Abstract
Invasive species are a regional and global threat to biological diversity. In order to evaluate an invasive predator species’ potential to harm populations of native prey species, it is critical to evaluate the behavioral responses of all life stages of the native prey species to the novel predator. The invasion of the African clawed frog (Xenopus laevis) into southern California provides an opportunity to evaluate the predation risk and behavioral responses of native amphibians. We performed predation trials and explored prey behavioral responses to determine how this invasive predator may impact native amphibian populations using Pacific chorus frogs (Pseudacris regilla) as a representative native California prey species. We found that X. laevis will readily prey upon larval and adult life stages of P. regilla. Behavior trials indicated that both larval and adult P. regilla exhibit prey response behaviors and will spatially avoid the novel invasive predator. The results suggest that native anurans may have a redundant predator response in both the larval and adult life stages, which could reduce the predatory impact of X. laevis but also drive emigration of native amphibians from invaded habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive species threaten biodiversity worldwide and reduce or even eliminate populations of native species (Mack et al. 2000; Bellard et al. 2016). Amphibians, in particular, have suffered severe population losses from invasive species owing to disease transmission, competition, habitat alterations, and direct predation (Kats and Ferrer 2003; Bucciarelli et al. 2014). Although many prey taxa have evolved the capacity to detect and respond to the presence of their natural predators to reduce predation risk, naïve native prey species may lack the ability to recognize or effectively respond to introduced predatory species (Sih et al. 2010). A native species may not respond appropriately, particularly if the exotic predator is not closely related to the animal’s native predators (Ferrari et al. 2010). Novel invasive predatory species such as introduced mammalian predators in New Zealand (Innes et al. 2010; Goldson et al. 2015) and introduced snakes in Guam (Fritts and Rodda 1998; Wiles et al. 2003) can cause precipitous loss of native species. Similarly, native amphibians in parts of the western United States have declined or disappeared owing to the introduction of predatory bullfrogs and fish species (Knapp and Matthews 2000; Adams and Pearl 2007).
Although studies have explored the responses of anurans to a suite of invasive species to determine if the native species respond to novel predators (e.g. (Gall and Mathis 2010; Nunes et al. 2012; Pease and Wayne 2013), these studies have focused on a single anuran life stage, either the adult or larval stage. But in each life stage the species may be adapted to detect and evade different predators. As a consequence, it is important to include both larval and adult anuran life stages when evaluating the impact of an invasive species because one life stage may be more vulnerable to predation or less able to respond to the novel predator than the other.
The African clawed frog (Xenopus laevis) invasion into southern California (Crayon 2005) offers an opportunity to investigate the responses of native anurans to a novel predatory species through their life cycle. As a generalist predator that preys on its own eggs and larvae (McCoid and Fritts 1980; Measey and Tinsley 1998) X. laevis adults could potentially attack and prey upon native amphibian eggs, larvae and adults. Larval X. laevis, on the other hand, are not considered a predatory threat to native amphibians because they are obligatory filter feeders (Seale 1982) and were therefore not included in this study. Native to sub-Saharan Africa (Tinsley et al. 1996), the invasion of X. laevis into southern California has coincided with declines in native amphibians (Mahrdt and Knefler 1972; McCoid and Fritts 1980); however, it is not clear if X. laevis predation was a factor in these declines (Crayon 2005).
Of the dietary studies involving invasive X. laevis populations (McCoid and Fritts 1980; Measey 1998; Lobos and Measey 2002; Faraone et al. 2008; Lillo et al. 2011), only one study has shown that X. laevis will consume native amphibians (Amaral and Rebelo 2012). Few of these studies, however, reported the presence of native amphibians in the ponds where X. laevis were collected (McCoid and Fritts 1980; Measey 1998; Lillo et al. 2011). One study in Italy showed that several amphibian species (Hyla intermedia, Pelophylax esculentus, Discoglossus pictus), but not all species (Bufo bufo), stopped reproduction in ponds after X. laevis had established (Lillo et al. 2011). This suggests that native anurans that are susceptible to predation either emigrate from invaded waters to avoid predation or are quickly consumed, resulting in local extirpation.
Southern California amphibians have co-evolved with a variety of native anuran predators. For example, adult California red-legged frogs (Rana draytonii) are known to prey upon Pacific chorus frogs (Pseudacris regilla) (Hayes and Tennant 1985) and southern mountain yellow-legged frogs (Rana muscosa) are thought to feed on P. regilla and Anaxyrus (Bufo) species (Pope and Matthews 2002). Xenopus laevis is comparable in size to these predatory anurans, but belongs to a family (Pipidae) that is not native to North America, which leaves a long evolutionary gap for potential prey recognition. Invasive X. laevis are also not typical of California frogs because they are nearly fully aquatic, leaving water only occasionally for dispersal (Lobos and Jaksic 2005). Their lack of tongue and suction feeding strategy are more typical of a fish than a native amphibian (Measey 1998). Pseudacris regilla do respond to fish predators (Pearl et al. 2003) but may not associate novel amphibian cues with a fish-like predatory threat. As a consequence, native amphibians may not recognize or appropriately respond to X. laevis in their adult or larval life stages.
This study used Pacific chorus frogs (Pseudacris regilla) as a representative of native California species to explore potential predation by and behavioral responses to X. laevis. Pseudacris regilla was chosen because it is highly palatable to most predators, is common throughout southern California, and has declined in areas X. laevis has invaded (Mahrdt and Knefler 1972; McCoid and Fritts 1980). Given that both larval and adult P. regilla occupy the slow-moving aquatic habitats favored by X. laevis (Crayon 2005), it is important to evaluate the potential effect of X. laevis on both larval and adult P. regilla life stages.
The following experiments were designed to determine (1) whether X. laevis prey upon P. regilla larvae and adults and (2) whether P. regilla, larvae or adults, respond to X. laevis with anti-predator behavior. Understanding the predation risks and responses will serve as a first step towards assessing the impact X. laevis may have on native anurans, and the potential for coexistence of native anurans with X. laevis.
Materials and methods
Experiments were performed over two summer field seasons using animals collected from the wild. The P. regilla and X. laevis were kept in plastic aquaria (18 cm wide, 17 cm tall, 28 cm long), filled with 4L of Nestle® bottled drinking water for adult X. laevis, larval X. laevis, and larval P. regilla, whereas adult P. regilla were supplied a water dish, unless otherwise stated. Animals were fed twice per week and tanks were cleaned weekly. Larval P. regilla were fed flake fish food and algal pellets; adult P. regilla were fed live crickets; and adult X. laevis were fed Xenopus-specific Nasco® frog brittle.
Xenopus laevis predation on larval P. regilla
Laboratory experiments were performed to determine if adult X. laevis would feed on larval P. regilla. The P. regilla larvae were raised from egg clutches deposited in the laboratory containers by amplexed adult pairs collected from Atascadero Creek, Santa Barbara County. Nine adult X. laevis were collected from a pond in the Hedrick Ranch Nature Area (HRNA), which is adjacent to the Santa Clara River, Ventura County, and kept in laboratory aquaria. One adult X. laevis was excluded from the study because it had not been observed feeding in the days leading up to the experiment and may have been sick at capture or particularly distressed from captivity.
The eight remaining X. laevis were fasted for 5 days and measured (snout-to-vent length, SVL) before the predation trials. A P. regilla larva (stages 32–41) (Gosner 1960) was haphazardly selected and placed in one of the eight X. laevis’ tanks. When the X. laevis consumed a P. regilla, another larva was placed in the tank within 5 min. The P. regilla were continuously replaced if consumed. Each trial ended when the X. laevis did not consume the P. regilla larva within 10 min of its introduction. We analyzed the relationship between the size of each X. laevis and the number of P. regilla it consumed using a linear regression.
Xenopus laevis predation on adult and juvenile P. regilla
A laboratory experiment was performed to determine if adult X. laevis would feed on adult and juvenile P. regilla. The eight adult P. regilla used in this experiment were collected from Atascadero Creek, Santa Barbara County. The juvenile P. regilla were the remaining metamorphosed larvae from the previous P. regilla larva predation experiment never exposed to a X. laevis. The sixteen X. laevis were collected from the isolated pond at the HRNA, eight of which had been used in the previous larval P. regilla predation experiment.
The X. laevis were fasted for 2 days before the trials. One X. laevis and one adult or juvenile P. regilla were measured and placed in an aquarium (18 cm wide, 17 cm tall, 28 cm long) filled with 5.5 L of bottled water with approximately 4 cm of space between the water surface and a fine mesh lid. The X. laevis were evaluated based on whether or not the P. regilla was consumed within a 24 h period. Two X. laevis that did not consume a P. regilla were re-tried with smaller P. regilla.
Behavioral response of larval P. regilla to X. laevis and a native invertebrate predator
A laboratory experiment was performed to determine if P. regilla larvae would exhibit an anti-predatory behavioral response in the presence of adult X. laevis (a non-native predator) and dragonfly nymphs (Aeshnidae; a native predator) as measured by larval activity levels and spatial avoidance. The P. regilla larvae were collected from a shallow isolated artificial pool lacking predators located in the HRNA, then kept in an aerated 20 gallon glass aquarium at 24 °C. Four of the X. laevis were collected from a pond on HRNA and four from an isolated pool on Piru Creek, a tributary of the Santa Clara River, in Ventura County. The four X. laevis collected from HRNA were later used in the P. regilla predation experiments. Eight dragonfly nymphs were collected from an isolated pool on the UCSB campus and kept in the laboratory in individual aerated plastic containers with 0.5 L of bottled water and fed bloodworms twice a week.
Trials were performed in clear plastic aquaria (18 cm wide, 17 cm tall, 28 cm long), each divided into two equal chambers by a clear plastic mesh divider (1.5 mm gauge). The tanks’ sides were covered in a white translucent screen to reduce shadows. Four liters of fresh bottled water at 25 ± 1.5 °C were used in each trial. Tanks were wiped down with 10% bleach and rinsed repeatedly (approximately five times) with DI water between each trial to remove residual animal cues.
A P. regilla larva was placed into each of the five treatments: X. laevis present (n = 22), X. laevis scent (n = 24), dragonfly present (n = 26), dragonfly scent (n = 18), or a control (no predator or predator scent; n = 25). In the X. laevis and dragonfly present treatments, the predator was placed in the trial aquaria for 30 min and the water was stirred prior to the introduction of the P. regilla larva to the opposing chamber. In the X. laevis and dragonfly scent treatments, the predator was placed in the aquarium for 30 min, removed, and the water stirred prior to the introduction of the P. regilla larva to the opposing chamber. Each P. regilla larva was staged following Gosner (1960) and used only once. The X. laevis adults and dragonfly nymphs were used repeatedly in trials.
Trials were conducted between 1100 and 1600 h and each ran for 11 min. The trials were videotaped from 20 cm above the water using a digital camera and later analyzed to evaluate P. regilla activity levels and spatial distribution within the aquarium. The first minute of each trial was discarded as an acclimation period for the P. regilla larva. In the following 10 min, we summed the number of seconds each P. regilla larva was active and the number of seconds it spent in the half of the aquarium closest to the mesh divider. Larvae were considered active if their tails were in motion. All time measurements were rounded to the nearest second for each bout of activity or move from one half of the aquarium to the other.
Separate ANOVAs and post hoc Tukey tests were performed on the number of seconds the P. regilla were active and the number of seconds they spent on the half of the tank closest to the mesh divider to compare treatment effects on activity levels and spatial avoidance, respectively.
Behavioral response of adult P. regilla to X. laevis
A field enclosure experiment was performed to determine if adult P. regilla would spatially avoid adult X. laevis. Thirty new adult P. regilla were collected from Isla Vista and Atascadero Creek, Santa Barbara County. Twenty new adult X. laevis were collected from a pond on HRNA.
Fifteen enclosures were used in the field experiment (Fig. 1). Each enclosure consisted of a rectangular 3-dimensional PVC frame (45 cm wide, 22 cm tall, 58 cm long) with small gauge (1 cm) plastic mesh on the top and on the four sides. The bottom of the enclosure was natural dirt with two aquaria (18 cm wide, 17 cm deep, 28 cm long) buried flush with the ground and filled with 7 L of well water, acting as separate water bodies. The two aquaria were arranged in the enclosure to create two halves, each with a water source and equal areas of substrate.
A diagram showing an overhead view of the inside of a treatment enclosure with a X. laevis penned in the right aquarium and the P. regilla on the opposite (non-X. laevis) side of the enclosure (a); a top view of a treatment enclosure (b); the 15 enclosures set up in the field (c). Bricks were used to hold the enclosures in place
Fifteen enclosures, five control without X. laevis and ten treatment enclosures with X. laevis were placed, single file, in an open area in HRNA, with every third enclosure a control. One adult P. regilla was included in each treatment and control enclosure. In each treatment enclosure, one X. laevis was placed in a mesh cube (20 cm by 15 cm by 15 cm) in the aquarium on one side of the enclosure, and an empty mesh cube was placed in the aquarium on the other side (alternating sides in each treatment enclosure). In this way, the X. laevis was confined to the mesh cube in one of the aquaria in the treatment enclosures but the P. regilla had free movement within the entire enclosure and could use either of the aquaria as a water source.
The experiment was run twice, each for 6 days, first with all male P. regilla and the second with all female P. regilla. Different X. laevis were used in each run of the experiment. At the beginning of the experiment, the P. regilla was placed in the center of each enclosure 30 min after a X. laevis had been placed in the mesh aquarium cube in each treatment enclosure. The position of each P. regilla was recorded three times per 24 h, once at dusk, once in the middle of the night at 0100 h and once at pre-dawn. The experiments began at 0100 h on the first day and ended with a pre-dawn observation on the sixth day, for a total of 17 observation periods. Each enclosure was checked in succession and no more than 3 min was spent locating the P. regilla in each enclosure.
The data were statistically analyzed with sign tests based on which side of the enclosure each individual P. regilla preferred. For each P. regilla, we summed the number of times it was observed on each side of the enclosure: the non-X. laevis or X. laevis side of the enclosure for the treatments; or the East or West side of the enclosure for the controls. We assigned a preference for one side or the other based on which side of the enclosure the P. regilla was observed on more often. Similarly, an additional sign test was performed to determine a preferential use of either of the water sources within the control and treatment enclosures using only the observation points when P. regilla were observed in the water.
Results
Xenopus laevis predation on larval P. regilla
There was a significant positive correlation between the size of the X. laevis and the number of P. regilla larvae consumed (linear regression, R2 = 0.83, p < 0.05) (Fig. 2). Seven out of the eight adult X. laevis consumed at least one P. regilla larva during the trial. One X. laevis individual consumed 25 P. regilla larvae in the time of the experiment, approximately 3.5 h.
Xenopus laevis predation on adult and juvenile P. regilla
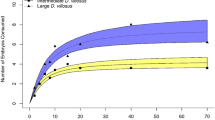
The X. laevis consumed 15 of the 18 adult and juvenile P. regilla within the 24-h feeding trials (Fig. 3). All juvenile P. regilla (SVL between 14 and 24 mm) were consumed by X. laevis (SVL 33–102 mm). The three largest adult P. regilla (SVL 34–35 mm) were not consumed.
Behavioral response of larval P. regilla to X. laevis and a native invertebrate predator
There was a significant effect of predator treatment on P. regilla larvae activity levels (ANOVA: F1,4 = 4.25, p < 0.01). However, no activity levels in any of the treatment were found to be significantly different from the control. The post hoc analysis found significant differences only between the X. laevis scent treatment and both the dragonfly nymph present and dragonfly nymph scent treatments (Fig. 4) (Tukey HSD, p < 0.05). For both X. laevis and dragonflies there is a trend for increased activity in the presence of the predator compared to the presence of only the scent of the predator; however, these differences are not statistically significant for either predator species.
There was a significant effect of predator treatment on spatial distribution of larval P. regilla within the aquaria (i.e. on the time spent in the half of the aquarium closest to the mesh divider vs. the back half of the aquarium; ANOVA: F1,4 = 3.57, p < 0.001). The post hoc analysis found significant differences between the control and the X. laevis-present treatment, with the P. regilla spending significantly less time in the half of that aquarium that was closer to the predator (Fig. 5; Tukey HSD, p < 0.05).
Spatial distribution of the P. regilla larvae when exposed to the scent cues or presence of a dragonfly nymph or adult X. laevis; number of seconds (mean ± SE) the P. regilla larvae spent on the half of the aquarium closest to the center mesh divider (near the predator). Letters above the SE bars distinguishing statistical differences between treatments and/or the control (Tukey pairwise comparison, p < 0.05)
Behavioral response of adult P. regilla to X. laevis
Adult P. regilla in the absence of X. laevis, displayed no preference for either side of the enclosures (binomial test: n = 10, p > 0.05) (blue dots in Fig. 6a). Five P. regilla in the control enclosure were observed more often on the East side and five were observed more often on the West side of the enclosure. All control P. regilla were observed at every survey period.
The number of times each adult P. regilla was observed on either side of the enclosure (a) or in the water sources (b). Pseudacris regilla in control enclosures were observed on either the East or West side and P. regilla in treatment enclosures were observed on either the non-X. laevis or the X. laevis side. Points were offset slightly to distinguish individuals with identical observation counts. The diagonal line represents an equal number of observations on either side of the enclosure or water source
Pseudacris regilla in the treatment enclosures displayed a significant preference for the side of the enclosure without the X. laevis (binomial test: n = 20, p < 0.001) (red triangles Fig. 6a). All 20 P. regilla in the treatment enclosures were observed more often on the non-X. laevis side of the enclosure than the X. laevis side. The P. regilla moved throughout the enclosure, rarely observed in the same location for two sequential time points in the treatment or control enclosures. On two occasions, a P. regilla in a treatment enclosure could not be located within the enclosure and those individual P. regilla have 16 rather than 17 observations.
The P. regilla also displayed a significant preference for the non-X. laevis water source in the treatment enclosures (binomial test: n = 16, p < 0.001) and showed no preference for the East or West water sources in the control enclosures (binomial test: n = 8, p > 0.05) (Fig. 6b). Pseudacris regilla that were not observed in water or were observed an equal number of times in both water sources were not included in this statistical analysis. There does not appear to be a pattern for when the P. regilla were observed in the X. laevis water source; some individuals were observed in the X. laevis water source in the beginning, middle, and end of the experiment. Only one female P. regilla was observed in a X. laevis water source, on one occasion, whereas five males were observed a total of seven times in X. laevis water source.
Discussion
Our predation experiments suggest that invasive X. laevis will prey upon larval and adult P. regilla. This is unsurprising given that larval P. regilla are a similar size to X. laevis larvae, which are cannibalized (Tinsley and McCoid 1996). Of greater concern for native amphibian populations is the ability of X. laevis to consume the adult P. regilla, which may have more profound population consequences. The loss of later life stages and older reproductive individuals may cause greater declines in populations than the loss of eggs or young juveniles (Doak et al. 1994; Vonesh and la Cruz 2002). Larval anurans suffer high mortality rates, some over 95% (Herreid and Kinney 1966), which means the few that survive to reproductive age become increasingly important to create the next generation. Only the largest adult P. regilla (34–35 mm SVL) avoided predation when matched up with small X. laevis (<70 mm SVL), presumably owing to gape limitation of the smaller adult X. laevis. However, gape limitation is unlikely to limit the larger X. laevis that reach >100 mm (SVL).
Although larval and adult P. regilla life stages were consumed by X. laevis in the laboratory, this does not necessarily imply that X. laevis predation will reduce or extirpate P. regilla populations in the field, although there is some evidence of this occurring (Mahrdt and Knefler 1972; McCoid and Fritts 1980). Amphibians can reduce their detection and capture by predators through a variety of anti-predator behaviors. Laval amphibians exhibit spatial avoidance, increased refuge use, as well as changes in morphology, timing of metamorphosis, and activity level (Skelly and Werner 1990; Pearl et al. 2003; Hossie et al. 2010). Similarly adult anurans are selective in where they lay their eggs and have been shown to avoid ovipositing in waters with predators (Rieger et al. 2004). These anti-predator responses should be expressed only in the presence of a threat because the behaviors or changes in morphology tend to be costly. For example, reduced larval activity reduces the amount of time larvae spend foraging and thereby adversely affects their later size, survivorship, growth, and development (Skelly 1992).
In this study, P. regilla displayed a spatial avoidance response to predator presence but did not display changes in activity levels. It is not surprising that the larvae displayed only one of the two response behaviors tested. Anuran larvae have been shown to have specific responses for different predators (Relyea 2001) and often the presence of more predator cues results in a stronger prey response than one predator cue alone (Hettyey et al. 2012). The X. laevis scent cue may trigger a response that was not measured in this study, such as changes in tail morphology or accelerated time to metamorphosis.
The larvae’s spatial avoidance response likely results from general predator cues that happen to fit X. laevis rather than cues specific only to X. laevis. The changes in water movement or an approaching dark shape could have caused the P. regilla to respond to the presence of X. laevis. There were multiple occasions when the predator appeared to have observed the P. regilla larva on the other side of the mesh and lunged towards it, which often caused the P. regilla to quickly swim away. This predator behavior could have influenced the spatial distribution of the P. regilla larvae because it only occurred near the mesh divider.
Predator motion could also explain the differences seen in the activity level between the predator present and predator scent-only treatments. For both X. laevis and dragonflies as predators, there was a tendency for P. regilla larvae to be more active in the presence of the predators than in the presence of just the predator scent. The motion of the predator appeared to be necessary for the P. regilla to initiate a behavioral response. The P. regilla did not appear to respond to the visual outline of the X. laevis as a potential threat; the larvae would often approach a motionless X. laevis or rest immediately next to the X. laevis on the opposite side of the mesh divider.
Xenopus laevis may be too far removed evolutionarily from P. regilla’s natural predators, for P. regilla to recognize its specific scent or other X. laevis-specific cues as a potential threat. Although P. regilla did evolve with native anuran predators (Hayes and Tennant 1985; Pope and Matthews 2002), more distantly related animals are thought to have more dissimilar scent cues that limit an amphibian’s ability to recognize novel predators (Ferrari et al. 2010). This has been shown with other amphibian species when exposed to novel predatory fishes with varying relatedness to their native predators (Gall and Mathis 2010). The Pipidae family, which includes X. laevis, is native to Africa and South America and Xenopus is endemic to sub-Saharan Africa (Tinsley and Kobel 1996). The olfactory scent cues may not be recognized by P. regilla larvae, which could force the larvae to rely upon more general predator cues that matched a native predator in some way. Native predatory anurans (R. draytonii or R. muscosa) were not included in this study due to their sensitive population statuses, and no other study was found to explore the behavioral responses of P. regilla to native anuran predatory species.
It is unclear why P. regilla larvae did not respond significantly to the native dragonfly nymphs, a native predator. Other anuran species have displayed reduced activity levels in the presence of native dragonfly nymphs (Nunes et al. 2012), and P. regilla larvae have been shown to spatially respond to dragonfly nymphs (Hammond et al. 2007). In our study, the data suggest that P. regilla larvae may increase their activity levels and spatially avoid dragonfly nymphs when in their presence, perhaps in effort to leave the area. However, the increased activity did not significantly differ from the control and may be a product of the attack motions of the nymphs eliciting flight reactions from the larvae.
Larval P. regilla might have exhibited stronger responses to the presence of the dragonfly and X. laevis predators were the scent of a consumed conspecific present. Anuran larvae sometimes respond to novel predators when a cue from consumed conspecifics is present, either through the diet of the predator or the presence of the larvae’s broken skin (Marquis et al. 2004; Mandrillon and Saglio 2005). This response could compensate for the lack of a species-specific predator response, allowing native larvae to avoid a range of predators without recognizing them individually. Neither predator in this experiment was fed anurans as part of their diet in order to eliminate this as a potential factor, but it may be key to stimulating a behavioral response.
Adult P. regilla displayed a significant spatial avoidance behavior when exposed to X. laevis. The adult behavioral response may be more important than the larval response because the adults can reduce the larvae’s exposure to the X. laevis by discriminating between invaded and non-invaded sites when selecting breeding sites. If the parents avoid depositing eggs in areas with aquatic predators, then the larvae have less need for an innate anti-predator response because they are not frequently exposed to those predators. Other naïve anuran species in Europe have been shown to stop reproduction in ponds after establishment of invasive X. laevis (Lillo et al. 2011). The data from the field enclosure experiment suggests that P. regilla avoid X. laevis invaded water sources when possible, because P. regilla were rarely observed in the water with a penned X. laevis. This experiment was performed during the breeding season of P. regilla, which suggests that P. regilla may avoid X. laevis areas when ovipositing, if alternative sites were available.
Xenopus laevis displays a clear ability to consume both larval and adult stages of native amphibians and may indirectly cause native amphibian emigration from local habitats through spatial predator avoidance. Native California anurans are absent from many areas that X. laevis has invaded but have never been found in the stomach contents of X. laevis (Crayon 2005), suggesting either quick consumption to extirpation or emigration of native anurans from those areas. These potential impacts may warrant X. laevis management to limit their current populations and prevent further invasions. Active management aimed at preventing their introduction is ideal because they are difficult to eradicate once established (Crayon 2005), although X. laevis populations have been shown to decline or go extinct on their own due to extreme cold or dry weather conditions (Rebelo et al. 2010; Tinsley et al. 2015). The recent drought in southern California may have eliminated some populations as semi-permanent water sources dried. Further study will be necessary to determine if co-existence between native amphibians and X. laevis occurs in the wild.
References
Adams MJ, Pearl CA (2007) Problems and opportunities managing invasive bullfrogs. Is there any hope? In: Gherardi F (ed) Biological invaders in inland waters—profiles, distribution and threats. Springer, Dordrecht, pp 679–693
Amaral P, Rebelo R (2012) Diet of invasive clawed frog Xenopus laevis at Lage stream (Oeiras, W Portugal). Herpetol J 22:187–190
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Lett 12:20150623
Bucciarelli GM, Blaustein AR, Garcia TS, Kats LB (2014) Invasion complexities: the diverse impacts of nonnative species on amphibians. Copeia 4:611–632
Crayon JJ (2005) Species account: Xenopus laevis. In: Lannoo MJ (ed) Amphibian declines: the conservation status of United States species, 1st edn. California, Berkeley, pp 522–525
Doak D, Kareiva P, Klepetka B (1994) Modeling population viability for the desert tortoise in the Western Mojave Desert. Ecol Appl 4:446–460
Faraone FP, Lillo F, Giacalone G, Valvo ML (2008) The large invasive population of Xenopus laevis in Sicily, Italy. Amphibia-Reptilia 29:405–412
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Fritts TH, Rodda GH (1998) The role of introduced species in the degradation of island ecosystems: a case history of Gaum. Ann Rev Ecol Syst 29:113–140
Gall BG, Mathis A (2010) Innate predator recognition and the problem of introduced trout. Ethology 116:47–58
Goldson SL, Bourdôt GW, Brockerhoff EG, Byrom AE, Clout MN, McGlone MS, Nelson WA, Popay AJ, Suckling DM, Templeton MD (2015) New Zealand pest management: current and future challenges. J R Soc N Z 45:31–58
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hammond JI, Luttbeg B, Sih A (2007) Predator and prey space use: dragonflies and tadpoles in an interactive game. Ecology 88:1525–1535
Hayes MP, Tennant MR (1985) Diet and feeding behavior of the California red-legged frog, Rana aurora draytonii (Ranidae). Southwest Nat 30:601
Herreid CF II, Kinney S (1966) Survival of Alaskan woodfrog (Rana sylvatica) larvae. Ecology 47:1039–1041
Hettyey A, Rölli F, Thürlimann N, Zuercher A, Van Buskirk J (2012) Visual cues contribute to predator detection in anuran larvae. Biol J Linn Soc 106:820–827
Hossie TJ, Ferland-Raymond B, Burness G, Murray DL (2010) Morphological and behavioral responses of frog tadpoles to perceived predation risk: a possible role for corticosterone mediation? Ecoscience 17:100–108
Innes J, Kelly D, Overton JC, Gillies C (2010) Predation and other factors currently limiting New Zealand forest birds. N Z J Ecol 34:86–114
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers Distrib 9:99–110
Knapp RA, Matthews KR (2000) Non-native fish introductions and the decline of the mountain yellow-legged frog from within protected areas. Conserv Biol 14:428–438
Lillo F, Faraone FP, Valvo Lo M (2011) Can the introduction of Xenopus laevis affect native amphibian populations? Reduction of reproductive occurrence in presence of the invasive species. Biol Invasions 13:1533–1541
Lobos G, Jaksic FM (2005) The ongoing invasion of African clawed frogs (Xenopus laevis) in Chile: causes of concern. Biodivers Conserv 14:429–439
Lobos G, Measey GJ (2002) Invasive populations of Xenopus laevis (Daudin) in Chile. Herpetol J 12:163–168
Mack RN, Simberloff D, Lonsdale MW, Evans H, Clout M (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Mahrdt C, Knefler FT (1972) Pet or pest? The African clawed frog. Environ Southwest 446:2–5
Mandrillon A-L, Saglio P (2005) Prior exposure to conspecific chemical cues affects predator recognition in larval common toad (Bufo bufo). Arch Hydrobiol 164:1–12
Marquis O, Saglio P, Neveu A (2004) Effects of predators and conspecific chemical cues on the swimming activity of Rana temporaria and Bufo bufo tadpoles. Arch Hydrobiol 160:153–170
McCoid MJ, Fritts TH (1980) Notes on the diet of a feral population of Xenopus laevis (Pipidae) in California. Southwest Nat 25:272–275
Measey GJ (1998) Diet of feral Xenopus laevis (Daudin) in South Wales, UK. J Zool Lond 246:287–298
Measey GJ, Tinsley RC (1998) Feral Xenopus in South Wales. Herpetol J 8:23–27
Nunes AL, Richter-Boix A, Laurila A, Rebelo R (2012) Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia 171:115–127
Pearl CA, Adams MJ, Schuytema GS, Nebeker AV (2003) Behavioral responses of anuran larvae to chemical cues of native and introduced predators in the Pacific Northwestern United States. J Herpetol 37:572–576
Pease KM, Wayne RK (2013) Divergent responses of exposed and naive Pacific tree frog tadpoles to invasive predatory crayfish. Oecologia 174:241–252
Pope KL, Matthews KR (2002) Influence of anuran prey on the condition and distribution of Rana muscosa in the Sierra Nevada. Herpetologica 58:354–363
Rebelo R, Amaral P, Bernardes M, Oliveira J, Pinheiro P, Leitão D (2010) Xenopus laevis (Daudin, 1802), a new exotic amphibian in Portugal. Biol Invasions 12:3383–3387
Relyea RA (2001) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Rieger JF, Binckley CA, Resetarits WJ (2004) Larval performance and oviposition site preference along a predation gradient. Ecology 85:2094–2099
Seale DB (1982) Obligate and facultative suspension feeding in anuran larvae: feeding regulation in Xenopus and Rana. Bio Bull 162:214–231
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Skelly DK (1992) Field evidence for a cost of behavioral antipredator response in a larval amphibian. Ecology 73:704–708
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322
Tinsley RC, Kobel HR (1996) The biology of Xenopus. Oxford University Press, Oxford
Tinsley RC, McCoid MJ (1996) Feral Populations of Xenopus outside of Africa. In: Tinsley RC, Kobel HR (eds) The biology of Xenopus. Oxford University Press, Oxford, pp 81–93
Tinsley RC, Loumont C, Kobel HR (1996) Geographical distribution and ecology. In: Tinsley RC, Kobel HR (eds) The biology of Xenopus. Oxford University Press, Oxford, pp 35–56
Tinsley RC, Stott LC, Viney ME et al (2015) Extinction of an introduced warm-climate alien species, Xenopus laevis, by extreme weather events. Biol Invasions 17:3183–3195
Vonesh J, la Cruz De O (2002) Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia 133:325–333
Wiles GJ, Bart J, Beck RE, Aguon CF (2003) Impacts of the brown tree snake: patterns of decline and species persistence in Guam’s avifauna. Conserv Biol 17:1350–1360
Acknowledgements
This research benefitted from access by Sandy Hedrick and Friends of the Santa Clara River to Hedrick Ranch Nature Area where experiments were performed and African clawed frogs were collected. We also thank Mike Booth and Steve Howard of United Water Conservation District for providing access to collect African clawed frogs. Thank you to Scott Cooper and Steve Rothstein for their input and advice. Funding provided by the Coastal Fund, University of California, Santa Barbara (WIN13-13).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, E.A., Dudley, T.L. & Briggs, C.J. Shared behavioral responses and predation risk of anuran larvae and adults exposed to a novel predator. Biol Invasions 20, 475–485 (2018). https://doi.org/10.1007/s10530-017-1550-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1550-x