Abstract
Understanding how biodiversity responds to fine-scale heterogeneity improves our ability to predict larger-scale diversity patterns and informs local-scale conservation practices. This information is important in the design of conservation set-asides in commercial forestry landscapes in the Maputaland-Pondoland-Albany biodiversity hotspot, in South Africa. We assessed how soil arthropod assemblages vary among biotopes with varying degrees of contrast in forestry landscape mosaics in this hotspot. The biotopes included dry and hydromorphic grasslands, indigenous forests and pine plantations. Assemblages were highly segregated among all biotopes for overall arthropods, and for all the feeding guilds, namely predators, herbivores, detritivores, and omnivores. There was a high degree of assemblage dissimilarity between structurally contrasting biotopes (grasslands vs. wooded biotopes) and biotopes that differ in their degree of transformation (natural biotopes vs. plantations). Yet, there was an equally high level of assemblage dissimilarity between dry and hydromorphic grasslands, which are similar in structure and undergo the same disturbance regimes, which emphasises the responsiveness of soil fauna to fine-scale habitat heterogeneity. Different biotopes favoured different feeding guilds and each biotope had species strongly associated with it, highlighting the complementarity of the biotopes. All natural biotopes had relatively high species richness, diversity, and species turnover. The diversity and turnover in pine plantations was as high as in the natural areas, suggesting that plantation conditions may favour certain soil arthropods.
Current delineation of conservation areas in timber estates that prioritize biodiversity-rich dry grasslands, wetlands and indigenous forests, greatly benefit soil arthropods. Approaches that promote a mix of biotopes in conservation areas and having biotopes represented across different landscapes would further maximize local heterogeneity and landscape-scale soil arthropod biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Priority conservation areas are generally identified at the regional scale (Margules and Pressey 2000). However, a high level of environmental heterogeneity can often be present at finer spatial scales (Pincebourde et al. 2016). Understanding how biodiversity responds to fine-scale heterogeneity is important for conservation planning, as it improves our ability to predict diversity patterns at larger spatial scales (Barton et al. 2010). Furthermore, as land management typically operates at finer spatial scales, such information can help inform practical conservation action.
Understanding the influence of finer-scale heterogeneity may be especially important for organisms that are smaller and less mobile, such as soil-dwelling arthropods, whose distribution is often closely related to environmental conditions at local scales (Eckert et al. 2022). Despite being a large and enormously diverse component of terrestrial ecosystems, soil arthropods are poorly known globally and in South Africa (Lavelle et al. 2006; Janion-Scheepers et al. 2016). Yet, they play major roles in ecosystem processes, such as macro-decomposition of litter, maintaining soil aggregation and porosity, and nutrient cycling (Lavelle et al. 2006). As soil arthropods are essential to the functioning of natural and managed ecosystems, they deserve greater attention in conservation planning (Barrios 2007).
In the Maputaland-Pondoland-Albany biodiversity hotspot of South Africa, high rates of habitat loss and degradation due to human activities greatly impact natural areas (CEPF 2010). One of the greatest historical impacts has been from commercial timber production which has transformed and fragmented large areas of primary, natural grasslands, leading to a major loss of biodiversity and impacting ecosystem hydrology through its intensive water usage (Neke and Du Plessis 2004). Mitigation of these impacts is essential, as grassland is the most threatened of all the South African biomes (Cadman et al. 2013). Less than 2% of grassland is formally conserved, and many key habitats are underrepresented in protected areas (Reyers et al. 2001). This makes grassland conservation on private and communally owned land critical in this region.
In the South African forestry sector, conservation areas consisting of remnant natural vegetation are set aside among the plantation compartments to mitigate the impact of plantation forestry on the environment, and to comply with environmental legislation and forestry certification regulations (Samways and Pryke 2015; FSA 2021). In the process of delineating conservation areas, valuable habitats such as species-rich natural grassland, rivers, wetlands, and indigenous forests are prioritized (Cadman et al. 2013). These areas of high natural value are often connected through large-scale conservation corridors, forming landscape scale ecological networks (ENs) that increase structural and functional connectivity throughout these working landscapes (Samways and Pryke 2015). When ENs are well-designed and appropriately managed though alien plant control and appropriate prescribed burning and grazing regimes, they support high levels of historical biodiversity and maintain ecosystem integrity of terrestrial and freshwater systems (Pryke and Samways 2012a; Kietzka et al. 2021). ENs thus contribute greatly to regional conservation in the biodiversity hotspot.
Previous work has emphasised the importance of large-scale structural aspects of the ENs in shaping above-ground arthropod assemblages. For example, corridor width (Pryke and Samways 2012b; Kietzka et al. 2021), connectivity (Theron et al. 2022) and surrounding land-use intensity (Pryke et al. 2022) all play important roles. However, there is growing evidence that smaller scale attributes such as within-corridor structural heterogeneity (Joubert et al. 2016; van Schalkwyk et al. 2021) and habitat diversity (Gaigher et al. 2021) also greatly influence arthropod assemblages. Research on soil arthropods in these systems have been less extensive, although their high level of responsiveness to local restoration (Eckert et al. 2019) and landscape context (Yekwayo et al. 2017) suggest that they are sensitive to environmental influences at different spatial scales.
Here, we assess whether soil arthropod assemblages vary among four dominant biotopes (dry grasslands, hydromorphic grasslands, indigenous forests, and pine plantations) in an EN-plantation landscape mosaic. Our first objective was to assess differences in species richness, diversity, and species turnover of soil arthropods among the biotopes. This allowed us to evaluate whether soil arthropods are negatively impacted by plantation forestry, which has been demonstrated for above-ground fauna in these systems (Pryke and Samways 2012b), likely due to low structural complexity and limited resources in these planted monocultures. It also enabled an assessment of the relative contribution of the different natural biotopes to soil arthropod diversity in the conservation areas. In addition, we compared species richness, diversity, and species turnover for each of the feeding guilds (predators, herbivores, detritivores and omnivores) separately. We expected the contrasting environmental conditions between the natural biotopes to favour different species groups, due to divergent resource needs of different species (Wiescher et al. 2012). Our second objective was to evaluate differences in assemblage composition between the biotopes for the overall assemblage, and for the feeding guilds. Based on previous work highlighting the responses of arthropods to contrasting biotopes (Yekwayo et al. 2017), we expected biotopes with a high degree of structural contrast (open vs. wooded biotopes) and biotopes that differ in their level of transformation (natural biotopes vs. pine plantations) to support highly segregated assemblages, whereas those that are similar in structure and land-use history (dry vs. hydromorphic grasslands) were predicted to support more similar assemblages. We supplemented these findings by determining whether any species are highly characteristic of individual biotope types, which would be a further indication of the uniqueness of assemblages in certain biotopes. To support the interpretation of the arthropod results, we compared the environmental conditions among the biotopes. Taken together, this information would aid in determining how soil arthropods respond to both large- and small-scale environmental differences and is important for guiding conservation planning in these landscapes.
Methods
Study area and design
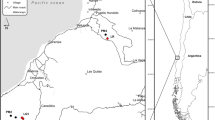
The Maputaland-Pondoland-Albany biodiversity hotspot is located along the southern African east coast. This hotspot is characterized by rich floristic diversity and endemism (Steenkamp et al. 2004). It also has an unusually high level of endemic vegetation types, including several types of grassland, bushveld, and thicket, and contains most of South Africa’s remaining tree-rich indigenous forests (CEPF 2010). Furthermore, the invertebrate fauna in the hotspot is highly diverse, with many charismatic, rare and localized species (Steenkamp et al. 2004). This study was conducted on the commercial plantation estates Good Hope (29.67095 S, 29.9639E) and Mount Shannon (29.68661 S, 29.97861E) (Fig. 1), in the KwaZulu-Natal Midlands of South Africa, which has a temperate climate (Mucina and Rutherford 2006). The estates are approximately 1 km apart, and are combined 6009 ha in size. Selected sites occurred within the elevation range of 1233 and 1599 m a.s.l. and the region lies at the foothills of the Drakensberg mountain range. Plantations comprise two-thirds exotic Pinus patula plantations and one-third unplanted, set aside conservation areas. Distances between sites ranged from 0.14 to 10 km. Minimum and maximum distances between sites within each biotope type can be seen in Table S1. Conservation areas consist of grasslands with small patches of indigenous Afromontane Forest. The grasslands occur on dry and hydromorphic soils, with the latter resulting in seasonally flooded grasslands and perennial wetlands. Ten sites were selected for each of the dominant natural biotope types, namely indigenous forests, dry grasslands, and hydromorphic grasslands. Sites were selected based on site availability and accessibility, and therefore three indigenous forest sites occurred just outside of the plantation boundary at Good Hope. For hydromorphic grasslands we included only seasonally flooded areas, as soil fauna are not easily sampled in perennial wetlands. We also included ten sites in the pine plantations to include a human-modified habitat. All these sites together totalled 40. To distinguish between dry and hydromorphic grasslands, we initially used soil GIS data provided by Mondi Group (www.mondigroup.com), which was then verified in the field by assessing general plant composition (Rountree et al. 2008). Fieldwork was conducted in the summer rainfall season during February and March 2016.
Arthropod sampling
Pitfall trapping was conducted at each site using four 300 ml plastic cups (9.5 cm diameter and 8 cm deep) which were placed in a 2 m2 grid with the rim of the traps flush with the soil surface. We attempted to avoid altered trap catches directly after placement (digging-in effects), by minimizing soil disturbance during pitfall setting (Greenslade 1973). To each trap, we added 50 ml 60% ethylene glycol with two drops of dish washing liquid to break the surface tension. Pitfall traps were immediately activated after placement and were left open for five days. Litter extractions using Winkler bags were used to complement pitfall trapping. Approximately 2 L of leaf litter was collected at each site, which was divided into two mesh bags (22 cm x 30 cm, with a mesh size of 0.5 cm), and were enclosed together within a single Winkler bag. A 130 ml plastic jar was attached to the bottom of the Winkler bag, and contained 50 ml of 70% ethylene glycol, with two drops of detergent. The leaf litter was allowed to dry out for five days, causing the arthropods to move downwards out of the leaf litter and into the collecting jar. After five days, the mesh bags were removed from the Winkler bag, and searched for any remaining arthropods within the leaf litter. To record any additional arthropods that are not easily captured by the other sampling methods, a 1 m2 quadrat was placed at random within a 5 m radius surrounding the pitfall trap area at each site, to actively search for leaf litter and topsoil arthropods. The random selection was done by throwing the quadrat in a random direction, and sampling where the quadrat landed. Active searching involved two people searching for arthropods for 10 min within the quadrat and collecting them with forceps or aspirators. Litter within the quadrat was turned over to detect arthropods under the litter layer. All collected arthropods were preserved in 75% ethanol.
Arthropods were sorted into morphospecies (from here on referred to as ‘species’), counted and identified to family level (Table S2) using relevant literature. We used Dirsh (1965) for Orthoptera, Keep and Ledger (1990) for Trombidiformes, Walker (1991) for Ixodida, Dippenaar-Schoeman and Harvey (2000) for Pseudoscorpiones, Haddad et al. (2006) for Opiliones and Amblypygi, Janion-Scheepers et al. (2015) for Collembola. We also used Picker et al. (2004) and Scholtz and Holm (1985) for Amphipoda, Blattodea, Coleoptera, Dermaptera, Hemiptera, Hymenoptera (Formicidae only), Isopoda, Lithobiomorpha, Oribatida, Orthoptera, Phasmatodea, Polydesmoidea, Scorpiones, Sphaerotheriidae, Spirostreptida and Thysanura. Species were grouped into feeding guilds (predators, herbivores, detritivores, or omnivores) based on the primary feeding mode of family it occurs in. Specimens of the order Araneae were sent to a spider specialist for identification. The collected spider specimens are now kept in the National Collection of Arachnida at the National Museum, Pretoria. All other reference specimens are maintained in the Stellenbosch University Entomological Museum.
Environmental variables
At each site, another 1 m2 quadrat was placed at random within a 5 m radius of the sampling area. Within the quadrat, a soil moisture and pH meter (Kelway, Inc.) and a soil penetrometer (Lang Penetrometer, Inc.) were used to measure soil moisture, pH, and soil compaction. Within the quadrat we also recorded percentage of total vegetation cover, herbaceous, shrub and grass cover, leaf litter cover, bare ground cover, vegetation height, and number of plant morphospecies. This was replicated three times within each site, the averages were used as representation of the site. In a 5 m radius surrounding the quadrat, the percentage shade and dead wood cover were recorded. Slope, elevation and aspect of each site (North or South-facing) were calculated in QGIS (version 2.18.0) (QGIS Development Team 2009). Means and standard errors of recorded variables are given in Table S3.
Statistical analyses
All data analyses were done in R version 3.6.3 (R Core Team 2016). Species accumulation curves were created using the iNEXT package to determine whether arthropod sampling was sufficient (Hsieh et al. 2020) (Fig. S1).
Shannon’s entropy (Hill’s number q1) was used as a measure of diversity and was calculated for the overall arthropod assemblage, as well as the four feeding guilds using the package HillR (Li 2018). To determine the effect of biotope type (indigenous forests, dry grasslands, hydromorphic grasslands, and pine plantations) on overall and functional guild species richness and Shannon’s entropy, linear mixed models (LMMs) and generalised linear mixed models (GLMMs) were performed using the lme4 package (Bates et al. 2014). Models that were non-normal based on Shapiro-Wilk tests (herbivore and omnivore Shannon’s entropy) fitted a gamma distribution. All remaining responses were normally distributed. To account for spatial autocorrelation in the models, sites were categorized into one of four spatial clusters based on farm (Good Hope or Mount Shannon) and latitude (north or south), resulting in a four-level factor called farm cluster (Good Hope North, Good Hope South, Mount Shannon North, and Mount Shannon South). This factor was included as a random variable in all models as it improved spatial autocorrelation, tested with correlograms in the ncf package (Bjørnstad 2020). Abundance-based species turnover, which together with nestedness differences drive changes in beta-diversity, was calculated per biotope for the overall assemblage and the four feeding guilds using the betapart package (Baselga et al. 2012). Analysis was conducted using Bray-Curtis similarity to include abundance, and species turnover was the balanced variation component of Bray-Curtis dissimilarity between pairs sites within the same biotope and sampled 55 times (the number of unique site combinations). To assess how species turnover differs between biotopes, linear models (LMs) were conducted with biotope type as the fixed parameter and farm cluster as random variable. Where significant effects occurred, Tukey post-hoc tests were conducted using the multcomp package (Hothorn et al. 2008) for species richness, Shannon’s entropy, and species turnover.
To determine the effect of biotope type on overall and functional guild assemblage composition, multivariate generalized linear models (manyGLMs) were performed using negative binomial distribution of abundance data with the mvabund package (Wang et al. 2012). Biotope type and farm cluster were included as a fixed parameter in each model. Pairwise models between each biotope were also calculated, where each biotope is defined as a character. Latent variable and residual variable models were made using the boral package (Hui 2016) for unconstrained ordination and visualizing differences in assemblage structures.
The indicspecies package was used (De Cáceres and Legendre 2009) to assess whether any species were highly associated with any of the biotopes. Multi-level pattern analysis was used to calculate an indicator value for each species with each biotope, which is based on species fidelity and specificity to each biotope.
Results
A total of 9 404 individuals were sampled, consisting of 24 different arthropod orders, 76 arthropod families and 204 species across all biotopes (Table S2). Omnivores were the most abundant feeding guild with 5200 individuals from 53 species, while predators were the most speciose guild with 1594 individuals from 85 species. We also sampled 2107 herbivore individuals from 46 species and 503 detritivore individuals from 20 species. The species accumulation curve indicated that the number of species were lowest in the pine plantations, but similar in the other biotopes (Fig. S1). Predators were abundant in the dry grasslands, while detritivores had higher species richness in the hydromorphic grasslands and indigenous forests, but higher abundances in the dry grasslands and pine plantations. Herbivores were very abundant and rich in the indigenous forests, while omnivores were abundant and rich in the dry grasslands (Figure S1).
Species richness had a significant response to biotopes for all species and each of the functional feeding groups (Table 1; Fig. S1). Overall species richness was significantly higher in dry grasslands than in pine plantations, whereas species richness in hydromorphic grasslands and indigenous forests did not differ from each other or from dry grasslands and pine plantations (Fig. 2, Fig. S2). Herbivore and omnivore species richness in dry grasslands was significantly richer in species than the indigenous forests and pine plantations, and omnivore species richness in dry grasslands was also higher than in hydromorphic grasslands (Fig. S2). Predators and detritivores had significantly more species in indigenous forests compared to the other biotopes, which did not differ from each other (Fig. 2, Fig. S2). For Shannon’s entropy, predators and detritivores were the only groups to show a response to biotope (Table 1). Shannon’s entropy of predators was significantly higher in the hydromorphic grasslands compared to pine plantations, while Shannon’s entropy for detritivores was significantly higher in the indigenous forests compared to both dry grasslands and pine plantations (Fig. S2). Species turnover was significantly different between biotopes for the overall and for each of the functional feeding guilds (Table 1). Overall, the highest species turnover was in the hydromorphic grasslands and lowest in the indigenous forests (Fig. 2). Predator species turnover was highest in the hydromorphic grasslands compared to the other biotopes. Detritivore species turnover was highest in indigenous forests and lowest in pine plantations. Herbivore and omnivore turnover was lower in indigenous forests than in the other biotopes (Fig. S2).
Pairwise test for the effects of biotope on overall number of species, Shannon’s entropy (Hill’s number = q1) and species turnover (calculated as the Bray-Curtis balanced variation). DryGrass = dry grasslands, HydroGrass = hydromorphic grasslands, IndFor = indigenous forests and Pine = pine plantations, while different letters above the medians represent significantly different pairs (p < 0.05)
Furthermore, we found a significant effect of biotope type on the overall assemblage composition (Table 2). All biotopes differed significantly from each other regarding overall assemblage composition (Table S4). Regarding the functional guilds, we also found a significant effect of biotope type on all feeding guilds (Table 2). The predator, detritivore and omnivore assemblage composition differed significantly across all biotope types (Table S4). Herbivore assemblage composition differed significantly across all biotopes, except between the dry and hydromorphic grasslands (Table S4).
The Indicspecies analyses showed that indigenous forests were the type of biotope with the most species (14 species), from a mixture of taxonomic groups and feeding guilds (Table S5). The dry grasslands had 12 species, mainly omnivores and predators, while hydromorphic grasslands had nine species, mainly predators, with some herbivores and detritivores (Table S5). Pine plantations had seven species associated with them, which was the lowest number of associated species and dominated by omnivores, two detritivores and a predator (Table S5).
Environmental variables also showed high variation between the biotope types (Table S3). Across all sites, high levels of variation occurred for variables such as site elevation and soil moisture, along with litter, vegetation, shade, herbaceous and grass cover sites (Table S3). As expected, the open habitats (dry and hydromorphic grasslands) had no shade cover compared to the wooded habitats (indigenous forests and plantations), although indigenous forests had greater mean shade cover compared to the pine plantations (Table S3). Variation in site elevation was greater for dry grasslands and indigenous forests, while showing less variation in the hydromorphic grasslands and pine plantations (Table S3). The mean vegetation cover was far greater within the open habitats compared to the wooded habitats (Table S3). Leaf litter cover was highest within the pine plantations, followed by indigenous forests, hydromorphic grasslands, and dry grasslands (Table S3). Hydromorphic grasslands had greater soil moisture, followed by indigenous forests, pine plantations and dry grasslands (Table S3).
Discussion
We found that assemblage composition was highly segregated among all biotope types for overall soil arthropods, as well as for the functional guilds. In addition, different functional guilds had affinities to different biotopes, and many species had high biotope fidelity. This dissimilarity in composition was expected where biotopes had a high level of structural contrast, i.e., open habitats (dry and hydromorphic grasslands) vs. wooded habitats (natural forests and plantations). Soil-dwelling arthropod assemblages can be strongly influenced by canopy cover (Yekwayo et al. 2017), which influences microhabitat conditions through its effect on temperature, humidity, soil moisture, light levels, and composition of the litter layer (Andersen 2019). Here, we recorded more canopy cover, shadier conditions, and greater amounts of leaf litter in the wooded biotopes (Table S3) which likely played important roles in structuring the assemblages.
The large differences we observed in assemblages between the natural habitats and pine plantations were also predicted, as the plantations are monocultures of exotic trees, which contrast starkly with the exceptionally high compositional diversity of both grasslands and indigenous forests. This is reflected in the low plant diversity and plant cover recorded in the pine plantations (Table S3). These highly altered conditions in plantations compared to the natural areas generally lead to divergent arthropod assemblages for above-ground and epigaeic fauna (Pryke and Samways 2012b). However, the segregation between the assemblages of dry and hydromorphic grasslands was almost as pronounced as between the other biotope combinations, even though they are superficially similar in structure and undergo similar disturbance regimes. This may have been due to variations in less obvious attributes like plant composition, soil moisture, and soil compaction (Table S3). Although seasonal wetlands share many attributes with terrestrial ecosystems, they can support terrestrial arthropods that do not occur in dry grasslands or other upland habitats (Spitzer and Danks 2006). The arthropods in these wet habitats are often uniquely adapted to avoid or tolerate inundation (Batzer and Wu 2020). In drier periods, hydromorphic soils also retain considerable moisture, which maintains a rich ground-dwelling fauna, and wetland plants that support characteristic, host-specific herbivorous arthropods (Batzer and Wu 2020). Although the dry and hydromorphic grasslands may appear to be very similar, small-scale habitat difference may translate into great differences in the associated soil fauna.
Our findings contribute to a growing body of research showing that subtle variations in habitat type can have distinct effects on arthropods. For example, small variations in vegetation structure can largely determine distribution of butterfly species, even in low-growing vegetation types such as heathland, dune vegetation, and grassland (de Vries et al. 2021). In grassy woodland in Australia, co-occurring and closely related trees in the same subgenus support highly distinct beetle assemblages (Barton et al. 2010). Furthermore, in other Afromontane grassland systems, beetle assemblages closely track subtle variations in vegetation composition that are only detectable with in-depth plant surveys (Foord et al. 2003). Considering the responsiveness of arthropods to these variations, incorporating small-scale heterogeneity into conservation planning may therefore greatly complement coarse-scale approaches to arthropod conservation (Hunter et al. 2017).
Overall species richness, diversity, and species turnover were relatively similar among all the biotopes, but there were greater differences when we considered the feeding guilds separately. The assemblage differences appear to be driven partly by different feeding guilds being favoured by different biotopes. For example, detritivores were well-supported by indigenous forests, omnivores were favoured by dry grasslands, and hydromorphic grasslands had high predator diversity and turnover. The different environmental conditions and resources in different biotopes likely met the requirements of different arthropod groups. For example, indigenous forest soils were moist with low compaction, high amounts of dead wood and shady conditions. These conditions generally promote detritivores in forest systems (Attignon et al. 2004). Similarly, omnivores, which were largely represented by ants in this study, likely benefitted from the open, sun-exposed conditions with high amounts of bare ground in the dry grasslands (Anderson 2019). Furthermore, spiders dominated the predator guild, and benefit from structurally and compositionally diverse vegetation (Midega et al. 2008). High plant cover, a variety of plant growth forms and tall vegetation in the hydromorphic grasslands likely promoted these predators. These patterns suggest that different ecological functions may be represented in varying degrees in the different biotopes. This further emphasises the complementarity of the natural biotopes.
Species turnover was also relatively high in most biotopes. The high species replacement among patches of the same biotope indicates high cumulative value of having representative patches of each biotope across different conservation areas and plantation estates (Riva and Fahrig 2022). The exception was indigenous forests, which had relatively low species turnover, mostly due to low turnover in herbivore and omnivore guilds among forest sites. A possible reason for this pattern may be that environmental conditions in the sheltered forest understory are more consistent among forest sites than in open grassland sites which experience greater environmental fluctuation (Anderson 2019). Forests are also not subjected to the same disturbances as grasslands, where grazing and fire create heterogeneous conditions across grassland sites. The lower species turnover among forests sites compared to open sites may thus reflect lower between-site heterogeneity. In addition, it is possible that our sampling of the forest floor only partially captured species turnover patterns, as arthropod species turnover in indigenous forests can vary depending on the vertical height considered (Xing et al. 2022).
Importantly, the pine plantations supported fairly high arthropod diversity and turnover, even though resource diversity and niche opportunities were expected to be low in the plantations. Indeed, plantations in this region are generally inhospitable to many arthropod taxa found in the natural areas (Pryke and Samways 2012b) and are perceived as structural barriers to mobile groups such as butterflies (Pryke and Samways 2001). It is possible that conditions in plantations promote certain soil-dwelling arthropods. For example, the plantations in this study had much deeper plant litter and a higher amount of dead wood than the other biotopes (Table S3), which generally promote arthropods in wooded habitats (Sandström et al., 2019). This would have important implications for continued functioning of the soil ecosystem in the production areas (Barrios 2007). However, more in-depth taxonomic and functional work is needed to determine the full effect of afforestation on the soil ecosystem.
Conservation implications
Our results highlight that the current landscape planning approach which prioritizes high conservation value habitats when delineating set-aside areas, greatly benefits soil arthropods by supporting species-rich and diverse assemblages. The high complementarity among habitat types, even among ones that vary in subtle ways, suggests that having a mix of biotopes together would help maintain heterogeneity in soil arthropod assemblages and their trophic functions within the conservation areas. Furthermore, high species turnover among sites indicates that representation of each biotope across different conservation areas in the landscape would maximize soil arthropod biodiversity at the landscape scale. By incorporating these principles into the conservation planning of forestry landscapes, we can promote the unseen soil biota that depend on these valuable conservation areas. Improving conservation in these working landscapes can make a highly meaningful contribution to conservation in this biodiversity hotspot, where timber production is a widespread activity.
Bayesian ordination and regression analysis of multivariate abundance data, showing latent variables 1 and 2. Illustrated is the pure latent biplot as well as the residual biplot with biotope type taken into account for (a) overall, (b) detritivore, (c) herbivore, (d) omnivore and (e) predator assemblages
References
Andersen AN (2019) Responses of ant communities to disturbance: five principles for understanding the disturbance dynamics of a globally dominant faunal group. J Anim Ecol 88:350–362. https://doi.org/10.1111/1365-2656.12907
Attignon SE, Weibel D, Lachat T, Sinsin B, Nagel P, Peveling R (2004) Leaf litter breakdown in natural and plantation forests of the Lama forest reserve in Benin. Appl Soil Ecol 27:109–124. https://doi.org/10.1016/j.apsoil.2004.05.003
Barrios E (2007) Soil biota, ecosystem services and land productivity. Ecol Econ 64:269–285. https://doi.org/10.1016/j.ecolecon.2007.03.004
Barton PS, Manning AD, Gibb H, Lindenmayer DB, Cunningham SA (2010) Fine-scale heterogeneity in beetle assemblages under co-occurring Eucalyptus in the same subgenus. J Biogeogr 37:1927–1937. https://doi.org/10.1111/j.1365-2699.2010.02349.x
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods in Ecol Evol 3:808–812. https://doi.org/10.1111/j.2041-210X.2012.00224.x
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 1:1–23. https://doi.org/10.48550/arXiv.1406.5823
Batzer D, Wu H (2020) Ecology of Terrestrial Arthropods in Freshwater Wetlands. Annu Rev Entomol 65:101–119. https://doi.org/10.1146/annurev-ento-011019-024902
Bjornstad ON (2020) ncf: Spatial covariance functions. R package version 1.2- 9. https://cran.r-project.org/web/packages/ncf/ncf.pdf. Accessed June 2022
Cadman M, deVilliers C, Lechmere-Oertel R, McCulloch D (2013) Grasslands ecosystem guidelines: Landscape interpretation for planners and managers. South African National Biodiversity Institute, Pretoria
CEPF (Critical Ecosystem Partnership Fund) (2010) Ecosystem profile. Maputaland-Pondoland-Albany biodiversity hotspot. Conservation International Southern African Hotspots Programme, Cape Town
De Caceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. https://doi.org/10.1890/08-1823.1
de Vries JPR, Koma Z, WallisDeVries MF, Kissling WD (2021) Identifying fine-scale habitat preferences of threatened butterflies using airborne laser scanning. Divers Distrib 27:1251–1264. https://doi.org/10.1111/ddi.13272
Dippenaar-Schoeman AS, Harvey MS (2000) A check list of the pseudoscorpions of South Africa (Arachnida: Pseudoscorpiones). Koedoe 43:89–102. https://doi.org/10.4102/koedoe.v43i2.201
Dirsh VM (1965) The african genera of Acridoidea. Cambridge University Press, London
Eckert M, Gaigher R, Pryke JS, Samways MJ (2019) Rapid recovery of soil arthropod assemblages after exotic plantation tree removal from hydromorphic soils in a grassland-timber production mosaic. Restor Ecol 27:1357–1368. https://doi.org/10.1111/rec.12991
Eckert M, Gaigher R, Pryke JS, Samways MJ (2022) Conservation of complementary habitat types and small-scale spatial heterogeneity enhance soil arthropod diversity. J Environ Manage 317:115482. https://doi.org/10.1016/j.jenvman.2022.115482
Foord SH, Ferguson JWH, van Jaarsveld AS (2003) Coleopteran assemblages in afromontane grasslands reflect fine-scale variation in vegetation. Environ Entomol 32:797–806. https://doi.org/10.1603/0046-225x-32.4.797
Forestry South Africa (2021) Environmental Guidelines for Commercial Forestry Plantations in South Africa. Available at: https://www.forestrysouthafrica.co.za/wp-content/uploads/2021/06/ENVIRONMENTAL-GUIDELINES-2021-June-email.pdf (accessed December 2022)
Gaigher R, Pryke JS, Samways MJ (2021) Habitat complementarity and butterfly traits are essential considerations when mitigating the effects of exotic plantation forestry. Biodivers Conserv 30:4089–4109. https://doi.org/10.1007/s10531-021-02293-6
Greenslade PJM (1973) Sampling ants with pitfall traps: digging in effects. Insectes Soc 20:343–353. https://doi.org/10.1007/BF02226087
Haddad CR, Dippenaar-Schoeman AS, Wesolowska W (2006) A checklist of the non-acarine arachnids (Chelicerata: Arachnida) of the Ndumo Game Reserve, Maputaland, South Africa. Koedoe 49:1–22. https://doi.org/10.4102/koedoe.v49i2.116
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hsieh TC, Ma KH, Chao A(2020) iNEXT: Interpolation and extrapolation for species diversity. R package version 2.0.20. https://cran.r-project.org/web/packages/iNEXT/iNEXT.pdf. Accessed June 2022
Hui FK (2016) Boral. Bayesian ordination and regression analysis of multivariate abundance data in R. Methods Ecol Evol 7:744–750. https://doi.org/10.1111/2041-210X.12514
Hunter ML, Acuña V, Bauer DM, Bell KP, Calhoun AJK, Felipe-Lucia MR et al (2017) Conserving small natural features with large ecological roles: a synthetic overview. Biol Conserv 211:88–95. https://doi.org/10.1016/j.biocon.2016.12.020
Janion-Scheepers C, Deharveng L, Bedos A, Chown S (2015) Updated list of Collembola species currently recorded from South Africa. ZooKeys 503:55–88. https://doi.org/10.3897/zookeys.503.8966
Janion-Scheepers C, Measey JJ, Steven B, Louise LC, Jonathan C, Joanna C et al (2016) Soil biota in a megadiverse country: current knowledge and future research directions in South Africa. Pedobiologia 59:129–174. https://doi.org/10.1016/j.pedobi.2016.03.004
Joubert L, Pryke JS, Samways MJ (2016) Positive effects of burning and cattle grazing on grasshopper diversity. Insect Conserv Divers 9:290–301. https://doi.org/10.1111/icad.12166
Keep ME, Ledger JA (1990) Lice (Phthiraptera), fleas (Siphonaptera) and mites (Trombidiformes, Sarcoptiformes and Mesostigmata) recorded from the larger game species in Natal. Lammergeyer 41:23–29
Kietzka GJ, Pryke JS, Samways MJ (2021) Webs of well-designed conservation corridors maintain river ecosystem integrity and biodiversity in plantation mosaics. Biol Conserv 254:108965. https://doi.org/10.1016/j.biocon.2021.108965
Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, Bureau F et al (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42:S3–S15. https://doi.org/10.1016/j.ejsobi.2006.10.002
Li D (2018) hillR: taxonomic, functional, and phylogenetic diversity and similarity through Hill numbers. J Open Source Softw 3:1041. https://doi.org/10.21105/joss.01041
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:243–253. https://doi.org/10.1038/35012251
Midega CAO, Khan ZR, Van den Berg J, Ogol CKPO, Dippenaar-Schoeman AS, Pickett JA, Wadhams LJ (2008) Response of ground-dwelling arthropods to a “push-pull” habitat management system: spiders as an indicator group. J Appl Entomol 132:248–254. https://doi.org/10.1111/j.1439-0418.2007.01260.x
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria
Neke K, Du Plessis M (2004) The threat of transformation: quantifying the vulnerability of grasslands in South Africa. Conserv Biol 18:466–477. https://doi.org/10.1111/j.1523-1739.2004.00157.x
Picker M, Griffiths C, Weaving A (2004) Field guide to insects of South Africa. Struik Nature, Cape Town
Pincebourde S, Murdock CC, Vickers M, Sears MW (2016) Fine-scale microclimatic variation can shape the responses of organisms to global change in both natural and urban environments. Integr Comp Biol 56:45–61. https://doi.org/10.1093/icb/icw016
Pryke JS, Roets F, Samways MJ (2022) Large african herbivore diversity is essential in transformed landscapes for conserving dung beetle diversity. J Appl Ecol 59:1372–1382. https://doi.org/10.1111/1365-2664.14152
Pryke JS, Samways MJ (2012a) Ecological networks act as extensions of protected areas for arthropod biodiversity conservation. J Appl Ecol 49:591–600. https://doi.org/10.1111/j.1365-2664.2012.02142.x
Pryke JS, Samways MJ (2012b) Conservation management of complex natural forest and plantation edge effects. Landsc Ecol 27:73–85. https://doi.org/10.1007/s10980-011-9668-1
Pryke SR, Samways MJ (2001) Width of grassland linkages for the conservation of butterflies in south african afforested areas. Biol Conserv 101:85–96. https://doi.org/10.1016/S0006-3207(01)00042-8
QGIS Development Team (2009) QGIS Geographic Information System. Open Source Geospatial Foundation. http://qgis.osgeo.org. Accessed March 2016
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org. Accessed March 2016
Reyers B, Fairbanks DHK, van Jaarsveld AS, Thompson M (2001) Priority areas for the conservation of south african vegetation: a coarse-filter approach. Divers Distrib 7:79–95. https://doi.org/10.1046/j.1472-4642.2001.00098.x
Riva F, Fahrig L (2022) The disproportionately high value of small patches for biodiversity conservation. Conserv Lett 15:e12881. https://doi.org/10.1111/conl.12881
Rountree M, Batchelor L, MacKenzie J, Hoare D (2008) Updated manual for the identification and delineation of wetlands and riparian areas. Department of Water Affairs and Forestry, Pretoria
Samways MJ, Pryke JS (2015) Large-scale ecological networks do work in an ecologically complex biodiversity hotspot. Ambio 45:161–172. https://doi.org/10.1007/s13280-015-0697-x
Sandström J, Bernes C, Junninen K, Lõhmus A, Macdonald E, Müller J, Jonsson BG (2019) Impacts of dead wood manipulation on the biodiversity of temperate and boreal forests. A systematic review. J Appl Ecol 56:1770–1781. https://doi.org/10.1111/1365-2664.13395
Scholtz CH, Holm E (1985) Insects of Southern Africa. Butterworths, Cape Town
Spitzer K, Danks HV (2006) Insect biodiversity of boreal peat bogs. Annu Rev Entomol 51:137–161. https://doi.org/10.1146/annurev.ento.51.110104.151036
Steenkamp Y, Van Wyk B, Victor J, Hoare D, Smith G, Dold T et al (2004) Maputaland-Pondoland-Albany. In: Mittermeier RA, Gill PR, Hoffman M, Pilgrim JD, Brooks TM, Mittermeier CG et al (eds) Hotspots revisited: Earth’s biologically richest and most endangered ecoregions. Monterrey, Mexico, pp 219–228
Theron KJ, Pryke JS, Samways MJ (2022) Maintaining functional connectivity in grassland corridors between plantation forests promotes high-quality habitat and conserves range restricted grasshoppers. Landsc Ecol 37:2081–2097. https://doi.org/10.1007/s10980-022-01471-3
van Schalkwyk J, Gaigher R, Pryke JS, Samways MJ (2021) Corridor heterogeneity is more important than corridor design for maintaining butterfly functional and taxonomic diversity. J Appl Ecol 58:2734–2746. https://doi.org/10.1111/1365-2664.14006
Walker JB (1991) A review of the ixodid ticks (Acari: Ixodidae) occurring in southern Africa. Onderstepoort J Vet Res 58:81–105. http://hdl.handle.net/2263/41388
Wang YI, Naumann U, Wright ST, Warton DI (2012) Mvabund – an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474. https://doi.org/10.1111/j.2041-210X.2012.00190.x
Wiescher PT, Pearce-Duvet JMC, Feener DH (2012) Assembling an ant community: species functional traits reflect environmental filtering. Oecologia 169:1063–1074. https://doi.org/10.1007/s00442-012-2262-7
Yekwayo I, Pryke JS, Roets F, Samways MJ (2017) Responses of ground living arthropods to landscape contrast and context in a forest-grassland mosaic. Biodivers Conserv 26:631–651. https://doi.org/10.1007/s10531-016-1262-z
Xing S, Hood ASC, Dial RJ, Fayle TM (2022) Species turnover in ant assemblages is greater horizontally than vertically in the world’s tallest tropical forest. Ecol Evol 12:e9158. https://doi.org/10.1002/ece3.9158
Acknowledgements
For assistance in the field, we thank J. Theron and S. Doarsamy. We also thank C. Deacon and A. Dippenaar-Schoeman for assisting with the identification of specimens. We thank Mondi Group for financial and technical support, and permitting sampling on their property. We also thank Ezemvelo KZN Wildlife for granting sampling permits and the support staff at the Department of Conservation Ecology and Entomology of Stellenbosch University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. M.E. collected data for the study. M.E. and J.S.P. performed statistical analyses. M.E., R.G. and J.S.P. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eckert, M., Gaigher, R., Pryke, J. et al. Soil arthropod assemblages reflect both coarse- and fine-scale differences among biotopes in a biodiversity hotspot. J Insect Conserv 27, 155–166 (2023). https://doi.org/10.1007/s10841-022-00449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-022-00449-5