Abstract
The breeding suppression hypothesis predicts that females of certain small mammal species will reduce reproduction as a response to the odour of a specialised mammalian predator. This was tested in a field experiment with grey-sided voles ( Clethrionomys rufocanus) during three summer seasons (1997–1999) in the subalpine tundra of northern Norway, which is a natural habitat of this species. In a first phase free-ranging voles in six unfenced 1-ha plots were monitored by live-trapping from June to August each year. In a second phase from August to September, three of the plots were sprayed with weasel ( Mustela nivalis) odour to simulate increased apparent predation risk, while the remaining three plots served as untreated controls. On all plots voles were individually marked with ear tattoos and were regularly live-trapped during the whole breeding season to follow their performance. On the treatment plots the recruitment rate of juveniles did not increase in late summer as it did on the control plots. The proportion of reproductively non-active adult females was significantly higher on the treatment plots for both old and young females. Our results thus verify the breeding suppression hypothesis for the first time under natural conditions. However, the response in overwintered females is in conflict with the original hypothesis because the assumed fitness benefits from breeding delayed until the next season are inaccessible to them. As an alternative explanation we propose a short-term response of reduced activity and interrupted breeding until the predator has exploited and left the feeding patch. Such a “duck and cover” strategy would increase the fitness of females of all age classes when prey habitats are patchy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the plus/minus interaction of fitness consequences, predators and their prey are bound together by an intimate relationship. For predators selection will favour optimised hunting abilities, while efficient predator avoidance will directly increase prey fitness. According to the life-dinner principle (Dawkins and Krebs 1979), stronger selective pressure is to be expected on the prey, providing the decisive lead in the resultant evolutionary arms race. Besides evolutionary consequences, a large body of literature has been published on the mutual effect on population dynamics and density, including the cyclic population changes of small rodents in the north with large amplitudes and sometimes extremely high densities during the peak phase [first described by Elton (1924)]. Although no single hypothesis could conclusively explain the phenomenon [see e.g. Krebs and Meyers (1974), and Stenseth and Ims (1993) for reviews], predator-prey interactions seem to play a major role (c.f. Korpimaki and Krebs 1996).
In addition to the direct reduction of prey numbers by predators, indirect effects on prey behaviour have also to be considered. Effects of perceived predation risk on mobility were demonstrated in laboratory experiments by Perrot-Sinal and Petersen (1997), in enclosures by Abramsky et al. (1996), and in field experiments by Norrdahl and Korpimäki (1998). Not only home range size, but also the preferred microhabitats within a given area can change under risk of predation (Bowers and Dooley 1993; Abramsky et al. 1996; Carlsen et al. 2000). Such microhabitat shifts are specific to the kind of predation: Korpimäki et al. (1996) induced field voles ( Microtus agrestis) to shift between microhabitats in an aviary by different experimental assemblages of mammalian (weasel, Mustela nivalis) and avian (kestrel, Falco tinnunculus) predators, which demonstrated the flexibility of behavioural responses to predation. Behavioural changes may also encompass activity timing: Jedrzejewska and Jedrzejewski (1990a) found bank voles ( Clethrionomys glareolus) in an enclosure to change their daily activity rhythm from activity peaks at dawn to diurnal activity when a weasel was present.
About 10 years ago H. Ylönen and co-workers described for the first time another possible trait of adaptive behavioural response to predation: in laboratory and enclosure experiments bank voles suppressed breeding when they perceived a high predation risk by small mustelids (Ylönen 1989; Ylönen et al. 1992; Ylönen and Magnhagen 1992; Ylönen 1994; Ylönen and Ronkainen 1994; Ylönen et al. 1995). This was interpreted as a strategy of maximising individual lifetime reproductive success under varying predation risk. The so-called “breeding suppression hypothesis” (BSH) is based on the fairly high predictability of population trends in areas with cyclic small mammal populations. After high population densities and—as a consequence—high predator activity during a peak summer, the prey population will probably crash during the following winter, and also the number of predators will decrease drastically. According to BSH assumptions, during the summer peak, young adult females, which are in their first year of maturity, may increase their survival chances by avoiding copulation and not getting pregnant. When actually surviving until the next summer they will find favourable conditions with few intraspecific competitors and low predation risk. Successful reproduction at low population density will result in a high fitness reward, but since winter survival is a precondition this would represent a high risk strategy because the females totally rely on the residual reproductive value (Pianka and Parker 1975) during their first summer. BSH also predicts that old females, which already have survived one winter, should produce a maximum of offspring even under high predation risk during the summer peak, because they do not have a realistic chance of surviving a second winter (Ylönen 1994; Ylönen and Ronkainen 1994).
In the field, voles (and other small mammals) can hardly estimate the risk of predation by the number of encounters with their predators, because such encounters are usually deadly. In order to allow for behavioural responses there must be other cues to get information about the predation pressure. Scent marks of the predators seem to fit this requirements, and responses of small rodents to the odour of their predators were indeed ascertained in several laboratory and enclosure studies (Stoddart 1976; Ylönen 1989; Jedrzejewska and Jedrzejewski 1990a; Ronkainen and Ylönen 1994; Koskela and Ylönen 1995; Koskela et al. 1996; Perrot-Sinal and Petersen 1997; Andelt and Beck 1998; Burwash et al. 1998). The picture is, however, not conclusive since other enclosure experiments have not shown any effects (Wolff and Davis-Born 1997; Jonsson et al. 2000). Mappes et al. (1998) criticised the positive findings as possibly being laboratory or methodological artefacts. It was further questioned whether the observed responses do in fact represent a specific anti-predator strategy, or can rather be interpreted as a general neophobic effect induced by any novel and pungent olfactory signal (Lambin et al. 1995; Kemble and Bolwahnn 1997; Mappes et al. 1998). However, field experiments with voles under natural conditions and removal or exclusion of predators have recently verified that at least some behavioural and physiological patterns of voles were directly influenced by the abundance of their mammalian predators (Steen 1994; Norrdahl and Korpimäki 1998; Carlsen et al. 2000).
In this study we conducted a field experiment to test for the first time whether evidence for the breeding suppression hypothesis can also be found in free-living populations in their natural environment. Indeed, field studies are burdened with a tremendous amount of uncontrollable variation and other methodological deficiencies. Nevertheless, experimental testing in the field is obviously the only way to judge whether or not findings from enclosures—and even more so from cages—are of any relevance for animals in the wild, where behaviour is affected by many extrinsic factors at the same time. Weak effects that are prevalent under laboratory conditions with reduced environmental complexity may be masked or even overwhelmed by other factors in the field, which would put into question the biological significance of findings from artificial habitats.
Materials and methods
Study area
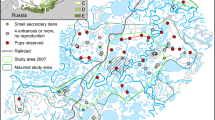
The experiment was conducted during three summer seasons (1997–1999) in Finnmarksvidda, northern Norway (Fig. 1). The study base was at Joatka Fjellstue (69°45′N, 23°58′E, 390 m altitude), situated about 45 km south-east of the city of Alta. The lower tundra between 400 and 500 m altitude south of the station is characterised by hills poorly covered with lichens ( Cladina, Cladonia) and heathland species ( Vaccinium, Empetrum), and by wet, lower areas with lakes, swamps ( Carex, Equisetum, Rubus) and thickets of birch ( Betula nana) and willow ( Salix ssp.). Resident grey-sided vole populations could only be found in the wet, lower areas. A detailed description of the study area is provided by Oksanen et al. (1999).
Map of the study area in Finnmarksvidda, northern Norway, the Joatka field station, and the surrounding tundra habitat. The lower map depicts the location of the base camp, and the three pairs of plots (1a/1b, 2a/2b, 3a/3b; 100×100 m with 100 Ugglan multiple-capture live-traps each). One of the two plots in a pair was treated with weasel ( Mustela nivalis) odour (a-plots in 1997 and 1999, b-plots in 1998), while the other served as a control. Treatment plots differed with respect to the concentration of weasel odour (plot 1, 25%; plot 2, 50%; plot 3, 100%; see text)
In the first year the experimental plots were chosen on the basis of whether they had established vole populations but a very low abundance of mammalian predators. Otherwise the treatment effects of experimentally applied weasel odour would have been confounded by natural scent marks. There were no fences or nets around our study sites, but from other extensive studies in the same area it was known that stoats and weasels visit the lower tundra habitat only occasionally (M. Aunapuu, T. Oksanen and L. Oksanen, personal communication). Also owls and raptors were only seen occasionally, but long-tailed scuas ( Stercorarius longicaudus) hunted quite frequently in the area. As another condition for the selection of study plots, differences in vegetation and topography had to be as small as possible, especially between the two plots which made up one pair of a treatment and control plot (a- and b-plots, see below). According to these standards, six plots—each of 1 ha in size—were selected within 2–5 km south of Joatka and 250–3,000 m from each other (see Fig. 1).
Trapping
On each plot 100 Ugglan multiple-capture live-traps baited with oats were set in a regular grid of ten rows with ten traps each and a trap mesh size of 10×10 m. The traps remained in their position for the whole time during summer and winter. Every year the arrangement of treatment and control plots within each pair was switched, i.e. in the summers of 1997 and 1999 the three a-plots served as controls and the three b-plots were treated with weasel odour, while in summer 1998 the three a-plots were treated and the b-plots served as controls. We trapped grey-sided voles on the plots from the end of June, i.e. the period of snow melt, until the first half of September when the first snow was falling again. Thus, the study period covered most of the grey-sided vole’s breeding season, which spans from June to October in that region (Henttonen and Viitala 1982). Due to the drastically changing photoperiod in high latitudes, the experiments started in the constant day light of the subarctic summer, while there was about 15 h light and 9 h darkness at the end of the field seasons in early September. With the exception of constant day light conditions during summer, the trapping schedule always covered the twilight periods of dawn and dusk, which are times of particularly high vole trappability.
We always trapped one pair of plots (a and b) in parallel, so that within 3 days all six plots were trapped once. On day 1 of a trapping session we activated the traps at 0500 hours on one plot, and at 0700 hours on the second plot of a pair. Traps were then checked after 4 and 8 h, respectively, continuously switching between the two plots every 2 h. After the last trap check the traps were deactivated. Starting from day 4, this schedule was repeated with traps activated at 1700 and 1900 hours, respectively. This second part of a trapping session was finished in the early morning of day 7. After a break of 2 or 3 days we started the next session and followed this trapping regime for the whole field season. In total this resulted in 18 trapping sessions (1997, n =4; 1998, n =8; 1999, n =6) with 288 trapping hours per plot (1997, 64 h; 1998, 128 h; 1999, 96 h).
At the first capture each vole was sexed, individually marked, and its age was estimated from body weight, fur characteristics and the visible state of vagina or scrotum. Marking was done with ear tattooing as described by Klimisch (1986) and Boye and Sondermann (1992). One ear was marked with a number (tattoo pliers with a revolving head; Ebeco, Germany), and the other ear with a letter (second pair of pliers that had to be changed manually; Hauptner, Germany). The ears were first perforated with the pins of the pliers, then a dark green or black tattoo colour (Hauptner) was rubbed into the skin with the fingertips. Markings were read with light from a halogen micro-torch held behind the ear. The reliability of this marking technique was estimated to 89.9% [see Lindner and Fuelling (2002) for a detailed report]. Body weight and reproductive state of an individual were noted again at every subsequent capture. Individuals that were recaptured at least twice on one plot were considered as residents.
Predator odour treatment
We started each year with monitor trapping on all six plots without applying any predator odour for about 1 month (hereafter called the “monitoring phase”), while the experimental treatment started in early August (hereafter called the “treatment phase”). The two phases of the experiment allowed us to consider three aspects: (1) comparison among plots and years during the monitoring phase, i.e. the unaffected early breeding season; (2) comparison between treatment and control plots during the treatment phase, i.e. the late breeding season; and (3) comparison between monitoring and treatment phase for each single plot. In 1997 there were only four trapping sessions (two during the monitoring and the treatment phase, respectively) and the treatment did not started before mid August. This delay was due to a late start of the field season caused by exceptionally late snow thaw, and the need for grown vegetation in the first year of the experiment to select the study plots according to the criterion of similar vegetation cover.
As predator odour we used bedding material from Least weasels ( Mustela nivalis) that were kept in cages at the University of Helsinki. The bedding—saw dust soaked with urine, faeces and gland extracts from weasels—was sent by parcel to Joatka. About 500 cm3 of the material was mixed with 5 l water, left for about 2 h and then filtered through coarse cloth to remove solid material. The resultant strongly smelling fluid was distributed with a spray bottle on the treatment plots every morning or evening before trapping started. On each spraying spot the spray bottle was activated 3 times which corresponded to approximately 3–5 ml fluid. Spraying spots were in the middle of the trap grid meshes, i.e. on the intersection of diagonals in the 10×10-m squares outlined by the surrounding four traps. To test whether the concentration of weasel odour had an effect on vole behaviour we applied three different densities of sprayed spots: for the lowest concentration (=25%, plots 1) only every fourth mesh was sprayed with three untreated grid meshes in between; for the second concentration (=50%, plots 2) we sprayed every second mesh; and for the highest concentration (=100%, plots 3), every mesh of the trap grid was sprayed.
Data analysis
We compared the reproductive output of females on treatment and control plots by means of the recruitment rate. In this open-field experiment it was not possible to determine the litter size of individual females since the trapping data did not reveal the matriline relationships reliably enough and we did not search for nests with young in order to keep the level of disturbance low. Therefore, we calculated the average per capita recruitment per plot from the total number of juveniles (animals between 10 and 20 g when they entered a trap for the first time) divided by the number of adult resident females (caught 3 times or more) on this particular plot. Juveniles trapped in the outermost trap lines only were not taken into account to diminish possible edge effects. Data were analysed with an ANOVA model using the four groups (control and treatment plots in the monitoring and the treatment phase, respectively), year, and treatment intensity as factors, and recruitment rate as the dependent variable.
Total recruitment rate per plot does not provide information on whether all females reduced their litter size, or whether some females stopped reproduction entirely while the others continued breeding at the same rate. Therefore, we compared the number of females that showed no signs of oestrus, pregnancy or lactation during the whole time of observation with the number of reproductively active females (plotwise comparison). The number of active and non-active females was compared between control plots and treatment plots for pooled data from the entire experiment. Additionally, we considered the body weight development of individual females. During pregnancy female body weight increases continuously and decreases in a sudden step at birth. We used this weight pattern to estimate the number of birth events for every resident adult female, searching for differences on control and treatment plots in the monitoring and the treatment phase. Finally, we looked for differences in the number of birth events of reproductively active young and overwintered females. All comparisons were tested by G -tests. Statistical analyses were done with Statview 4.5 (Haycock et al. 1994) and SuperANOVA 1.11 (Haycock et al. 1989).
Results
Trapping records
According to long-term monitoring in the Joatka area (L. Oksanen and T. Oksanen, personal communication) the grey-sided vole population showed a slight increase in 1997 and fairly high numbers in 1998. Density went down in winter 1998/1999, but vole numbers in summer 1999 were still high. So neither a major outbreak nor a severe population crash occurred during the 3 years of our study. Thus the experimental design was not seriously burdened with cyclic density fluctuations as a confounding variable.
The view that the vole densities only changed moderately was also supported by our trapping records (Table 1). During the three field seasons a total of 2,662 captures of 726 individuals was made, and the number of individuals marked divided by the number of trapping sessions (i.e. a measure of trapping intensity) remained fairly constant over the years (1997, 44.5; 1998, 37.0; 1999, 42.0). The overall average recapture rate was 3.7 captures per individual, but trappability differed markedly among individuals. One hundred and fifty-two adult voles (=20.9%) were captured at least 3 times and were thus classified as residents, while a few adults (20=2.8%) were only captured once or twice and were considered transients. The vast majority of 555 individuals (=76.4%) were marked as subadults or juveniles and disappeared or died before they reached maturity.
Considering the open-field situation and the long and harsh winter conditions in the subarctic tundra, a surprisingly high proportion of individuals from the previous field season was recaptured when trapping resumed in 1998 and 1999 (Table 1). With 14 out of 60 (1998) and 13 out of 55 (1999), respectively, overwintered voles made up >1/5 of the overall resident population, and many of them could be followed until August and September of their second summer. Out of the 27 overwintered voles, 14 were males (1998, nine; 1999, five) and 13 were females (1998, five; 1999, eight).
Reproduction
To test for effects of the predator odour on reproduction we compared the number of resident females (caught 3 times or more on a plot) and the number of juveniles (recruits) when they were caught the first time (Table 2). Neither the year of observation (ANOVA, F =0.05, df =2, P =0.949) nor the different densities of spraying spots on the 25%, 50% or 100% treatment plots (ANOVA, F =2.25, df =2, P =0.138) had a significant effect on average recruitment rate as the dependent variable, which allowed us to pool the data for statistical analyses. We found weasel odour to have a statistically significant effect on the recruitment rate on treatment plots as compared to the control plots (ANOVA, F =6.30, df =2, P =0.010; Fig. 2). During the monitoring phase, recruitment rates were comparable on the monitoring and treatment plots (Fisher’s protected LSD, P =0.833). Recruitment rate increased considerably from early to late summer on the control plots (Fisher’s protected LSD, P =0.003), but remained low or even slightly decreased on the plots treated with weasel odour (Fisher’s protected LSD, P =0.795).
Mean recruitment (+SD) of grey-sided voles ( Clethrionomys rufocanus) on control and treatment plots. Recruitment was estimated plotwise as the average number of juveniles per adult resident female, based on the pooled trapping data (1997–1999) during the monitoring phase ( m, early summer) and the treatment phase ( t, late summer). The black column indicates recruitment under treatment with weasel odour
Apart from the obvious overall trend in the pooled data set, breaking down data to single plots and years revealed a considerable amount of variance in the seasonal dynamics of recruitment rates (Fig. 3). Out of the nine control plots, six showed an increasing recruitment rate in late summer (Fig. 3a). On one control plot the recruitment rate decreased, while estimates for the remaining two plots were not possible because data sets were incomplete (no adult females observed during the monitoring phase on plot 2 in 1997, and during the treatment phase on plot 1 in 1999). A decreasing or stagnating recruitment rate in late summer was found in six out of the nine treatment plots (Fig. 3b), while two plots showed an increase, and on plot 3 no adult females were trapped during the treatment phase in 1999.
Changes in recruitment (average number of juveniles per adult resident female) of grey-sided voles between the m phase (early summer) and t phase (late summer) on control and treatment plots. Data as in Fig. 2, but split by year and plot to show the degree of variance in the observed pattern. Concentrations of weasel odour: plots 1 ( diamonds)=25% sprayed spots, plots 2 ( triangles)=50% sprayed spots, plots 3 ( squares)=100% sprayed spots. Three data points are missing because no adult resident females were captured (1997, control plot 2, m phase; 1999, control plot 1, t phase; 1999, treatment plot 3, t phase). For abbreviations, see Fig. 2
We trapped significantly ( G-test, P=0.024) more reproductively non-active females on the treatment plots (55 active, 15 non-active) than on control plots (59 active, five non-active). Based on the curves of individual long-term weight development which allowed us to exclude phases of increased body weights due to pregnancies, the non-active females were always among the lightest of all adult females and gained very little weight over the whole summer. Among the reproductively active females on treatment and control plots there was no significant ( G -test, P =0.841) difference in numbers between the monitoring (control plots 29, treatment plots 26) and the treatment phase (control plots 30, treatment plots 29). Likewise, no significant difference (G-test, P =0.196) could be found between the number of litters (birth events recorded as a sudden drop in body weight) of females which were born the previous year and had overwintered (control plots 19, treatment plots 11) as compared to adult females in their first summer (control plots 12, treatment plots 14).
Discussion
Breeding suppression under field conditions
Effects of predation pressure on home range size, habitat preferences, activity patterns, and breeding are expressions of anti-predatory behavioural responses related to two crucial aspects of the prey’s life, i.e. foraging and reproduction. Several studies have shown that small mammal foraging is risk-sensitive (e.g. Brown et al. 1988; Holmes 1991; Kotler et al. 1991; Kotler et al. 1993; Otter 1994). Oksanen and Lundberg (1995) have analysed the fitness consequences of maximum individual survival (i.e. foraging), maximum reproductive output, and intermediate strategies at different levels of predation risk by a modelling approach. Reduced reproductive activity in the face of high predation pressure may increase individual survival, but will—on the other hand—inevitably decrease the number of produced offspring. Hence, an awkward evolutionary trade-off with respect to the reproductive value and lifetime reproductive success has to be solved in order to maximise individual fitness.
Predator odour, which serves as a cue for prey to estimate predator abundance, was applied in this experiment to simulate high apparent predation risk in the natural tundra habitat of grey-sided voles. Although we intentionally selected plots with no or very little actual predator presence to avoid a confusing experimental design, weasels are a very common source of predation risk for grey-sided voles in tundra habitats. Hence strategic responses of predator avoidance are to be expected as general behavioural traits even in populations that do not suffer from acute predation pressure. In accordance with the breeding suppression hypothesis, we found lower average per capita recruitment rates on the plots treated with weasel odour as compared to untreated control plots. During the monitoring phase, the plots that later became the treatment plots showed recruitment rates similar to the control plots, but in most cases the rates did not increase in the later breeding season, which is the natural pattern in the area (L. Oksanen, personal communication) and which was observed on the control plots. Since predation was only simulated with no actual removal of individuals, and since the lack of recruitment increase was independent of the year of observation or particular plots, it is reasonable to assume that the lower recruitment rate on treatment plots was a response of the voles to the olfactory signal alone.
Only two exceptions from the general pattern occurred, i.e. per capita recruitment rate increasing in late summer on treatment plots (see Fig. 3b). Interestingly, both exceptions occurred on plots with the lowest spraying spot density (plots 1, 25%), but since an influence of treatment intensity could not been proven statistically with the ANOVA model used, these exceptions might also have been stochastic variations due to low vole densities on these plots. Note that the recruitment rate also decreased on one control plot in late summer, suggesting some defective variation occurring in our field experiments which indeed were impeded by many uncontrollable factors. Taking these deficiencies into consideration, the pattern of responses depicted in Fig. 3 was fairly conclusive.
For the interpretation of the general pattern it is crucial to consider the underlying processes that resulted in reduced recruitment rates on the treatment plots in late summer. We found more non-breeding adult females on the treatment plots, while the number of reproductively active females on control and treatment plots did not differ between the monitoring and the treatment phase. This indicates that the mechanism behind lower recruitment rates was probably not a general reduction in litter size. Rather, some individual females responded with a total suppression of breeding, whereas the majority of the females continued to breed unaffected by the olfactory cue.
A question that could not be answered by our study is whether breeding suppression was a specific response to apparent predation risk or a general response to a novel smell in the environment. Koskela et al. (1996) found an equal weight reduction in non-breeding females in response to weasel and rabbit odour, so Mappes et al. (1998) and Korpimäki and Krebs (1996) hypothesised that breeding suppression may simply be a side-effect of reduced food intake when voles are confronted with unfamiliar olfactory signals. Several findings in the literature confirm a response to novel olfactory cues per se, but the responses are in general stronger with predator scent marks as compared to non-predator odours (Kemble and Bolwahnn 1997; Perrot-Sinal and Petersen 1997; Bramley et al. 2000). A stronger response to predator odours was even found in Norway rats unfamiliar with mammalian predators in their natural habitat (Bramley et al. 2000), suggesting that the responses are triggered by sulphurous odours, a signal for carnivore digestion (Nolte et al. 1994). Other studies (e.g. Calder and Gorman 1991; Perrot-Sinal et al. 1996) failed to show any effect of non-predator odours on prey behaviour.
Still, the possibility of a neophobic effect has to be tested properly by experiments with predator, non-predator, and no odours. In our study we decided not to include a non-predator odour as a neophobic control due to the limitation of plots and replicates. No treatment and predator-odour treatment are the two extremes, while effects of non-predator odours are to be expected somewhere in between. Since field experiments are already burdened with much environmental noise, experiments under more controlled conditions in enclosures seem to be more appropriate to solve this question. However, from a theoretical point of view, breeding suppression as a response to novelty as such does not seem to be an adequate explanation, because there would be no direct fitness benefit compensating for a considerable loss in actual reproductive output. Breeding suppression as a specific response to predator odours, however, would provide such a payback and would fit into the scenario of an evolutionary “arms race” in which the prey minimises predation risk by every means accessible. Interestingly, the only positive finding for a neophobic effect in voles so far (Koskela et al. 1996) was related to feeding and weight development, while reproduction, measured as the number and length of oestrous cycles, in the same experiment, was significantly lower when females were confronted with Least weasels than with rabbits. Since controls for no odour were not included in the study of Koskela et al. (1996), the claim for the existence of a neophobic effect (Mappes et al. 1998) is questionable anyway.
Breeding suppression—a new interpretation
Although numbers were small we found clear indications that non-breeders were not only young adult females in their first summer, but also old females from the previous year. This is in obvious conflict with the predictions from Ylönen’s breeding suppression hypothesis (Ylönen and Ronkainen 1994) and with the originally assumed selective benefit from breeding delayed until the next summer. Interestingly, Ylönen and Ronkainen (1994) also found breeding suppression in young as well as in old females in a laboratory experiment, and the assumed lifetime reproductive benefit from long-term breeding suppression has always been controversial (Hansson 1995; Lambin et al. 1995; Kokko and Ranta 1996; Kaitala et al. 1997; Prevot-Julliard et al. 1999; Kokko and Ruxton 2000).
Therefore, we want to put forward an alternative idea for explaining the breeding suppression phenomena on a much smaller spatial and shorter temporal scale. In our experiment we exposed the vole populations to a constant odour treatment from late July/early August until the end of the breeding season in early September, which simulated continually high predation pressure over a period of 6 or 7 weeks. However, this might have been an unrealistic assumption. The tundra of northern Fennoscandia is not a continuous habitat (Wielgolaski 1975), but rather shows considerable heterogeneity with respect to patches of high and low productivity, and totally barren ground. This also applies to the Joatka area where our experiment was conducted. In this kind of landscape, voles are not continuously distributed, but reside in patches with uneven abundance. Mustelid predators will visit profitable foraging patches occasionally and change to another patch when the first one is exploited to the threshold of profitability (Oksanen and Schneider 1995). Hence the time a predator spends on a particular patch will depend on prey density (Charnov 1976; Oksanen et al. 1981).
Under these conditions, voles might not suppress breeding for the whole season, but only for the limited time interval of high predation pressure, i.e. as long as a predator is actually present in a particular patch. The observed breeding response was combined with reduced growth rates during summer and a significant reduction of activity ranges on the plots treated with weasel odour (Fuelling 2001). Females in oestrus increase their activity (Cushing 1985), and predation risk is generally higher in wide-ranging voles (Norrdahl and Korpimäki 1998; Banks et al. 2000). So the reduction of female mobility under predation risk might be part of a complex behavioural strategy of generally reduced activity, comprising both reproductive activity and use of space. Such a short-term “duck and cover” strategy would be advantageous for both young and old females, which is more consistent with our findings than the original breeding suppression hypothesis. In accordance with this short-term view, our adult voles did not leave the area contaminated with weasel odour, as observed by Jedrzejewski and Jedrzejewska (1990b). However, immigration into and emigration from the treatment plots may have been decreased and increased, respectively, in juveniles, which could have contributed to the pattern that we have observed. But since we have no data on the unmarked juveniles, this additional or even alternative explanation must remain speculative.
Obviously the intrusion of a mustelid predator is unpredictable for the voles, so some females might already be pregnant or have to take care of a litter when predation risk increases. Consequently, not all of the females can stop breeding immediately in the presence of a predator, and the option of the duck and cover strategy will be restricted to a relatively small proportion of the patch residents. Therefore, an observable decrease in breeding which affects the population level will only be prevalent under experimental conditions or in high density patch populations that cause the predator to be present for a longer time interval. This might also contribute to the still enigmatic rapid decline of vole densities during the crash phase of a population cycle: high prey density during the peak phase will increase the number of predators visiting a particular patch and the time intervals of their presence. We predict that the longer predation pressure remains high the more females—young and old—will switch to the duck and cover strategy and cease breeding. In this situation the duck and cover strategy would have an even stronger effect on density dynamics than the original breeding suppression hypothesis, according to which only young females should delay breeding.
However, an alternative explanation of the duck and cover strategy would be that it is an evolutionary trait driven by frequency-dependent selection, i.e. that it is not evolutionarily stable unless the trait is restricted to a minority. These conflicting assumptions have to be tested by a combination of laboratory studies, enclosure and field experiments as well as by modelling approaches, which have to explicitly consider the fitness consequences of breeding suppression and the patchy habitat structure. Our results suggest that breeding suppression under high predation risk is a natural behavioural pattern, and not a mere laboratory artefact. It might be primarily based on the patchiness of small mammal abundance and would, therefore, not necessarily be limited to cyclic populations. However, the underlying physiological mechanisms and the individual fitness consequences in particular have to be further investigated in detail.
References
Abramsky Z, Strauss E, Subach A, Kotler BP, Riechman A (1996) The effect of barn owls ( Tyto alba) on the activity and microhabitat selection of Gerbillus allenbyi and G. pyramidum. Oecologia 105:313–319
Andelt WF, Beck TDI (1998) Effect of black-footed ferret odors on behavior and reproduction of prairie dogs. Southwest Nat 43:344–351
Banks PB, Norrdahl K, Korpimäki E (2000) Nonlinearity in the predation risk of prey mobility. Proc R Soc Lond B 267:1621–1625
Bowers MA, Dooley JLJ (1993) Predation hazard and seed removal by small mammals: microhabitat versus patch scale effects. Oecologia 94:247–254
Boye P, Sondermann D (1992) Ohrtätowierungen zur individuellen Kennzeichnung von Nagetieren im Freiland. Säugetierkd Inf 3:425–430
Bramley GN, Waas JR, Henderson HV (2000) Responses of wild Norway rats ( Rattus norvegicus) to predator odours. J Chem Ecol 26:705–719
Brown JS, Kotler BP, Smith RJ, Wirtz WO (1988) The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia 76:408–415
Burwash MD, Tobin ME, Woolhouse AD, Sullivan TP (1998) Laboratory evaluation of predator odors for eliciting an avoidance response in roof rats ( Rattus rattus). J Chem Ecol 24:49–66
Calder CJ, Gorman ML (1991) The effects of red fox Vulpes vulpes faecal odours on the feeding behaviour of Orkney voles, Microtus arvalis. J Zool Lond 224:599–606
Carlsen M, Lodal J, Leirs H, Jensen TS (2000) Effects of predation on temporary autumn populations of subadult Clethrionomys glareolus in forest clearings. Z Säugetierkd 65:100–109
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theor Pop Biol 9:129–136
Cushing BS (1985) Estrous mice and vulnerability to weasel predation. Ecology 66:1976–1978
Dawkins R, Krebs JR (1979) Arms races between and within species. Proc R Soc Lond B 205:489–511
Elton CS (1924) Periodic fluctuations in the numbers of animals: their causes and effects. Br J Exp Biol 2:119–163
Fuelling O (2001) Behavioural response of grey-sided voles ( Clethrionomys rufocanus) to mustelid predation—a field study in the Finnmarksvidda of northern Norway. Doctorate thesis. Friedrich-Schiller-University Jena, Jena
Hansson L (1995) Is the indirect predator effect a special case of generalized reactions to density-related disturbances in cyclic rodent populations? Ann Zool Fenn 32:159–162
Haycock KA, Roth J, Gagnon J, Feldman DSJ, Finzer WF, Hofmann R, Simpson J (1989) SuperANOVA 1.11. Abacus Concepts
Haycock KA, Roth J, Gagnon J (1994) StatView 4.5. Abacus Concepts
Henttonen H, Viitala J (1982) Clethrionomys rufocanus (Sundevall, 1846)—Graurötelmaus. In: Niethammer J, Krapp F (eds) Handbuch der Säugetiere Europas, Rodentia II, vol 2/II. Akademische Verlagsgesellschaft, Wiesbaden, pp 147–164
Holmes WG (1991) Predator risk affects foraging behavior of pikas: observational and experimental evidence. Anim Behav 42:111–120
Jedrzejewska B, Jedrzejewski W (1990a) Antipredator behaviour of bank voles and prey choice of weasels—enclosure experiments. Ann Zool Fenn 27:321–328
Jedrzejewski W, Jedrzejewska B (1990b) Effect of a predator’s visit on the spatial distribution of bank voles: experiments with weasels. Can J Zool 68:660–666
Jonsson P, Koskela E, Mappes T (2000) Does risk of predation by mammalian predators affect the spacing behaviour of rodents? Two large-scale experiments. Oecologia 122:487–492
Kaitala V, Mappes T, Ylönen H (1997) Delayed female reproduction in equilibrium and chaotic populations. Evol Ecol 11:105–126
Kemble ED, Bolwahnn BL (1997) Immediate and long-term effects of novel odors on risk assessment in mice. Physiol Behav 61:543–549
Klimisch HJ (1986) Ohrtätowierungstechnik zur individuellen Kennzeichnung von weissen Kleintiernagern. Z Versuchstierkd 28:277–281
Kokko H, Ranta E (1996) Evolutionary optimality of delayed breeding in voles. Oikos 77:173–175
Kokko H, Ruxton GD (2000) Breeding suppression and predator-prey dynamics. Ecology 81:252–260
Korpimäki E, Krebs CJ (1996) Predation and population cycles of small mammals—a reassessment of the predation hypothesis. BioScience 46:754–764
Korpimäki E, Koivunen V, Hakkarainen H (1996) Microhabitat use and behavior of voles under weasel and raptor predation risk: predator facilitation? Behav Ecol 7:30–34
Koskela E, Ylönen H (1995) Suppressed breeding in the field vole ( Microtus agrestis): an adaptation to cyclically fluctuating predation risk. Behav Ecol 6:311–315
Koskela E, Horne TJ, Mappes T, Ylönen H (1996) Does risk of small mustelid predation affect the oestrous cycle in the bank vole, Clethrionomys glareolus? Anim Behav 51:1159–1163
Kotler P, Brown JS, Hasson O (1991) Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72:2249–2260
Kotler BP, Brown JS, Slotow RH, Goodfriend WL, Strauss M (1993) The influence of snakes on the foraging behavior of gerbils. Oikos 67:309–316
Krebs CJ, Meyers JH (1974) Population cycles in small mammals. Adv Ecol Res 8:267–399
Lambin X, Ims RA, Steen H, Yoccoz NG (1995) Vole cycles. Trends Ecol Evol 10:204
Lindner E, Fuelling O (2002) Marking methods in small mammals—ear-tattoo as an alternative to toe-clipping. J Zool Lond 256:159–163
Mappes T, Koskela E, Ylönen H (1998) Breeding suppression in voles under predation risk of small mustelids: laboratory or methodological artifact? Oikos 82:365–369
Nolte DL, Mason JR, Epple G, Aronov E, Campbell DL (1994) Why are predator urines aversive to prey? J Chem Ecol 20:1505–1516
Norrdahl K, Korpimäki E (1998) Does mobility or sex of voles affect risk of predation by mammalian predators? Ecology 79:226–232
Oksanen L, Lundberg P (1995) Optimization of reproductive effort and foraging time in mammals: the influence of resource level and predation risk. Evol Ecol 9:45–56
Oksanen T, Schneider M (1995) The influence of habitat heterogeneity on predator-prey dynamics. In: Lidicker WZ (ed) Landscape approaches in mammalian ecology and conservation. University of Minnesota Press, Minneapolis, Minn., pp 122–150
Oksanen L, Fretwell SD, Arruda J, Niemela P (1981) Exploitation ecosystems in gradients of primary productivity. Am Nat 118:240–261
Oksanen T, Schneider M, Rammul U, Hamback P, Aunapuu M (1999) Population fluctuations of voles in north Fennoscandian tundra: contrasting dynamics in adjacent areas with different habitat composition. Oikos 86:463–478
Otter K (1994) The impact of potential predation upon the foraging behaviour of eastern chipmunks. Can J Zool 72:1858–1861
Perrot-Sinal T, Petersen K (1997) Exposure to predator odor reduces locomotor activity levels in adult male rats: lack of effect of hippocampal lesion. J Chem Ecol 23: 2175–2186
Perrot-Sinal T, Heale VR, Kavaliers M, Ossenkopp K-P (1996) Sexually dimorphic aspects of spontaneous activity in meadow voles Microtus pennsylvanicus: effects of exposure to fox odor. Behav Neurosci 110:1126–1132
Pianka ER, Parker WS (1975) Age-specific reproductive tactics. Am Nat 109: 453–464
Prevot-Julliard AC, Henttonen H, Yoccoz NG, Stenseth NC (1999) Delayed maturation in female bank voles: optimal decision or social constraint? J Anim Ecol 68:684–697
Ronkainen H, Ylönen H (1994) Behaviour of cyclic bank voles under risk of mustelid predation: do females avoid copulations? Oecologia 97:377–381
Steen H (1994) Spatial aspects of population dynamics in small rodents. V. Do predators determine the survival rates of voles? An experiment. PhD thesis. University of Oslo, Oslo
Stenseth NC, Ims RA (1993) Population dynamics of lemmings: temporal and spatial variation—an introduction. In: Stenseth NC, Ims RA (eds) The biology of Lemmings. Linnean Society Symposium Series. Academic Press, London, pp 61–96
Stoddart DM (1976) Effect of the odour of weasels ( Mustella nivalis L) on trapped samples of their prey. Oecologia 22:439–441
Wielgolaski FE (1975) Primary production of alpine meadow communities. In: Wielgolaski FE (ed) Fennoscandian tundra ecosystems. Part 1. Plants and microorganisms. (Ecological Studies, vol 16) Springer, Berlin Heidelberg New York, pp 121–128
Wolff JO, Davis-Born R (1997) Response of gray-tailed voles to odours of a mustelid predator: a field test. Oikos 79:543–548
Ylönen H (1989) Weasels Mustela nivalis suppress reproduction in cyclic bank voles Clethrionomys glareolus. Oikos 55:138–140
Ylönen H (1994) Vole cycles and antipredatory behaviour. Trends Ecol Evol 9:426–430
Ylönen H, Magnhagen C (1992) Antipredatory behaviour: a “hot spot” of evolutionary ecology of the nineties. Ann Zool Fenn 29:179–182
Ylönen H, Ronkainen H (1994) Breeding suppression in the bank vole as antipredatory adaption in a predictable environment. Evol Ecol 8:1–9
Ylönen H, Jedrzejewska B, Jedrzejewski W, Heikkilä J (1992) Antipredatory behaviour of Clethrionomys voles—”David and Goliath” arms race. Ann Zool Fenn 29:207–216
Ylönen H, Koskela E, Mappes T (1995) Small mustelids and breeding suppression of cyclic microtines: adaptation or general sensitivity? Ann Zool Fenn 32:171–174
Acknowledgements
We thank Tarja and Lauri Oksanen for their invitation to work in their study area and for many fruitful discussions, and Britta and Helge Romsdal for their hospitality at Joatka Fjellstue. We are especially grateful to Janne Sundell for providing us with the disreputable output of his weasels, and to the post office at Alta for storing the smelly packages until we could collect them. Michael Depka, Katre Eplor, Oskar Eriksen, Helmut Fuelling, Michael Hühn, Elke Lindner, Jonas Richter, Sönke Weinert, and Alexander Wolf assisted the field work and helped us fighting the mosquitoes. Particular thanks go to Hannu Ylönen for helpful comments and discussions about breeding suppression, and to Andrew Davis, John Slogett, Vicky Temperton, Marcio Fattorini, Blandine Massonnet and anonymous reviewers who commented on the manuscript. The study was financially supported by the German Research Council (DFG, grants HA 1513/9-1 and HA 1513/9-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuelling, O., Halle, S. Breeding suppression in free-ranging grey-sided voles under the influence of predator odour. Oecologia 138, 151–159 (2004). https://doi.org/10.1007/s00442-003-1417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1417-y