Abstract
Antipredator behaviour is an important fitness component in most animals. A co-evolutionary history between predator and prey is important for prey to respond adaptively to predation threats. When non-native predator species invade new areas, native prey may not recognise them or may lack effective antipredator defences. However, responses to novel predators can be facilitated by chemical cues from the predators’ diet. The red swamp crayfish Procambarus clarkii is a widespread invasive predator in the Southwest of the Iberian Peninsula, where it preys upon native anuran tadpoles. In a laboratory experiment we studied behavioural antipredator defences (alterations in activity level and spatial avoidance of predator) of nine anurans in response to P. clarkii chemical cues, and compared them with the defences towards a native predator, the larval dragonfly Aeshna sp. To investigate how chemical cues from consumed conspecifics shape the responses, we raised tadpoles with either a tadpole-fed or starved crayfish, or dragonfly larva, or in the absence of a predator. Five species significantly altered their behaviour in the presence of crayfish, and this was largely mediated by chemical cues from consumed conspecifics. In the presence of dragonflies, most species exhibited behavioural defences and often these did not require the presence of cues from predation events. Responding to cues from consumed conspecifics seems to be a critical factor in facilitating certain behavioural responses to novel exotic predators. This finding can be useful for predicting antipredator responses to invasive predators and help directing conservation efforts to the species at highest risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behaviour plays a decisive role in shaping the outcome of predator–prey interactions (Lima and Dill 1990; Lima 1998; Ferrari et al. 2010). Predation is an important selective force acting on the behaviour of prey species and, in order to minimise predation risk, many organisms have evolved a variety of predator-avoidance behavioural defences (reviews in Lima and Dill 1990; Kats and Dill 1998). A decrease in activity level is one of the most common and effective behavioural antipredator responses that reduces vulnerability to predation (Lima and Dill 1990; Kats and Dill 1998). Spatial avoidance of predators also acts as an antipredator defence by reducing the rate of predator–prey encounters and, consequently, predation risk (Laurila et al. 1997; Relyea 2001; Nicieza et al. 2006). However, these behavioural shifts often incur costs to animals due to reduced resource acquisition, which can alter growth, development and life-history patterns (Werner and Anholt 1996; Lima 1998; but see Steiner 2007). Therefore, there should be strong selection on prey to recognise dangerous predators, to accurately determine predation risk and to adjust antipredator responses accordingly.
Invasive predators are a worldwide threat to biodiversity (Blackburn et al. 2010). Freshwater ecosystems are amongst the most invaded and are particularly vulnerable to introduced predators (Lodge et al. 1998; Cox and Lima 2006). For instance, many amphibian population declines have been associated with the introduction of exotic aquatic predators (review in Kats and Ferrer 2003). When predators invade areas outside their historical geographic range, native prey species are likely to suffer heavy predation due to the lack of a common evolutionary history with the exotic predator (Cox and Lima 2006; Gall and Mathis 2010). This is because the evolutionary naïveté of prey may either cause a failure to recognise and respond to novel predation threats, or result in inappropriate or ineffective antipredator defences (Cox and Lima 2006; Strauss et al. 2006; Sih et al. 2010). The degree of naïveté can depend on the similarity of the invader to native predators, since phylogenetic relatedness is often associated with ecological similarity (Cox and Lima 2006; Sih et al. 2010). For instance, distantly related aquatic predator species are likely to have dissimilar chemical signatures. Hence, a novel predator with no related species in the native community may pose a higher threat, as it is less likely to be recognised as dangerous by native prey (Strauss et al. 2006; Gall and Mathis 2010; Sih et al. 2010). Several studies have reported a lack of native prey responses to aquatic invasive predators, which may cause profound changes in the invaded ecosystems (e.g. Gamradt and Kats 1996; Knapp 2005). A classic example is the introduction of the Nile perch (Lates niloticus) in Lake Victoria, which caused severe declines in native cichlids, probably due to lack of predator recognition (Witte et al. 1992).
In aquatic ecosystems, predator chemical cues are particularly important for prey in assessing predation risk (Kats and Dill 1998). The chemicals to which prey respond may be predator-specific odours, cues that are actively or passively released by injured or consumed conspecifics or, more frequently, a combination of both (Chivers and Smith 1998; Schoeppner and Relyea 2009; Fraker et al. 2009; Hettyey et al. 2010). Several studies have shown that fed predators commonly elicit strong antipredator defences, while starved predators often do not (Stirling 1995; Slusarczk 1999; Schoeppner and Relyea 2005, 2009; but see Petranka and Hayes 1998; Van Buskirk and Arioli 2002). As a predator may become chemically ‘labelled’ by its diet, recognition of a novel predator can be facilitated if diet chemical cues are associated with it (reviewed in Ferrari et al. 2010). However, studies on prey responses to invasive predators generally do not consider the role of chemical cues originating from consumed conspecifics and their potential importance for enabling predator recognition (but see Marquis et al. 2004).
Freshwater crayfishes, which have been widely introduced outside their native range, can have major impacts on native prey species and cause severe population declines (Hobbs et al. 1989; Lodge et al. 1998; Cox and Lima 2006; Larson and Olden 2010). In the southwest Iberian Peninsula, most freshwater habitats have been invaded by Procambarus clarkii (red swamp crayfish), an exotic crayfish endemic to northeastern Mexico and south-central USA (Hobbs et al. 1989; Gherardi 2006). This crayfish was introduced in Spain in 1973, and by the 1990s it was already abundant in southwestern Portugal, an area previously devoid of freshwater crayfishes or functionally similar species (Habsburgo-Lorena 1983; Almaça 1991). This area holds a remarkable endemic anuran community; out of nine species, three are endemic to the Iberian Peninsula and three others have a restricted distribution outside Portugal and Spain (Gasc et al. 1997). P. clarkii readily preys upon eggs and larvae of all these anurans (Cruz and Rebelo 2005). Due to its high fecundity and rapid growth, it can quickly build extremely large populations, especially in fish-free habitats, and become a serious threat to amphibian populations (Gherardi 2006; Larson and Olden 2010).
The ability of anuran tadpoles to detect and respond to P. clarkii is largely unknown, despite the importance of this information for understanding the extent to which native amphibians are able to cope with this invasive predator. In this study, our main goal was to understand if anuran tadpoles from a community in the southwest Iberian Peninsula that was invaded by P. clarkii approximately 25 years ago are able to exhibit behavioural defences in the presence of chemical cues from this crayfish. For this, we performed a laboratory experiment in which we assessed changes in tadpole activity level and spatial avoidance of the predator in the presence of chemical cues from the invasive crayfish, and compared them with those elicited in the presence of a common native predator, the larval dragonfly Aeshna sp. We also investigated the role of chemical cues from consumed conspecifics by comparing behavioural responses in the presence of starved and conspecific-fed predators. A previous study has shown that many of our study species may change their behaviour under direct predation risk by P. clarkii (Cruz and Rebelo 2005), but no study has compared these responses to those elicited in the presence of a native predator, nor investigated the role of predator chemical cues or cues from predated conspecifics. Finally, we evaluated how these behavioural responses change over larval development.
The anuran community in southwestern Portugal consists of nine species: Iberian water frog (Pelophylax perezi), Mediterranean tree frog (Hyla meridionalis), European tree frog (Hyla arborea), common toad (Bufo bufo), natterjack toad (Bufo calamita), Iberian parsley frog (Pelodytes ibericus), Western spadefoot toad (Pelobates cultripes), Iberian painted frog (Discoglossus galganoi) and Iberian midwife toad (Alytes cisternasii). Except the two Bufo species, these species (or very closely related species) show behavioural plasticity in the presence of caged aeshnid dragonflies (Van Buskirk 2002; Nicieza et al. 2006; Richter-Boix et al. 2007; Gomez-Mestre and Díaz-Paniagua 2011), and we expect them to show behavioural responses in the presence of this native predator. Behavioural responses to aeshnids have not been studied for P. cultripes; we predict that behavioural defences may be unnecessary, at least late in development, because the large body size of these tadpoles acts as an important antipredator mechanism (Tejedo 1993). Concerning responses to the exotic crayfish, we predict most of the species to not detect and respond to this novel predator; however, chemical cues from consumed conspecifics may elicit responses. Cruz and Rebelo (2005) have shown that these anuran species differ in vulnerability to predation by P. clarkii, the two Bufo species, P. cultripes and D. galganoi being the most susceptible species. This may indicate that these species lack appropriate defences towards this crayfish. Finally, we predict antipredator defences to be strongest in the beginning of tadpole development because prey vulnerability and responses to predators decrease as prey size increases (e.g. Eklöv and Werner 2000; Hettyey et al. 2010).
Materials and methods
Study species and its maintenance
This study was conducted in the surroundings of the Sado River basin (southwest Portugal), an area that was colonised by P. clarkii in the 1980s (R. Rebelo pers. obs.). In this area, P. clarkii can be found in all types of water bodies (e.g. streams, temporary ponds), including small and shallow ones (Cruz and Rebelo 2007). Aeshna larvae are voracious native predators of tadpoles, widely abundant in water bodies throughout this area. All anuran species included in this study co-occur with both these predators.
The experiment took place in the laboratory of the Centro de Biologia Ambiental field station (Grândola, southwest Portugal, 38°06.482′N, 8°34.140′W), from 15th December 2007 to 15th November 2008. This extended experimental period was due to differences in breeding phenology of the study species. Several egg masses of each species were collected from ponds located in the Alentejo region, southwest Portugal (Table 1). In A. cisternasii, males show parental care until tadpoles become free-swimming, and the tadpoles used in the experiment had been very recently released in the ponds. All the ponds had established crayfish populations, with evidence of crayfish reproduction (juveniles or brooding females). Clutches were kept in several species-specific 5 L aquaria filled with spring water from the field station, until tadpoles reached Gosner stage 25 (operculum closure over gills; Gosner 1960). Larvae were fed commercial fish food and boiled lettuce ad libitum every 2 days. Throughout the experiment, water was changed every 5 days. Water temperature was 18.0 ± 0.17 °C (mean ± SE) and the photoperiod was 12L:12D.
Predators were collected from local streams or ponds. Adult crayfish were captured using baited funnel traps and late instar dragonfly larvae using dip-nets. Predators were transferred to the laboratory and kept either in 40 L aquaria (crayfish) or individually in 1.2 L plastic boxes (dragonflies). We fed crayfish with commercial fish food and small invertebrates, and dragonflies with tadpoles and Ephemeroptera larvae.
Experimental setup
We performed a factorial experiment using the nine anuran species and five predator treatments, each combination being replicated once in each of five spatial blocks. This resulted in a total of 225 experimental units, each consisting of a plastic tank (39 × 28 × 28 cm) filled with 10 L of water. At the start of the experiment (day 0), ten tadpoles at developmental stage 25 and a predator cage were added to each tank. The five predator treatments were: fed crayfish, fed dragonfly, starved crayfish, starved dragonfly and control (no predator). We used both fed and starved predator treatments in order to investigate whether behavioural responses towards the exotic predator are facilitated by the presence of chemical cues emitted by consumed conspecifics. The predators were placed in the cages 1 day after the tadpoles. A single predator was placed in a cylindrical opaque cage, tethered at the surface in the middle of the container. The sides of the cage were covered with fine mesh netting, allowing chemical cues to flow out of the cage. Only chemical predator cues (no visual or tactile cues) were available for the tadpoles. Crayfish and dragonfly cages had a diameter of 85 and 62 mm, respectively. In the control treatments, we randomly used empty predator cages from one of the two sizes. While inside the predator cage in each experimental container, predators from the ‘fed’ treatments were fed three focal species tadpoles every other day. Starved predators were not fed during the experiment. Prior to entering the experiment, all predators were starved for at least 48 hours. All the predators were replaced every 2 weeks so that the starved predators could be fed.
Response variables and statistical analyses
We recorded tadpole behaviour (activity level and spatial avoidance) at the beginning (time period 1), middle (time period 2) and close to the end (time period 3) of larval development. As the length of larval period differs among different species, the dates of behavioural observations—as well as head/body length and Gosner developmental stage (Gosner 1960) at each date—also varied (Table 2). On each observation day, tadpole activity and spatial avoidance were estimated by counting the number of active tadpoles and the number of tadpoles close to the predator cage in each tank at five repeated occasions between 8 a.m. and 12 p.m., separated by at least 30 minutes. A tadpole was considered active when it was actively swimming (either slowly or with fast bursts of speed), feeding (even if not substantially altering position) or simply undulating its tail (without actively swimming). Tadpoles were considered to be close to the predator cage if they were either in physical contact with the cage or not more than 1 cm away from it.
When making comparisons across taxa, there is a need to examine whether the focal phenotypic traits are correlated with the phylogenetic history of the focal species. Since nine different anuran species were used in this study, we followed Abouheif’s (1999) recommendation of testing for phylogenetic trait independence prior to analysing our data. If no significant phylogenetic autocorrelation is detected among species (failure to reject the null hypothesis of phylogenetic independence), phylogenetically comparative methods do not have to be used and conventional statistical analyses can be applied (Abouheif 1999). To test for the assumption of phylogenetic independence within our data set, we first calculated the extent of predator-induced behavioural plasticity for each species and predator treatment separately. This was estimated as the proportional change in either activity level or spatial avoidance in each of the four predator environments relative to the no predator environment [e.g. (behaviour in fed dragonfly presence—behaviour in predator absence)/behaviour in predator absence; Richardson (2001); Van Buskirk (2002). Using these values, a Test For Serial Independence (TFSI) was performed for each treatment, using the program “Phylogenetic Independence, Version 2.0” (Abouheif 1999; Reeve and Abouheif 2003). The phylogenetic tree used was constructed based on Duarte et al. (2012) (Online Resource 1). Phylogenetic autocorrelation was calculated in the form of a C statistic and topology of the original data was randomised 10,000 times to estimate the null hypothesis.
Since we did not find a significant phylogenetic autocorrelation for behavioural plasticity either in activity level (C ≤ 0.177, P ≥ 0.22) or spatial avoidance (C ≤ 0.353, P ≥ 0.09) in any of the predator treatments, subsequent statistical analyses were not phylogenetically corrected. To examine differences in species behavioural responses to the exotic and native predators over their development, we used a Generalised Linear Mixed Model (GLMM) with a Binomial error distribution and a logit link function. As behaviour was recorded in three different time periods, the tank (i.e., the experimental container) was considered a random factor in the model to restrict the independence of tanks in the different time observations. For the GLMM, the proportion of active tadpoles or tadpoles close to the predator cage per container, estimated as the number of tadpoles active/close to the predator cage divided by their total number in a tank, was used as a response variable. This value was the average of the five measurements recorded per container in each time period. The analyses were performed at the community level, using all nine species. They were followed by multiple comparisons, in which the responses of each species in each time period were compared. The specific comparisons used were chosen in order to answer the following questions: (1) which species are responding to the native and/or to the invasive predators (control treatment vs. all others); (2) do cues from consumed conspecifics play a role in this (fed vs. starved treatments); and (3) how similar is the response to the two predators (fed dragonfly vs. fed crayfish and starved dragonfly vs. starved crayfish).
In order to understand if species responding more strongly to the native predator also respond more to the exotic predator and if these responses are mediated by cues from consumed conspecifics, Pearson correlations between predator-induced behavioural plasticity in the two starved and in the two fed predator treatments were calculated (see above for calculations of behavioural plasticity). We used data from time period 1, as this was the period when the strongest responses were observed. If species antipredator responses are mainly mediated by consumed conspecifics cues, we expect to find a positive correlation between the two fed predator treatments and no correlation between the two starved treatments. Alternatively, if species require the presence of predator-specific cues to respond, we expect a strong response to the dragonfly and a weak or no response to the crayfish (due to the lack of recognition of crayfish kairomones), resulting in either a negative or no correlation between the two fed and the two starved treatments. To investigate which amount of predator-induced behavioural plasticity in the fed treatments was due to the presence of cues from consumed conspecifics (hereafter plasticity due to CC cues) we estimated, for each predator and species, the proportional change in activity level or spatial avoidance in the fed predator treatment relative to the starved predator treatment [(behaviour in presence of fed predator—behaviour in presence of starved predator)/behaviour in presence of starved predator]. We then performed a correlation between the plasticity due to the CC cues in the presence of dragonfly and crayfish. If all plasticity is attributed to the CC cues, plasticity should be the same in the presence of the two predators and a near-perfect correlation should be observed, since the number of tadpoles fed to the two different predators was equal. All the analyses were performed using IBM SPSS statistics 20.
Results
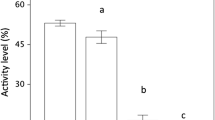
Time period was an important factor in determining activity level among the nine anuran species (Table 3). In general, species increased their overall activity with time, and D. galganoi was the only species that decreased activity over time (Fig. 1). A significant interaction between time period, species and predator treatment was found, indicating species-specific complex alterations in tadpoles’ behavioural responses to different predator regimes throughout their development (Table 3). The multiple comparisons between different predator treatments performed for each species showed that dragonfly presence (together with predated conspecifics) strongly affected tadpole activity level, with activity decreasing in all species except P. perezi, B. calamita and H. arborea. In most species, this was observed in all time periods (Table 4; Fig. 1). For P. cultripes, an initial response of reduced activity to fed dragonfly later disappeared, while for P. perezi a higher proportion of active tadpoles was observed in the starved dragonfly treatment early in ontogeny (Table 4; Fig. 1). B. bufo was the only species showing a continuous and strong response to both dragonfly (fed and unfed) and crayfish (fed) predators, although this response was stronger in the presence of the native predator (Table 4; Fig. 1). An initial significant response to the fed crayfish and a later response to the starved dragonfly arose in A. cisternasii, while in D. galganoi these responses appeared later in development and were stronger in the fed treatments. Both hylid species were less active in the presence of the crayfish predator late in their development, either when the predator was fed (H. meridionalis) or unfed (H. arborea) (Table 4; Fig. 1). For B. calamita, no differences in activity level were detected between any predator treatments.
Proportion of active tadpoles (mean ± SE) of the nine anuran species a Alytes cisternasii, b Bufo bufo, c Discoglossus galganoi, d Hyla meridionalis, e Hyla arborea, f Pelobates cultripes, g Pelophylax perezi, h Pelodytes ibericus and i Bufo calamita) in presence of five predator treatments during three different time periods. Plots of different species, top to bottom, are organised from the most to the least responsive species. Note different y axis scales in the graphs due to species variable baseline activity levels
The overall response across species in predator spatial avoidance did not vary significantly across time periods (Table 3; Fig. 2). Still, we again found a significant interaction between time period, species and predator treatment, indicating among-species differences in antipredator responses through ontogeny (Table 3; Fig. 2). For all species, significant responses of spatial avoidance consisted of moving away from the predator cage. D. galganoi, B. bufo and H. meridionalis strongly avoided the cage with either fed or unfed dragonfly predators, while H. arborea and P. ibericus avoided the predator cage only in the presence of the fed dragonfly (Table 4; Fig. 2). Tadpoles of A. cisternasii showed continuous and strong avoidance behaviour to both the native and the exotic predators (both fed and starved) throughout development. This was the only species significantly avoiding the predator cage in the crayfish treatment (Table 4; Fig. 2). Tadpoles of P. cultripes, P. perezi and B. calamita did not show spatial avoidance of the predator cages in the presence of any predator (Table 4; Fig. 2).
No correlation was detected between species-level plasticity in activity level in the starved dragonfly and starved crayfish treatments (r = 0.269, N = 9, P = 0.484; Fig. 3a). However, there was a significant positive correlation between species plasticity in the fed dragonfly and fed crayfish treatments (r = 0.717, N = 9, P = 0.03; Fig. 3b). A positive correlation was also found between the two predator treatments for plasticity in activity attributed to the CC cues (r = 0.699, N = 9, P = 0.036; Fig. 3c), suggesting that the correlation between plasticity in the two fed treatments was due to among-species variation in plasticity to CC cues. Concerning behavioural plasticity in spatial avoidance of the predator, no significant correlations were found between fed (r = 0.317, N = 9, P = 0.406) or starved treatments (r = 0.09, N = 9, P = 0.889), or for plasticity to the CC cues (r = 0.039, N = 9, P = 0.920).
Relationship between behavioural plasticity in activity level of the nine anuran species in the presence of dragonfly and crayfish, in time period 1 (solid black line): a plasticity in presence of the starved predators: for each predator axis data points represent [(activity with starved predator present—activity with predator absent)/activity with predator absent]; b plasticity in presence of the fed predators: for each predator axis data points represent [(activity with fed predator present—activity with predator absent)/activity with predator absent]; c plasticity attributed to the consumed conspecifics chemical cues: for each predator axis data points represent [(activity with fed predator present—activity with starved predator present)/activity with starved predator present]. The dashed line represents the expected relationship if species respond to cues exactly in the same way in the dragonfly and crayfish treatments. Plasticity values below zero indicate a decrease in activity in the presence of predators and positive plasticity values reflect an increase in activity with predators present. Ac stands for Alytes cisternasii, Bb for Bufo bufo, Dg for Discoglossus galganoi, Hm for Hyla meridionalis, Ha for Hyla arborea, Pc for Pelobates cultripes, Pp for Pelophylax perezi, Pi for Pelodytes ibericus and Bc for Bufo calamita
Discussion
This study provides the first community-wide assessment of antipredator behaviour of anuran larvae in response to chemical cues of the invasive crayfish P. clarkii, the most cosmopolitan crayfish species in the world (Gherardi 2006). Although the introduction of P. clarkii into our study area is relatively recent, we found that five out of nine prey species responded behaviourally to this predator. As expected, these responses were greatly mediated by chemical cues resulting from predation events.
Behavioural responses to the native and introduced predators
In the presence of dragonfly, five species had a strong behavioural response (A. cisternasii, D. galganoi, B. bufo, H. meridionalis and P. ibericus), three had a moderate response (P. cultripes, H. arborea and P. perezi), while one species had no significant response (B. calamita). In general, activity levels were greatly reduced and strong spatial avoidance of the predator was observed in the presence of the native predator. These are common and usually effective antipredator responses (reviews in Lima and Dill 1990; Kats and Dill 1998), often reported for larval amphibians exposed to odonate predators (e.g. Werner and Anholt 1996; Relyea 2001; Richardson 2001; Nicieza et al. 2006). The strong responses observed here indicate that, as expected, most of these anuran species do have the potential of exhibiting behavioural strategies to avoid predation. The significant behavioural response of B. bufo to the dragonfly was somewhat unexpected, since most studies report a lack of behavioural antipredator responses in this species (e.g. Laurila et al. 1997; Richter-Boix et al. 2007). However, although bufonids are known to rely on chemical toxic deterrents as defences against predators, several invertebrate predators (including P. clarkii) are resistant to them, which may cause tadpoles to use alternative defences (Semlitsch and Gavasso 1992; Cruz and Rebelo 2005). Laurila et al. (1998) also reported a weak decrease in B. bufo activity in the presence of a dragonfly.
We found that five tadpole species (A. cisternasii, B. bufo, D. galganoi, H. meridionalis and H. arborea) significantly altered activity level and/or showed spatial avoidance in the presence of P. clarkii. Although these responses were generally weaker than the ones elicited towards the native predator, this indicates that the ability to respond to the invasive crayfish is quite pervasive in this amphibian community. These results are consistent with previous studies demonstrating that amphibians alter their behaviour in response to chemical cues of non-native predators, such as P. clarkii (e.g. Pearl et al. 2003; Marquis et al. 2004; Gall and Mathis 2010). Pearl et al. (2003) found different responses in two native anurans to cues from an introduced predator which, together with our study, show that species from the same community may commonly respond differently to exotic predators.
Differences in the ability of prey species to respond behaviourally to the crayfish could have arisen due to several aspects of species ecology, such as their habitat use or body size (and associated swimming abilities). Anuran species face different selective environments along the pond permanency gradient, ranging from permanent lakes and ponds to ephemeral pools (Wellborn et al. 1996). Since permanent water bodies have a higher predator abundance and diversity than ephemeral ones, species using the former habitats (P. perezi, H. arborea, B. bufo and A. cisternasii in our case) are likely to have more generalised (as opposed to predator-specific) antipredator defences that allow responses to a wide array of predators, likely facilitating responses to novel invasive predators (Richter-Boix et al. 2007; Smith and Awan 2009; Gómez and Kehr 2011). Indeed, in this study, three of the four species typically inhabiting permanent water bodies responded to the exotic predator (A. cisternasii, B. bufo and H. arborea). Nonetheless, P. perezi, also a permanent pond species, did not respond to the crayfish, indicating that the hydroperiod gradient does not explain all the differences in species antipredator responses. Alternatively, prey that have small body size and relatively weak swimming ability, such as the bufonids and D. galganoi, should elicit antipredator defences in the presence of many different predators. Two of these species (the exception being B. calamita) indeed responded to the crayfish predator. However, species with larger body size and good swimming ability, such as the hylids, also responded to the exotic crayfish. The evolutionary history of a species can also be important in defining differences in species antipredator responses (Richardson 2001); however, we did not find any phylogenetic autocorrelations in the behavioural responses of these species, indicating that evolutionary history does not seem to play an important role here (see also Richter-Boix et al. 2007). On the whole, since none of the previously mentioned factors entirely explains differences in behavioural responses to the exotic predator, it is possible that all these factors interact to define the final species-specific responses.
The strong behavioural responses by B. bufo and D. galganoi towards P. clarkii are surprising, considering that an earlier study found that these species were the most vulnerable to P. clarkii’s predation (Cruz and Rebelo 2005). This might indicate that in Cruz and Rebelo’s study, tadpoles did not have enough time to develop behavioural defences against the crayfish (it only lasted 48 hours) or, alternatively, that the behavioural responses shown here may be a non-effective response towards this exotic predator. For example, because crayfish rely much more on chemical rather than on visual cues to detect prey, decreased activity in response to crayfish may render inactive tadpoles to a risk equal to that experienced by active tadpoles (Aquiloni et al. 2005).
The importance of chemical cues from consumed conspecifics
In this study, most species significantly changed behaviour in the presence of fed predators, suggesting that cues from consumed conspecifics play a very important role in inducing anuran behavioural responses. However, six out of nine species also altered behaviour when exposed to chemical cues from the starved dragonfly, indicating that predator-specific odours from a native predator are, in many cases, sufficient to launch behavioural defences.
In the three species that responded strongly to the exotic predator (A. cisternasii, B. bufo and D. galganoi), behavioural plasticity (considering activity level) to the fed crayfish was mostly explained by plasticity to the CC cues (cues from consumed conspecifics), indicating that the response to the exotic predator was mediated by cues resulting from predation events. These results are in accordance with those by Marquis et al. (2004), who suggested the presence of conspecific alarm cues to be crucial in chemical detection of predators by B. bufo. Injured tadpoles of this species are known to release alarm cues—chemicals released by specialised cells in the skin—that induce antipredator responses, such as a reduction in activity, in conspecifics (Chivers and Smith 1998; Hagman 2008; Fraker et al. 2009). Responding to cues from injured conspecifics is frequent among anuran species (Chivers and Smith 1998; Hagman 2008; Ferrari et al. 2010) and may also be the case for A. cisternasii and D. galganoi (S. Amaral, M.J. Cruz and R. Rebelo, unpublished data). Responding to broad and general alarm cues seems to be a critical factor in allowing tadpoles to elicit behavioural defences to novel invasive predators, and makes common evolutionary history between predator and prey unnecessary for responses to be elicited (Sih et al. 2010).
A. cisternasii and H. arborea showed a significant behavioural response in the presence of the starved crayfish. This indicates that these species can launch defences towards the exotic crayfish even when only information about the predator identity is present, which likely reflects the detection of a threat. This may indicate a simple reaction to an unknown chemical stimulus, but it can also indicate predator recognition since, given enough time, native species may develop the ability to recognise and respond to cues from novel invasive predators (Strauss et al. 2006). This can be highly beneficial because it would allow species to respond to novel predators even when predation events have not yet occurred (Kats and Dill 1998; Schoeppner and Relyea 2005).
Behavioural responses across ontogeny
As predicted, anuran species showed different antipredator responses to predator chemical cues over ontogeny. All the species responding to the native predator elicited behavioural defences in the first developmental period, indicating an early development of antipredator behaviour, which is in accordance with other studies (e.g. Petranka and Hayes 1998; Laurila et al. 2004). However, not all these species elicited defences in time period 3; at times, the antipredator response disappeared as the tadpoles grew larger. This indicates that, as tadpole size increased, vulnerability to predation probably decreased, reducing the importance of behavioural defences (e.g. Eklöv and Werner 2000; Hettyey et al. 2010). Alternatively, it may also indicate that tadpoles altered their defensive strategies over ontogeny, shifting from behavioural to morphological defences later in development (Relyea 2003). In the case of P. cultripes, overall activity level greatly increased at the same time as the antipredator response disappeared, suggesting that in this case large body size and high activity levels are linked with decreased behavioural responses towards predators. On the other hand, since predation risk did not increase with time in this experiment, we cannot exclude the possibility that, in some of the species, tadpoles might have stopped responding due to habituation (Magurran 1990).
Behavioural plasticity correlations
We found a positive correlation between predator-induced behavioural plasticity in activity level in the presence of the fed native and exotic predators. Since there was no relationship between plasticity in the two starved treatments, the former correlation was probably a result of the correlation between plasticity to the CC cues (cues from consumed conspecifics) in the two predator treatments. This reinforces the idea that the antipredator responses were greatly mediated by chemical signals resulting from predation events upon conspecifics. Further, it suggests that the magnitude of antipredator responses to native predators, when greatly mediated by CC cues, can be a good proxy for predicting the potential antipredator responses to novel predators in amphibians and possibly even in other species. Still, the correlation between plasticity to the CC cues in the two predator treatments was not close to perfect, as expected if all the observed plasticity was due to the CC cues. Instead, species plasticity to the CC cues was stronger in the presence of dragonfly than in the presence of crayfish, indicating that additional information from the predator contributed to an increased plastic response when the dragonfly was present. Once again, there seems to be a difference in species responses depending on the length of coexistence with the predator.
The fact that we found no significant correlations between predator treatments for plasticity in predator spatial avoidance was probably due to many species not altering this trait in predator presence and to the wide variation in responses often observed. This suggests that plasticity in this trait is less widespread among species from this community than plasticity in activity level. Alternatively, it may also indicate that, for some species, chemical cues are not enough to induce alterations in this behavioural trait, maybe because it implies more risk or is more costly.
Conclusions
Since P. clarkii arrived in southwest Portugal in the 1980s, larval anurans have been exposed to high predation pressure imposed by this exotic predator (Cruz and Rebelo 2005). Our experiment showed that five out of nine anuran species present in this area exhibited behavioural responses to this novel predator. We suggest that these species, provided the behavioural responses are effective, will be better able to persist with the continued expansion and establishment of this introduced predator. However, since failure to recognise an exotic species as a predator is probably one of the most damaging forms of prey naiveté (Cox and Lima 2006), the remaining four species—unless eliciting alternative defences—will be highly vulnerable, which may lead to population declines or even local extinctions. Since one of these species, P. ibericus, is an Iberian endemic with a very limited distribution area (Loureiro et al. 2008), serious conservation problems may arise in the near future. Evaluating which species rely on chemical cues from predation events in their responses to predators may be a useful tool for understanding the potential of native prey species to respond to invasive alien predators and help direct conservation efforts to the most susceptible species.
References
Abouheif E (1999) A method to test the assumption of phylogenetic independence in comparative data. Evol Ecol Res 1:895–909
Almaça C (1991) L’ecrevisse a pieds blancs, Astacus pallipes Lereboullet 1858, au Portugal. L’Astaciculteur de France 28:11–16
Aquiloni L, Ilhéu M, Gherardi F (2005) Habitat use and dispersal of the invasive crayfish Procambarus clarkii in ephemeral water bodies of Portugal. Mar Freshw Behav Physiol 38:225–236
Blackburn TM, Pettorelli N, Katzner T, Gompper ME, Mock K, Garner TWJ, Altwegg R, Redpath S, Gordon IJ (2010) Dying for conservation: eradicating invasive alien species in the face of opposition. Anim Conserv 13:227–228
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5:338–352
Cox JG, Lima SL (2006) Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680
Cruz MJ, Rebelo R (2005) Vulnerability of southwest Iberian amphibians to an introduced crayfish, Procambarus clarkii. Amphibia-Reptilia 26:293–303
Cruz MJ, Rebelo R (2007) Colonization of freshwater habitats by an introduced crayfish, Procambarus clarkii, in Southwest Iberian Peninsula. Hydrobiologia 575:191–201
Duarte H, Tejedo M, Katzenberger M, Marangoni F, Baldo D, Beltrán JF, Martí DA, Richter-Boix A, Gonzalez-Voyer A (2012) Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Global Change Biol 18:412–421
Eklöv P, Werner EE (2000) Multiple predator effects on size-dependent behavior and mortality of two species of anuran larvae. Oikos 88:250–258
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Fraker M, Hu E, Cuddapah V, McCollum S, Relyea R, Hempel H, Denver R (2009) Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm Behav 55:520–529
Gall BG, Mathis A (2010) Innate predator recognition and the problem of introduced trout. Ethology 116:47–58
Gamradt SC, Kats LB (1996) Effect of introduced crayfish and mosquitofish on California newts. Conserv Biol 10:1155–1162
Gasc JP, Cabela A, Crnobrnja-Isailovic J, Dolmen D, Grossenbacher K, Haffner P, Lescure J, Martens H, Martínez Rica JP, Maurin H, Oliveira ME, Sofianidou TS, Veith M, Zuiderwijk A (1997) Atlas of amphibians and reptiles in Europe, Collection Patrimoines Naturels, 29. SPN/IEGB/MNHN, Paris 496 pp
Gherardi F (2006) Crayfish invading Europe: the case study of Procambarus clarkii. Mar Freshw Behav Physiol 39:175–191
Gómez VI, Kehr AI (2011) Morphological and developmental responses of anuran larvae (Physalaemus albonotatus) to chemical cues from the predators Moenkhausia dichoroura (Characiformes: Characidae) and Belostoma elongatum (Hemiptera: Belostomatidae). Z. Zool Stud 50:203–210
Gomez-Mestre I, Díaz-Paniagua C (2011) Invasive predatory crayfish do not trigger inducible defences in tadpoles. Proc Royal Soc Lond B 278:3364–3370
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Habsburgo-Lorena AS (1983) Socioeconomic aspects of the crawfish industry in Spain. Freshw Crayfish 5:552–554
Hagman M (2008) Behavioral responses by tadpoles of six Australian species to chemical cues from other tadpoles. Herpetol Conserv Biol 3:239–246
Hettyey A, Zsarnóczai S, Vincze K, Hoi H, Laurila A (2010) Interactions between the information content of different chemical cues affect induced defences in tadpoles. Oikos 119:1814–1822
Hobbs HH, Jass JP, Huner JV (1989) A review of global crayfish introductions with particular emphasis on two North-American species (Decapoda, Cambaridae). Crustaceana 56:299–316
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers Distrib 9:99–110
Knapp RA (2005) Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biol Conserv 121:265–279
Larson ER, Olden JD (2010) Latent extinction and invasion risk of crayfishes in the southeastern United States. Conserv Biol 24:1099–1110
Laurila A, Kujasalo J, Ranta E (1997) Different antipredator behaviour in two anuran tadpoles: effects of predator diet. Behav Ecol and Sociobiol 40:329–336
Laurila A, Kujasalo J, Ranta E (1998) Predator-induced changes in life-history in two anuran tadpoles: effects of predator diet. Oikos 83:307–317
Laurila A, Järvi-Laturi M, Pakkasmaa S, Merilä J (2004) Temporal variation in predation risk: stage-dependency, graded responses and fitness costs in tadpole antipredator defences. Oikos 107:90–99
Lima SL (1998) Nonlethal effects in the ecology of predator–prey interactions: what are the ecological effects of anti-predator decision-making? Bioscience 48:25–34
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lodge DM, Stein RA, Brown KM, Covich AP, Bronmark C, Garvey JE, Klosiewski SP (1998) Predicting impact of freshwater exotic species on native biodiversity: challenges in spatial scaling. Aust J Ecol 23:53–67
Loureiro A, Ferrand de Almeida N, Carretero MA, Paulo OS (eds) (2008) Atlas dos Anfíbios e Répteis de Portugal. Instituto da Conservação da Natureza e da Biodiversidade, Lisboa 257 pp
Magurran AE (1990) The adaptive significance of schooling as an anti-predator defence in fish. Ann Zool Fennici 27:3–18
Marquis O, Saglio P, Neveu A (2004) Effects of predators and conspecific chemical cues on the swimming activity of Rana temporaria and Bufo bufo tadpoles. Arch Hydrobiol 160:153–170
Nicieza AG, Álvarez DA, Atienza EMS (2006) Delayed effects of larval predation risk and food quality on anuran juvenile performance. J Evol Biol 19:1092–1103
Pearl CA, Adams MJ, Schuytema GS, Nebeker AV (2003) Behavioral responses of anuran larvae to chemical cues of native and introduced predators in the Pacific Northwestern United States. J Herpetol 37:572–576
Petranka JW, Hayes LJ (1998) Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. B. Behav Ecol Sociobiol 42:263–271
Reeve J, Abouheif E (2003) Phylogenetic Independence. Version 2.0. Computer Program
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2003) Predators come and predators go: the reversibility of predator-induced traits. Ecology 84:1840–1848
Richardson JML (2001) A comparative study of activity levels in larval anurans and response to the presence of different predators. Behav Ecol 12:51–58
Richter-Boix A, Llorente GA, Montori A (2007) A comparative study of predator-induced phenotype in tadpoles across a pond permanency gradient. Hydrobiologia 583:43–56
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Schoeppner NM, Relyea RA (2009) Interpreting the smells of predation: how alarm cues and kairomones induce different prey defences. Funct Ecol 23:1114–1121
Semlitsch RD, Gavasso S (1992) Behavioural responses of Bufo bufo and Bufo calamita tadpoles to chemical cues of vertebrate and invertebrate predators. Ethol Ecol Evol 4:165–173
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naiveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Slusarczk M (1999) Predator-induced diapause in Daphnia magna may require two chemical cues. Oecologia 119:159–165
Smith GR, Awan AR (2009) The roles of predator identity and group size in the antipredator responses of American toad (Bufo americanus) and bullfrog (Rana catesbeiana) tadpoles. Behaviour 146:225–243
Steiner UK (2007) Investment in defense and cost of predator-induced defense along a resource gradient. Oecologia 152:201–210
Stirling G (1995) Daphnia behavior as a bioassay of fish presence or predation. Funct Ecol 9:778–784
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:354–371
Tejedo M (1993) Size-dependent vulnerability and behavioral responses of tadpoles of two anuran species to beetle larvae predators. Herpetologica 49:287–294
Van Buskirk J (2002) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am Nat 160:87–102
Van Buskirk J, Arioli M (2002) Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology 83:1580–1585
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Werner EE, Anholt BR (1996) Predator-induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology 77:157–169
Witte F, Goldschmidt T, Wanink J, Van Oijen M, Goudswaard K, Witte-Maas E, Bouton N (1992) The destruction of an endemic species flock: quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environ Biol Fishes 34:1–28
Acknowledgments
We thank Pedro Andrade, Erika Almeida, Susana Alves and Cátia Guerreiro for their invaluable help in the field and experimental work, Hélder Duarte for providing data on species phylogeny and Jesús Díaz-Rodríguez for providing an insight on the Pelodytes taxonomy. Permits were provided by the Portuguese Instituto da Conservação da Natureza e da Biodiversidade (ICNB). This research was funded by the FCT Project POCI/BIA-BDE/56100/2004, FCT grant SFRH/BD/29068/2006 and by Stiftelsen för Zoologisk Forskning.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nunes, A.L., Richter-Boix, A., Laurila, A. et al. Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia 171, 115–127 (2013). https://doi.org/10.1007/s00442-012-2389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2389-6