Abstract
Current meta-community theories postulate that the structure of local communities depends on dispersal, environmental filtering, and biotic interactions. However, disentangling the relative effects of these factors in the field and for diverse assemblages is a major challenge. A solution is to address natural but simple communities (i.e. with low numbers of species in few trophic levels), wherein one of these factors is predominant. Here, we analyse the micro-arthropod community of a moss-turf habitat typical of the Antarctic Peninsula region, and test the widely accepted hypothesis that this system is abiotically driven. In the austral summers 2006/7 and 2007/8, we sampled nearly 80 units of moss from four islands in the Argentine Islands. Using variance partitioning, we quantified the relative contribution of: (1) multiple scale spatio-temporal autocorrelation; (2) environmental effects; (3) the island effect. Little variance (1 %) was accounted for by sources 1 (1 %, significant) and 2 (<1 %, not significant). The island effect significantly accounted for the largest amount of variation (8 %). There was a relatively large effect of spatially structured environmental variation (7 %). Null models demonstrated that species co-occurred less frequently than expected by chance, suggesting the prevalence of negative interactions. Our data support the novel hypothesis that negative biotic interactions are the most important structuring force of this micro-arthropod community. The analysed system is a good proxy for more complex communities in terms of taxonomic composition and the functional groups present. Thus, biotic interaction might be a predominant factor in soil meta-community dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a meta-community framework, local communities are driven by at least three interacting forces: dispersal, environmental filtering, and biotic interactions (Leibold et al. 2004). Current research in community ecology aims to disentangle the relative effects of each of these factors (Dumbrell et al. 2010; Ofiteru et al. 2010; Bell 2010; Caruso et al. 2011). However, it is very challenging to quantify the importance of these three key factors in the field and for typically very diverse assemblages. In the current study, we exploit the idea that simplified but natural communities can be used as model or proxy systems (e.g. Bowker et al. 2010) to provide insight into fundamental processes underlying community assembly. More specifically, a general hypothesis is that in such simple systems only one of the three proposed major interacting forces (Leibold et al. 2004) may be the predominant factor structuring communities. This hypothesis might then be tested observationally and/or experimentally, thereby contributing to unravelling the interactions between meta-community factors. The background knowledge available on such systems, coupled with their inherent simplicity, allows the formulation and testing of robust hypotheses through examination of the statistical properties of species co-occurrence matrices (Gotelli and Ulrich 2012) and/or the application of multivariate variance partitioning techniques (Borcard et al. 1992, 2004; Legendre and Legendre 1998; Cottenie 2005).
Soil is one of the most diverse environments in terms of its contained invertebrate communities (Wardle 2002; Bardgett 2005). However, fundamental questions relating to the causes and maintenance of this diversity remain only partially answered (Bardgett 2002; Lindo and Winchester 2009; Caruso et al. 2012). Dispersal, environmental filtering, and biotic interactions are all known to play a key role, although their relative importance is expected to vary depending on the system analysed. For example, the high spatio-temporal complexity of the soil environment in terms of chemistry and physical architecture has been hypothesised to offer a vast array of ecological niches to soil species (Wardle 2002). In addition, the high complexity of soil food webs allows many and relatively narrowly defined taxa (e.g. oribatid mites) to be partitioned into several and continuous trophic levels (Maraun et al. 2011). However, mechanistic or robust observational studies that support this hypothesis for entire communities rather than selected taxonomic assemblages are unavailable, while environmental variables only partially and then sometimes weakly correlate with soil animal community structure. This is especially true when correlations are controlled for the effect of spatial autocorrelation (Legendre and Legendre 1998; Ettema and Wardle 2002; Lindo and Winchester 2009; Caruso et al. 2012).
Spatial patterns due to dispersal processes are known to be a dominant source of community variation in the soil, regardless of the effect of environment variables (Ettema and Wardle 2002), in particular in systems that are highly fragmented and have a complex paleogeographic history. Arthropods in Antarctic ice-free areas provide paradigmatic examples of such systems (Booth and Usher 1984; Usher and Booth 1984, 1986; Adams et al. 2006; Convey et al. 2008; McGaughran et al. 2008): on the one hand, local physical constraints such as water availability structure species distributions (Booth and Usher 1984; Usher and Booth 1984; Convey et al. 2003; Caruso et al. 2007); on the other hand, broad-scale patterns in community structure and population genetics clearly suggest that the history of glaciation events is an important determinant of species distributions (Convey et al. 2008; McGaughran et al. 2008; Caruso et al. 2009).
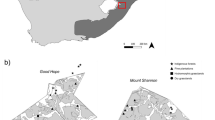
Our study focuses on the region of the Argentine Islands, a small archipelago on the west coast of the Antarctic Peninsula, representative of the terrestrial ecosystems of this region. These islands have been largely unexplored in terms of the detail of their soil animal community, with the exception of a small number of samples obtained in the late 1960s (Tilbrook 1967). We targeted the conspicuous moss-turf habitat that represents one of the most common terrestrial habitats on the four islands sampled in this study and, more generally, throughout the maritime Antarctic (Tilbrook 1967). Here, we analyse the arthropods of this system, which is known to contain representatives of the most important groups in soil animal communities (Wardle 2002; Bardgett 2005).
We tested the widely accepted hypothesis (Tilbrook 1967; Booth and Usher 1984; Usher and Booth 1986; Convey 1996; Sinclair et al. 2006; Hogg et al. 2006) that these Antarctic assemblages are abiotically driven and should therefore display the community patterns expected under the species sorting meta-community model (Leibold et al. 2004; Cottenie 2005). This means that we expect environmentally-driven patterns to play the major role. We partitioned the variance of the “species by sample” matrix obtained into the unique and shared effects of three sources of variation (Borcard et al. 1992, 2004): spatio-temporal effect, environmental effect, island effect. Additionally, we ran null model analysis (Gotelli 2000; Gotelli and Ulrich 2010) of species co-occurrence to test for patterns of species segregation and aggregation, which would, respectively, suggest either the presence of negative interactions or common response(s) to an environmental factor and/or the indirect effect of interactions with other species.
Materials and methods
Study area, sampling strategy and sample processing
The region of the Argentine Islands is characterised by the presence of numerous islands of a range of sizes located at about 65°15′S, 64°16′W. The samples analysed in the current study were collected during the 12th Ukrainian Antarctic expeditions in 2006/7 and 2007/8. Due to logistic constraints and the distribution of the targeted moss-turf habitat, the 74 samples obtained were collected unevenly across four major islands: Peterman Island (44 samples), Galindez Island (11), Irizar Island (5) and Great Yalour Island (16). The moss-turf habitat included a small number of dominant moss species (e.g. Polytrichum, Brachythecium, Sanionia) and several lichens. This habitat is common and widespread in maritime Antarctic ice-free areas (Smith 1972; Longton 1988) and hosts a community of invertebrates which is macroscopically dominated by mites and collembolans (Tilbrook 1967; Booth and Usher 1984; Usher and Booth 1986). The richness of this community is very limited both regionally and locally. For instance, Convey and Smith (1997) reported 16 species of mites and 4 collembolans in the region of Marguerite Bay. Similar numbers have been reported in other Antarctic regions (e.g. Richard et al. 1994; Convey and Quintana 1997; Adams et al. 2006). Within an area, smaller scale habitat patches (e.g. an area of moss-turf) typically host a small fraction of this diversity (Booth and Usher 1984). The trophic structure of these systems is also simple, including only one or two species of predatory mite (e.g. Lister et al. 1988), while the majority of other species are assumed to be either generalist or more specific microbivores or detritivores (Fitzsimons 1971; Davidson and Broady 1996; Worland and Lukešová 2000).
Our field sampling design was based on identifying areas of well-developed moss-turf and randomly sampling a total of 72 15 cm × 15 cm × 5 cm blocks (including the top 5 cm of the mineral layer upon which the turf was growing). Each sampling location was georeferenced using GPS. Sampling sites were classified subjectively in three groups to account for allochtonous nutrient inputs: (1) sites surrounded by penguin (Pygoscelis adeliae and P. papua) nesting sites (high nutrient input); (2) sites relatively close to penguin nesting sites but more directly influenced by the nests of the south polar skua Catharacta maccormicki (medium nutrient input); and (3) sites relatively close to the nests of C. maccormicki but far from penguin nesting sites (low nutrient input). We used this information because in this system birds are a important source of nutrients for polar soils (Barrett et al. 2006; Bokhorst et al. 2007; Zmudczyńska et al. 2012). Micro-arthropods were extracted using bulb lamp extractors and 1/3 of each moss sample was also carefully washed in Petri dishes to recover as many individuals as possible. The dry mass of moss examined was recorded after completion of extractions. All animals were preserved in 75 % ethanol, identified to species level and counted in each sample. Forty-seven of the samples from Peterman Island were collected in March 2007, while all other samples were collected between November 2007 and March 2008.
Data analyses
We applied a multivariate regression approach based on canonical correspondence analysis (CCA) and redundancy analysis (RDA; Borcard et al. 1992, 2004; Legendre and Legendre 1998) to model the response of the arthropod community to three potential sources of variation: (1) multiple-scale spatio-temporal autocorrelation; (2) environmental effects; (3) the island effect. The spatio-temporal component represents spatial and temporal patterns in species distribution that are virtually independent of environmental effects (Borcard et al. 1992). At the same time, variation shared by this component and the environment must be interpreted as spatially-structured environmental effects (Legendre et al. 2005). In principle, if one can measure the most relevant environmental variables, then the variation accounted for by the spatio-temporal component largely represents those patterns that arise from intrinsic dynamics such as dispersal (Legendre et al. 2005; Smith and Lundholm 2010). In order to quantify the spatio-temporal component, we used the powerful method of principal coordinate analysis of neighbour matrices (PCNM; Borcard and Legendre 2002). We applied this analysis to the sample spatial distance matrix and to the sample temporal distance matrix (based on numbers of days after the first sampling date). The analysis produces a number of eigenvectors that account for those spatial and temporal patterns that can be solved by the sampling design in terms of extent (spatio-temporal span of the study), interval (average distance between two samples) and grid (Borcard and Legendre 2002; Borcard et al. 2004; Dray et al. 2006). The vectors differ in terms of the scale of the patterns they account for, and relatively large distance matrices (76 by 76 in the current study) can produce many vectors. Then, a multivariate extension of the AIC criterion can be used to select the linear combination of vectors that describes the largest amount of variation in the species matrix with the lowest possible number of vectors (Dray et al. 2006). We did not use the more general class of Moran’s eigenvector mapping (MEM; Dray et al. 2006), of which PCNM is a particular case, since preliminary results showed the PCNM was performing as well as generalised MEMs.
Once the combination of vectors that best accounts for spatio-temporal patterns is defined, the categorical factor “island” can be used as an additional explanatory variable. When controlling for the partial effect of the measured environmental variables and spatio-temporal autocorrelation, this factor may account for patterns in species distributions that, for example, depend on the environmental variables that play a role at the island scale but that were not measured.
Finally, we defined environmental effects based on the following parameters: moss water content, moss dry mass, and avian influence (high, medium, low). Water is the most important physical constraint in many Antarctic terrestrial ecosystems (Booth and Usher 1984; Convey et al. 2003). Moss dry mass is known to be a strong correlate of micro-arthropod species distribution in Antarctic moss-turf (Booth and Usher 1984). Avian influence is a key factor affecting soil nutrients in Antarctic and comparable Arctic regions (Bokhorst et al. 2007; Zmudczyńska et al. 2012). More detailed analyses of chemical variables such as the concentration of P, N, Na and K, or pH, have in some cases (Booth and Usher 1984) been found to correlate well with species distributions, although a clear mechanistic understanding is still missing. Given the conditions of our sampling sites, we consider that the categorical factor “birds”, while being a coarse and simplistic measure, is a good and pragmatic proxy for the nutrient status in our specific system. We also recognise that the effect of variables we did not measure (e.g. pH, Na, K) is known to be spatially structured in the moss-turf habitat (Booth and Usher 1984). Should such effects be present in our study locations, they would be included in the “spatio-temporal components” and/or the island component and we interpreted our results taking into account this limitation (see “Discussion” below).
We used variance partitioning to quantify the amount of variation accounted for by spatio-temporal components, environment, and the island factor. The significance of each source of variation was tested by a permutation test executed on the relevant partial RDA, which statistically controlled (“partial”) for the effect of the other sources of variation (Oksanen et al. 2009). All multivariate analyses were performed with the R package vegan (Oksanen et al. 2009).
This multivariate approach allows quantification of the relative effects of the environment and spatio-temporal autocorrelation in species distributions. However, the effect of biotic interactions can remain unidentified by this approach (Smith and Lundholm 2010; Caruso et al. 2011, 2012). Indeed, environmental filtering can cause correlations between species distribution and environmental parameters. However, the environment being relatively constant, negative biotic interactions (e.g. predation, competition for resources) would be expected to cause spatial patterns such as segregation. In such circumstances, species will co-occur less frequently than expected in the absence of any interaction (Gotelli 2000; Leibold et al. 2004).
In order to provide an initial description of patterns of species covariation, we calculated all pair-wise Pearson correlation coefficients and corresponding p values with Bonferroni correction. Then, we performed a formal null model analysis (Gotelli 2000; Gotelli and Ulrich 2012) testing whether patterns of species co-occurrence in the overall matrix showed the deterministic signal of non-random processes. Many indices exist that quantify patterns of species co-occurrence and we used the C-score (Gotelli 2000; Gotelli et al. 2010; Gotelli and Ulrich 2012) because it provides good statistical power and low Type I error rates, especially when coupled with the randomisation scheme employed in our analysis (below). The C-score quantifies checkerboard distributions (Stone and Roberts 1992): the higher it is the more the species matrix is characterised by species that “avoid each other” (segregation). Since we wanted to test for non-random patterns that may arise from species interactions, the most conservative approach to randomise the matrix and build a null distribution of the C-score is to create matrices that have the same row (species) and column (site) totals as the true matrix. This corresponds to algorithm SIM9 in Gotelli (2000), who states that it ensures species co-occurrences are random with respect to one other. We calculated 5,000 random matrices and compared the central tendency of the null distribution to the observed C-score. The C-score is an aggregate index that describes the average behaviour of a metric which is calculated on a species pair basis. Thus, important information can be obtained by testing for non-random patterns in species association on a species pair basis. However, this creates the statistical problem that, even for small matrices such as that analysed here (with few species overall), the number of possible pairs is high (in our case, 21 pairs). This increases considerably the risk of Type I error (Gotelli and Ulrich 2010). Thus, we followed the method recently proposed by Gotelli and Ulrich (2010), which is based on building confidence limits using the empirical Bayes approach. Null model analysis was performed using the FORTRAN program Pairs (Ulrich 2008).
Results
We collected 5,189 individuals that belonged to seven micro-arthropod species (Table 1). The most abundant and frequent species were the isotomid collembolan Cryptopygus antarcticus and the oribatid mite Alaskozetes antarcticus. The predatory mesostigmatid mite Gamasellus racovitzai, whose main prey is C. antarcticus, had typically low abundance but could locally be represented by relatively high numbers (n ≤ 21). All species were found on all islands excepting the isotomid collembolan Isotoma (Folsomotoma) octooculata and the prostimatid mite Stereotydeus villosus. The former was not found on two islands and the latter on one. These two species were typically present at low density but were very abundant (several tens of ind.) in a few samples. Larvae of the chironomid midge Belgica antarctica were present at very low abundance although were widespread. Finally, the neanurid collembolan Friesea grisea had a patchy distribution, in some cases being abundant (up to 33 ind.). Overall, the high number of collected individuals (>5,000) and the low number of species (<10) suggests that the sampling effort was sufficient to describe the overall richness of this system, as also confirmed by rarefaction curves (not shown).
Variance partitioning based on partial RDA showed that the three sets of variables (spatio-temporal effects, environmental effects, island factor) that were used to predict multivariate species distributions accounted for 16 % of the overall variance in the species matrix (Table 2). The island factor represented nearly half of this, while the remainder was accounted for by the spatially structured effect of the environment + the island (i.e. autocorrelation within a certain island). The individual effect of spatio-temporal autocorrelation was low but statistically significant while that of the environment alone was not significant.
The matrix of species pair-wise correlations showed that, after Bonferroni correction at α = 0.05, only two pairs of species were significantly (in both cases positively) associated: F. grisea and I. octooculata, and F. grisea and G. racovitzai.
Null model analysis performed on the entire species matrix (7 species, 76 sites, fixed row–fixed column sums) showed that species co-occurred significantly less often than expected by chance. The observed C-score was 174.3 while the 95 % confidence limit of the null distribution was 163–173.2. Pair-wise-based co-occurrence analysis showed that 8 out of 21 species pairs co-occurred more often than expected by chance while 13 out of 21 co-occurred less often. These numbers account for the fact that the overall matrix resulted in a pattern of segregation. Nevertheless, the conservative empirical Bayes approach could solve only 1 out of the 8 positive associations and this was again the collembolans F. grisea and I. octooculata. Of the 13 pairs of species that co-occurred less often than expected by chance, again only 1 unequivocally achieved statistical significance, the mites A. antarcticus and S. villosus.
Discussion
The extreme environmental conditions of Antarctica, which constrain life in terms of water availability, and the patchy distribution of vegetation and resources have been postulated and in some cases shown to be a major structuring force of soil micro-arthropod communities at local scales (Tilbrook 1967; Booth and Usher 1984; Usher and Booth 1986; Convey et al. 2003; Sinclair et al. 2006). The strong limits in dispersal that characterise polar microarthropod species have been considered a fundamental factor structuring species distributions from medium to very broad (i.e. regional) spatial scales. A range of recent studies based on both spatially explicit analyses of species distributions and molecular data have confirmed the fundamental importance of dispersal limitation in this group (Sinclair et al. 2006; Chown and Convey 2007; Convey et al. 2008; McGaughran et al. 2008; Caruso et al. 2010). Overall, these findings suggest that the species sorting meta-community dynamics and perhaps patch dynamics can prevail in the assembly of terrestrial Antarctic communities, which have until now been assumed to be abiotically driven (e.g. Hogg et al. 2006). This would imply that environmental factors should be the major correlate of multivariate species distribution.
Our analyses demonstrate that the community of microarthropods in the moss-turf of four islands in the region of the Argentine islands archipelago depends on spatial and temporal effects that are independent of the measured environmental variables. Indeed, a relatively low but nevertheless significant fraction of variation was accounted for by these effects. At the same time, a much larger amount of variation was accounted for by spatially structured environmental variation, while environment alone could not explain a significant amount of variation. In addition, a relatively large and significant proportion of community variation was accounted for by the factor “island”. This was neither a spatial effect in the sense of dispersal limitation, which is accounted for by the spatio-temporal component based on PCNMs, nor an environmental effect in terms of the environmental variables measured. With reference to the potential importance of other non-measured environmental variables, clearly there are other relevant variables (Booth and Usher 1984). Thus, the island effect could incorporate such unmeasured variables. Given the approach we used to model spatio-temporal patterns, these missing environmental variables are not climatic or meteorological (e.g. winds), for which each island might differ due to their different position in the archipelago. Thus, we propose that more subtle differences in the geological nature of the substratum or other components of the targeted habitat (e.g. moss species composition) are likely to contribute to the island effect.
Alternatively, or complementarily, we cannot exclude stochastic fluctuations in population size due to local, highly context-dependent and thereby unpredictable environmental effects (Chown and Convey 2007). These local demographic stochastic effects, when coupled with dispersal dynamics, might imply neutral dynamics of community structure at larger scales (Hubbell 2001). This would be consistent with the fact that a relatively low but nevertheless significant fraction of variation was accounted for by spatial and temporal patterns that were independent of the measured environmental variables. Overall, the multivariate approach based on variance partitioning showed that both local environmental factors and spatio-temporal processes (e.g. dispersal) independent of these factors contribute to the structure of the micro-arthropod community. However, environmental effects are relatively weak and account for a rather small proportion of data variance. Thus, the fundamental hypothesis that Antarctic terrestrial systems are abiotically driven is not supported by our analyses. Key additional insights are provided by combination of null model analysis coupled with knowledge of the biology of the species under investigation. Based on a range of previous studies (Fitzsimons 1971; Lippert 1971; Booth and Usher 1984; Burn 1984; Usher and Booth 1984, 1986; Lister et al. 1988; Block and Convey 1995), the network of interactions illustrated in Fig. 1 provides a plausible model. This suggests that negative interactions due to competition for shared resources could outweigh multitrophic interactions. The results of our null model analysis on the overall species matrix provide support for this hypothesis, with species co-occurring less often than expected by chance, and hence being segregated spatially. Thus, our study provides the first statistically significant evidence supporting the hypothesis that negative interactions due to competition can be a fundamental structuring force in such communities (Hogg et al. 2006). The fact that the overall matrix resulted in a pattern of segregation does not mean that there cannot also be positive association. For example, the pair-wise null model analysis based on the conservative empirical Bayes approach (Gotelli and Ulrich 2010) detected a positive association between the two collembolans F. grisea and I. octooculata. We interpret the observed pattern as a combination of environmental filtering and competition for resource, which might also involve a third collembolan species, C. antarcticus. In comparison to C. antarcticus, F. grisea can cope better with dry conditions (Usher and Booth 1984; Convey et al. 2003; Hayward et al. 2004). The two species are thought to target different food resources (Fig. 1) and it is therefore unlikely they compete for resources. At the same time, C. antarcticus and I. octooculata feed on algae and differ in terms of several traits of the life cycle (Burn 1984) so that they could spatially segregate due to competition dynamics. Indeed, they did so, but the conservative empirical Bayes test could not support a decisive conclusion on this. Given the observed patterns, we speculate that these two different processes (environmental filtering and competition with respect to a third species) may indirectly cause and reinforce the aggregation of F. grisea and I. octooculata, which could therefore be detected even by a very conservative statistical approach.
Postulated species interactions in a food web as derived from existing studies. Solid grey lines indicate predator–prey or resource–consumer trophic links that have been demonstrated or are considered very probable, while dotted lines represent links postulated with less certainty. Black lines indicate competitive interactions known to take place as they utilise shared resources (as distinct from processes such as physical competition for space)
Overall, our study rejects the widely-held hypothesis that biotic pressures are very unimportant relative to abiotic (physical environmental) drivers in Antarctic terrestrial ecosystems (Convey 1996; Hogg et al. 2006). This adds a novel perspective to the interpretation of dynamics underlying the structure of soil animal communities in the Antarctic region (Hogg et al. 2006; Chown and Convey 2007; Convey et al. 2008).
More generally, the system studied here contains representatives of many of the major arthropod groups and other invertebrates of a typical soil animal community (Wardle 2002; Bardgett 2005). Thus, our conclusions may be relevant to and apply more generally within soil communities, in the contexts both of the dynamics linking taxonomically defined functional groups and of those describing interactions between species within any one of these groups.
The last decade has been characterised by a lively and on-going debate on the relative role of niche and neutral determinants of community structure (e.g. Hubbell 2001; Leibold et al. 2004; Leibold and McPeek 2006; Levine and HilleRisLambers 2009). However, very few studies have addressed this topic in the remarkably diverse and functionally important animal communities of soil systems, while those that are available have focused on narrowly defined taxonomic assemblages (Lindo and Winchester 2009; Caruso et al. 2012). Beyond the specific debate on niche and neutral assembly processes, ecologists have been seeking to define a more unifying framework using the conceptual umbrella of meta-community dynamics (e.g. Leibold et al. 2004). Our analyses give a clear demonstration of how a multitrophic and meta-community perspective applied to model and natural systems such as moss communities (Lindo and Gonzalez 2010) allows us to address patterns of species distribution expected under biotic interaction (e.g. competition). The relatively limited diversity of these systems coupled with the relatively good background knowledge of species ecology allows us to gain more mechanistic insights on the processes that cause community patterns. This is a fundamental if little-explored subject that will in the future allow the unravelling of the complex processes that determine and maintain soil animal biodiversity.
References
Adams BJ, Bardgett RD, Ayres E, Wall DH, Aislabie J, Bamforth S, Bargagli R, Cary C, Cavacini P, Connell L, Convey P, Fell JW, Frati F, Hogg ID, Newsham KK, O’Donnell A, Russell N, Seppelt RD, Stevens MI (2006) Diversity and distribution of Victoria Land biota. Soil Biol Biochem 38:3003–3018
Bardgett RD (2002) Causes and consequences of biological diversity in soil. Zoology 105:367–375
Bardgett RD (2005) The biology of soil. A community and ecosystem approach. Oxford University Press, New York
Barrett JE, Virginia RA, Hopkins DW, Aislabie J, Bargagli R, Bockheim JG, Campbell IB, Lyons WB, Moorhead DL, Nkem JN, Sletten RS, Steltzer H, Wall DH, Wallenstein MD (2006) Terrestrial ecosystem processes of Victoria Land, Antarctica. Soil Biol Biochem 38:3019–3034
Bell T (2010) Experimental tests of the bacterial distance-decay relationship. ISME J 4:1357–1365
Block W, Convey P (1995) The biology, life cycle and ecophysiology of the Antarctic mite Alaskozetes antarcticus. J Zool 236:431–449
Bokhorst S, Huiskes A, Convey P, Aerts R (2007) External nutrient inputs into terrestrial ecosystems of the Falkland Islands and the maritime Antarctic region. Polar Biol 30:1315–1321
Booth RG, Usher MB (1984) Arthropod communities in a maritime Antarctic moss-turf habitat: effects of the physical and chemical environment. J Anim Ecol 53:879–893
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Model 153:51–68
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2004) Dissecting the spatial structure of ecological data at multiple scales. Ecology 85:1826–1832
Bowker MA, Maestre FT, Escolar C (2010) Biological crusts as a model system for examining the biodiversity–ecosystem function relationship in soils. Soil Biol Biochem 42:405–417
Burn AJ (1984) Life cycle strategies in two Antarctic Collembola. Oecologia 64:223–229
Caruso T, Borghini F, Bucci C, Colacevich A, Bargagli R (2007) Modelling local-scale determinants and the probability of microarthropod species occurrence in Antarctic soils. Soil Biol Biochem 39:2949–2956
Caruso T, Hogg ID, Carapelli A, Frati F, Bargagli R (2009) Large-scale spatial patterns in the distribution of Collembola (Hexapoda) species in Antarctic terrestrial ecosystems. J Biogeogr 36:879–886
Caruso T, Hogg ID, Bargagli R (2010) Identifying appropriate sampling and modelling approaches for analysing distributional patterns of Antarctic terrestrial arthropods along the Victoria Land latitudinal gradient. Antarct Sci 22:742–748
Caruso T, Chan Y, Lacap DC, Lau MCY, McKay CP, Pointing SB (2011) Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. ISME J 5:1406–1413
Caruso T, Taormina M, Migliorini M (2012) Relative role of deterministic and stochastic determinants of soil animal community: a spatially explicit analysis of oribatid mites. J Anim Ecol 81:214–221
Chown SL, Convey P (2007) Spatial and temporal variability across life’s hierarchies in the terrestrial Antarctic. Philos Trans R Soc Lond B 362:2307–2331
Convey P (1996) The influence of environmental characteristics on the life history attributes of Antarctic terrestrial biota. Biol Rev 71:191–225
Convey P, Quintana RD (1997) The terrestrial arthropod fauna of Cierva Point SSSI, Danco Coast, northern Antarctic Peninsula. Eur J Soil Biol 33:19–29
Convey P, Smith RIL (1997) The terrestrial arthropod fauna and its habitats in northern Marguerite Bay and Alexander Island, maritime Antarctic. Antarct Sci 9:12–26
Convey P, Block W, Peat HJ (2003) Soil arthropods as indicators of water stress in Antarctic terrestrial habitats? Glob Change Biol 9:1718–1730
Convey P, Gibson JAE, Hillenbrand C-D, Hodgson DA, Pugh PJA, Smellie JL, Stevens MI (2008) Antarctic terrestrial life—challenging the history of the frozen continent? Biol Rev 83:103–117
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182
Davidson M, Broady P (1996) Analysis of gut contents of Gomphiocephalus hodgsoni carpenter (Collembola: Hypogastruridae) at Cape Geology, Antarctica. Polar Biol 16:463–467
Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Model 196:483–493
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345
Ettema CH, Wardle DA (2002) Spatial soil ecology. Trends Ecol Evol 17:177–183
Fitzsimons JM (1971) On the food habits of certain Antarctic arthropods from coastal Victoria Land and adjacent islands. Pacific Insects Monograph 25:121–125
Gotelli NJ (2000) Null model analysis of species co-occurence patterns. Ecology 81:2606–2621
Gotelli N, Ulrich W (2010) The empirical Bayes approach as a tool to identify non-random species associations. Oecologia 162:463–477
Gotelli NJ, Ulrich W (2012) Statistical challenges in null model analysis. Oikos 121:171–180
Gotelli NJ, Graves GR, Rahbek C (2010) Macroecological signals of species interactions in the Danish avifauna. Proc Natl Acad Sci USA 107:5030–5035
Hayward SAL, Worland MR, Convey P, Bale JS (2004) Effects of moisture on the local distribution of the Antarctic Collembola Cryptopygus antarcticus and Friesea grisea. Soil Biol Biochem 36:927–934
Hogg ID, Craig Cary S, Convey P, Newsham KK, O’Donnell AG, Adams BJ, Aislabie J, Frati F, Stevens MI, Wall DH (2006) Biotic interactions in Antarctic terrestrial ecosystems: are they a factor? Soil Biol Biochem 38:3035–3040
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Legendre P, Borcard D, Peres-Neto PR (2005) Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecol Monogr 75:435–450
Leibold MA, McPeek MA (2006) Coexistence of the niche and neutral perspectives in community ecology. Ecology 87:1399–1410
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Levine JM, HilleRisLambers J (2009) The importance of niches for the maintenance of species diversity. Nature 461:254–257
Lindo Z, Gonzalez A (2010) The bryosphere: an integral and influential component of the Earth’s biosphere. Ecosystems 13:612–627
Lindo Z, Winchester N (2009) Spatial and environmental factors contributing to patterns in arboreal and terrestrial oribatid mite diversity across spatial scales. Oecologia 160:817–825
Lippert G (1971) Occurrence of arthropods in mosses at Anvers Island, Antarctic Peninsula. Pacific Insects Monograph 25:137–144
Lister A, Block W, Usher MB (1988) Arthropod predation in an Antarctic terrestrial community. J Anim Ecol 57:957–970
Longton RE (1988) Biology of polar bryophytes and lichens. Cambridge University Press, Cambridge
Maraun M, Erdmann G, Fischer BM, Pollierer MM, Norton RA, Schneider K, Scheu S (2011) Stable isotopes revisited: their use and limits for oribatid mite trophic ecology. Soil Biol Biochem 43:877–882
McGaughran A, Hogg ID, Stevens MI (2008) Patterns of population genetic structure for springtails and mites in southern Victoria Land, Antarctica. Mol Phylogenet Evol 46:606–618
Ofiteru ID, Lunn M, Curtis TP, Wells GF, Criddle CS, Francis CA, Sloan WT (2010) Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci USA 107:15345–15350
Oksanen J, Kindt R, Legendre P, O’Hara RB, Gavin L, Simpson GL, Solymos P, Stevens MH, Wagner H (2009) vegan: Community Ecology Package. R package version 1.15–4. http://CRANR-projectorg/package=vegan
Richard KJ, Convey P, Block W (1994) The terrestrial arthropod fauna of the Byers Peninsula, Livingston Island, South Shetland Islands. Polar Biol 14:371–379
Sinclair BJ, Scott MB, Klok CJ, Terblanche JS, Marshall DJ, Reyers B, Chown SL (2006) Determinants of terrestrial arthropod community composition at Cape Hallett, Antarctica. Antarct Sci 18:303–312
Smith RIL (1972) Vegetation of the South Orkney Islands with special reference to Signy Island. Br Antarct Surv Sci Rep 68:1–124
Smith TW, Lundholm JT (2010) Variation partitioning as a tool to distinguish between niche and neutral processes. Ecography 33:648–655
Stone L, Roberts A (1992) Competitive exclusion, or species aggregation? Oecologia 91(419):424
Tilbrook PJ (1967) Arthropod ecology in the maritime Antarctic. In: Gressitt JL (ed) Entomology of Antarctica. American Geophysical Union, Washington, pp 307–320
Ulrich W (2008) Pairs—a FORTRAN program for studying pairwise species associations in ecological matrices. http://wwwhomeumkpl/~ulrichw/?Research:Software:Pairs
Usher MB, Booth RG (1984) Arthropod communities in a maritime Antarctic moss-turf habitat: three-dimensional distribution of mites and Collembola. J Anim Ecol 53:427–441
Usher MB, Booth RG (1986) Arthropod communities in a maritime Antarctic moss-turf habitat: multiple scales pattern in the mites and Collembola. J Anim Ecol 55:155–170
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princeton University Press, New Jersey
Worland MR, Lukešová A (2000) The effect of feeding on specific soil algae on the cold-hardiness of two Antarctic micro-arthropods. Polar Biol 23:766–774
Zmudczyńska K, Olejniczak I, Zwolicki A, Iliszko L, Convey P, Stempniewicz L (2012) Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biol 35:1233–1245
Acknowledgments
This work is a contribution to the EBESA IPY project n1 452 and was supported by the Italian PNRA (Programma azionale di Ricerche in Antartide). T. Caruso was supported by the Alexander von Humboldt Foundation. P. Convey is a member of the British Antarctic Survey’s Ecosystems Programme. The study also contributes to the SCAR Evolution and Biodiversity in Antarctica programme. The authors thank the Ukrainian National Antarctic Scientific Centre and Taras Shevchenko National University of Kyiv for comprehensive support of this research. We thank one anonymous reviewer and the editor Roland Brandl for their valuable comments on this article. The authors declare no conflict of interest. Experiments comply with the current laws of the country in which experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Rights and permissions
About this article
Cite this article
Caruso, T., Trokhymets, V., Bargagli, R. et al. Biotic interactions as a structuring force in soil communities: evidence from the micro-arthropods of an Antarctic moss model system. Oecologia 172, 495–503 (2013). https://doi.org/10.1007/s00442-012-2503-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2503-9