a differences between hosts and sites (data points are mean exploration type composition of a seedling provenance) and b differences between exploration types. Bi-plot indicates direction of increase of mean annual temperature (correlated with axis 2, r = −0.545). Full solution is 2-dimensional (Stress = 18.09, P < 0.05)
Multivariate analysis of species and exploration type dissimilarity indices confirmed that the observed differences between fungal communities of each host and site were significant (Table 5). Host was the strongest factor in determining community composition, with the significant interaction effect supporting the observed differences in host by site interactions (Figs. 3 and 4). This indicated that both host and habitat filtering was taking place amongst the fungal communities. Pairwise analysis of community composition by site indicated different trends in each host (Table 5). Douglas-fir seedling communities were most similar in the two southernmost sites (VN-DF and PG-DF) with all other pairs of sites showing significant differences in composition. In terms of resource acquisition strategies, there were no significant differences in exploration type composition within the contemporary Douglas-fir range. All pairwise comparisons with and between sites outside of this range indicated that functional composition was significantly different. Two lodgepole pine seedling communities (VN-DF and PG-DF) displayed compositional similarity to that of the transitional JP-DF site; all other pairs of sites indicated significant differences in composition. However, resource acquisition strategies tended to be more similar between sites than those of Douglas-fir, and the differences were unrelated to the Douglas-fir range boundary. Analysis of the homogeneity of multivariate dispersions revealed no significant difference in pairwise dispersion between groups (for species or exploration type data), providing support for the significance of the PERMANOVA analyses.
Discussion
We used a common-garden greenhouse approach to examine the hypothesis that the degree of compatibility with fungal symbionts could facilitate or limit interior Douglas-fir migration northwards in response to climate change. This approach enabled assessment of the fungal communities formed by two host species, from multiple seed provenances, in soils taken from an ecosystem gradient that crossed the distribution boundary of interior Douglas-fir. Both host and habitat filtering of fungal communities were observed.
Soil inoculum potential
This experiment focused on the potential of the soil to form root-associated fungal communities. We determined that soil from different habitats can influence colonisation, and we found evidence that selection takes place between host seedlings and the available pool of soil-residing symbiont propagules (with selection potentially driven by either partner). Our seedlings were grown from seed in a pooled and homogenised mixture of natural forest organic horizon, and thus the available symbiont species pool was derived from spores plus vegetative colonisation via surviving fungi attached to mature root tips excised during soil coring and collection (e.g. Alexander et al. 1992). Some of the species detected here are known to form long-lived resistant spores (e.g. Rhizopogon spp.; Bruns et al. 2009; Nara 2009; Nguyen et al. 2012) or sclerotia (e.g. Cenococcum spp.; Massicotte et al. 1992). The fungi derived from these sources are likely to be the most important source of the root-associated fungal community during seedling establishment as tree migration proceeds. Other potential sources excluded from this analysis are the living mycorrhizal networks present in forests (e.g. Simard et al. 2012). Our study focused exclusively on soil-derived fungal symbionts, and we found significant differences based on host and habitat filtering that we expect will play an important role in the field during seedling establishment.
Host filtering
Lodgepole pine and Douglas-fir root-associated fungal communities were significantly different in all soils except one: located 50 km north of the currently recognised distribution boundary of Douglas-fir (MZ-LP) which may be considered an ecological transition zone for this host. In every other soil, only a small fraction of the symbiont species were shared, and observed community differences between hosts were 2–8 standard deviations from the expected mean (where all fungal species colonise each host equally; as per the co-occurrence analysis). The magnitude of the differences between host communities was greater in soils that were collected within the distribution of both hosts (i.e. inside the current distribution of Douglas-fir). This indicates that each host was associating with different fungal species from the same species pool present in the soil, indicating selection by hosts for symbionts or vice versa. Multivariate community analyses strongly supported the observation of host filtering within the current Douglas-fir distribution. Because the experiment was performed in greenhouse containers over a single growing season, preferential selection either by host or fungus, as well as priority effects (e.g. Kennedy et al. 2009) appear the most likely processes responsible for the observed communities. Lodgepole pine provenances showed similar levels of root colonisation in each soil, whereas Douglas-fir provenances varied in the proportion of their roots that were colonised. Importantly, for assisted migration operations with Douglas-fir, one of the ‘improved’ provenances showed low colonisation in all soils and a further three were generally less colonised by EMF than the ‘wild’ provenances. Thus, host genetics appear to be a factor in the overall level of mycorrhizal colonisation, suggesting that ‘improved’ Douglas-fir provenances may generally be less likely to form mycorrhizas during establishment than ‘wild’ provenances.
EMF species were more abundant on Douglas-fir seedlings. The division of the fungal community between Douglas-fir and lodgepole pine (31.0 and 44.8 %, respectively, 24.1 % shared) was similar to that observed between ectomycorrhizal hosts in other two-host studies (Twieg et al. 2007; Timling et al. 2012), but only half as many species were shared here as between Douglas-fir and ponderosa pine in an earlier greenhouse experiment (Massicotte et al. 1999). Interestingly, Suillus lakei was not observed frequently despite that: (a) it is regarded as a specialist of Douglas-fir, and (b) its sporocarps were frequently observed throughout the host range (specifically at each of the VN-DF, PG-DF and JP-DF sites). Yet, S. lakei only occurred as a seedling root symbiont in soils where Douglas-fir was not native. This was counter to our expectations, suggesting that S. lakei must have dispersed to these sites in the past and is able to persist in these soils, or it formed associations with an, as yet unknown, alternate host. In a study of Douglas-fir EMF communities along a chronosequence, Twieg et al. (2007) did not encounter S. lakei in seedling (≤5 year old) communities, whereas it made up ∼10 % of the community for 26-year-old trees. Thus, the Douglas-fir/S. lakei association seems to prefer mature hosts or be less competitive on seedlings. Those Douglas-fir/S. lakei associations encountered in our study were likely formed due to a lack of alternative options for the host (in terms of symbionts available in the species pool) or symbiont (in terms of host age or species), particularly in the furthest soil outside its current range where overall percent colonisation of Douglas-fir roots was lowest.
Habitat filtering
Significant habitat filtering was observed in the fungal communities of interior Douglas-fir seedlings, with strong compositional differences primarily between soils collected from inside and outside the contemporary host distribution. This was particularly evident when the Douglas-fir communities were examined as exploration types. Communities of interior lodgepole pine also displayed habitat filtering along the ecosystem gradient, although this was much less pronounced amongst their exploration types and did not show any relationship to the range of Douglas-fir. Factors related to BEC zone temperature regime, frost-free period and soil nutrient status were strongly correlated with compositional divisions in seedling fungal communities, implying that they may be drivers of habitat filtering in soil fungal communities. However, the primary difference in community composition was between Douglas-fir communities from seedlings grown in Douglas-fir soil vs. all other communities. Thus, historical host distribution (i.e. the current availability of Douglas-fir compatible fungal symbionts may be influenced by the historical distribution of the host) appears to be the most significant factor in seedling EMF community composition. Douglas-fir exhibited less root colonisation in the FS-LP site soil, and lodgepole pine root colonisation was lowest in both the FS-LP and VN-DF site soils, indicating lower compatibility between hosts and the symbiont assemblage of these soils. The extensive distribution of lodgepole pine beyond the study sites suggests that it is not affected by reduced compatibility with symbiont assemblages. Whereas for interior Douglas-fir, variation in the ability to form fungal associations could be functionally relevant to survival, especially under the intense competitive interactions in non-managed stands and in areas where environmental conditions fluctuate widely and/or are at the extremes of host tolerance.
Where symbionts show a strong degree of host preference, it is likely that their distribution will closely follow the current distribution(s) of the host(s), unless they are able to disperse widely or else form long-lived resistant propagules (e.g. Rhizopogon and Suillus spp.). This latter ability appears an important one, given the presence of the host specialist Suillus lakei in soils collected tens and hundreds of km outside of the host’s current distribution (see above). For symbionts that display a wide host tolerance, those considered to be generalists rather than specialists (Bruns et al. 2002; Smith et al. 2009), it is less likely that their distribution will reflect the distribution of any single host species and may instead represent the legacy of their evolutionary history, dispersal ability and the biogeography of all possible hosts.
Taxonomy vs. function
Fungal community composition varied between BEC zone soils for both taxonomic diversity (TD) and functional diversity (FD) assessments but to different extents. The major pairwise TD and FD compositional differences were between BEC zone soils within Douglas-fir’s current distribution and those beyond. Roots of Douglas-fir seedlings grown in soils inside its natural distribution generally displayed a higher abundance of Rhizopogon spp. (ectomycorrhizal), whereas soils beyond the natural distribution produced a higher abundance of Meliniomyces sp. (fungal endophyte: mycorrhizal status unknown) and uncolonised roots. The long-range exploration types expressed by Rhizopogon spp. are associated with increased translocation of water and N to the host (e.g. Bingham and Simard 2011), via thick rhizomorphs that can extend for 10s of centimetre through the soil (Agerer 2001). Reduced availability of fungi utilizing this resource acquisition strategy could potentially lead to increased drought stress following seed dispersal. Although Douglas-fir seedlings did form symbioses with fungi in soils from outside their current distribution, their exploration types shifted from long-range and contact types to short-range and fungal endophyte types. While there may be functional benefits in having roots colonised by fungal endophytes such as DSE (Newsham 2011), on balance, these associations appear more likely to be neutral to detrimental (Mayerhofer et al. 2013). Regardless, fungal endophyte symbionts are widely distributed associates of ectomycorrhizal and ericoid mycorrhizal hosts (Mandyam and Jumpponen 2005; Newsham et al. 2009; Upson et al. 2009; Timling et al. 2012) so further investigation into their ecological importance is warranted. Importantly, this points to functional differences in the available pool of symbionts that will associate with Douglas-fir outside of its current distribution, leading to initial symbioses that may be of little benefit to the host. For lodgepole pine seedlings compositional differences in TD were observed between pairs of sites, whereas differences in FD were less common and unrelated to the distribution boundary of Douglas-fir. Since all lodgepole pine communities were dominated by uncolonised roots, short-range exploration types and fungal endophytes, it may be that Pinus symbiont community assembly is inherently different to that of Pseudotsuga. An interesting follow-up experiment would be to test whether lodgepole pine seedlings display similar patterns when grown outside of their current southern boundary where Douglas-fir thrives.
It should be noted that the ecological challenges faced by a migrating host species during periods of rapid climatic change are expected to be different from those encountered following translocation to an entirely different region (e.g. exotic species forestry; Nuñez et al. 2009; Gundale et al. 2014). Migration across a host boundary within the same geographical region does not negate the impacts of historical biogeographic processes (Zobel et al. 2011) and coevolution (Johnson and Stinchcombe 2007) faced during natural range expansion, even if performed as a management operation. As a consequence, experiments that mimic short migratory distances are more realistic explorations of the initial dispersal processes leading to natural changes in the distribution of host-symbiont systems, rather than deliberate long-distance anthropogenic changes. The use of a control host species taken from within the same family appears to be a viable method for assessing dispersal effects across a host boundary and, although more soils and more host species are warranted, forms a solid basis for future investigations.
Conclusions
Here, we examined the response of organic horizon fungal symbionts to seedlings of two common western North American ectomycorrhizal host species (interior Douglas-fir and interior lodgepole pine) across an ecosystem gradient. Seedling root-symbiont community composition along the ecosystem gradient indicated significant host filtering (different species composition associated with each host) at all but one site, and significant habitat filtering (different species composition associated with each site) was observed for both hosts. Host species shared 24 % of fungi, which corresponded well with other multi-host studies. Host and habitat filtering of symbiont communities was indicated by co-occurrence and multivariate ordination techniques for both host species. Beyond the northern distribution boundary of Douglas-fir, fewer symbioses were formed, which involved different fungal species and fungal resource acquisition strategies compared to seedlings grown in soils from the contemporary host distribution. The benefit of these associations to newly dispersed seedlings requires further investigation, because even if seedlings are capable of growing with a novel community of EMF, it is possible that their success, in terms of growth potential and long-term survival, may be negatively affected. The lodgepole pine fungal communities did not display such strong patterns in the distribution of fungal exploration types. In this system, the regional-scale processes affecting host biogeography appeared to have an important impact on symbiont community structure. We suggest that a reduction in symbiont colonisation, coupled with a switch in the identity of symbionts, may have an important filtering effect on successful seedling dispersal outside of current distributions for interior Douglas-fir. This is an important consideration for future forest management under rapidly changing climatic conditions.
References
Addy HD, Piercey MM, Currah RS (2005) Microfungal endophytes in roots. Can J Bot 83:1–13
Agerer R (2001) Exploration types of ectomycorrhizae. Mycorrhiza 11:107–114
Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S (2008) Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl 1:95–111
Alexander I, Ahmad N, Su See L (1992) The role of mycorrhizas in the regeneration of some Malaysian forest trees. Philos T Roy Soc B 335:379–388
Bingham MA, Simard SW (2011) Do mycorrhizal network benefits to survival and growth of interior Douglas-fir seedlings increase with soil moisture stress? Ecol Evol 1:306–316
Bogar LM, Kennedy PG (2013) New wrinkles in an old paradigm: neighborhood effects can modify the structure and specificity of Alnus-associated ectomycorrhizal fungal communities. FEMS Microbiol Ecol 83:767–777
Bruns TD, Bidartondo MI, Taylor DL (2002) Host specificity in ectomycorrhizal communities: what do the exceptions tell us? Integr Comp Biol 42:352–359
Bruns TD, Peay KG, Boynton PJ, Grubisha LC, Hynson NA, Nguyen NH, Rosenstock NP (2009) Inoculum potential of Rhizopogon spores increases with time over the first 4 yr of a 99-yr spore burial experiment. New Phytol 181:463–470
Coops NC, Waring RH (2011) A process-based approach to estimate lodgepole pine (Pinus contorta Dougl.) distribution in the Pacific Northwest under climate change. Clim Change 105:313–328
R Core Team (2014) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. URL http://www.R-project.org/
Diamond JM (1975) Assembly of species communities. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Harvard University Press, Cambridge, pp 342–444
Dormann CF (2007) Promising the future? Global change projections of species distributions. Basic Appl Ecol 8:387–397
Elias SA (2013) The problem of conifer species migration lag in the Pacific Northwest region since the last glaciation. Quat Sci Rev 77:55–69
Elmqvist T, Folke C, Nyström M, Peterson G, Bengtsson J, Walker B, Norberg J (2003) Response diversity, ecosystem change, and resilience. Front Ecol Environ 1:488–494
Fernando AA, Currah RS (1996) A comparative study of the effects of the root endophytes Leptodontidium orchidicola and Phialocephala fortinii (Fungi Imperfecti) on the growth of some subalpine plants in culture. Can J Bot 74:1071–1078
Gotelli NJ, Entsminger GL (2009) EcoSim: Null models software for ecology. Version 7. Acquired Intelligence Inc. & Kesey-Bear. Jericho, VT 05465. URL: http://garyentsminger.com/ecosim.htm
Griesbauer HP, Green DS (2010) Assessing the climatic sensitivity of Douglas-fir at its northern range margins in British Columbia, Canada. Trees 24:375–389
Gundale MJ, Kardol P, Nilsson M-C, Nilsson U, Lucas RW, Wardle DA (2014) Interactions with soil biota shift from negative to positive when a tree species is moved outside of its native range. New Phytol. doi:10.1111/nph.12699
Hamann A, Wang TL (2005) Models of climatic normals for genecology and climate change studies in British Columbia. Agr Forest Meteorol 128:211–221
Hamann A, Wang TL (2006) Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 87:2773–2786
Hambleton S, Sigler L (2005) Meliniomyces, a new anamorph genus for root-associated fungi with phylogenetic affinities to Rhizoscyphus ericae (≡ Hymenoscyphus ericae), leotiomycetes. Stud Mycol 53:1–27
Hoeksema JD, Hernandez JV, Rogers DL, Mendoza LL, Thompson JN (2012) Geographic divergence in a species-rich symbiosis: interactions between Monterey pines and ectomycorrhizal fungi. Ecology 93:2274–2285
IPCC (2013) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G-K et al (eds) Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Ishida TA, Nara K, Hogetsu T (2007) Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer-broadleaf forests. New Phytol 174:430–440
Iverson LR, Schwartz MW, Prasad AM (2004) How fast and how far might tree species migrate in the eastern United States due to climate change? Glob Ecol Biogeogr 13:209–219
Ji B, Gehring CA, Wilson GW, Miller RM, Flores-Rentería JNC (2013) Patterns of diversity and adaptation in Glomeromycota from three prairie grasslands. Mol Ecol 22:2573–2587
Johnson MT, Stinchcombe JR (2007) An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol 22(5):250–257
Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci U S A 107:2093–2098
Karst J, Jones MD, Turkington R (2009) Ectomycorrhizal colonization and intraspecific variation in growth responses of lodgepole pine. Plant Ecol 200:161–165
Kennedy PG, Peay K, Bruns TD (2009) Root tip competition among ectomycorrhizal fungi: are priority effects a rule or an exception? Ecology 90:2098–2107
Kõljalg U, Nilsson RH, Abarenkov K et al (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Kolotelo D, van Steenis E, Peterson M, Bennett R, Trotter D, Dennis J (2001) Seed handling guidebook. British Columbia Ministry of Forests. Tree Improvement Branch, Victoria
Kranabetter JM, Stoehr MU, O’Neill GA (2012) Divergence in ectomycorrhizal communities with foreign Douglas-fir populations and implications for assisted migration. Ecol Appl 22:550–560
Liimatainen K, Niskanen T, Dima B, Kytövuori I, Ammirati JF, Frøslev TG (2014) The largest type study of Agaricales species to date: bringing identification and nomenclature of Phlegmacium (Cortinarius) into the DNA era. Persoonia - Mol Phylogeny Evol Fungi 33:98–140
Lim S, Berbee ML (2013) Phylogenetic structure of ectomycorrhizal fungal communities of western hemlock changes with forest age and stand type. Mycorrhiza 23:473–486
Little EL (1978) Atlas of United States trees, volume 5, U.S. Department of Agriculture Miscellaneous Publication 1361, Florida
Jull MJ (1999) Douglas-fir silviculture “on the edge”: silvicultural systems at the northern range of the species. In: Lousier JD, Kessler WB (eds) Ecology and management of interior Douglas-fir (Pseudotsuga menziesii var. glauca) at the northern extreme of its range. Proceedings of a workshop held 7–9 October 1996 in Fort St. James, British Columbia. University of Northern British Columbia, Prince George, pp 34-44
Mandyam K, Jumpponen A (2005) Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud Mycol 53:173–189
Martin KJ, Rygiewicz PT (2005) Fungal specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol 5:28
Marx DH, Ruehle JL, Kenney DS, Cordell CE, Riffle JW, Molina RJ, Pawuk WH, Navratil S, Tinus RW, Goodwin OC (1982) Commercial vegetative inoculum of Pisolithus tinctorius and inoculation techniques for development of ectomycorrhizae on container-grown tree seedlings. For Sci 28:373–400
Massicotte HB, Trappe JM, Peterson RL, Melville LH (1992) Studies on Cenococcum geophilum. II. Sclerotium morphology, germination, and formation in pure culture and growth pouches. Can J Bot 70:125–132
Massicotte HB, Molina R, Tackaberry LE, Smith JE, Amaranthus MP (1999) Diversity and host specificity of ectomycorrhizal fungi retrieved from three adjacent forest sites by five host species. C J Bot 77:1053–1076
Mayerhofer MS, Kernaghan G, Harper KA (2013) The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza 23:119–128
Mikola P (1970) Mycorrhizal inoculation in afforestation. Int Rev For Res 3:123–196
Nara K (2009) Spores of ectomycorrhizal fungi: ecological strategies for germination and dormancy. New Phytol 181:245–248
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793
Newsham KK, Upson R, Read DJ (2009) Mycorrhizas and dark septate root endophytes in polar regions. Fungal Ecol 2:10–20
Nguyen NH, Hynson NA, Bruns TD (2012) Stayin’ alive: survival of mycorrhizal fungal propagules from a 6-yr-old forest soil. Fungal Ecol 5:741–746
Nilsson RH, Hyde KD, Pawlowska J et al (2014) Improving ITS sequence data for identification of plant pathogenic fungi. Fungal Divers 67:11–19
Nuñez MA, Horton TR, Simberloff D (2009) Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90:2352–2359
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013). Vegan: community ecology package. R package version 2.0-10. http://CRAN.R-project.org/package=vegan
Peay KG, Schubert MG, Nguyen NH, Bruns TD (2012) Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Mol Ecol 21:4122–4136
Pedlar JH, McKenney DW, Aubin I, Beardmore T, Beaulieu J, Iverson L, O'Neill GA, Winder RS, Ste-Marie C (2012) Placing forestry in the assisted migration debate. Biosci 62:835–842
Peterson RL, Bradbury SM (1999) Use of plant mutants, intraspecific variants, and non-hosts in studying mycorrhiza formation and function. In: Varma A, Hock B (eds) Mycorrhiza, 2nd edn. Springer, Berlin, pp 153–176
Pickles BJ, Egger KN, Massicotte HB, Green DS (2012) Ectomycorrhizas and climate change. Fungal Ecol 5:73–84
Pojar J, Klinka K, Meidinger DV (1987) Biogeoclimatic ecosystem classification in British Columbia. For Ecol Manag 22:119–154
Rehfeldt GE, Jaquish BC, Lopez-Upton J, Saenz-Romero C, St Clair JB, Leites LP, Joyce DG (2014) Comparative genetic responses to climate for the varieties of Pinus ponderosa and Pseudotsuga menziesii: Realized climate niches. For Ecol Manag 324:126–137
Reithmeier L, Kernaghan G (2013) Availability of ectomycorrhizal fungi to black spruce above the present treeline in Eastern Labrador. PLoS One 8:e77527
Rineau F, Courty P-E (2011) Secreted enzymatic activities of ectomycorrhizal fungi as a case study of functional diversity and functional redundancy. Ann For Sci 68:69–80
Rosado SCS, Kropp BR, Piché Y (1994) Genetics of ectomycorrhizal symbiosis. I. Host plant variability and heritability of ectomycorrhizal and root traits. New Phytol 126:105–110
Roy M, Rochet J, Manzi S, Jargeat P, Gryta H, Moreau P-M, Gardes M (2013) What determines Alnus-associated ectomycorrhizal community diversity and specificity? A comparison of host and habitat effects at a regional scale. New Phytol 198:1228–1238
Ruotsalainen AL, Kytöviita M-M (2004) Mycorrhiza does not alter low temperature impact of Gnaphalium norvegicum. Oecologia 140:226–233
Simard SW (2009) The foundational role of mycorrhizal networks in self-organisation of interior Douglas-fir forests. For Ecol Manag 258S:S95eS107
Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste FP (2012) Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biol Rev 26:39–60
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, San Diego
Smith ME, Douhan GW, Fremier AK, Rizzo DM (2009) Are true multihost fungi the exception or the rule? Dominant ectomycorrhizal fungi on Pinus sabiniana differ from those on co-occurring Quercus species. New Phytol 182:295–299
Stone L, Roberts A (1990) The checkerboard score and species distributions. Oecologia 85:74–79
Tedersoo L, Pärtel K, Jairus T, Gates G, Põldmaa K, Tamm H (2009) Ascomycetes associated with ectomycorrhizas: molecular diversity and ecology with particular reference to the Helotiales. Environ Microbiol 11:3166–3178
Thompson K (2000) The functional ecology of soil seed banks. In: Fenner M (ed) Seeds the ecology of regeneration in plant communities. CABI Publishing, Wallingford, pp 215–235
Timling I, Dahlberg A, Walker DA, Gardes M, Charcosset JY, Welker JM, Taylor DL (2012) Distribution and drivers of ectomycorrhizal fungal communities across the North American Arctic. Ecosphere 3:111
Twieg BD, Durall DM, Simard SW (2007) Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol 176:437–447
Upson R, Read DJ, Newsham KK (2009) Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 20:1–11
Verhoeven KJF, Simonsen KL, McIntyre LM (2005) Implementing false discovery rate control: increasing your power. Oikos 108:643–647
Wang T, Hamann A, Spittlehouse D, Aitken SN (2006) Development of scale-free climate data for western Canada for use in resource management. Int J Climatol 26:383–397
Wang T, Campbell EM, O’Neill GA, Aitken SN (2012) Projecting future distributions of ecosystem climate niches: uncertainties and management applications. For Ecol Manag 279:128–140
Weiher E, Keddy PA (1999) Ecological assembly rules: perspectives, advances, retreats. Cambridge University Press, Cambridge
White TJ, Bruns TD, Lee S, Taylor J (1990) Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Zobel M (1997) The relative role of species pools in determining plant species richness: an alternative explanation of species coexistence? Tree 12:266–269
Zobel M, van der Maarek E, Dupré C (1998) Species pool: the concept, its determination and significance for community restoration. Appl Veg Sci 1:55–66
Zobel M, Otto R, Laanisto L, Naranjo-Cigala A, Pärtel M, Fernández-Palacios JM (2011) The formation of species pools: historical habitat abundance affects current local diversity. Glob Ecol Biogeogr 20:251–259
Acknowledgments
We thank A. Harris, J. Lawyer, J. Lee, C. Lim, J. Orlowsky, S. Robertson, N.-A. Rose and J. Salokannel for the field or lab assistance. Seeds were kindly provided by G. O’Neill and S. Reitenbach at the BC Ministry of Forests, Lands and Natural Resources. Two anonymous reviewers gave extremely helpful feedback on the manuscript. Funding was provided by the Forest Genetics Council of BC.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 255 kb)
Rights and permissions
About this article
Cite this article
Pickles, B.J., Gorzelak, M.A., Green, D.S. et al. Host and habitat filtering in seedling root-associated fungal communities: taxonomic and functional diversity are altered in ‘novel’ soils. Mycorrhiza 25, 517–531 (2015). https://doi.org/10.1007/s00572-015-0630-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0630-y