Abstract
Introducing N-fixing species in the understorey of fast-growing plantations might be an attractive option to improve soil N status. Intensive fine root sampling was performed in a complete randomized block design to investigate the ability of Eucalyptus grandis and Acacia mangium seedlings in monospecific stands and mixed-species plantations to take up complementary resources from niche exploration of soil layers. The same soil layers were explored by the two species down to a depth of 2 m in monospecific stands. Whilst the development of E. grandis fine roots was not affected by A. mangium trees in mixed-species plantations, A. mangium fine roots were excluded from the upper soil layer from 18 months after planting onwards, despite the paramount importance of that horizon for tree nutrition in highly weathered soils, and were only found deeper and close to A. mangium trees 30 months after planting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root interactions in a plant community can affect species diversity through competitive exclusion, niche partitioning and facilitation (Schenk 2006). Pot experiments with herbaceous species showed that roots interact with their biotic and abiotic environments using a variety of mechanisms, through effects on resource availability, exchanges of various kinds of signals and allelochemical interactions (Callaway 2002; Hierro and Callaway 2003; Semchenko et al. 2007). The finding that some roots can detect other roots, or inert objects, and can distinguish between self and non-self roots creates experimental challenges to assess the effects of root competition on plant development (Semchenko et al. 2007). Information on the mechanisms driving root growth in forest soils is much more limited (Kueffer et al. 2007). Most studies have been conducted in monospecific forest stands (Jackson et al. 1996; Schmid and Kazda 2005) or in agroforestry systems associating tree species with annual (Allen et al. 2004; Moreno et al. 2005; Mulia and Dupraz 2006) or perennial crops (Schaller et al. 2003; Van Kanten et al. 2005). A high degree of plasticity in tree roots has been demonstrated, leading to a partitioning of the soil space between crops and trees (Schaller et al. 2003; Mulia and Dupraz 2006). However, little is known about below-ground competition in mixed-species forests. A better understanding of the effects of inter- and intraspecific competition on root development is necessary to improve the control of invasive tree species and to model forest dynamics (Leuschner et al. 2001; Kueffer et al. 2007).
The sustainability of fast-growing tropical plantation forests is of concern since nutrient reserves are low in highly weathered soils and large amounts of biomass removal by harvesting can lead to unbalanced nutrient budgets (Corbeels et al. 2003; Laclau et al. 2005). It has been demonstrated that multi-species plantations are likely to generate greater productivity than monospecific stands when the different species improve the capture of natural resources or when a facilitation process occurs between them (Binkley et al. 2003, Forrester et al. 2004, 2006; Erskine et al. 2006). Planting N-fixing trees in commercial plantations of non-fixing trees might therefore be an attractive option to improve soil N status. Nitrogen-fixing trees are expected to be quickly suppressed by genetically improved non-fixing trees and might lead to more complete use of site resources through the development of a stratified canopy and niche partitioning of soils by fine roots of the different species (Kelty 2006; Forrester et al. 2006). Other benefits, including soil C sequestration (Resh et al. 2002), improved nutrient cycling, reduced risk of pest damage, and a larger range of products, have been reported (Forrester et al. 2004; Kelty 2006). Whilst the influence of a stratified canopy on light interception is well documented in mixed-species plantations (Hunt et al. 2006), the ability of different species to improve the capture of below-ground resources remains unclear (Bauhus et al. 2000; Kelty 2006; Bakker et al. 2006; Leuschner et al. 2001; Kueffer et al. 2007).
An experiment combining an additive and a replacement series between Eucalyptus grandis (Hill ex Maiden) and Acacia mangium (Wild) was set up in Brazil to investigate the effects of inter- and intraspecific competition on tree development and nutrient cycling (Laclau et al. 2008; Bouillet et al. 2008). The hypothesis tested was that the plasticity of tree roots observed in response to competition with crops in agroforestry systems also occurred in mixed-species forests and led to niche partitioning of soils by the different species. The objective was to characterize the dynamics of soil exploration by the fine roots of these species, which are widely planted in tropical countries, and to gain insight into the effects of inter- and intraspecific competition on the capacity of the two species to use complementary below-ground resources.
Materials and methods
Study area
Ecological situation
The study was carried out at the Itatinga experimental station (University of São Paulo). The area was located at latitude 23°02′S and longitude 48°38′W, and the height above sea level was 860 m. The mean annual rainfall over the last 15 years was 1,360 mm with a cold season from June to September (Fig. 1).
The average annual temperature was 19°C with an absolute minimum of −4°C recorded in July 2000. Minimum temperature values below 5°C have been recorded for a few days each year. The relief of the study area was typical of the São Paulo Western Plateau, with a gentle undulating topography. The experiment was located on the top of a hill (slope <3%). The soils were Ferralsols (FAO classification) developed on Cretaceous sandstone, Marília formation, Bauru group. Textural uniformity was high (clay content around 12% in the A1 horizon and ranging from 20% to 25% between the depths of 1 m and 6 m). The pH was acidic (between 4.5 and 5), and the amounts of available nutrients were quite low. The effective cation exchange capacity (ECEC) ranged from 0.5 to 3 cmolc kg−1 between the upper layer and a depth of 3 m, and the amounts of exchangeable bases were lower than 0.2 cmolc kg−1 below a depth of 5 cm (Maquère 2008).
The experiment was set up in a former Eucalyptus saligna (Sm.) plot managed as a coppice, without fertilizer application from 1940 to 1998. The stumps were devitalized by glyphosate application and E. grandis seedlings were planted in 1998 with low fertilizer inputs (300 kg ha−1 NPK 10:20:10). High levels of nutrient exports with the boles, and the lack of fertilization from 1940 to 1998, made this a suitable area for expecting a eucalypt response to N inputs.
Experimental design
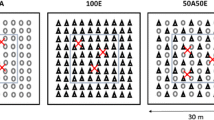
The E. grandis stand was harvested in December 2002. Only the boles (stemwood + stembark) were removed from the plot and harvest residues were spread uniformly in the field. A complete randomized block design was set up in May 2003, with seven treatments (900 m2 per plot) and four blocks, in order to assess the influence of an Acacia mangium understorey on the growth of Eucalyptus grandis seedlings (mono progeny from the Suzano Company). A complete description of the experimental design is given by Laclau et al. (2008). The study of fine root development was carried out in three treatments: 100A:0E (Acacia mangium planted at a stocking density of 1,111 trees ha−1), 0A:100E (Eucalyptus grandis planted at a stocking density of 1,111 trees ha−1) and 50A:100E (Idem 0A:100E, + Acacia mangium planted at a density of 555 trees ha−1).
The eucalypt seedlings were planted in the interrow after subsoiling (depth 40 cm), at a spacing of 3 m × 3 m. Acacia seedlings were inoculated with Rhizobium strains selected by Embrapa/Agrobiologia for their N fixation capacities, and exhibited high levels of nodulation in the nursery. They were planted at mid-distance between eucalypts in the same planting rows in 50A:100E. Such a design did not disturb mechanized silvicultural operations and could be used in commercial plantations. Fertilizer applications were representative of the commercial silviculture in that region and previous experiments showed that they were not limiting for tree growth. Two tons per hectare of dolomitic limestone were applied on planting and 40 g P plant−1 were dug in 20 cm from the plants, as well as 9 g K plant−1, 3 g B plant−1, 6 g Fe plant−1, 3 g Zn plant−1, and 1 g Mn plant−1. Three complementary fertilizations were applied, with 25 kg K ha−1 at 6, 12 and 18 months after planting in all treatments. By contrast, nitrogen fertilizers were not applied in 100A:0E, 0A:100E and 50A:100E. Complete weeding was carried out by repeated herbicide applications (glyphosate) the first year after planting. The lack of grasses and shrubs in this experiment made it possible to study the development of eucalypt and acacia roots without confusion with roots from other species.
Measurements and sampling
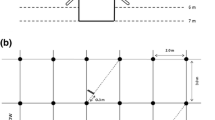
Fine root density (FRD) was quantified 6, 12, 18 and 30 months after planting, in 100A:0E, 0A:100E and 50A:100E, using a soil corer approach. Roots <3 mm in diameter were classified as fine roots, as in previous studies carried out in Brazilian Eucalyptus plantations (e.g. Mello et al. 2007). We acknowledge a non-usual classification since fine roots in most studies are roots <2 mm in diameter. Fine roots <1 mm in diameter were weighed in all the samples collected 18 and 30 months after planting and amounted on average to 77% and 67% of the total mass of fine roots <3 mm in diameter for A. mangium and E. grandis, respectively (data not shown). Livesley et al. (1999) showed that the sieve of 550 µm mesh size used in the present study recovered between 93 and 96% of grevillea and maize root biomass and between 73 and 98% of their root length, depending on the sample location. Fine roots were sampled in 5–16 positions (depending on the treatment) at different distances from a tree with a mean basal area in 12 plots at each age (3 treatments × 4 blocks). A different area (without missing neighbouring trees) was sampled on each date in the plots, in order to measure fine root densities at each age independently of the previous measurements. Fine roots were sampled down to a depth of 1 m 6 months (11/2003) and 12 months (05/2004) after planting and a depth of 2 m 18 months (11/2004) and 30 months (11/2005) after planting (Fig. 2). Intensive sampling was carried out 6 and 12 months after planting (8 and 16 positions per plot in the monospecific and mixed-species treatments, respectively). We subsequently cored only five and eight positions per plot in the monospecific and mixed-species treatments, respectively. Cores were taken with a steel soil corer driven into the soil by a sledgehammer. We used a soil corer with a small internal diameter (45 mm) because the separation of the roots of each species was labour intensive and the time required was directly dependent on the soil volume sampled. Cores were sampled stepwise at the following depths: 0–15 cm, 15–50 cm, 50–100 cm on all the sampling dates, as well as 100–150 cm and 150–200 cm 18 and 30 months after planting.
All the roots of each species were washed free of soil with tap water using a sieve of 550 µm mesh size and separated carefully by hand (sight, tactile sense and flotation if required) into living roots and dead roots. Living roots were sorted according to various criteria such as living stele, bright colour and resilient aspect. While living roots were clear and pliable, dead roots were breakable and dark. Reference roots sampled in monospecific stands were used to facilitate the identification of each species for roots collected in the 50A:100E treatment. E. grandis fine roots had a higher degree of branching and were darker than A. mangium fine roots. The samples of each component were dried at 65°C to constant weight. After carefully removing the last adherent soil particles by hand, samples were weighed (accuracy 10−4 g). The methodology used to remove soil particles was tested in an adjacent area and samples of fine roots carbonized at 450°C for 4 h exhibited ash contents close to values observed for aerial tree components, uncontaminated by soil particles (Jourdan et al. 2008). Fine root dry matter values in this article were therefore not corrected.
Among the total set of samples of roots <3 mm in diameter collected at all the depths and positions, 36, 39 and 39 samples were randomly selected in the 100A:0E treatment 6, 12 and 18 months after planting, respectively, and 49, 69 and 39 samples in the 0A:100E treatment. The roots were placed in vessels containing a 70% alcohol solution, which allowed root structure fixation and preservation at the original sampling dimensions (Mello et al. 2007). Thereafter, digitized images of the roots were obtained with a flatbed scanner, over which a glass tray containing the roots immersed in a water film was placed. The images were recorded at 100 dpi resolution in the pcx format, at 256 shades of grey and the total root lengths were measured using the SIARCS (Integrated System for Root and Soil Cover Analysis) software, developed by EMBRAPA (Jorge et al. 1996). The same samples were dried at 65°C to constant weight and weighed. Linear regressions between the length and mass of fine roots showed that specific root lengths (SRL) and standard errors over the sampling period were 70.1 ± 1.7 m g−1 for A. mangium (R2 = 0.93, n = 114) and 63.6 ± 1.4 mg−1 for E. grandis (R2 = 0.93, n = 157). We expressed the results of fine root densities (FRDs) in g dm−3 because the weights of all the root samples were measured.
Statistical analysis
The different positions were weighted 6 and 12 months after planting to estimate mean FRDs per soil layer as they represented different soil areas. The FRDs were calculated for an imaginary 2.25 m2 area of soil in 100A:0E and 0A:100E (1.5 m * 1.5 m). The 2.25 m2 area of soil was subdivided into four regions indicated in different shades of grey in Fig. 2, representing different distances from a tree. The areas of these regions were 0.09, 0.4725, 1.0 and 0.6875 m2 from the closer to the farther distance from a tree, respectively. These regions were weighted according to their proportion of the total 2.25 m2 soil area. The weighting factor for each position of fine root sampling was half of the weighting factor of the corresponding region since two positions were sampled in each region (one in the planting-row and one in the inter-row). The weighting factors were then 0.020, 0.105, 0.222, 0.153 for the positions at 15 cm, 50 cm, 100 cm and 150 cm from the tree, respectively. In 50A:100E, the FRDs were calculated for an imaginary 4.5 m2 area of soil: a 2.25 m2 area close to an E. grandis tree and a 2.25 m2 area close to an A. mangium tree (Fig. 2). The weighting factors were 0.010, 0.052, 0.111, and 0.077 for the positions at 15 cm, 50 cm, 100 cm and 150 cm from the A. mangium tree, respectively. The same weighting factors were used for the positions at 15 cm, 50 cm, 100 cm and 150 cm from the E. grandis tree in the inter-row, and in the planting-row on the side without A. mangium tree (see Fig. 2). The sampling design 18 and 30 months after planting made it possible to calculate non-weighted means of FRDs (same soil volume sampled for each position). We compared the FRDs of E. grandis and A. mangium by two-way ANOVAs. The effects of treatments and blocks on mean FRD values for all the sampled positions per plot, at each age, were tested in each soil layer (SAS 1999). The fine roots of A. mangium in 50A:100E were excluded from the ANOVAs because the stocking density of A. mangium trees in that treatment was only half the tree stocking density in 100A:0E and 0A:100E. The effects of stand age and blocks were tested by two-way ANOVAs for the positions sampled, on all the dates. The repeated measurements option was not used to compare FRDs at different ages because we sampled roots in different areas of the plots at each age. Homogeneity of variances was tested at each age and original values were transformed when variances were unequal (log or sqrt transformation). The significant differences between the levels of each factor were checked with the Newman Keuls’ multiple comparison test. The level of significance was P < 0.05.
Results
Vertical distribution of fine roots
Six months after planting, FRDs in the 0–10 cm and 10–30 cm soil layers were significantly lower for A. mangium trees in 50A:100E than for E. grandis trees in 0A:100E and 50A:100E (Fig. 3a). Fine roots of the two species were found down to a depth of 1 m at age 6 months but FRDs were <0.1 g dm−3 below a depth of 30 cm, whatever the treatment. The FRDs of A. mangium trees in 50A:100E were about half those in 100A:0E, where the stocking density of A. mangium trees was double.
Fine root densities 6 months after planting (a), 12 months after planting (b), 18 months after planting (c) and 30 months after planting (d). The horizontal bars show the LSD values when the differences between treatments were significant (P < 0.05). A. mangium fine roots in 50A:100E were excluded from the ANOVA (the stocking density of A. mangium trees was half that of E. grandis in this treatment)
Twelve months after planting, FRDs were >0.1 g dm−3 within the upper 30 cm of soil in 100A:0E, 0A:100E, and for E. grandis in 50A:100E (Fig. 3b). In the 0–10 cm layer, the FRD of E. grandis trees in the monospecific stand was significantly higher than the FRD of A. mangium trees in 100A:0E. The FRD of A. mangium trees in the upper layer was about three times higher in 100A:0E than in 50A:100E 1 year after planting.
A trend of decreasing FRD with soil depth was observed 18 months after planting in all treatments except for A. mangium trees in 50A:100E (Fig. 3c). The FRD of A. mangium trees in 100A:0E was significantly lower in the upper soil layer (0–10 cm) than the FRD of E. grandis trees in 0A:100E and 50A:100E. The same feature was observed in deep soil layers (100–150 cm and 150–200 cm) with FRDs significantly lower in 100A:0E than in 0A:100E. The FRDs remained stable from a depth of 50 cm to a depth of 200 cm in all treatments, suggesting that deeper soil layers were also explored by the fine roots of the two species.
The FRDs of A. mangium and E. grandis trees were no longer significantly different in the 0–10 cm soil layer at age 30 months, as a result of a decrease in E. grandis FRD from 18 to 30 months after planting (Fig. 3d). The trend of lower FRDs for A. mangium trees than E. grandis trees in the deep soil layers observed 18 months after planting was also found at age 30 months (significant difference in the 150–200 cm layer only).
A. mangium fine roots were almost absent in the upper soil layer of the mixed-species treatment 18 months after planting, and the highest FRDs were found in the 10-30 cm soil layer (Fig. 3c). The amounts of A. mangium fine roots observed below a depth of 50 cm were very low in 50A:100E (< 0.05 g dm−3). A similar pattern was observed 30 months after planting with very low FRDs for A. mangium in 50A:100E. The highest FRDs were no longer observed in the 10–30 cm soil layer but in the 30–50 cm layer.
Horizontal distribution of fine roots
The total amount of fine roots in the uppermost 1 m layer was relatively uniform for the two species in monospecific stands and E. grandis trees in the mixed-species treatment. The only significant differences in FRD between the sampling locations in monospecific stands were a higher FRD close to A. mangium trees 30 months after planting in 100A:0E (Table 1). The development of A. mangium fine roots was greatly modified by planting E. grandis trees in a mixture, with slower exploration of the interrow and a narrowing trend for A. mangium trees throughout stand development. The development of E. grandis fine roots in 50A:100E was uniform from 12 to 30 months after planting, as observed in the monospecific stand.
Close to trees
A. mangium FRD increased significantly from 18 to 30 months after planting in the upper soil layer (0–10 cm) close to trees in 100A:0E (Position 1, Fig. 4a). A. mangium fine roots were not observed the first year after planting in the 50–100 cm soil layer at that position, but a significant increase in FRD occurred between the ages of 12 and 18 months. The FRDs close to trees in 100A:0E were not significantly different at 18 and 30 months after planting, between a depth of 10 cm and 200 cm.
Fine root densities depending on the sampling position in the planting row (indicated in Fig. 2). P1 position in 100A:0E close to A. mangium trees (a); P1 position in 0A:100E close to E. grandis trees (b); A. mangium fine roots in the P4 position in 50A:100E, close to A. mangium trees (c); E. grandis fine roots in the P4 position in 50A:100E, close to A. mangium trees (d); A. mangium fine roots in the P1 position in 50A:100E, close to E. grandis trees (e) and E. grandis fine roots in the P1 position in 50A:100E, close to E. grandis trees (f). The horizontal bars show the LSD values when the differences between treatments were significant (P < 0.05)
As observed in 100A:0E, the FRDs close to E. grandis trees in 0A:100E increased significantly from 12 to 18 months after planting in the 50–100 cm soil layer (Position 1, Fig. 4b). Large inter-block variability at each sampling age led to non-significant variations in mean FRD over the E. grandis growth period in 0A:100E, except in the 50–100 cm and the 150–200 cm layers. However, an increasing FRD trend was observed from 6 to 18 months after planting in the upper soil, then a decrease from 18 to 30 months of age. The FRD values were about 0.2 g dm−3 between the depths of 30 cm and 200 cm, at 18 and 30 months after planting.
The higher stocking density in 50A:100E increased the competition between trees. The dynamics of soil exploration by A. mangium fine roots close to A. mangium trees was consistent with the general pattern observed in Fig. 3 for the whole stand (Position 4, Fig. 4c). The A. mangium FRD was influenced by the position in 50A:100E, with the highest FRDs found in the planting row (Fig. 4c and e, Fig. 5c and e). Maximum FRDs close to A. mangium trees were observed in the 10–30 cm layer 18 months after planting and in the 30–50 cm layer 30 months after planting.
Fine root densities depending on the sampling position in the interrow (indicated in Fig. 2). P3 position in 100A:0E in the middle of the interrow (a); P3 position in 0A:100E in the middle of the interrow (b); A. mangium fine roots in the P5 position in 50A:100E, in the middle of the interrow close to A. mangium trees (c); E. grandis fine roots in the P5 position in 50A:100E, in the middle of the interrow close to A. mangium trees (d); A. mangium fine roots in the P3 position in 50A:100E, in the middle of the interrow close to E. grandis trees (e) E. grandis fine roots in the P3 position in 50A:100E, in the middle of the interrow close to E. grandis trees (f). The horizontal bars show the LSD values when the differences between treatments were significant (P < 0.05)
A. mangium FRDs were very low whatever the soil depth close to E. grandis trees, except 12 months after planting in 50A:100E (Position 1, Fig. 4e). The upper soil layer was not completely explored by E. grandis fine roots at age 12 months and A. mangium FRDs in the 0–10 cm and 10–30 cm layers amounted to about 50% of those for E. grandis. A sharp decrease in A. mangium FRDs close to E. grandis trees occurred between ages 12 and 18 months (Position 1, Fig. 4e). Whilst the dynamics of A. mangium FRD were highly influenced by the proximity of E. grandis and A. mangium trees in 50A:100E, the dynamics of E. grandis FRDs were little influenced by the position from 12 months after planting onwards (Table 1; Fig. 4d and f). The highest FRDs for E. grandis in the upper soil layer were observed 18 months after planting close both to E. grandis trees and to A. mangium trees in 50A:100E.
Middle of the interrow
The FRD in the 0–10 cm soil layer was multiplied by a factor of about 6 from 6 to 12 months after planting in the middle of the interrow of treatment 100A:0E (Position 3, Fig. 5a). In the underlying layer (10–30 cm), the highest FRD was observed at age 18 months and a significant decrease occurred from 18 to 30 months after planting. In the 30–50 cm and 50–100 cm layers, the FRDs 18 and 30 months after planting were significantly higher than at age 6 months. A similar pattern was observed in the interrow of treatment 0A:100E, with FRDs of the same order of magnitude as in 100A:0E down to a depth of 2 m (Position 3, Fig. 5b).
A. mangium fine roots were not found below a depth of 30 cm (or only in extremely small amounts) in the middle of the interrow of treatment 50A:100E over the study period (Positions 3 and 5, Fig. 5c and e). Even in the upper soil layers, A. mangium FRDs were much lower than E. grandis FRDs in the middle of the interrow, from age 12 months onwards (Positions 3 and 5, Fig. 5d and f). A significant increase in E. grandis FRDs was observed from 6 to 12 months after planting in the interrow of treatment 50A:100E, for most of the soil layers. FRDs remained unchanged thereafter down to a depth of 2 m, up to 30 months of age.
Discussion
Below-ground competition was studied through the dynamics of fine root distribution, but other factors playing integral parts of root interactions between species were not studied, such as mycorrhizal associations that affect the availability of resources with low mobility, or species-specific associations between soil fungi, microbes and plant roots (Zobel et al. 1997). Moreover, interactions between root and shoot competition occur frequently (Schenk 2006), and our experiment was not designed to separate above- and below-ground competition. The above-ground development of the two species studied over the same period (Laclau et al. 2008) was therefore likely to influence the dynamics of fine root development considerably.
Inter- and intraspecific competition
The reduced growth of A. mangium trees in 50A:100E was a result of interspecific competition. Above-ground competition between A. mangium and E. grandis trees occurred from 9 months after planting, with the closure of the planting row by the canopy of the two species, and led to a stratified canopy (Laclau et al. 2008). The reduction in A. mangium fine root density in 50A:100E, compared to 100A:0E, in the upper soil layer from 6 to 12 months after planting showed that above- and below-ground competition started roughly at the same time in the 50A:100E treatment. The productivity of A. mangium stands can be very high in southeastern Asia (15–30 Mg ha−1 year−1), where large areas of plantations are managed for pulpwood production (Kaimowitz and Barr 2002; Cossalter and Pye-Smith 2003). The poor competitiveness of A. mangium trees with E. grandis trees in our study might be a consequence of contrasting degrees of genetic improvement for the two species in Brazil, as well as environmental conditions too cold for optimum development of A. mangium trees at the study site (Bouillet et al. 2008).
The highest E. grandis FRD in the upper soil layers was observed at age 18 months and the subsequent decrease in FRD might indicate a decrease in intraspecific root competition. Fine root densities are highly dependent on soil water content in eucalypt plantations (Mello et al. 2007) and slight changes in rainfall distribution between 18 and 30 months after planting might account for the differences in FRD observed in the uppermost soil layers (Fig. 1). However, other factors might be involved in the changes in FRD after canopy closure. The decrease in E. grandis FRD from 18 to 30 months after planting occurred proportionally to the decrease in E. grandis leaf biomass, but the driving processes are unknown (Laclau et al. 2008).
Competition for below-ground resources tends to be more intense in forests on infertile soils than on nutrient-rich soils (Coomes and Grubb 2000). The above-ground net primary production (ANPP) from 18 to 30 months after planting in 0A:100E was 37 Mg ha−1 year−1 (Laclau et al. 2008). This high ANPP, in comparison with most forest ecosystems worldwide, shows that fertilizer applications and well-distributed rainfall led to high availability of resources at the study site. High root competition, despite high availability of natural resources, was therefore observed in our study, as reported in other productive forests between understorey plants and trees (Coomes and Grubb 2000; Bakker et al. 2006; Coelho et al. 2007), or in agroforestry systems associating Eucalyptus deglupta Blume with Coffea arabica L. (Schaller et al. 2003).
Location of fine roots in monospecific and mixed-species stands
Most fine root distribution studies in forest ecosystems are limited to the top layers of the soil. Interspecific root competition in mixed-species forests has been studied down to a depth of 30 cm in plantations of Eucalyptus globulus Labill. and Acacia mearnsii de Wild (Bauhus et al. 2000), 10 to 60 cm (except 2 profiles down to 160 cm) in mixed temperate beech-oak forest (Leuschner et al. 2001), 100 cm in mature stands of Fagus sylvatica L. and Picea abies (L.) Karst. (Schmid 2002), and 120 cm in mixed forests of Pinus pinaster Ait. and understorey species (Bakker et al. 2006). However, some studies of fine root distributions in temperate and tropical agroforestry systems have highlighted that the intercrop may displace tree roots from the topsoil to layers below a depth of 1 m (Moreno et al. 2005; Mulia and Dupraz 2006). Fine roots have been observed down to depths >3 m one year after planting eucalypts in highly weathered tropical soils (Bouillet et al. 2002) and eucalypt species can exploit soil water to depths of at least 8–10 m within 7 years of planting (Robinson et al. 2006). In our study, a small decrease in FRD from 10 to 100 cm in depth 12 months after planting, and uniform fine root profiles between 30 and 200 cm at 18 and 30 months of age, suggested that not insubstantial amounts of fine roots were present beyond the sampling depths. However, sampling fine roots down to a depth of 2 m should be sufficient to study root competition that is likely to occur preferentially in the upper soil the first years after planting.
High FRD variability was observed at each sampling age, whatever the position in the stands, and made it difficult to detect significant differences between treatments or stand ages. The low volume of soil sampled by the soil corer (internal diameter of 45 mm) was partially responsible for the large spatial variability observed. Moreover, patches of contrasting nutrient availability and a high degree of spatial and temporal heterogeneity of water content in forest soils might be involved in the large FRD variability observed (Jackson et al. 1996). Fine roots respond in general to nutrient-enriched soil patches by enhanced growth and greater root density (Morris 1996; Schmid and Kazda 2005). Despite the high variability of FRD found at each sampling age, the results showed clearly that the whole soil volume down to a depth of 1 m was colonized by E. grandis fine roots from 12 months after planting onwards. Uniform distribution of tree roots was observed in Mediterranean agroforestry systems down to a depth of 200 cm (Moreno et al. 2005), as in the E. grandis stands between the depths of 10 cm and 200 cm in our study, at age 30 months. By contrast, A. mangium FRDs were largely influenced by E. grandis trees in mixed-species stands. Therefore, tree root densities differed greatly from the negative exponential decrease of fine root distribution with distance from the trunk currently used in tree growth models. The current models would fail to describe correctly the rooting patterns of A. mangium trees in the mixed-species stands, as reported by Mulia and Dupraz (2006) for tree roots in agroforestry systems.
Root plasticity as a response to interspecific competition
Root competition can affect the availability of a resource for plants either by resource depletion or by mechanisms that inhibit access to the resource for other roots. The horizontal distribution of E. grandis and A. mangium fine roots in 50A:100E suggested competition, with a sharp decrease in A. mangium roots with the distance from A. mangium trees, whereas the E. grandis roots where not influenced by the presence of A. mangium trees. The gradual exclusion of A. mangium fine roots from the upper soil layers in 50A:100E was also consistent with competition. Competition for resources between the two species did not lead to aggregative placement of E. grandis root towards A. mangium trees in the 50A:100E treatment, as observed for grasses in nutrient-poor inland sand-dune habitats (Bartelheimer et al. 2006). Moreover, the lack of influence of A. mangium trees on E. grandis fine root distribution in 50A:100E suggests that facilitative interaction between the two species, through the release of N-rich compounds by A. mangium roots, was weak over the study period. That feature was consistent with biomass accumulation and N concentration in E. grandis tree components that were not significantly different in 0A:100E and 50A:100E (Bouillet et al. 2008). Plants can interact by modifying resource availability and exchanging various kinds of signals (Hierro and Callaway 2003). Eucalyptus litter leachates are characterized by high polyphenol contents (Chapuis-Lardy et al. 2002) and many studies have shown that they are likely to suppress root and shoot growth of crops and weeds (Lisanework and Michelsen 1993; Moradshahi et al. 2003; Espinosa-García et al. 2008). It has been shown in Portugal that litter decomposition but also root exudates of Eucalyptus, are likely to enhance soil water repellency (Doerr et al. 1998). Whether competition for water and nutrients, or biotic or chemical interactions were the dominant processes occurring between E. grandis and A. mangium in the 50A:100E treatment remains an open question.
Schenk (2006) suggested that root competition for soil resources with a directional component provides an analogue to shoot competition for directional light. Soil resources have a strong vertical dimension, with a directional component provided by water infiltration that is particularly strong in highly weathered tropical soils, where most available nutrients are located in the upper soil layer (released by organic matter mineralization and fertilizers) and transported by gravitational solutions. Roots that are closer to the surface have a competitive advantage over deeper roots, because, on average, they have access to larger amounts of water and nutrients (Laclau et al. 2003; 2004). The changes in vertical distribution of A. mangium fine roots in response to competition with E. grandis in 50A:100E follow a pattern found in other studies where superior competitors had a larger proportion of their roots in the uppermost soil layers (Moreno et al. 2005, Mulia and Dupraz 2006; Forrester et al. 2006). However, this trend might depend on the availability of water and nutrients in deep soil layers. The underground competitiveness of beech in a mixture with other species has been reported to push the root system of other species towards the surface, with beech fine roots occupying a large proportion of the rooting zone (Leuschner et al. 2001; Schume et al. 2004). The vertical distribution of resource-acquiring organs in our study was also shaped above ground by the competition for vertically distributed resources. Interspecific competition increased the early vertical growth in 50A:100E compared to 100A:0E, and decreased the number of stems per A. mangium tree, increasing the length of the period where A. mangium trees were able to compete with E. grandis trees for light acquisition (Laclau et al. 2008).
Soil exploration by fine roots of the two species was similar in monospecific stands, and root competition in mixed-species stands displaced the dominated species into non-optimum parts of its fundamental niche (close to the A. mangium trees and excluded from the upper soil layers). The capacity of different species in mixed forests to improve the capture of soil resources by niche-partitioning has been suggested to explain the enhancement of net primary production compared to monospecific stands in numerous experiments (Kelty 1992; Kelty 2006; Forrester et al. 2006). Such behaviour has been observed in agroforestry systems where tree root systems turn downward in the presence of herbaceous crops and can lead to inverted root profiles (Lehmann et al. 1998; Moreno et al. 2005; Mulia and Dupraz 2006). Deep roots are likely to provide a safety-net service taking up nutrients leached from the topsoil and transferring nutrients available in deep layers to the soil surface (Allen et al. 2004; Jobbagy and Jackson 2004; Mulia and Dupraz 2006). However, a mineralogical study down to a depth of 15 m at the study site showed the absence of primary minerals likely to release nutrients by weathering (Maquère 2008). Moreover, the sum of base cations <0.1 cmolc kg−1 between the depths of 10 cm and 15 m at the study site indicated that complementarity between the two species for nutrient uptake in deep soil layers was unlikely. Losses of nutrients by deep drainage were very low in an adjacent stand of the same E. grandis progeny in the first 2 years after planting (Maquère 2008), as observed for eucalypt plantations in Congolese sandy soils (Laclau et al. 2005). Sap flow measurements and monitoring of soil water content in adjacent stands of the same E. grandis progeny showed that actual evapotranspiration in monospecific stands amounted roughly to the rainfall from the second year after planting onwards (Unpublished data). The displacement of A. mangium roots due to competition with E. grandis in our study was therefore unlikely to enhance the capture of soil resources. A lack of complementary niches was also observed by Bauhus et al. (2000) in mixed-species plantations of E. globulus and A. mearnsii. Asymmetric interactions between species in temperate mixed forests led to significant reductions in fine root biomass of the dominated species (Bi et al. 1992; Fredericksen and Zedaker 1995; Leuschner et al. 2001), as observed in our study. Differences in root distribution between species in mixed forests might therefore mainly result from differences in competitiveness between species to capture natural resources.
References
Allen SC, Jose S, Nair PKR, Brecke BJ, Nkedi-Kizza P, Ramsey CL (2004) Safety-net role of tree roots: evidence from a pecan (Carya illinoensis K. Koch)-cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. For Ecol Manage 192:395–407. doi:10.1016/j.foreco.2004.02.009
Bakker MR, Augusto L, Achat DL (2006) Fine root distribution of trees and understory in mature stands ofmaritime pine (Pinus pinaster) on dry and humid sites. Plant Soil 286:37–51. doi:10.1007/s11104-006-9024-4
Bartelheimer M, Steinlein T, Beyschlag W (2006) Aggregative root placement: a feature during interspecific competition in inland sand-dune habitats. Plant Soil 280:101–114. doi:10.1007/s11104-005-2612-x
Bauhus J, Khanna PK, Menden N (2000) Aboveground and belowground interactions in mixed plantations of Eucalyptus globulus and Acacia mearnsii. Can J For Res 30:1886–1894. doi:10.1139/cjfr-30-12-1886
Bi H, Turvey ND, Heinrich P (1992) Rooting density and tree size of Pinus radiata (D.Don) in response to competition from Eucalyptus obliqua (L’Herit). For Ecol Manage 49:31–42. doi:10.1016/0378-1127(92)90158-6
Binkley D, Senock R, Cromack KJ (2003) Phosphorus limitation on nitrogen fixation by Falcataria seedlings. For Ecol Manage 186:171–176. doi:10.1016/S0378-1127(03)00240-8
Bouillet JP, Laclau JP, Arnaud M, Thongo M’bou A, Saint-André L, Jourdan C (2002) Changes with age in the spatial distribution of roots of a Eucalyptus clone in the Congo. Impact on water and nutrient uptake ability. For Ecol Manage 171:43–57. doi:10.1016/S0378-1127(02)00460-7
Bouillet JP, Laclau JP, Gonçalves JLM, Moreira MZ, Trivelin PCO, Jourdan C, Silva EV, Piccolo MC, Tsai SM, Galiana A (2008) Mixed-species plantations of Acacia mangium and Eucalyptus grandis in Brazil. 2. Nitrogen accumulation in the stands and N2 biological fixation. For Ecol Manage 255:3918–3930. doi:10.1016/j.foreco.2007.10.050
Callaway RW (2002) The detection of neighbors by plants. Trends Ecol Evol 17:104–105. doi:10.1016/S0169-5347(01)02438-7
Chapuis-Lardy L, Contour-Ansel D, Bernhard-Reversat F (2002) High-performance liquid chromatography of water-soluble phenolics in leaf litter of three Eucalyptus hybrids (Congo). Plant Sci 163:217–222. doi:10.1016/S0168-9452(02)00099-7
Coelho SRF, Gonçalves JLM, Laclau JP, Mello SLM, Moreira RM, Silva EV (2007) Crescimento, nutrição e fixação biológica de nitrogênio em plantios mistos de eucalipto e leguminosas arbóreas. Pesquisa Agropecu Bras 42:759–768. doi:10.1590/S0100-204X2007000600001
Coomes DA, Grubb PJ (2000) Impacts of root competition in forests and woodlands: a theoretical framework and review of experiments. Ecol Monogr 70:171–207
Corbeels M, O’Connell AM, Grove TS, Mendham DS, Rance SJ (2003) Nitrogen release from eucalypt leaves and legume residues as influenced by their biochemical quality and degree of contact with soil. Plant Soil 250:15–28. doi:10.1023/A:1022899212115
Cossalter C, Pye-Smith C (2003) Fast-wood forestry: myths and realities. CIFOR, Jakarta, p 50
Doerr SH, Shakesby RA, Walsh RPD (1998) Spatial variability of soil hydrophobicity in fire-prone Eucalyptus and pine forests, Portugal. Soil Sci 163:313–324. doi:10.1097/00010694-199804000-00006
Erskine PD, Lamb D, Bristow M (2006) Tree species diversity and ecosystem function: can tropical multi-species plantations generate greater productivity? For Ecol Manage 233:205–210. doi:10.1016/j.foreco.2006.05.013
Espinosa-García FJ, Martínez-Hernández E, Quiroz-Flores A (2008) Allelopathic potential of Eucalyptus spp plantations on germination and early growth of annual crops. Allelopathy J 21:25–38
Forrester DI, Bauhus J, Khanna PK (2004) Growth dynamics in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. For Ecol Manage 193:81–95
Forrester DI, Bauhus J, Cowie AL, Vanclay JK (2006) Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: a review. For Ecol Manage 233:211–230
Fredericksen TS, Zedaker SM (1995) Fine root biomass, distribution, and production in young pine-hardwood stands. New For 10:99–110
Hierro JL, Callaway RM (2003) Allelopathy and exotic plant invasion. Plant Soil 256:29–39
Hunt MA, Battaglia M, Davidson NJ, Unwin GL (2006) Competition between plantation Eucalyptus nitens and Acacia dealbata weeds in northeastern Tasmania. For Ecol Manage 233:260–274
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distribution for terrestrial biomes. Oecologia 108:389–411
Jobbagy EG, Jackson RB (2004) The uplift of soil nutrients by plants: biogeochemical consequences across scales. Ecology 85:2380–2389
Jorge LAC, Ralisch R, Abi-Saab OJG, Medina CC, Guimarães MF, Neves CSVJ, Crestana S, Cintra FLD, Bassoi LH, Fernandes SBV (1996) Aquisicão de imagens de raízes. In: Jorge LAC (ed) Recomendacões práticas para aquisição de imagens digitais através do SIARCS. EMBRAPA-CNPDIA (Circular técnica 1), São Carlos, pp 2–28
Jourdan C, Silva EV, Gonçalves JLM, Ranger J, Moreira RM, Laclau JP (2008) Fine root production and turnover in Brazilian Eucalyptus plantations under contrasting nitrogen fertilization regimes. For Ecol Manage 256:396–404
Kaimowitz D, Barr C (2002) Heartrots in plantation hardwoods in Indonesia and Australia. ACIAR Tech Rep 51:1–2
Kelty MJ (1992) Comparative productivity of monocultures and mixed-species stands. In: Kelty MJ, Larson BC, Oliver CD (eds) The ecology and silviculture of mixed-species forests. Klumer Academic, Dordrecht, pp 125–141
Kelty MJ (2006) The role of species mixtures in plantation forestry. For Ecol Manage 233:195–204
Kueffer C, Schumacher E, Fleischmann K, Edwards PJ, Dietz H (2007) Strong below-ground competition shapes tree regeneration in invasive Cinnamomum verum forests. J Ecol 95:273–282
Laclau JP, Ranger J, Nzila JD, Bouillet JP, Deleporte P (2003) Nutrient cycling in a clonal stand of Eucalyptus and an adjacent savanna ecosystem in Congo. 2. Chemical composition of soil solutions. For Ecol Manage 180:527–544
Laclau JP, Toutain F, Thongo A, Arnaud M, Joffre R, Ranger J (2004) The function of the superficial root mat in the biogeochemical cycles of nutrients in Congolese Eucalyptus plantations. Ann Bot 93:249–261
Laclau JP, Ranger J, Deleporte P, Nouvellon Y, Saint-André L, Marlet S, Bouillet JP (2005) Nutrient cycling in a clonal stand of Eucalyptus and an adjacent savanna ecosystem in Congo. 3. Input–output budgets and consequences for the sustainability of the plantations. For Ecol Manage 210:375–391
Laclau JP, Bouillet JP, Gonçalves JLM, Silva EV, Jourdan C, Cunha MCS, Moreira MR, Saint-André L, Maquère V, Nouvellon Y, Ranger J (2008) Mixed-species plantations of Acacia mangium and Eucalyptus grandis in Brazil. 1. Growth dynamics and net primary production. For Ecol Manage 255:3905–3917
Lehmann J, Peter I, Steglich C, Gebauer G, Huwed B, Zech W (1998) Below-ground interactions in dryland agroforestry. For Ecol Manage 111:157–169
Leuschner C, Hertel D, Coners H, Büttner V (2001) Root competition between beech and oak: a hypothesis. Oecologia 126:276–284
Lisanework N, Michelsen A (1993) Allelopathy in agroforestry systems: the effects of leaf extracts ofCupressus lusitanica and three Eucalyptus spp. on four Ethiopian crops. Agroforestry Systems 21:63–74
Livesley SL, Stacey CL, Gregory PJ, Buresh RJ (1999) Sieve size effects on root length and biomass measurements of maize (Zea mays) and Grevillea robusta. Plant Soil 207:183–193
Maquère V (2008) Dynamics of mineral elements under a fast-growing Eucalyptus plantation in Brazil. Implications for soil sustainability. PhD thesis, Agroparitech, Paris, p 369
Mello SLM, Gonçalves JLM, Gava JL (2007) Pré- and post harvest fine root growth in Eucalyptus grandis stands installed in sandy and loamy soils. For Ecol Manage 246:186–195
Moradshahi A, Ghadiri H, Ebrahimikia F (2003) Allelopathic effects of crude volatile oil and aqueous extracts of Eucalyptus camaldulensis Dehnh. Leaves on crops and weeds. Allelopathy J 12:189–195
Moreno G, Obrador JJ, Cubera E, Dupraz C (2005) Fine root distribution in Dehesas of Central-Western Spain. Plant Soil 277:153–162
Morris EC (1996) Effect of localized placement of nutrients on root competition in self-thinning populations. Ann Bot 78:353–364
Mulia R, Dupraz C (2006) Unusual fine root distributions of two deciduous tree species in southern France: what consequences for modelling of tree root dynamics? Plant Soil 281:71–85
Resh SC, Binkley D, Parrota JA (2002) Greater soil carbon sequestration under nitrogen-fixing trees compared with Eucalyptus species. Ecosystems 5:217–231
Robinson N, Harper NJ, Smettem KRJ (2006) Soil water depletion by Eucalyptus spp. integrated into dryland agricultural systems. Plant Soil 286:141–151
Sas Institute (1999) SAS/STAT software and enhancement, release 6.11 edition. Cary
Schaller M, Schroth G, Beer J, Jiménes F (2003) Species and site characteristics that permit the association of fast growing trees with crops: the case of Eucalyptus deglupta as coffee shade in Costa Rica. For Ecol Manage 175:205–215
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739
Schmid I (2002) The influence of soil type and interspecific competition on the fine root system of Norway spruce and European beech. Basic Appl Ecol 3:339–346
Schmid I, Kazda M (2005) Clustered root distribution in mature stands of Fagus sylvatica and Picea abies. Oecologia 144:25–31
Schume H, Jost G, Hager H (2004) Soil water depletion and recharge patterns in mixed and pure forest stands of European beech and Norway spruce. J Hydrol 289:258–274
Semchenko M, Hutchings MJ, John EA (2007) Challenging the tragedy of the commons in root competition: confounding effects of neighbour presence and substrate volume. J Ecol 95:252–260
Van Kanten R, Schroth G, Beer J, Jiménez F (2005) Fine-root dynamics of coffee in association with two shade trees in Costa Rica. Agrofor Forum 63:247–261
Zobel M, Moora M, Haukioja E (1997) Plant coexistence in the interactive environment: Arbuscular mycorrhiza should not be out of mind. Oikos 78:202–208
Acknowledgements
Our thanks to Forestry Science and Research Institute (IPEF), CIRAD, State of São Paulo Research Foundation (FAPESP) (No. 2002/11827-9), the USP-COFECUB project (2003/1.10895.1.3), and the European Integrated Project “Ultra Low CO2 Steelmaking” (ULCOS—Contract n°515960) for their financial support. We thank Brazilian Agricultural Research Corporation (EMBRAPA-Agrobiology) for providing selected Rhizobium strains and the entire staff of the Itatinga experimental station for field measurements. We are particularly grateful to Maria Rosa Gonçalves and Estevão Araújo for their contribution to this study and Peter Biggins for the revision of the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
da Silva, E.V., de Moraes Gonçalves, J.L., de Freitas Coelho, S.R. et al. Dynamics of fine root distribution after establishment of monospecific and mixed-species plantations of Eucalyptus grandis and Acacia mangium . Plant Soil 325, 305–318 (2009). https://doi.org/10.1007/s11104-009-9980-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-9980-6