Abstract
Breeding suppression hypothesis (BSH) predicts that, in several vole species, females will suppress breeding in response to high risk of mustelid predation; compared to breeding females, suppressing females would gain higher chances of survival. Seminal evidence for BSH was obtained in the laboratory, but attempts to replicate breeding suppression under field conditions were less conclusive. We tested whether breeding suppression occurs in common voles (Microtus arvalis), and how population density and predation risk combined affect voles’ reproductive activity. We found that, in contrast to males, female common voles suppress reproductive activity when faced with high predation risk. Population size was not reduced despite breeding suppression. A model of the interaction between predation risk and population density revealed that predator-induced breeding suppression depends on the density of conspecifics. We concluded that breeding suppression is a viable adaptation only at low vole densities, when per capita predation risk is high. Finally, we identified the key issues of experimental design required for the consistency of future studies on breeding suppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Odors of predators modify the behavior of many mammals (for an extensive review, see Apfelbach et al. 2005). Small rodent prey, voles in particular, have received much attention in this matter. Responses of voles fall into several categories: suppressed feeding (Calder and Gorman 1991; Borowski 1998), decreased activity (Gorman 1984), and changes in spacing (Jedrzejewski et al. 1993). Some interesting sex-specific effects were found: males or non-breeding females fled in reaction to the cues of predator proximity, whereas breeding females and juveniles did not (Jedrzejewski and Jedrzejewska 1990).
Responses of voles to predation risk are not limited to obvious behavioral effects. Ylönen (1989) was first to show that the presence of predators or predator odors suppresses females’ reproductive activity. This reaction inspired further studies, which discovered such intricate effects as copulation avoidance (Ronkainen and Ylönen 1994; Ylönen and Ronkainen 1994; Koskela and Ylönen 1995), delayed maturation and hindered development of gonads (Heikkilä et al. 1993), and decreased frequency of estrus (Koskela et al. 1996). Except for the study of Heikkilä et al., no clear effects of predation risk were found in male voles. Intriguingly, female responses were induced only by specialist predators (i.e., mustelids, namely weasels and stoats). By contrast, generalist terrestrial predators, or avian predators, do not cause those effects (Wolff and Davis-Born 1997; Klemola et al. 1998; Jonsson et al. 2000).
Based on the accumulated evidence, the breeding suppression hypothesis (BSH) was coined. BSH predicts that, when faced with high predation risk from mustelid predators, the females will attempt to minimize it by suppressing reproduction (Ronkainen and Ylönen 1994). Females are preyed upon by mustelids more than males (Norrdahl and Korpimäki 1998). Moreover, females in estrus are particularly attractive to mustelid predators (Cushing 1985). This indicates that, in a population of voles, breeding females are at the highest risk of predation. Suppressing females are rewarded with increased chances of survival. Still, it is unclear why only part of the population employs this adaptation, while some females continue to breed in spite of predatory threat (Fuelling and Halle 2004). For them, unfulfilled breeding opportunities may be too high a price for the relative safety.
BSH remains controversial, despite a fair amount of research. Initial studies revealed acute behavioral or physiological effects of predator odor, but their validity was criticized on the grounds of artificial conditions (Mappes et al. 1998; Norrdahl and Korpimäki 2000; Wolff 2003) and lack of control for novelty (Lambin et al. 1995). The controversy prompted experiments under natural conditions. The attempts to verify breeding suppression in the field brought inconsistent results: some studies contradicted breeding suppression (e.g., Wolff and Davis-Born 1997; Mappes et al. 1998; Sullivan et al. 2004), but others confirmed it (e.g., Mappes and Ylönen 1997; Fuelling and Halle 2004). In contrast to laboratory studies, field studies are burdened with uncontrollable factors, which may hamper the traceability of breeding suppression. We think that carefully designed experiments will bring conclusive evidence for BSH. We address the key methodological issues in this paper.
Female reproduction is not only affected by predation risk but also by factors such as photoperiod and population density. Photoperiod limits breeding to the long daylight season (Lecyk 1962; Breed and Clarke 1970), while high population density induces social breeding suppression (Saitoh 1981; Ostfeld et al. 1993). In autumn, reproductive activity is reduced by coinciding high densities of vole populations and shortening daylength. The effect of predation risk is similar; regardless of the root cause, the resulting numbers of breeding females are reduced. It was concluded by Kaitala et al. (1997) that both population density and predation risk determine the proportion of reproductively active females. Ylönen et al. (1995) proposed that these two factors act additively; Hansson (1995) implied that they may be additive, but only at high vole densities. We aim to verify these statements.
We tested whether common voles (Microtus arvalis) suppress breeding when challenged with mustelid odor. To differentiate the influences of population density and predation risk, we modeled the effect of those factors on reproductive activity of females.
Materials and methods
Field site and time frame
The experiment was carried out at Remderoda Field Research Station, located in central Germany (50°56′N, 11°32′E; 320 m a.s.l.) near the city of Jena. The field site was protected from terrestrial predators with a 2-m-high wire-mesh fence; the access of avian predators was unrestricted. The study area comprised six enclosures, organized as shown in Fig. 1. Each plot was a square of 0.25 ha area (50 × 50 m), delimited by a vole-proof barrier made of sheet metal. In the growing season, the interior of the plots was covered with grasses and tall herbs. To emulate habitat edge perimeter of each plot, a 2.5-m-wide strip was mown regularly. Within the habitat, 25 live traps (Oos trap with shelter box; Halle 1994) were placed in a 5 × 5 square grid, at distances of 10 m. We arranged 16 odor sources in a 4 × 4 square grid; an odor source was placed in the center of a square delimited with four adjacent traps (see Fig. 1). To let the voles habituate to the setup, the odor sources were installed 4 weeks before the odor application period.
The study site at the Remderoda Field Research Station. Traps are marked with filled squares. Odor sources are marked with open dots. Dotted lines denote access paths to traps and odor sources. Gray zone indicates the area devoid of vegetation. During the treatment phase in 2007, eight additional multiple-capture traps were used at the perimeter of each plot (not shown)
We ran the experiment during the vole breeding season, following the same schedule in 2007 and 2008. In early spring, vegetation cover in the plots was removed. Before the start of the experiment, we trapped out the voles remaining from the previous year. Some of the overwintered voles were kept to repopulate the plots in mid-May. In 2007, we released 4 males and 7 non-pregnant females in each plot except for plot 1, where 6 females were released. Due to low abundance in spring 2008, we limited the number of released females to 6 per plot. To imitate spring population structures, we included both young and overwintered individuals, with the majority being in their first season. By the end of June, we had started the first phase of the experiment wherein we monitored the dynamics of the populations. The monitoring phase was followed by the treatment phase, during which we manipulated the perceived risk of predation with olfactory cues.
Population monitoring
Each year, we sampled the six populations throughout both monitoring and treatment phases on a weekly basis. We trapped within each plot on 2 days a week, usually with 1 day inbetween. Traps were checked in the morning and in the late afternoon. We activated the traps at sunset on the preceding day to allow trapping overnight. The traps were baited with pieces of fresh apple, barley, and commercial rodent chow. Upon first capture, individuals with body mass of 15 g or higher were sexed and marked under light anesthesia with a passive integrated transponder (PIT; Trovan); the tags allowed future identification of recaptured voles. We regarded individuals weighing less than 15 g as too small for marking; they were classed as juveniles and released. Upon recapture of a tagged individual, we recorded its identity, body mass, and reproductive status. Based on these observations, we qualified each adult vole as either reproductively active or inactive in a given week. Not being part of the reproductively active subpopulation, juveniles were excluded from the counts until qualified for tagging.
Males with small or withdrawn testes were recorded as reproductively inactive; males with enlarged testes were recorded as reproductively active (Reichstein 1964). To count reproductively active females, we invented a simple scoring system based on their reproductive biology. Gestation in the common vole typically lasts for 20–21 days (Reichstein 1964). In the last 3–5 days preceding parturition, body mass of the female increases and the abdomen is enlarged. With regard to breeding suppression, reproductive activity materializes in ongoing gestations; hence, we deemed an antepartum female captured in week t 0 as reproductively active in weeks t 0, t −1, and t −2; the 3 weeks corresponded to the gestation period. In cases of missing capture data or overlooked gestations, we inferred the female’s reproductive status from sudden drops of body mass coinciding with a record of lactation in subsequent weeks. Reproductive activity was only interpolated when the individual’s capture record contained sufficient information, and never for gaps longer than 2 weeks. Non-pregnant females counted as reproductively inactive; females with non-perforated vaginas were classified as juveniles, or as reproductively inactive if already marked. In cases of multiple captures within a week, the most frequent reproductive status was decisive, in both males and females.
Odor application
We applied olfactory cues to simulate two levels of perceived predation risk during the second phase of the experiment (i.e., the treatment phase). We used the odor of domestic ferrets (Mustela putorius furo) as a cue of high mustelid predation risk. Ferrets are not specialized in hunting for voles, but the composition of scent marks of species in the genus Mustela is very similar (Brinck et al. 1983; Zhang et al. 2003). Furthermore, ferret body odor acts as a very potent stressor in rodents (Masini et al. 2005). Each year, we applied the ferret odor in three of the six plots: plots 3, 5, and 6 in 2007, and plots 1, 2, and 4 in 2008 (Fig. 1). This arrangement minimized potential odor carryover to the adjacent plots. Plots receiving ferret odor are referred to cumulatively as ‘predator treatment’, for both phases of the experiment. The remaining plots served as controls with low level of predation risk (‘non-predator treatment’). Here, we applied two non-predator odors: of a herbivore, i.e. the European rabbit (Oryctolagus cuniculus), and of fresh cage bedding. We used these odors to control for the effect of novelty. Rabbit odor was applied in plots 1 and 4 in 2007, and in plot 3 in 2008; cage bedding odor was applied in plot 2 in 2007, and plots 5 and 6 in 2008 (Fig. 1). In the analyses, we tested for differences in the effect of the control odors.
The odors were prepared as water extracts of cage bedding. Both ferret and rabbit material was acquired from captive male−female pairs. It comprised a mixture of feces, urine, and fur. Fresh cage bedding was obtained commercially. To prepare the extracts, one part of bedding was soaked for 24 h in five parts of water. Afterwards, the liquid fraction was strained, filtered, and frozen at −18 °C until use. To the human nose, the ferret extract had a pungent smell. The smell of the rabbit extract was milder and clearly different, while the extract of fresh cage bedding smelled pleasantly of wet wood. As odor sources, we used 0.5-L glass jars, placed on the ground amid vegetation. Jar lids were perforated to allow continuous odor release and refilling. We used manually operated, pressurized spray bottles to distribute the thawed extracts among the odor sources. We applied about 30 mL of the extract per odor source, totaling approx. 500 mL per plot. The odors were applied sequentially, starting with the non-predator plots and finishing in the predator plots. We used separate spraying equipment for each odor. As an extra precaution, we rinsed the impermeable protective clothing after each spraying session. The odors were applied throughout the whole treatment phase, three times a week at 2-day intervals. In 2007, the treatment phase started in week 36 (early September) and lasted for 12 weeks; in 2008, it started in week 32 (early August) and lasted for 13 weeks.
Population parameters
Capture data were used to gauge population densities and reproductive activity. We estimated population densities with the Minimum Number Alive (MNA; Krebs 1999). This estimate was based solely on the marked portion of the population, i.e., individuals with body mass of at least 15 g. However, it was improbable that we would capture every single individual at exactly the 15 g threshold; in fact, some voles clearly exceeded it at first capture. Hence, our MNA estimates in raw form were negatively biased. To minimize this bias, we applied a moderate correction to the capture data. The correction was based on a model of mean increase of vole body mass over time. We fit this model to a data subset which contained individuals weighing approximately 15 g at first capture. Females in advanced gestation were excluded from the model, as their body masses exceeded the baseline, and would have introduced positive bias. Using separate fit curves for males and females, we approximated the number of weeks that elapsed since a given individual had passed the 15 g mark. We extrapolated the presence of each such individual backwards in time, thus improving the accuracy of the MNA estimate.
Reproductive activity was measured as the proportion of reproductively active individuals to the total of adult (i.e., reproductively mature) individuals of the given sex. To minimize overlap between the two experimental phases, female activity derived from gestations conceived near the end of the monitoring phase (i.e., first week of September in 2007, and first week of August in 2008) was excluded from the analyses of treatment effects.
Data analysis
We analyzed the data using R, version 2.10.1 (R Development Core Team 2009) with package lme4 (Bates and Maechler 2009) installed. Unless otherwise noted, we used linear mixed-effects models (LMM) for population densities, and generalized linear mixed-effects models (GLMM) with binomial error family and logit link function for proportions of reproductively active individuals. Likelihood ratio tests (LR) were used to determine the significance of model terms. To account for any differences between the plots, potentially affecting the measured population parameters, identity of the plots was included as a random effect in all instances of mixed models. The two study years differed with regard to vole densities. In order not to overlook the year effect, and having expected density-dependence of the proportions of reproductively active individuals, the analyses included year as a fixed effect.
The year variable was ignored in the analysis of the interaction of predation risk and population density. As an indirect result of the year effect, the pooling of 2007 and 2008 datasets granted a fourfold range of densities. The model included the following explanatory variables: predation risk and experimental phase (binary factors), and population density (continuous variable). Proportion of reproductively active females was the response variable.
Results
The analyses are based on 16,219 captures, 12,251 in 2007, and 3,968 in 2008. We captured and marked 366 females and 327 males in 2007, and 164 females and 178 males in 2008; 12.6 % of marked individuals was never recaptured. Among recaptured voles, the mean number of recaptures per individual was 17.6 (median 12), but females (mean = 18.8, median = 14) were captured more often than males (mean = 16.4, median = 10; t test on square-root transformed data: t = 2.88, df = 903, p = 0.0041). Manipulation of predation risk did not affect trappability (ANOVA on square-root-transformed data: F = 1.25, df = 2, p = 0.287).
Of the total of 9,463 weekly observations, 1,363 were extrapolated using the model of body mass increase (15.7 % of observations in 2007, and 11.2 % of observations in 2008); this difference between the years was parallel with the difference in the number of captures per year. The vast majority of the extrapolated observations (997 of 1,363) was added to the monitoring phase. As far as the treatment phase is concerned, the extrapolated data were applied mainly to male individuals (233 of 386 added observations). Of 326 males present in the treatment phase, 182 were recruits; 95 required the correction, which ranged from 1 to 12 weeks (mean = 2.9, SD = 2.10). In females, the correction had less impact. Of 383 females present in the treatment phase, 153 were recruited during that phase; of those, 89 had the correction applied: 1, 2, or 3 weeks were prepended ahead of first capture (mean = 1.5, SD = 0.70). The observations added to the estimates of female population size in the treatment phase equalled 1.6 % of the weekly observations total.
Population dynamics
The populations showed different dynamics in the two years. In 2007, vole numbers increased rapidly in the summer and peaked in mid-autumn, with maximum densities of 260–480 voles/ha. The seasonal increase in population size was not nearly as evident in 2008, when maximum densities reached only 120–200 voles/ha (Fig. 2). In the analysis (LMM), we allowed for a random intercept and random slope over time for each plot. Initially, we tested for the differences between the two non-predator treatments during the treatment phase, but we found none significant (LR: χ 2 = 1.57, df = 1, p = 0.21).
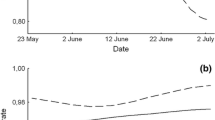
Population density of Microtus arvalis over time in a 2007 and c 2008. Gray areas indicate the treatment phase. Solid lines represent plots assigned to the predator treatment; dashed lines represent non-predator treatment plots. b Differences in mean population density between plots and between years. Dots mark the means and whiskers indicate the range of values. Gray dots predator treatment, open dots non-predator treatment. Lines connecting the dots visualize the pattern of population densities consistent across the 2 years. Statistics are given in the text
Predator treatment had no significant effect on population density (predation risk × experimental phase interaction; LR: χ 2 = 0.78, df = 2, p = 0.68). The difference between 2007 and 2008 in the rate of weekly density change was confirmed (LR: χ 2 = 224.00, df = 1, p < 0.001). In addition to the year effect, we tested for the effect of the plot. We fit a model with a common intercept, corresponding to similar initial population sizes, and linear, quadratic, and cubic terms for time; the latter two accounted for non-linear pattern of the population density changes. This analysis revealed significant differences between the plots (LR: χ 2 = 392.63, df = 9, p < 0.001) with a strikingly consistent pattern in both years (Fig. 2, inset). The linear estimates of mean weekly change in population density (MNA) ranged from 3.0 ± 1.5 (SE) in plot 1 to 7.1 ± 1.1 (SE) in plot 3. Additionally, we found significant differences in both cubic (LR: χ 2 = 20.42, df = 4, p < 0.001) and quadratic terms (LR: χ 2 = 17.03, df = 4, p = 0.002) describing the dynamics of our study populations.
Proportion of reproductively active males
The model (GLMM) for the proportion of reproductively active males included a random effect for the plots, with random intercept and random slope over time. We found no effect of the predator treatment on the proportion of reproductively active males during the treatment phase in both 2007 (LR: χ 2 = 1.48, df = 2, p = 0.48) and 2008 (LR: χ 2 = 2.07, df = 2, p = 0.36).
Proportion of reproductively active females
Reproductive activity of females was generally lower later in the season; this coincided with the treatment phase. During the treatment phase of the first year, we observed relatively little reproductive activity. In the non-predator treatment, reproductive activity seemed evenly distributed between age groups. In the predator treatment, most of the activity appeared among a few older females; a majority of the older females remained reproductively inactive throughout the treatment phase. In comparison with the non-predator treatment, very few of the females recruited during the treatment phase produced litters. In the second year, almost all of the females recruited during the treatment phase displayed some reproductive activity, regardless of the treatment. Some of the females, both younger and older, abandoned reproductive activity roughly halfway through the treatment phase.
The proportions of reproductively active females reflected the differences between treatments, experiment phases, and years. The decrease of this parameter was significantly stronger in the predator treatment, as compared with the non-predator treatment (predation risk × experimental phase interaction; LR: χ 2 = 25.56, df = 1, p < 0.001); this effect was consistent in both years (Fig. 3). In addition, we found a significant difference in the effect size between the 2 years (LR: χ 2 = 61.75, df = 1, p < 0.001). No significant difference was found between rabbit and fresh cage bedding during the treatment phase (LR: χ 2 = 3.16, df = 2, p = 0.20), justifying the integration of the two olfactory cues into one non-predator control.
Mean proportion of reproductively active females in 2007 and 2008. P predator treatment, NP non-predator treatment, M monitoring phase, T treatment phase. Gray bars correspond to periods of increased predation risk (T:P). Error bars ±1 binomial SE of the means; P values (GLMM) apply to comparisons between treatments within a phase
Interaction of population density and predation risk
We tested the interaction between predation risk and population density in both phases of the experiment (Fig. 4). The model (GLMM) included a random effect for the plots. Comparing the predator and non-predator treatments, we found that the model slopes of the relationship between proportion of reproductively active females and population density (predation risk × population density interaction) were not significantly different during both the monitoring phase (LR: χ 2 = 0.45, df = 1, p = 0.50) and the treatment phase (LR: χ 2 = 1.15, df = 1, p = 0.28); model intercepts (simple effect of the treatment) for the predator and non-predator treatments were not different in the monitoring phase, when no odors were applied (LR: χ 2 = 3.30, df = 1, p = 0.070); in the treatment phase, however, the intercept for the predator treatment was smaller than the intercept for the non-predator treatment (LR: χ 2 = 23.54, df = 1, p < 0.001), indicating the negative effect of predation risk on the proportion of reproductively active females (Fig. 4).
Relationship between the proportion of reproductively active females and population density at two levels of predation risk in monitoring and treatment phases. P predator treatment, NP non-predator treatment. Points mark weekly values for plots. Lines represent the GLMM models’ fits back-transformed from a logit scale
Comparing the monitoring and treatment phases, we found that in the non-predator treatment, model intercepts did not change (LR: χ 2 = 0.37, df = 1, p = 0.54), while in the predator treatment, the intercept decreased significantly (LR: χ 2 = 22.27, df = 1, p < 0.001). In the non-predator treatment, the model slope was only marginally steeper during the treatment phase than during the monitoring phase (LR: χ 2 = 4.08, df = 1, p = 0.043); in contrast, this difference was evident in the predator treatment (LR: χ 2 = 13.96, df = 1, p < 0.001).
Discussion
Population dynamics
Frequent recaptures provided us with a detailed dataset covering the demography and reproductive activity of 12 common vole populations. The populations developed in a similar way, starting from low initial size, increasing rapidly during the summer, and peaking in early autumn. After the annual peak, the densities declined slowly. Such a pattern corresponds to the typical density development of free-living populations (Nabaglo 1981).
Regardless of a similar course of development, the densities of our study populations varied substantially. Despite almost identical initial sizes, the populations reached much higher maximum densities in 2007 than in 2008. No cause for the year effect (e.g., overuse of vegetation in the first year) was evident; interestingly, the difference in density between the years was apparent in free-living common vole populations in the area (personal observation). In addition to the year effect, the mean densities differed markedly between the plots. These differences were consistent across years, but independent of the experimental treatment. Factors like variable habitat quality, soil structure, vegetation cover, or food supply could explain the effect of the plot; however, there were no obvious visual differences between the plots. As far as simulated predation risk is concerned, we observed no apparent effect on population densities—contrary to the anticipated consequences of breeding suppression. Instead, the variation of population densities reflected obscure differences between years and between plots.
According to the BSH, an increase in predation risk induces breeding suppression; consequently, it should result in relatively lower population size (Norrdahl and Korpimäki 2000). Yet, we found no differences in population size between predator and non-predator treatments. Breeding females may increase reproductive effort under high predation risk (Norrdahl and Korpimäki 1995; Mappes and Ylönen 1997). Increased effort could, at least in part, compensate for the litters unrealized by the suppressing females. Alternatively, the overall reproductive output may have indeed decreased. Since recruitment substantially lags behind reproduction (6–12 weeks between conception and recruitment of the resultant individual; personal estimation), a treatment period of 12 or 13 weeks could be too short for vole numbers to reflect the lower reproductive output.
Breeding suppression could result in an increase in population size reaching beyond one breeding season. To balance the missed reproduction opportunities, females surviving to their second year (after suppressing reproduction in the first year) could increase their reproductive output then (Ylönen et al. 1992). In our experiment, however, the time frame of each replicate was limited to a single season. Hence, we could not verify the long-term effects of suppressed breeding.
Proportion of reproductively active males
We found no effect of increased predation risk on the proportion of reproductively active males, which is in accord with existing evidence (e.g., Ronkainen and Ylönen 1994; Klemola et al. 1997; Mappes and Ylönen 1997). It is commonly agreed that predator olfactory cues induce stress in male murids (for some contradicting evidence, see Fletcher and Boonstra 2006). For instance, stress response was reported in laboratory rats exposed to fox odor (Thomas et al. 2006). In male meadow voles (Microtus pennsylvanicus), fox odor reduced their general activity (Perrot-Sinal et al. 2000). The anal gland scent of the Siberian weasel (Mustela sibirica) resulted in elevated stress hormone levels in rat-like hamsters (Cricetulus triton) and golden hamsters (Mesocricetus auratus), but did not affect reproductive physiology (Zhang et al. 2003). Only acute exposures to predator odors affect reproduction in male rodents (Vasilieva et al. 2000; Wang and Liu 2002). It seems that this reaction is part of a general physiological or behavioral complex evoked by severe stress. Very high intensities of predatory stimuli are unlikely to imitate natural levels of predation risk. Therefore, occurrence of breeding suppression in male rodents is probably restricted to a laboratory setting.
In contrast to females, breeding suppression in males is not a viable adaptation to predation risk. Males merely escape from areas tainted with weasel odors, whereas reproductively active females do not (Jedrzejewski and Jedrzejewska 1990). For males, immediate escape is a simple yet effective response to predation risk, but it is unavailable to pregnant or pup-rearing females. Moreover, mustelid predators prefer to hunt females (Norrdahl and Korpimäki 1998). Consequently, males are exposed to lower mustelid predation risk; in their situation, breeding suppression would be inadequate and unnecessary.
Proportion of reproductively active females
The decreased proportion of reproductively active females, observed in populations facing high mustelid predation risk, is in agreement with the BSH. Earlier studies on captive voles demonstrated clear effects of predation risk on female reproduction. Ylönen (1989) reported that none of the four bank vole (Myodes glareolus) females kept in presence of a weasel (Mustela nivalis) had been in breeding condition, while three of four females unexposed to predator had reproduced. In a scaled-up experiment, Ylönen and Ronkainen (1994) exposed breeding pairs of bank voles to stoat (Mustela erminea) odor. Only 6 of 34 females were reproductively active under predation risk, whereas 23 of 34 females reproduced in the control group. The same effect was found in field voles (Microtus agrestis). In pairs challenged with mixed weasel and mink (Mustela vison) odor, only 2 of 16 females bred; by contrast, 14 of 17 females bred in control pairs (Koskela and Ylönen 1995).
In opposition to the laboratory studies, experiments under more natural conditions brought less clear-cut results. Mappes and Ylönen (1997) placed cages with pairs of bank voles in outdoor enclosures and simulated predation risk with stoat odor. In the predator treatment, only 16 of 51 females reproduced, while in the control, the corresponding figure equaled 25 of 49. Here, the effect of simulated predation risk was not as strong as in laboratory trials, but still quite clear. However, subsequent study failed to produce the same effect in the field, despite the use of an acute predatory stimulus (Mappes et al. 1998). Several experiments with North American Microtus species produced further evidence against breeding suppression. Wolff and Davis-Born (1997) exposed gray-tailed voles (Microtus canicaudus) to mink feces and urine over a period of 4 weeks, but found no difference in the proportion of pregnant and lactating females between treatment and control areas. Jonsson et al. (2000) replicated this experiment, reaching the same conclusion. More recently, Sullivan et al. (2004) simulated predation risk with a mixture of two synthetic components present in the anal gland secretion of several mustelids. The treatment had no effect on the number of pregnant females of montane vole (Microtus montanus) and meadow vole (M. pennsylvanicus).
As accumulating negative reports increased the uncertainty of the BSH, a field study in Scandinavia brought surprising evidence in its favor. Over three breeding seasons, Fuelling and Halle (2004) simulated predation risk by applying weasel odor in an open field. Three populations of gray red-backed voles (Myodes rufocanus) were exposed to the odor; three others served as controls. Here, the average proportion of reproductively active females was lower in areas with predator treatment (0.79), as compared to control (0.92). Despite considerable variation—inherent in field experiments—the reduction of female reproductive activity under predation risk was clear.
Existing data indicate substantial geographic and taxonomic variation in responses of female voles to predation risk. The majority of Scandinavian studies on breeding suppression was positive, but these experiments were limited to voles of the genus Myodes. Conversely, North American studies focused on Microtus species, and produced no evidence for breeding suppression. Our study is the first to reveal breeding suppression in Microtus arvalis, a Eurasian species absent in most of Scandinavia (for distribution range, see Haynes et al. 2003). Geographical differences between Europe and North America seem to go deeper than differences between genera. In addition, co-existing species of the same genus may vary in response to predator odors (Heikkilä et al. 1993); breeding suppression among vole species could thus be less than a common strategy.
Kokko and Ranta (1996) suggested that, for breeding suppression to have an adaptive effect, the females must produce at least one female surviving to puberty. Such minimal reproductive output would ensure continuity, but it implies that breeding suppression is a genetically determined trait. However, it may not necessarily be heritable; all females may carry genes for both suppressing and breeding responses to predation risk, thus allowing phenotypic diversity. Employing either strategy may result from a combination of environmental factors other than predation risk; different conditions (e.g., low or high density of populations) could produce either of the two responses in a given female.
Instead of genetical heritability, the effect of predation risk may extend to the next generation of females through maternal effects. The impact of environmental factors on a pregnant female indirectly shapes the phenotype of the offspring during prenatal development (Sheriff et al. 2010). Exposure of a pregnant female to predation risk, among other stressors, could affect her female offspring and their response to the stressor. How exactly the strategy of breeding suppression pertains in a population constitutes an interesting problem for future research.
Methodological issues
Breeding suppression was never rejected in a laboratory study. Clear effects obtained in the laboratory may have been due to the proximity of the predator or high concentrations of its olfactory cues, but the consistency of the findings is striking. Attempts to reproduce the effects of predator odor in field conditions either revealed much weaker responses or failed altogether. It may seem that traceability of breeding suppression decreases with increasingly natural conditions. In reality, field experiments varied in design and protocol, making definite generalizations difficult. To ensure comparability of data, future field studies should apply more consistent methodology. We identify some of the key issues below.
Wherever predation risk is simulated temporarily, care should be taken to avoid bias of reproductive activity estimates. Occurrence of breeding suppression in field studies is inferred from the reproductive states of females, so it is not instantly detectable. Females mating soon before the start of treatment period will be recorded as pregnant during it. If predation risk is simulated for a relatively short time, the counts of reproductively active females will be biased. Additionally, treatment period spanning a little over the duration of a single gestation (e.g., Wolff and Davis-Born 1997; Jonsson et al. 2000) could be too short for suppressed breeding to become apparent. Experimenters should allow enough time to account for the delay.
Studying breeding suppression requires an accurate definition of reproductive activity. Some of the existing studies (e.g., Wolff and Davis-Born 1997; Jonsson et al. 2000; Fuelling and Halle 2004) regarded lactating females as reproductively active. In common voles, dams normally nurse the sucklings for over 3 weeks (personal observation); in other species, the length of the lactation period is probably similar. Predator-induced breeding suppression may already take effect during this period, postponing further litters. Hence, counting lactating but not parturient females as reproductively active will cause positive bias.
The origin and composition of predator odors are an important aspect of studies on breeding suppression. Apfelbach et al. (2005) emphasized that odors of a generalist predator (e.g., mink) may not be as significant for voles as odors of a specialist (i.e., weasel or stoat). Mink odor may be too weak an impulse to affect reproduction. In fact, trials using mink odors (Wolff and Davis-Born 1997; Jonsson et al. 2000) failed to induce breeding suppression. In contrast, studies using weasel or stoat odor brought positive results (reviewed in Apfelbach et al. 2005), except for a case where two synthetic components were involved (Sullivan et al. 2004). In our experiment, breeding suppression was induced with the full body odor of the ferret, a generalist predator. Ferret body odor is a potent stress agent in rats (Masini et al. 2005), marking its relevance for rodents. Further, odor of European polecat (Mustela putorius, the feral form of the ferret), modifies vole behavior (Jedrzejewski et al. 1993). Even though ferrets and polecats are generalists, their odors may affect voles in a similar way as odors of specialists. The age of the olfactory cues could play an additional role. The chemical composition of the cue may change as it gradually decays, thus affecting its intensity and significance. However, we are not aware of studies dealing with this aspect. Existing evidence indicates that only ecologically significant, complete cues of predation risk induce breeding suppression, but the understanding of their function is limited.
Manipulation of the actual predation pressure is an alternative to simulated predation risk. Klemola et al. (1997) surveyed the reproductive performance of sibling voles (Microtus rossiaemeridionalis) and field voles (M. agrestis) under reduced density of weasels and stoats. The proportion of reproductively active females decreased in 2 out of 6 study areas after predators had been removed, while it decreased in 5 out of 6 areas where predation risk remained high; here, the numbers of reproductively active females declined sharply. This outcome was interpreted as a consequence of selective predation rather than suppressed breeding. Incidentally, this interpretation supports the BSH: if most reproductively active females were indeed killed off by the predators, then surviving non-breeders would have gained the benefit of relaxed competition. Nonetheless, the study of Klemola et al. shows that coexisting predators may interfere with population dynamics of prey. Selective killing of reproductively active females changes the age structure, sex ratio, and particularly, the proportion of reproductively active females. In addition, the result of selective killing could be mistaken for breeding suppression, as Klemola et al. pointed out. To avoid this pitfall and afford control over perceived predation risk, actual predators should have no access to the study sites.
Apart from ambient predator pressure, also unrestrained vole migration may disturb field studies. Since voles avoid predator odors (Jedrzejewski and Jedrzejewska 1990; Jedrzejewski et al. 1993), some individuals could escape from the study area. In addition, immigration may dilute the study population, thus weakening any potential effects. In our opinion, outdoor enclosures provide an optimal balance between controlled and uncontrolled environment. Large enclosures offer almost natural conditions, while they keep away ground predators and unsolicited cues of predation risk, as well as eliminate vole migration.
Interaction of population density and predation risk
In natural vole populations, the proportion of reproductively active females decreases in the course of the breeding season. This is a result of two main factors: photoperiod and population density. The length of daylight controls breeding of Microtus voles (Lecyk 1962; Breed and Clarke 1970), limiting the bulk of reproduction to the growing season. In Central Europe, reproduction of common voles begins in March and ceases in November; in wintertime, the voles remain reproductively inactive (Reichstein 1964). Hence, the proportion of reproductively active females decreases towards winter. We found a hint of seasonal breeding in our data: in the monitoring phase, spanning from late June until early August or September, the mean proportions of reproductively active females ranged between 0.65 and 0.80. In the treatment phase (lasting till November), the corresponding figures were always lower, regardless of the level of simulated predation risk.
Population density, the second factor affecting reproductive activity, increases with advancing breeding season. Reproductive activity in a vole population is inversely related to its density: at high density, social breeding suppression occurs (Ostfeld et al. 1993). A detailed account of density dependence of female reproductive activity in the common vole was provided by Reichstein (1964); our data are in accord with Reichstein’s findings. Low densities of the early populations concurred with high proportions of reproductively active females; the proportions decreased as the populations grew in numbers. Mean densities in our populations were higher in 2007 than in 2008, while the reverse was true for the coinciding proportions of reproductively active females. We suppose that, in the first year, some density-dependent breeding suppression occurred; this effect was weaker in the second year. Furthermore, maturation of some females recruited during the treatment phase of 2007 was delayed; as a result, they produced no litters. Maturation of young, lower-ranking females is suppressed in presence of reproductively active females (Saitoh 1981); a similar effect was put forward as a potential response to predation risk (e.g., Heikkilä et al. 1993). It seems, however, that in our case delayed maturation was caused by high population densities rather than by predation risk: the second year was characterized by much lower population densities, and, regardless of the treatment, almost all the younger females did produce a litter at some point.
The effect of increasing density is inseparable from the effect of shortening photoperiod; in the course of the breeding season, both factors result in decreased reproductive activity. Photoperiod follows a fixed annual pattern and, to some extent, it pre-determines reproductive activity. Fluctuations of population density add further variability—both within and between years—as indicated in our data. Kaitala et al. (1997) put forward that predation risk and population density may collectively affect female reproductive activity. Like population density, predation risk has a negative impact; since both factors can occur simultaneously, one can easily confuse their effects.
To resolve this issue, we modeled the combined effect of population density and predation risk on the proportion of reproductively active females. As anticipated, the response variable was inversely related to vole density, regardless of treatment and experimental phase (Fig. 4). Moreover, high predation risk negatively affected the proportion of reproductively active females, but this effect was much weaker than the effect of high density. Finally, the effect of predation risk was evident only at low population densities. We conclude that predator-induced breeding suppression is conditional on population density. The effects of predation risk and population density are not additive at high vole densities, where social suppression is dominant. Our conclusion is in partial agreement with the concept of general additivity (Ylönen et al. 1995), but it contradicts the claim of additivity at high densities (Hansson 1995).
Not to breed is a rewarding strategy under high predation risk, albeit only at low densities of conspecifics. At high densities, voles are abundant prey. Resulting per capita predation risk is low, making breeding suppression devoid of purpose. Conversely, breeding females become most conspicuous when prey are scarce. For a non-breeding female, the chances of survival increase to a level comparable with male voles. Therefore, at low vole density, the benefit of breeding suppression may outweigh the costs of reduced reproductive output.
Relative effects of population density and predation risk on vole reproductive activity have been investigated in the past. Based on long-term field data, Norrdahl and Korpimäki (1995) inferred that the effect of predator pressure was stronger. The contradiction with our finding may be caused by the difference in perceived predation risk. Norrdahl and Korpimäki measured the actual density of mustelid predators, which varied over a range of values. By contrast, we mimicked the presence of predators with olfactory cues. We cannot rule out that our stimulus was weak, compared with the real risk of predation. Mappes and Ylönen (1997) found a minimal effect of population density on reproductive activity in bank voles. Again, the effect of predation risk was strong, regardless of vole abundance. The authors simulated high density with pre-collected vole odors, but vole pairs had no physical contact with conspecifics. Inaccurate perception of density could partially explain the overwhelming effect of predation risk. In light of the contrasting evidence, the interaction of predation risk and population density is open to further research.
Ylönen and Ronkainen (1994) suggested that the age of the female may determine whether she will suppress breeding; the role of age was disputed by Fuelling and Halle (2004). Our results support the interpretation that breeding suppression is independent of female age, but density effects also seem to play a role here. The effect of predation risk did seem independent of age at relatively low densities. In contrast, almost all reproductive activity was suppressed at high densities, except for a few, mostly relatively old, individuals; the remaining long-lived females became reproductively inactive towards the end of their lifetime. The actual role of female age, a potential trait determining female response to predation risk, requires verification.
Mappes and Ylönen (1997) observed that mean litter size increased both under high predation risk and high abundance of conspecifics. Apparently, females breeding in spite of poor survival perspectives or fierce competition try to make the most of a bad situation. This indicates a universal, dichotomous adaptation to unfavorable conditions: either maximize the breeding effort, or put it on hold in hope of better tomorrow. It is intriguing what individual trait or environmental factor determines the female’s choice of strategy.
References
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav R 29:1123–1144. doi:10.1016/j.neubiorev.2005.05.005
Bates D, Maechler M (2009) lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-32. http://CRAN.R-project.org/package=lme4
Borowski Z (1998) Influence of predator odour on the feeding behaviour of the root vole (Microtus oeconomus Pallas, 1776). Can J Zool 76:1791–1794. doi:10.1139/cjz-76-9-1791
Breed WG, Clarke JR (1970) Effect of photoperiod on ovarian function in vole, Microtus agrestis. J Reprod Fertil 23:189–192. doi:10.1530/jrf.0.0230189
Brinck C, Erlinge S, Sandell M (1983) Anal sac secretion in mustelids—a comparison. J Chem Ecol 9:727–745. doi:10.1007/BF00988779
Calder CJ, Gorman ML (1991) The effects of red fox Vulpes vulpes fecal odors on the feeding behavior of Orkney voles Microtus arvalis. J Zool 224:599–606. doi:10.1111/j.1469-7998.1991.tb03788.x
Cushing BS (1985) Estrous mice and vulnerability to weasel predation. Ecology 66:1976–1978. doi:10.2307/2937393
Fletcher QE, Boonstra R (2006) Do captive male meadow voles experience acute stress in response to weasel odour? Can J Zool 84:583–588. doi:10.1139/Z06-033
Fuelling O, Halle S (2004) Breeding suppression in free-ranging grey-sided voles under the influence of predator odour. Oecologia 138:151–159. doi:10.1007/s00442-003-1417-y
Gorman ML (1984) The response of prey to stoat (Mustela erminea) scent. J Zool 202:419–423. doi:10.1111/j.1469-7998.1984.tb05092.x
Halle S (1994) Eine einfache und effektive Falle für den Lebendfang von Kleinsäugern. Säugetierkd Inf 18:647–649
Hansson L (1995) Is the indirect predator effect a special case of generalized reactions to density-related disturbances in cyclic rodent populations?. Ann Zool Fenn 32:159–162
Haynes S, Jaarola M, Searle JB (2003) Phylogeography of the common vole (Microtus arvalis) with particular emphasis on the colonization of the Orkney archipelago. Mol Ecol 12:951–956. doi:10.1046/j.1365-294X.2003.01795.x
Heikkilä J, Kaarsalo K, Mustonen O, Pekkarinen P (1993) Influence of predation risk on early development and maturation in 3 species of Clethrionomys voles. Ann Zool Fenn 30:153–161
Jedrzejewski W, Jedrzejewska B (1990) Effect of a predators visit on the spatial distribution of bank voles—experiments with weasels. Can J Zool 68:660–666. doi:10.1139/z90-096
Jedrzejewski W, Rychlik L, Jedrzejewska B (1993) Responses of bank voles to odors of 7 species of predators—experimental data and their relevance to natural predator-vole relationships. Oikos 68:251–257. doi:10.2307/3544837
Jonsson P, Koskela E, Mappes T (2000) Does risk of predation by mammalian predators affect the spacing behaviour of rodents? Two large-scale experiments. Oecologia 122:487–492. doi:10.1007/s004420050970
Kaitala V, Mappes T, Ylönen H (1997) Delayed female reproduction in equilibrium and chaotic populations. Evol Ecol 11:105–126. doi:10.1023/A:1018491630846
Klemola T, Koivula M, Korpimäki E, Norrdahl K (1997) Small mustelid predation slows population growth of Microtus voles: a predator reduction experiment. J Anim Ecol 66:607–614. doi:10.2307/5914
Klemola T, Korpimäki E, Norrdahl K (1998) Does avian predation risk depress reproduction of voles? Oecologia 115:149–153. doi:10.1007/s004420050501
Kokko H, Ranta E (1996) Evolutionary optimality of delayed breeding in voles. Oikos 77:173–175
Koskela E, Horne TJ, Mappes T, Ylönen H (1996) Does risk of small mustelid predation affect the oestrous cycle in the bank vole, Clethrionomys glareolus? Anim Behav 51:1159–1163. doi:10.1006/anbe.1996.0117
Koskela E, Ylönen H (1995) Suppressed breeding in the field vole (Microtus agrestis) – an adaptation to cyclically fluctuating predation risk. Behav Ecol 6:311–315. doi:10.1093/beheco/6.3.311
Krebs CJ (1999) Ecological methodology, 2nd edn. Benjamin/Cummings, Menlo Park
Lambin X, Ims RA, Steen H, Yoccoz NG (1995) Vole cycles. Trends Ecol Evol 10:204–204. doi:10.1016/S0169-5347(00)89055-2
Lecyk M (1962) The effect of the length of daylight on reproduction in the field vole (Microtus arvalis Pall). Zool Pol 12:189–221
Mappes T, Koskela E, Ylönen H (1998) Breeding suppression in voles under predation risk of small mustelids: laboratory or methodological artifact? Oikos 82:365–369. doi:10.2307/3546977
Mappes T, Ylönen H (1997) Reproductive effort of female bank voles in a risky environment. Evol Ecol 11:591–598. doi:10.1007/s10682-997-1514-1
Masini CV, Sauer S, Campeau S (2005) Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci 119:280–292. doi:10.1037/0735-7044.119.1.280
Nabaglo L (1981) Demographic processes in a confined population of the common vole. Acta Theriol 26:163–183
Norrdahl K, Korpimäki E (1995) Does predation risk constrain maturation in cyclic vole populations? Oikos 72:263–272. doi:10.2307/3546228
Norrdahl K, Korpimäki E (1998) Does mobility or sex of voles affect risk of predation by mammalian predators?. Ecology 79:226–232. doi:10.2307/176877
Norrdahl K, Korpimäki E (2000) The impact of predation risk from small mustelids on prey populations. Mammal Rev 30:147–156. doi:10.1046/j.1365-2907.2000.00064.x
Ostfeld RS, Canham CD, Pugh SR (1993) Intrinsic density-dependent regulation of vole populations. Nature 366:259–261. doi:10.1038/366259a0
Perrot-Sinal T, Ossenkopp KP, Kavaliers M (2000) Influence of a natural stressor (predator odor) on locomotor activity in the meadow vole (Microtus pennsylvanicus): modulation by sex, reproductive condition and gonadal hormones. Psychoneuroendocrinology 25:259–276. doi:10.1016/S0306-4530(99)00054-2
R Development Core Team (2009) R: a language and environment for statistical computing. Version 2.10.1. R Foundation for Statistical Computing, Vienna, Austria, ISBN 3-900051-07-0. http://www.R-project.org
Reichstein H (1964) Untersuchungen zum Körperwachstum und zum Reproduktionspotential der Feldmaus, Microtus arvalis (Pallas, 1779). Z Wiss Zool 170:112–222
Ronkainen H, Ylönen H (1994) Behavior of cyclic bank voles under risk of mustelid predation—do females avoid copulations? Oecologia 97:377–381. doi:10.1007/BF00317328
Saitoh T (1981) Control of female maturation in high density populations of the red-backed vole, Clethrionomys rufocanus bedfordiae. J Anim Ecol 50:79–87. doi:10.2307/4032
Sheriff MJ, Krebs CJ, Boonstra R (2010) The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology 91:2983–2994. doi:10.1890/09-1108.1
Sullivan TP, Sullivan DS, Reid DG, Leung MC (2004) Weasels, voles, and trees: influence of mustelid semiochemicals on vole populations and feeding damage. Ecol Appl 14:999–1015. doi:10.1890/02-5284
Thomas RM, Urban JH, Peterson DA (2006) Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol 201:308–315. doi:10.1016/j.expneurol.2006.04.010
Vasilieva NY, Cherepanova EV, von Holst D, Apfelbach R (2000) Predator odour and its impact on male fertility and reproduction in Phodopus campbelli hamsters. Naturwissenschaften 87:312–314. doi:10.1007/s001140050728
Wang ZL, Liu JK (2002) Effects of steppe polecat (Mustela eversmanni) odor on social behaviour and breeding of root voles (Microtus oeconomus). Acta Zool Sin 48:20–26
Wolff JO (2003) Laboratory studies with rodents: facts or artifacts?. BioScience 53:421–427. doi:10.1641/0006-3568(2003)053[0421:LSWRFO]2.0.CO;2
Wolff JO, Davis-Born R (1997) Response of gray-tailed voles to odours of a mustelid predator: a field test. Oikos 79:543–548. doi:10.2307/3546898
Ylönen H (1989) Weasels Mustela nivalis suppress reproduction in cyclic bank voles Clethrionomys glareolus. Oikos 55:138–140. doi:10.2307/3565886
Ylönen H, Jedrzejewska B, Jedrzejewski W, Heikkilä J (1992) Antipredatory behavior of Clethrionomys voles—‘David and Goliath’ arms race. Ann Zool Fenn 29:207–216
Ylönen H, Koskela E, Mappes T (1995) Small mustelids and breeding suppression of cyclic microtines—adaptation or general sensitivity? Ann Zool Fenn 32:171–174
Ylönen H, Ronkainen H (1994) Breeding suppression in the bank vole as antipredatory adaptation in a predictable environment. Evol Ecol 8:658–666. doi:10.1007/BF01237848
Zhang JX, Cao C, Gao H, Yang ZS, Sun LX, Zhang ZB, Wang ZW (2003a) Effects of weasel odor on behavior and physiology of two hamster species. Physiol Behav 79:549–552. doi:10.1016/S0031-9384(03)00123-9
Zhang JX, Ni J, Ren XJ, Sun LX, Zhang ZB, Wang ZW (2003b) Possible coding for recognition of sexes, individuals and species in anal gland volatiles of Mustela eversmanni and M. sibirica. Chem Senses 28:381–388. doi:10.1093/chemse/28.5.381
Acknowledgments
We would like to thank Magda Bereza, Daniel Bergelt, Volkmar Haus, Kirsten Parker, and Amelie Zander for their effort put into the fieldwork. Thanks also go to Eric Allan for the hints on statistical analysis. This project was co-funded by the Max Planck Society (International Max Planck Research School by the Institute of Chemical Ecology in Jena, Germany), and the Institute of Ecology, Friedrich Schiller University Jena, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Sundell.
Rights and permissions
About this article
Cite this article
Jochym, M., Halle, S. To breed, or not to breed? Predation risk induces breeding suppression in common voles. Oecologia 170, 943–953 (2012). https://doi.org/10.1007/s00442-012-2372-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2372-2