Abstract
The consequences of diversity on belowground processes are still poorly known in tropical forests. The distributions of very fine roots (diameter <1 mm) and fine roots (diameter <3 mm) were studied in a randomized block design close to the harvest age of fast-growing plantations. A replacement series was set up in Brazil with mono-specific Eucalyptus grandis (100E) and Acacia mangium (100A) stands and a mixture with the same stocking density and 50 % of each species (50A:50E). The total fine root (FR) biomass down to a depth of 2 m was about 27 % higher in 50A:50E than in 100A and 100E. Fine root over-yielding in 50A:50E resulted from a 72 % rise in E. grandis fine root biomass per tree relative to 100E, whereas A. mangium FR biomass per tree was 17 % lower than in 100A. Mixing A. mangium with E. grandis trees led to a drop in A. mangium FR biomass in the upper 50 cm of soil relative to 100A, partially balanced by a rise in deep soil layers. Our results highlight similarities in the effects of directional resources on leaf and FR distributions in the mixture, with A. mangium leaves below the E. grandis canopy and a low density of A. mangium fine roots in the resource-rich soil layers relative to monospecific stands. The vertical segregation of resource-absorbing organs did not lead to niche complementarity expected to increase the total biomass production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The role of species diversity in ecosystem functioning is an important issue in functional ecology attracting increasing attention over the last decade. A tendency towards higher production in mixed-species communities than in mono-specific ecosystems (i.e. ‘over-yielding’) has been documented in grasslands (Tilman et al. 1996; Hector et al. 1999; Marquard et al. 2009) and more recently in forests (e.g. Binkley et al. 2003; Vilà et al. 2003; Forrester et al. 2006; Pretzsch and Schütze 2009; Paquette and Messier 2011). Even though a ‘sampling-effect’ may have biased some results (Fridley 2001), most of the studies suggest that inter-specific interactions are likely to enhance total biomass production through an increase in resource availability, resource use and/or more efficient use of resources in producing biomass compared to monocultures (Richards et al. 2010; Cardinale et al. 2011). Two types of inter-specific interactions have been proposed to explain the positive effects of biodiversity on productivity: (1) facilitation processes may lead to an increase in the availability of resources for one species due to other species or to modifications in the environment (Callaway 2002), and (2) complementarity between species, including niche differentiation, making it possible to increase the amount of available resources (Fridley 2001; Cardinale et al. 2007). However, other studies have shown no impact or a depressive effect of mixtures on aboveground forest production (e.g. Forrester et al. 2006).
The vast majority of studies investigating the relationships between diversity and productivity in forest ecosystems have been restricted to aboveground tree components (Binkley et al. 2003; Vilà et al. 2003; Kelty 2006; Paquette and Messier 2011). Whilst the influence of a stratified canopy on light interception is well documented (e.g. Bauhus et al. 2004; Hunt et al. 2006), the ability of different species to improve the capture of belowground resources remains unclear (Leuschner et al. 2001; Kelty 2006; Kueffer et al. 2007; Meinen et al. 2009). Studies dealing with fine root densities in mixed-species forest ecosystems are scarce and mainly restricted to the top soil (often 0–30 cm) in boreal and temperate regions. As usually observed for aboveground tree components, an overall trend of fine root over-yielding has been shown in mixed-species forests (e.g. Schmid 2002; Brassard et al. 2011). However, some studies did not detect significant differences between fine root biomasses in mixed- and single-species stands (Bauhus et al. 2000; Meinen et al. 2009; Lei et al. 2012). Size-asymmetric interspecific root competition has been shown in temperate forests (Leuschner et al. 2001; Rewald and Leuschner 2009; Lei et al. 2012). Belowground competition matching aboveground competition can lead to the displacement of the dominated species’ fine roots towards soil layers with fewer resources at both nutrient-rich and nutrient-poor sites (Schmid 2002; Schmid and Kazda 2002). Scramble competition (through resource depletion) or contest competition (that inhibits access of other roots to resources through species-specific mechanisms) are likely to affect the development of the competitors’ roots in mixed-species stands (Schenk 2006).
The aim of our study was to investigate the effects of interspecific interactions between highly productive tree species on their rooting patterns in deep tropical soils. We used an experiment combining an additive and a replacement series between Eucalyptus grandis Hill ex Maid. and Acacia mangium (Willd.) trees in southern Brazil (Laclau et al. 2008). Extensive data collection in this experiment showed that aboveground net primary production (ANPP) from planting to harvesting was significantly higher in monospecific E. grandis (100E) than A. mangium (100A) stands, with the mixture (50A:50E) in an intermediate position (Nouvellon et al. 2012). Gross primary production of about 4.2 kg C m−2 year−1 in 100E (Nouvellon et al. 2012) was in the highest range of world forest production (Luyssaert et al. 2007). Higher vertical growth rates for E. grandis trees than for A. mangium trees led to a stratified canopy in 50A:50E, from 2 years after planting onwards (Laclau et al. 2008). Total belowground carbon fluxes (TBCF) estimated from soil CO2 efflux measurements over the last 2 years before harvesting showed that belowground carbon (C) allocation was significantly greater in mixed stands than in monospecific plantations (Nouvellon et al. 2012). Silva et al. (2009) showed in the additive series of this experiment that E. grandis fine root distributions were not influenced by A. mangium trees. By contrast, A. mangium fine roots were excluded from the upper soil layer in the mixture with E. grandis trees. The conclusions of their study carried out over the early growth period (tree age <2.5 years) were hampered by higher stocking densities in a mixture than in monospecific stands. Our study was carried out in the replacement series of the same experiment (100A, 100E and 50A:50E stands), 1 year before the harvest (5-year-old trees). To our knowledge, it is the first study investigating the effects of interspecific interactions on fine root development close to the harvest age of tropical forests, in replicated treatments manipulating species diversity. Although studies in agroforestry systems have shown that trees and crops can explore deep soil layers differently (Lehmann 2003; Allen et al. 2004; Mulia and Dupraz 2006), the effects of species mixing on root distributions below a depth of 1 m are little documented in mixed-species forests. We put forward the hypotheses that: (1) mixing E. grandis and A. mangium trees significantly increases fine root biomass down to a depth of 2 m in comparison with single-species stands (transgressive over-yielding hypothesis), as suggested by TBCF estimations, and (2) interspecific interactions in mixed-species stands lead to the exclusion of A. mangium roots from the soil layer with the highest availability of water and nutrients, which matches the superiority of Eucalyptus in aboveground competition (competitive exclusion hypothesis; Schenk 2006).
Materials and methods
Study site
The study was carried out at the Itatinga Experimental Station (University of São Paulo) located at 23°02′S, 48°38′W. The long-term average rainfall from 1990 to 2010 was 1,390 mm with a cold season from June to September. The average annual temperature was 19 °C with minimum temperature values below 5 °C for a few days each year.
The experiment was located on a hilltop (slope <3 %) 860 m above sea level. The soils were Ferralsols according to the FAO classification developed on cretaceous sandstone. Textural uniformity was high (clay content around 15 %). The pH was acidic (about 5.5) and the amounts of bioavailable nutrients in this soil were low. Effective cation exchange capacity ranged from 0.2 to 1.8 cmolc kg−1 in the upper 3 m of soil and the amounts of exchangeable base cations (K+, Na+, Ca2+, Mg2+) were <0.1 cmolc kg−1 beyond a depth of 5 cm (Table 1).
The experiment was set up in a former Eucalyptus saligna Sm. plot managed as a coppice without fertiliser applications from 1940 to 1998. The stumps were killed by glyphosate application and E. grandis seedlings were planted in 1998 with low fertilizer inputs (30, 26 and 25 kg ha−1 of N, P and K, respectively). Only the boles of the E. grandis stand were harvested in December 2002 and harvest residues were spread uniformly in the field.
Experimental layout
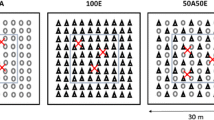
A complete randomized block design was set up in May 2003 with seven treatments and four blocks in order to assess the influence of A. mangium trees on the growth of E. grandis seedlings. Each plot had a total area of 30 × 30 m and an inner plot of 18 × 18 m with two buffer rows. A complete description of the experimental layout can be found in Laclau et al. (2008). Our study was carried out in three treatments and three blocks:
-
100A, monospecific A. mangium stand;
-
100E, monospecific E. grandis stand;
-
50A:50E, mixture in a proportion of 1:1 between E. grandis and A. mangium trees.
The seedlings were planted at a spacing of 3 × 3 m after cultivation with a ripping tine down to a depth of 40 cm, without N fertilisation. Acacia mangium seedlings were inoculated with Rhizobium strains selected for their N2 fixation capacities. In the 50A:50E treatment, the two species were planted alternately in the row, and between adjacent rows (Fig. 1). Two tons per hectare of dolomitic limestone were applied at planting and 40 g P plant−1 were buried 20 cm from the plants, as well as 9 g K plant−1, 3 g B plant−1, 6 g Fe plant−1, 3 g Zn plant−1, and 1 g Mn plant−1. Three complementary fertilisations were applied with 25 kg K ha−1 at 6, 12 and 18 months after planting in the three treatments. Another treatment with a total application of 120 kg ha−1 of N (ammonitrate fertiliser) in monospecific E. grandis stands in this experiment showed that N fertilisation significantly enhanced tree growth in the first 2 years after planting, but aboveground biomass was no longer significantly affected by N fertiliser addition at age 3 years (Laclau et al. 2008).
Sampling positions in the 50A:50E treatment. In each inner plot (excluding two buffer rows), nine positions were randomly sampled close to Acacia trees (full circle) and nine positions close to Eucalyptus trees (open circle). The same design was used in treatments 100A and 100E with nine positions per plot. The centres of the nine squares (0.5 × 0.5 m) were sampled for each species
Our study was carried out 5 years after planting. At this age, the stocking density of living trees was similar in the three treatments (about 1,100 trees per hectare) and E. grandis tree height was 10–12 m higher than A. mangium tree height in single- and mixed-species stands (Table 2). Mixing the two species led to slight aboveground over-yielding with 6 % more total aboveground biomass in 50A:50E than the average of the two single-species stands. However, the aboveground biomass at age 5 years was 11 % lower in 50A:50E than in 100E. A detailed description of the dynamics of biomass accumulation within tree components over the full rotation is given by Nouvellon et al. (2012).
Root sampling
Fine roots <3 mm in diameter were sampled down to a depth of 2 m in the three treatments (100A, 100E and 50A:50E) and three blocks. Two root classes were distinguished within each sample: very fine roots <1 mm in diameter (FR1) and roots with a diameter between 1 and 3 mm (FR1-3). We used a threshold of 3 mm to allow comparisons of fine root mass with previous studies in E. grandis and A. mangium plantations at our study site (Silva et al. 2009). A further separation of fine roots smaller than 1 mm diameter was carried out to study the development of the roots directly involved in the physiological processes of water and nutrient uptake. Nine positions per plot were sampled in the single-species treatments (100A and 100E) and 18 positions in 50A:50E (Fig. 1). Cores were taken with a steel soil corer driven into the soil by a sledgehammer. We used a soil corer with an internal diameter of 8 cm down to a depth of 50 cm and another soil corer with an internal diameter of 4.5 cm between 50 and 200 cm. Cores were sampled stepwise to avoid soil compaction, at the following depths: 0–10, 10–30, 30–50, 50–100, 100–150 and 150–200 cm.
All the roots of each species were washed free of soil and separated carefully (sight, touch and flotation if required) into living roots and dead roots. Dead roots were discarded and only live rootlets were studied. Whilst living roots were pale and pliable, dead roots were breakable and dark. Living roots were sorted according to various criteria such as living stele, bright colour and elasticity. The colour of the roots was a good indication of the species to which they belonged. Reference roots sampled in monospecific stands were used to facilitate the identification of each species for roots collected in the 50A:50E treatment. Eucalyptus grandis fine roots had a higher branching proportion and were darker than A. mangium fine roots. The samples of each component were dried at 65 °C to constant weight. After carefully removing the last adherent soil particles by hand, samples were weighed (±0.0001 g). Root densities were calculated by dividing the dry matter of roots by the volume of the soil sample. Biomasses of FR1 and FR1-3 were computed in each soil layer multiplying the volume of the layer by the mean density of roots <1 mm in diameter, and with a diameter between 1 and 3 mm, respectively.
Soil water contents
A pit was dug manually down to a depth of 3.0 m at the centre of the 100A and 100E plots of block 1, 2.5 years after planting. Three TDR probes (Trase; Soilmoisture, Santa Barbara, USA) were horizontally buried at depths of 0.15, 1.50 and 3.0 m (9 probes per plot) in an undisturbed area at different distances from trees. The trenches were back-filled with soil horizons in their natural arrangement after installation. Probe calibration was checked by gravimetric soil water content and bulk density measurements. Volumetric soil water contents were measured every 1–2 weeks from September 2005 to April 2008 (except during periods of equipment failure).
Data analyses
Sampling positions were located close to different trees in each plot and individual root biomass measurements within a given soil layer were thus considered independent. Three-way analyses of variance (ANOVAs) were used to test the effects of treatments, blocks, and sampling positions, as well as the interaction between positions and treatments on total FR1 and FR1-3 densities (irrespective of the species) in each soil layer (0–10, 10–30, 30–50, 50–100, 100–150, 150–200 and 0–200 cm). Root densities of each species in 50A:50E were compared with 50 % of the root density at the same spatial position in the monoculture of the same species, to test the effects of interspecific interactions on root densities. The main factors of the ANOVAs were treatment, block, sampling position (from 1 to 9), species of the closest tree (see Fig. 1) and the interactions between species of the closest tree and sampling positions, as well as between treatments and sampling positions. A residual analysis was performed to check whether the residuals met the assumptions of the ANOVA and, if necessary, raw data were log- or square root-transformed so that residuals were homoscedastic and normally distributed. The probability level used to determine significance was P < 0.05. When significant differences were detected between treatment levels, the Bonferroni multiple range test was used to compare treatment means. Pearson correlation coefficients between root densities in each soil layer, and the distance from the nearest trunk of each species, were computed for each treatment. All the data were processed using the software package SAS v.9.2 (SAS, Cary, NC, USA).
Results
Dynamics of soil water content
Soil water contents during the dry season were about 50 % higher in 100A than in 100E at depths of 150 and 300 cm the third year after planting (Fig. 2). Even though differences between the two monocultures declined as stands developed, the time-course of soil water contents showed lower amounts of water withdrawn from the upper 3 m of soil in 100A than in 100E. Whilst gravitational solutions transferred in deep soil layers by drainage only reached 3 m in depth three times from 2.5 to 5 years after planting in 100E, and during very short periods, nine increases in soil water contents occurred at a depth of 3 m in 100A.
Fine root over-yielding
Mixing A. mangium trees with E. grandis trees led to a 27–28 % higher total FR biomass (diameter <3 mm) down to a depth of 2 m in 50A:50E than in 100A and 100E. Total FR1 biomass was 269.2, 239.4 and 302.3 g m−2 in 100A, 100E and 50A:50E, respectively (Table 3), and FR1-3 biomass was 82.8, 107.5 and 143.0 g m−2 in 100A, 100E and 50A:50E, respectively (Table 4). FR1 comprised 68–76 % of the biomass of total fine roots in the three treatments. Mean FR1 density in the 0–2 m soil layer was significantly higher close to trees (position 1) than far from trees (position 9), but spatial positions did not significantly influence the FR1-3 density (data not shown). The interaction between position and treatment was not significant, whatever the diameter class.
Fine root density distributions down to a depth of 2 m exhibited different patterns of soil space occupation in the three treatments (Fig. 3). A tendency towards higher fine root densities in 50A:50E than in 100A was found at depths >50 cm, but the differences were only significant for FR1-3. Fine root over-yielding in 50A:50E relative to 100E was a result of 20–100 % higher root densities in all the soil layers (except the 0–10 cm layer for FR1-3). Only soil layers at depths >50 cm were involved in fine root over-yielding in 50A:50E relative to 100A (Fig. 3). Fine root over-yielding in the mixture relative to monospecific A. mangium stands was accounted for by 25-63 % higher FR1 densities in 50A:50E, along with FR1-3 densities that were more than double those in 100A in the 50–100, 100–150 and 150–200 cm soil layers.
Mean densities of fine roots <1 mm in diameter (a) and between 1 and 3 mm in diameter (b) down to a depth of 2 m in treatments 100A (filled black bars), 100E (open bars) and 50A:50E (filled grey bars, irrespective of species). Standard errors between blocks are indicated (n = 3). When differences between treatments were significant in the same soil layer (P < 0.05), they are indicated by different letters
Root distributions of each species in a mixture vs. monocultures
Comparisons of fine root vertical distributions for each species in 50A:50E and in monospecific stands showed that fine root development was largely affected by intra- and interspecific interactions. For a stocking density of A. mangium and E. grandis trees that was 50 % lower in 50A:50E than in single-species stands, the A. mangium fine root biomass cumulated down to a depth of 2 m in 50A:50E amounted to 46 and 33 % of the amounts in 100A for FR1 and FR1-3, respectively, and the E. grandis fine root biomass in 50A:50E was 75 % and 108 % of the amounts in 100E for FR1 and FR1-3, respectively (Tables 3, 4). Eucalyptus grandis FR1 biomass in 50A:50E was significantly more than 50 % of the biomass in 100E for the 10–30 and 30–50 cm soil layers (Fig. 4a). The same pattern was found for E. grandis FR1-3 biomass in the 50–100 and 100–150 cm soil layers (Fig. 4b), and the trend was similar for the two classes of fine roots in other soil layers even though the differences were not significant. Mixing A. mangium with E. grandis trees led to a drop in A. mangium fine root biomass in the upper 50 cm of soil relative to 100A, roughly balanced by a rise in deep soil layers. However, A. mangium FR densities in 50A:50E were only significantly lower than 50 % of the values in 100A in the 0–10 and 30–50 cm soil layers (both for FR1 and FR1-3).
Percentages of FR1 biomass (<1 mm in diameter) (a), and FR1-3 biomass (diameter 1–3 mm) (b), in the 50A:50E treatment relative to the amounts in each soil layer for the single-species stands. The dotted line indicates the percentage expected for Eucalyptus roots (open bars) and Acacia roots (filled bars) if root development was similar to that in monospecific stands (with a half stocking density for each species). Standard errors between the three blocks are indicated and * denotes a significant difference (P < 0.05) between the root biomass of a particular species in 50A:50E and 50 % of the biomass at the same positions in the pure stands of the same species
Acacia mangium and E. grandis fine root distributions did not exhibit a clear pattern of horizontal soil exploration. Fine root densities of the two species were not significantly correlated with the distance from trees whatever the soil layer (except in the 30–50 cm layer for A. mangium roots), in mixed- and single-species stands. The sampling position effect was not significant in the ANOVAs performed between A. mangium fine root densities in 50A:50E and 50 % of the densities in 100A for most of the soil layers. Mixing E. grandis with A. mangium trees did not significantly modify the densities of E. grandis FRs at the sampled positions, whatever the soil layer. Neither A. mangium nor E. grandis FR1 were totally excluded from any soil layer in the mixture, whatever the sampled distances from each tree species (data not shown).
Discussion
Fine root over-yielding
In agreement with our first hypothesis, mixing A. mangium with E. grandis trees led to transgressive over-yielding in FR biomass close to the harvest age. It exclusively resulted from a sharp increase in E. grandis fine root densities, since the total A. mangium fine root biomass down to a depth of 2 m in 50A:50E was slightly less than 50 % of the biomass in 100A. Transgressive over-yielding in our 5-year-old mixture was consistent with TBCF from 4 to 6 years after planting, which was significantly higher in 50A:50E (1.13 g C m−2 year−1) than the mean between 100E and 100A (0.94 g C m−2 year−1) (Nouvellon et al. 2012). Even though a large share of TBCF is allocated to fine root production, respiration, exudation and to mycorrhiza (Maier et al. 2004; Cairney 2012), fine root respiration contributes to 30–55 % of total soil CO2 effluxes in tropical Eucalyptus plantations (Nouvellon et al. 2008; Marsden et al. 2008). Consequently, the larger fine root biomass in 50A:50E than in single-species stands probably contributed to the differences in TBCF between stands. Higher fine root biomass and fine root production in mixed-species stands than in single-species stands were also shown in Pinus taeda L. and Robinia pseudoacacia L. plantations (Fredericksen and Zedaker 1995) and in forests with Populus tremuloides Michx. trees in two boreal regions (Brassard et al. 2011). Likewise, FR over-yielding was reported in mixed-species forests of Tsuga heterophylla (Raf.) Sarg. and Thuja plicata Donn. ex D. Don stands (Wang et al. 2002), as well as in Fagus sylvatica L. and Picea abies (L.) Karst. stands at both nutrient-rich and nutrient-poor sites (Schmid and Kazda 2002). However, other studies did not find significant differences in FR biomass between single- and mixed-species forests down to a depth of 30–40 cm. Fine root biomass in a mixture of Eucalyptus globulus Labill. and Acacia mearnsii de Wild was similar to monospecific plantations despite transgressive over-yielding above ground (Bauhus et al. 2000), and similar FR densities were also found in 12 temperate deciduous forests representing a gradient in tree species mixing in central Europe (Meinen et al. 2009). Further investigations are needed to disentangle the effects of contrasting rooting traits (in particular growth and turnover rates) between co-existing species and biotic/abiotic factors on FR over-yielding in mixed-species forests.
Consequences of directional resources on soil space occupation
Rainfall and fertiliser applications in the top soil led to a strong vertical gradient of water and nutrient availabilities in our experiment. Most of the available nutrients are located in the upper soil layer in plantation forests established on highly weathered soils (released by fertilisers over the early growth period and throughout the biological cycle of nutrients after canopy closure) and carried by gravitational solutions (Lilienfein et al. 2000; Laclau et al. 2003, 2010). Soil coring showed that the depth of the water table was about 16 m in our experiment (Christina et al. 2010). Soil water content monitoring, as well as sap flow and eddy covariance measurements in nearby E. grandis stands, showed that the total annual rainfall was evapotranspired from the third year after planting onwards and the amount of available water stored in deep soil layers after clear-cutting had already been taken up close to the harvest age (Laclau et al. 2010; Nouvellon et al. 2011). The same pattern probably occurred in the 50A:50E plots where leaf and fine root biomasses were higher than in 100E (Table 2), as reported in another eucalypt and acacia mixture experiment in Australia (Forrester et al. 2010). Experiments manipulating water and nutrient availabilities in Brazilian Eucalyptus plantations under similar environments confirmed that tree growth was limited by water availability in the last years before harvesting (Stape et al. 2010). Consequently, the downward fluxes of water and nutrients after rainfall events led to a strong competitive advantage for trees with the highest density of superficial roots. The capacity to take up gravitational solutions in the top soil rapidly after rainfall events was thus probably a key component of inter-tree competition driving fine root distributions in our experiment.
The strong vertical directional component of natural resources might account for a similar behaviour of the two species aboveground for light capture, and belowground for water and nutrient uptake. Despite a 50 % lower E. grandis stocking density in 50A:50E than in 100E, branch biomass at age 2.5 years (Laclau et al. 2008) and fine root densities in most soil layers (present study) for this species were not significantly different between the two treatments. This feature, as well as only a 21 % lower total area of E. grandis leaves in 50A:50E than in 100E over the whole rotation (le Maire et al. 2012), suggested a similar behaviour of space occupation above- and belowground for the dominant species in the mixture. Interspecific competition with A. mangium trees was weak for E. grandis trees which exhibited strong plasticity making it possible to develop almost the same biomass of absorbing organs (leaves and fine roots) as in monocultures, despite a half stocking density. By contrast, crown development in the A. mangium trees below the E. grandis canopy to capture light (le Maire et al. 2012), as well as the rise in fine root density in deep soil layers offsetting their partial exclusion in the top soil, were greatly influenced by interspecific competition. This pattern shows that A. mangium trees grew using natural resources that E. grandis trees had not used above ground (i.e. light) or that they had not the capacity to take up rapidly in the upper soil layers (i.e. water and nutrients). A modelling approach showed that mean light use efficiency (ratio of stem biomass increment divided by absorbed photosynthetically active radiation) over the full rotation in our plots was significantly correlated with tree height for A. mangium trees but not for E. grandis trees, in both single- and mixed-species plots (le Maire et al. 2012). This pattern suggests size-symmetric access to soil resources for E. grandis trees in both monospecific plots and in a mixture. By contrast, the positive correlation between A. mangium tree height and light use efficiency over the rotation suggests that the tallest Acacia trees captured proportionally more soil resources than small Acacia trees, leading to more efficient use of the absorbed photosynthetically active radiations. This feature might be explained by a predominance of A. mangium roots belonging to the tallest trees in the upper soil layer where the water and nutrient availabilities were highest. Our results thus support the analogy between root competitions for soil resources with a directional component and shoot competition for directional light reported in other studies (Schenk 2006).
Belowground competitive exclusion
Even though the results were in agreement with our second hypothesis, the dominated species’ fine roots were only partially excluded from the resource-rich upper soil layer and the shift of A. mangium fine roots to deeper layers might also result from an earlier occupation of surface soil horizons by E. grandis fine roots. Future experiments are needed to disentangle the causes of fine root stratification in mixed plantations of A. mangium and E. grandis. In particular, root growth chamber experiments (Leuschner et al. 2001) might be useful to investigate competition between tree fine roots experimentally in the field.
Our systematic soil sampling representative of all the distances from the two tree species in 50A:50E showed a broad overlap of the root systems of neighbouring trees. The lack of significant influence of the distance from trees on the fine root densities of each species in the mixture suggests that competition was less than in mixed plantations of the same species with a double stocking density of Eucalyptus trees (Silva et al. 2009). Studies of fine root distributions in agroforestry systems have shown that tree root systems can turn downwards in the presence of herbaceous crops and interspecific interactions can lead to inverted root profiles (Lehmann et al. 1998; Moreno et al. 2005; Mulia and Dupraz 2006). However, this trend might depend on the availability of water and nutrients in deep soil layers. The competitiveness of beech in mixtures has been reported to push the root system of other species towards the surface, with fine roots of beech occupying a large proportion of the deep soil layers when water availability was limiting tree growth during the summer (Schmid 2002; Schume et al. 2004). Studies in agroforestry systems and forest ecosystems therefore suggest that roots of the weakest competitor can be partially excluded from soil areas where the resources limiting tree growth are concentrated. Whilst belowground competition may be considered to decrease when soil resource availability increases (Newman 1973; Coomes and Grubb 2000), the contrasting root distributions for the same species growing in mixed- and single-species stands in our study suggest high levels of competition. These fast-growing plantations require large amounts of water and nutrients throughout their development (Cabral et al. 2010; Laclau et al. 2010; Stape et al. 2010). The lack of aboveground transgressive over-yielding in our mixed plantations suggests that facilitation processes were limited in comparison with interspecific competition, which followed the general trend of competition dominating over facilitation in environments with high biomass production (Warren et al. 2009; Paquette and Messier 2011).
Further research will be needed to gain insight into the effects of interspecific interactions on mycorrhizal status, FR architecture and FR development down to the root front for the two species. Such studies should be carried out in areas highly depleted in N, where a mixture with N-fixing trees should improve the growth of non-N-fixing trees through complementarity and facilitation mechanisms. Stable isotopes or analogues of nutrients would be useful for comparing root activities in different soil layers for trees in single- and mixed-species stands (Silva et al. 2011). They might be used to quantify the uptake of water and nutrients for each species, which would help to provide insight into the effects of species mixing on size-(a)symmetric competition in tropical forests.
References
Allen SC, Jose S, Nair PKR, Brecke BJ, Nkedi-Kizza P, Ramsey CL (2004) Safety-net role of tree roots: evidence from a pecan (Carya illinoensis K. Koch)-cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. For Ecol Manag 192:395–407
Bauhus J, Khanna PK, Menden N (2000) Aboveground and belowground interactions in mixed plantations of Eucalyptus globulus and Acacia mearnsii. Can J For Res 30:1886–1894
Bauhus J, van Winden AP, Nicotra AB (2004) Aboveground interactions and productivity in mixed-species plantations of Acacia mearnsii and Eucalyptus globulus. Can J For Res 34:686–694
Binkley D, Senock R, Cromack KJ (2003) Phosphorus limitation on nitrogen fixation by Falcataria seedlings. For Ecol Manag 186:171–176
Brassard BW, Chen HYH, Bergeron Y, Paré D (2011) Differences in fine root productivity between mixed- and single-species stands. Funct Ecol 25:238–246
Cabral OMR, Rocha HR, Gash JHC, Ligo MAV, Freitas HC, Tatsch JD (2010) The energy and water balance of a Eucalyptus plantation in southeast Brazil. J Hydrol 388:208–216
Cairney JWG (2012) Extramatrical mycelia of ectomycorrhizal fungi as moderators of carbon dynamics in forest soil. Soil Biol Biochem 47:198–208
Callaway RW (2002) The detection of neighbors by plants. Trends Ecol Evol 17:104–105
Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, Weis JJ (2007) Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA 104:18123–18128
Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy E, Gamfeldt L, Balvanera P, O’Connor MI, Gonzalez A (2011) The functional role of producer diversity in ecosystems. Am J Bot 98:572–592
Christina M, Laclau J-P, Gonçalves JLM, Jourdan C, Nouvellon Y, Bouillet J-P (2010) Almost symmetrical vertical growth rates above and below ground in one of the world’s most productive forests. Ecosphere 2(3):1–10
Coomes DA, Grubb PJ (2000) Impact of root competition in forests and woodlands: a theoretical framework and review of experiments. Ecol Monogr 70:171–208
Forrester DI, Bauhus J, Cowie AL, Vanclay JK (2006) Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: a review. For Ecol Manag 233:211–230
Forrester DI, Theiveyanathan S, Collopy JJ, Marcar NE (2010) Enhanced water use efficiency in a mixed Eucalyptus globulus and Acacia mearnsii plantation. For Ecol Manag 259:1761–1770
Fredericksen TS, Zedaker SM (1995) Fine root biomass, distribution, and production in young pine-hardwood stands. New For 10:99–110
Fridley JD (2001) The influence of species diversity on ecosystem productivity: how, where, and why? Oikos 93:514–526
Hector A, Schmid B, Beierkuhnlein C, Caldeira MC, Diemer M, Dimitrakopoulos PG, Finn JA, Freitas H, Giller PS, Good J, Harris R, Hogberg P, Huss-Danell K, Joshi J, Jumpponen A, Körner C, Leadley PW, Loreau M, Minns A, Mulder CPH, O’Donovan G, Otway SJ, Pereira JS, Prinz A, Read DJ, Scherer-Lorenzen M, Schulze ED, Siamantziouras ASD, Spehn EM, Terry AC, Troumbis AY, Woodward FI, Yachi S, Lawton JH (1999) Plant diversity and productivity experiments in European grasslands. Science 286:1123–1127
Hunt MA, Battaglia M, Davidson NJ, Unwin GL (2006) Competition between plantation Eucalyptus nitens and Acacia dealbata weeds in northeastern Tasmania. For Ecol Manag 233:260–274
Kelty MJ (2006) The role of species mixtures in plantation forestry. For Ecol Manag 233:195–204
Kueffer C, Schumacher E, Fleischmann K, Edwards PJ, Dietz H (2007) Strong below-ground competition shapes tree regeneration in invasive Cinnamomum verum forests. J Ecol 95:273–282
Laclau J-P, Ranger J, Nzila JD, Bouillet J-P, Deleporte P (2003) Nutrient cycling in a clonal stand of Eucalyptus and an adjacent savanna ecosystem in Congo. 2. Chemical composition of soil solutions. For Ecol Manag 180:527–544
Laclau J-P, Bouillet J-P, Gonçalves JLM, Silva EV, Jourdan C, Cunha MCS, Moreira MR, Saint-André L, Maquère V, Nouvellon Y, Ranger J (2008) Mixed-species plantations of Acacia mangium and Eucalyptus grandis in Brazil. 1. Growth dynamics and net primary production. For Ecol Manag 255:3905–3917
Laclau J-P, Ranger J, Gonçalves JLM, Maquère V, Krusche AV, M’Bou AT, Nouvellon Y, Saint-André L, Bouillet J-P, Piccolo CM, Deleporte P (2010) Biogeochemical cycles of nutrients in tropical Eucalyptus plantations: main features shown by intensive monitoring in Congo and Brazil. For Ecol Manag 259:1771–1785
le Maire G, Nouvellon Y, Christina M, Gonçalves JLM, Bouillet JP, Laclau JP (2012) Tree and stand light use efficiencies over a full rotation of mixed and pure Eucalyptus grandis and Acacia mangium plantations. For Ecol Manag, in press. doi:10.1016/j.foreco.2012.03.005
Lehmann J (2003) Subsoil root activity in tree-based cropping systems. Plant Soil 255:319–331
Lehmann J, Peter I, Steglich C, Gebauer G, Huwed B, Zech W (1998) Below-ground interactions in dryland agroforestry. For Ecol Manag 111:157–169
Lei P, Scherer-Lorenzen M, Bauhus J (2012) Belowground facilitation and competition in young tree species mixtures. For Ecol Manag 265:191–200
Leuschner C, Hertel D, Coners H, Büttner V (2001) Root competition between beech and oak: a hypothesis. Oecologia 126:276–284
Lilienfein J, Wilcke W, Ayarza MA, Vilela L, Lima SC, Zech W (2000) Soil acidification in Pinus caribaea forests on Brazilian savanna oxisols. For Ecol Manag 128:145–157
Luyssaert S et al (2007) CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob Change Biol 13:2509–2537
Maier CA, Albaugh TJ, Allen HL, Dougherty PM (2004) Respiratory carbon use and carbon storage in mid-rotation loblolly pine (Pinus taeda L) plantations: the effect of site resources on the stand carbon balance. Glob Change Biol 10:1335–1350
Marquard E, Weigelt A, Temperton VM, Roscher C, Schumacher J, Buchman N, Fischer M, Weisser W, Schmid I (2009) Plant species richness and functional composition drive overyielding in a six-year grassland experiment. Ecology 90:3290–3302
Marsden C, Nouvellon Y, Thongo A, Saint-André L, Jourdan C, Kinana A, Epron D (2008) Two independent estimations of stand-level root respiration on clonal Eucalyptus stands in Congo: up scaling of direct measurements on roots versus the trenched-plot technique. New Phytol 177:676–687
Meinen C, Hertel D, Leuschner C (2009) Biomass and morphology of fine roots in temperate broad-leaved forests differing in tree species diversity: is there evidence of below-ground overyielding? Oecologia 161:99–111
Moreno G, Obrador JJ, Cubera E, Dupraz C (2005) Fine root distribution in Dehesas of Central–Western Spain. Plant Soil 277:153–162
Mulia R, Dupraz C (2006) Unusual fine root distributions of two deciduous tree species in southern France: what consequences for modelling of tree root dynamics? Plant Soil 281:71–85
Newman EI (1973) Competition and diversity in herbaceous vegetation. Nature 244:310
Nouvellon Y, Epron D, Kinana A, Mabiala A, D’Annunzio R, Deleporte P, Saint-André L, Marsden C, Roupsard O, Bouillet J-P, Hamel O, Laclau J-P (2008) Soil CO2 efflux and soil carbon balance following savannah afforestation in Congo: comparison of two site preparation treatments. For Ecol Manag 255:1926–1936
Nouvellon Y, Stape JL, Le Maire G, Epron D, Gonçalves JLM, Bonnefond JM, Campoe O, Loos R, Chavez R, Bouillet J-P, Laclau J-P (2011) Factors controlling carbon and water balances on fast growing Eucalyptus plantations. Proceedings IUFRO 2011, Brazil, Improvement and culture of Eucalypts, pp 43–46
Nouvellon Y, Laclau J-P, Epron D, le Maire G, Bonnefond J-M, Gonçalves JLM, Bouillet J-P (2012) Carbon allocations throughout the development of monospecific and mixed-species plantations of Eucalyptus grandis and Acacia mangium in Brazil. Tree Physiol 32:680–695
Paquette A, Messier C (2011) The effect of biodiversity on tree productivity: from temperate to boreal forests. Glob Ecol Biogeogr 20:170–180
Pretzsch H, Schütze G (2009) Transgressive overyielding in mixed compared with pure stands of Norway spruce and European beech in Central Europe: evidence on stand level and explanation on individual tree level. Eur J For Res 128:183–204
Rewald B, Leuschner C (2009) Belowground competition in a broad-leaved temperate mixed forest: pattern analysis and experiments in a four-species stand. Eur J For Res 128:387–398
Richards AE, Forrester DI, Bauhus J, Scherer-Lorenzen M (2010) The influence of mixed tree plantations on the nutrition of individual species: a review. Tree Physiol 30:1992–1208
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739
Schmid I (2002) The influence of soil type and interspecific competition on the fine root system of Norway spruce and European beech. Basic Appl Ecol 3:339–346
Schmid I, Kazda M (2002) Root distributions in monospecific and mixed stands on different soils. For Ecol Manag 159:37–47
Schume H, Jost G, Hager H (2004) Soil water depletion and recharge patterns in mixed and pure forest stands of European beech and Norway spruce. J Hydrol 289:258–274
Silva EV, Gonçalves JLM, Coelho SRF, Moreira RM, Mello SLM, Bouillet J-P, Jourdan C, Laclau J-P (2009) Dynamics of fine root distribution after establishment of monospecific and mixed-species plantations of Eucalyptus grandis and Acacia mangium. Plant Soil 325:305–318
Silva EV, Bouillet J-P, Gonçalves JLM, Abreu Junior CA, Trivelin PCO, Hinsinger P, Jourdan C, Nouvellon Y, Stape JL, Laclau J-P (2011) Functional specialization of Eucalyptus fine roots: contrasting potential uptake rates for nitrogen, potassium and calcium tracers at varying soil depths. Funct Ecol 25:996–1006
Stape JL, Binkley D, Ryan MG, Fonseca S, Loos RA, Takahashi EN, Silva CR, Silva SR, Hakamada RE, Ferreira JMA, Lima AMN, Gava JL, Leite FP, Andrade HB, Alves JM, Silva GGC, Azevedo MR (2010) The Brazil Eucalyptus potential productivity project: influence of water, nutrients and stand uniformity on wood production. For Ecol Manag 259:1684–1694
Tilman D, Wedin D, Knops J (1996) Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379:718–720
Vilà M, Vayreda J, Gracia C, Ibañez JJ (2003) Does tree diversity increase wood production in pine forests? Oecologia 135:299–303
Wang XL, Klinka K, Chen HYH, de Montigny L (2002) Root structure of western hemlock and western redcedar in single- and mixed-species stands. Can J For Res 32:997–1004
Warren J, Topping C, James P (2009) A unifying evolutionary theory for the biomass–diversity–fertility relationship. Theor Ecol 2:119–126
Acknowledgments
Our thanks to Agence Nationale de la Recherche (Intens&fix, ANR-2010-STRA-004), FAPESP Thematic Project (2010/16623-9), CIRAD (ATP Neucapalm), the USP-COFECUB project (No. 22193PA), and the European Integrated Project Ultra Low CO2 Steelmaking (ULCOS-Contract n515960) for their financial support. We thank EMBRAPA-Agrobiologia for providing selected Rhyzobium strains and the entire staff of the Itatinga experimental station for field measurements. We are particularly grateful to Rildo Moreira e Moreira (ESALQ) and Eder Araujo da Silva (http://www.floragroapoio.com.br) for their contribution to this study, as well as to Peter Biggins (CIRAD) for the revision of the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Koerner.
Rights and permissions
About this article
Cite this article
Laclau, JP., Nouvellon, Y., Reine, C. et al. Mixing Eucalyptus and Acacia trees leads to fine root over-yielding and vertical segregation between species. Oecologia 172, 903–913 (2013). https://doi.org/10.1007/s00442-012-2526-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2526-2