Abstract
We hypothesized that the greater competitive ability of invasive exotic plants relative to native plants would increase under elevated CO2 because they typically have traits that confer the ability for fast growth when resources are not limiting and thus are likely to be more responsive to elevated CO2. A series of competition experiments under ambient and elevated CO2 glasshouse conditions were conducted to determine an index of relative competition intensity for 14 native-invasive exotic species-pairs. Traits including specific leaf area, leaf mass ratio, leaf area ratio, relative growth rate, net assimilation rate and root weight ratio were measured. Competitive rankings within species-pairs were not affected by CO2 concentration: invasive exotic species were more competitive in 9 of the 14 species-pairs and native species were more competitive in the remaining 5 species-pairs, regardless of CO2 concentration. However, there was a significant interaction between plant type and CO2 treatment due to reduced competitive response of native species under elevated compared with ambient CO2 conditions. Native species had significantly lower specific leaf area and leaf area ratio under elevated compared with ambient CO2. We also compared traits of more-competitive with less-competitive species, regardless of plant type, under both CO2 treatments. More-competitive species had smaller leaf weight ratio and leaf area ratio, and larger relative growth rate and net assimilation rate under both ambient and elevated CO2 conditions. These results suggest that growth and allocation traits can be useful predictors of the outcome of competitive interactions under both ambient and elevated CO2 conditions. Under predicted future atmospheric CO2 conditions, competitive rankings among species may not change substantially, but the relative success of invasive exotic species may be increased. Thus, under future atmospheric CO2 conditions, the ecological and economic impact of some invasive exotic plants may be even greater than under current conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past two decades, the potential for invasive exotic plants to alter ecosystem structure and function has been increasingly recognized (Levine et al. 2003; Dassonville et al. 2008). The invasion of ecosystems by exotic plants has been identified as a major threat to biodiversity (Mack et al. 2000; van der Wal et al. 2008; Roura-Pascual et al. 2009) and is considered a significant management and economic concern (Pimentel et al. 2005; Beck et al. 2009).
When exotic plants become dominant in the vegetation communities they invade, this dominance is generally attributed to their superior competitive ability (Maron and Connors 1996; Hamilton et al. 1999; Callaway and Aschehoug 2000; Ewing 2002; Groves et al. 2003; Miller and Duncan 2004; White and Holt 2005; Coleman and Levine 2007; Pfeifer-Meister et al. 2008). However, there are relatively fewer studies that have measured competitive ability of native and exotic species directly. These studies have generally found that invasive exotic plants are competitively superior to their native counterparts in both terrestrial (Barney et al. 2009; Werner et al. 2010) and marine environments (Bando 2006; Wang et al. 2006; Rhazi et al. 2009), although there are exceptions (Vila et al. 2003; Corbin and D’Antonio 2004; Li et al. 2008).
An important applied question in ecological research is whether interactions between invasive exotic and native species will be affected by predicted future climate change (Dukes and Mooney 1999; Hellmann et al. 2008). One critical component of global change that will directly affect plant species’ interactions is increasing atmospheric CO2 concentration. Over the last two decades, the amount of CO2 available to plants has increased significantly and this increase is predicted to continue under a range of emission scenarios (IPCC 2007). Plants with fast growth have been shown to be favored by this increased availability of CO2 (LaDeau and Clark 2001; Tangley 2001; Poorter and Navas 2003). Leaf traits that are associated with carbon capture strategies resulting in fast growth when resources are not limited are strongly correlated with invasiveness (Smith and Knapp 2001; Grotkopp et al. 2002; Burns 2006; Grotkopp and Rejmanek 2007; Leishman et al. 2007; Leishman et al. 2010). Therefore, it can be hypothesized that invasive exotic plants will have a greater positive response to elevated CO2 conditions than their native counterparts, resulting in an increase in their competitive ability relative to slower growing species. Open top chamber (Dukes 2002; Hattenschwiler and Korner 2003), controlled environment glasshouse (Smith et al. 1987), environmental controlled growth chamber (Sasek and Strain 1991; Ziska 2003; Baruch and Jackson 2005; Ziska et al. 2005; McPeek and Wang 2007; Song et al. 2009), and FACE (Smith et al. 2000; Huxman and Smith 2001; Belote et al. 2003; Nagel et al. 2004) experiments that have manipulated CO2 levels have provided evidence to support this hypothesis (but see Taylor and Potvin 1997; Dukes 2000; Bradford et al. 2007 for exceptions).
We employed an experimental design that measured the relative competitive ability of native and exotic species grown in pairs, rather than inferring competitive ability based on abundance and growth data. Such a measure of competitive ability has the advantage that it encompasses a suite of potential mechanisms, e.g., increased photosynthetic rates (Poorter and Navas 2003; Long et al. 2004; Song et al. 2009), and decreased leaf construction costs (Nagel et al. 2004; Baruch and Jackson 2005) and expresses them as an outcome of the interaction between two species. However, this method has the disadvantage of being based on interactions of only two plant species. We attempted to overcome this by measuring competitive outcomes for 14 native-invasive exotic species-pairs that encompassed a range of growth forms and families. We are unaware of any previous study that has utilized a competitive index for measuring competitive interactions between plants with CO2 concentration as a factor. As the competition index is based on biomass, it is reasonable to assume that the more competitive species will become relatively more abundant than the less-competitive species. However, it should be noted that our measure of competitive ability does not include some mechanisms, such as increased reproductive output (Smith et al. 2000; Nagel et al. 2004; McPeek and Wang 2007) or enhancement in germination rates (Baruch and Jackson 2005; McPeek and Wang 2007), that may also be affected by elevated CO2.
The general question addressed in this study is: are competitive interactions between native and invasive exotic plant species altered under projected future CO2 conditions? We grew co-occurring common native and invasive exotic species of the Cumberland Plain Woodland of western Sydney in non-limiting water and nutrient conditions in a series of paired competition experiments. The hypothesis we tested was that the superior competitive ability of invasive exotic plant species relative to co-occurring native plant species of the same functional type will be enhanced under elevated CO2 levels. We then asked: what growth and allocation traits contribute to changes in competitive ability under elevated CO2?
Materials and methods
Experimental design
Fourteen native and invasive exotic plant species-pairs were grown in a series of competition experiments under ambient (380–420 ppm) and elevated (675–715 ppm) CO2 concentrations. The ambient treatment represents the atmospheric CO2 concentration during the turn of the twenty-first century (IPCC 2007). The elevated treatment represents the predicted atmospheric CO2 concentration by 2100 (IPCC 2007). Target plants were grown either singly in pots, or surrounded by three neighbors of the other species from the species-pair. For each species-pair, the competitive response of the target native and invasive exotic species was determined. There are a number of methods that have been developed to measure competitive ability, including the relative competition index (Grace 1995), relative neighbor effect (Markham and Chanway 1996) and logarithm of response ratio (Goldberg et al. 1999), that have varying advantages and disadvantages (Goldberg et al. 1999; Oksanen et al. 2006). We chose to measure the corrected index of relative competition intensity (CRCI) following the method of Oksanen et al. (2006).
There were eight replicates of each of the four competition treatments for each species-pair (each species within the pair grown singly and in competition). This design resulted in a total of 896 pots (i.e. 2 CO2 treatments × 4 competition treatments × 8 replicates × 14 species-pairs). Each CO2 treatment was split between two glasshouses to ensure that the CO2 treatments were not confounded with the glasshouse. The temperature of the glasshouses was set for a maximum of 28°C and a minimum of 21°C. Within each glasshouse, treatments were randomly assigned to pots. On a fortnightly basis for the duration of the experiment, the pots within each glasshouse were randomly assigned to new positions to reduce bias caused by variation across the different areas within each glasshouse.
Species selection, seed collection and germination
The plant species used in this study are common co-occurring species of the Cumberland Plain Woodland, western Sydney, Australia. Cumberland Plain Woodland typically consists of open eucalypt woodland with a diverse grassy and herbaceous ground cover (Little 2003). All the exotic species are considered to be successful invaders rather than simply exotics that have become naturalized in Cumberland Plain Woodland. Species pairs were selected based on three criteria: the species within each pair were (1) from the same functional group (grass, vine, herb or shrub/tree); (2) utilized the same photosynthetic pathway (C3 or C4); and (3) had the same life history (i.e. annual or perennial). Seeds for each of the 28 plant species were collected from a range of individual plants from sites in the Hawkesbury region of western Sydney or were obtained from a commercial supplier (Nindethana Seed Service, Albany, WA, Australia). All species and their traits are shown in Table 1. Once collected, the seeds for each of the 28 plant species were germinated on moist filter paper within petri dishes. To spread the risk of germination failure, each plant species was germinated in a number of different petri dishes.
Planting and growth
The seedlings were transplanted at the stage of cotyledon emergence into the treatments described above, with all pots for each species-pair being planted within 24 h of each other. This removed the effects of differences between species in time of germination. For each individual target or neighbor plant, multiple seedlings were transplanted as insurance against seedling mortality. After 3 days, the remaining excess seedlings were removed from the pots.
The seedlings were grown in pots with a diameter of 175 mm and a depth of 195 mm. The pots contained 2.4 L of a soil mixture consisting of Cumberland Plain Woodland soil, organic garden mix and coarse river sand in a ratio of 2:1:1. The Cumberland Plain Woodland soil was obtained from Mt Annan Botanical Gardens while the organic garden mix and river sand were obtained from a commercial supplier (Australian Native Landscapes, Terrey Hills, NSW, Australia). To prevent any soil being lost from the holes in the bottom of the pots during the experiment, the pots were lined with newspaper.
The plants were grown for a period of 12 weeks under the specified glasshouse conditions. The plants were mist watered for 2 min three times daily to ensure that they were not water-limited. To counteract the nutrient loss resulting from this daily watering, 6.5 ± 0.2 g of slow release native plant fertilizer (23N:2P:17K; J.R. Somplo, Lathrop, CA, United States) was added to each pot. After 4 weeks of growth, lattices were placed around the perimeter of the pots that contained vine species which allowed them to climb. This ensured that the vine species were localized to their own pots so that they did not influence the growth of plants in neighbouring pots.
Harvesting and measuring competition
After the 12-week growth period the target plants were harvested into the following components: (1) three fully expanded outer canopy leaves, (2) the remaining leaf biomass, (3) the belowground biomass, and (4) the stem biomass. All plant parts were washed free of soil before being oven-dried at 80°C for 48 h and weighed using a Mettler Toledo B-S electronic balance. The weights of the different components were then added together to give the total biomass of the target plant. Using this data the relative neighbor effect (RNE) was calculated by randomly pairing target plants grown in competition with those grown without competitors within each species-pair.
where X is the total biomass of plants grown without competitors and Y is the total biomass of plants grown in competition.
Subsequently, using the RNE value, the corrected index of relative competition intensity (CRCI) was calculated (Oksanen et al. 2006).
Therefore a CRCI value = 0 indicates there is no effect of competition on the target plant, >0 indicates that competition has a negative effect, and <0 indicates that competition has a positive effect on the target plant. Thus low CRCI values indicate a greater competitive response.
Data analysis of CRCI values
To determine if competitive interactions between the native and invasive exotic species were affected by elevated CO2, a mixed model nested ANOVA was performed. The factors in the model were CO2 concentration (i.e. elevated or ambient), plant type (i.e. invasive exotic or native) and species pair nested within plant type. Plant type and CO2 concentration were treated as fixed factors and species-pair as a random factor. The response variable was the calculated CRCI. We did an initial analysis to determine if there was a significant difference between the two glasshouses within each CO2 treatment, using the same mixed model nested ANOVA as above but with the additional factor ‘glasshouse’ added. Glasshouse was found to be not significant (P = 0.769) and so was removed from subsequent models. We then used paired t tests to test for significant differences between all possible combinations of plant type and CO2.
Measuring growth and allocation traits
To determine the contribution of growth and allocation traits to the biomass outcomes analyzed as the CRCI values, a range of traits that reflect allocation at the leaf-level or whole-plant level were measured or calculated for each species on an individual plant basis, using the target plants grown in competition. We measured relative growth rate (RGR) and its components specific leaf area (SLA), leaf mass ratio (LMR) and net assimilation rate (NAR), as well as leaf area ratio (LAR) and root weight ratio (RWR). The measurement and calculation of each trait is described in Table 2.
Data analysis of growth and allocation traits
We used paired t tests to examine if there were differences in trait values for each plant type × CO2 pair-wise combination that was found to differ significantly in CRCI values. We then wanted to examine what traits contributed to competitive ability at both ambient and elevated CO2, irrespective of plant type. To do this, we used paired t tests to compare the trait values of the more-competitive species with the less-competitive species within each species-pair.
All statistical analyses were performed using Minitab 15 statistical software (Minitab 2007) with the significance level set at 0.05.
Results
CRCI analysis
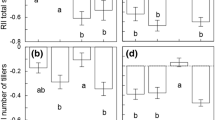
There was no significant interaction between species-pairs (within plant type) and CO2 treatment (F 26,447 = 1.05, P = 0.394; Online Resource 3), indicating that the competitive rankings within each species pair were not altered by CO2 treatment (Online Resource 2). In 9 of the 14 species-pairs the invasive exotic species was more competitive and in 5 of the 14 species-pairs the native species was more competitive, under both ambient and elevated CO2 conditions (Online Resource 2). There was a significant difference in CRCI between species-pairs nested within plant type (F 26,447 = 10.89, P < 0.001; Online Resource 3).
There was a significant interaction between plant type and CO2 treatment (F 1,447 = 4.45, P = 0.045; Online Resource 3), suggesting that the native and invasive exotic species’ competitive response varied with CO2 treatment. Paired t tests showed that on average the competitive response of the native species decreased under elevated CO2 compared to ambient CO2 (\( \bar{y}_{{\rm Ambient\, CO}_{2}} \) = 0.377, \( \bar{y}_{{\rm Elevated \, CO}_{2}} \) = 0.554; t 13 = 2.417, P = 0.031), while no other plant type × CO2 treatment contrasts were significant (Table 3; Fig. 1).
Corrected relative competition index (CRCI) of 14 native and exotic species-pairs (mean ± SE) grown under ambient (380–420 ppm) and elevated CO2 (675–715 ppm) treatments. CRCI is a measure of competitive response based on biomass, with values >0 indicating that competition has a negative effect on plant biomass. CRCI values closer to 0 indicate a stronger competitive response
Growth and allocation trait analysis
We used paired t tests to examine if there were trait differences between native species under ambient and elevated CO2 treatments as these were the only plant type × CO2 combinations that were found to differ significantly in competitive response. Both SLA and LAR of native species were significantly lower under elevated CO2 compared with ambient CO2 (Table 4). There were no significant differences between CO2 treatments for LWR, NAR, RGR or RWR (Table 4).
We then examined if there were differences in trait values of more-competitive and less-competitive species within species-pairs, irrespective of plant type, at both ambient and elevated CO2. Within species-pairs, more-competitive species had significantly smaller LWR and LAR and significantly larger RGR and NAR than less-competitive species, under both ambient and elevated CO2 treatments (Table 5).
Discussion
In this study, we examined whether competitive interactions between native and invasive exotic plant species are affected by CO2 conditions. We found that competitive rankings within species-pairs were not altered by CO2 level. In 9 out of 14 species-pairs, the invasive exotic species was more competitive while in the remaining 5 species-pairs the native species was more competitive, under both ambient and elevated CO2 treatments.
Although the competitive rankings within species-pairs were not affected by CO2 level, the strength of the competitive interactions was affected. The corrected index of relative competition intensity (CRCI; Oksanen et al. 2006) of native species on average was significantly increased under elevated compared with ambient CO2, indicating that the competitive response of natives under elevated CO2 was reduced. The CRCI is based on the competitive response of the target plant grown in competition with neighbors but can also be interpreted as a measure of the competitive effect of the neighbor plants. Thus our results show that native species had on average a reduced competitive response under elevated compared with ambient CO2 but this may also be interpreted as an increased competitive effect of invasive exotic species under elevated CO2. These results suggest that under predicted future atmospheric CO2 conditions, competitive rankings among species may not change substantially, but the relative success of invasive exotic species may be increased (Smith et al. 2000; Huxman and Smith 2001; Belote et al. 2003; Nagel et al. 2004).
It is often assumed that invasive exotic plants are superior competitors to native species. An interesting outcome of this study was that native and invasive exotic plants did not differ overall in their competitive ability under either ambient or elevated CO2 conditions. Although similar results have previously been reported for ambient CO2 conditions (e.g., Corbin and D’Antonio 2004; Suding et al. 2004), the majority of studies have shown that invasive exotic species are better competitors than native species (e.g., Hager 2004; Miller and Duncan 2004; White and Holt 2005; Coleman and Levine 2007; Pfeifer-Meister et al. 2008). Our results suggest that there are species-specific attributes that play an important role in determining the competitive interactions between native and invasive exotic plants, and that an understanding of these traits may be more informative than knowledge of a plant’s status as native or exotic for predicting the outcome of interactions between species.
Invasive exotic plants were predicted to respond more strongly than native plants to elevated CO2 levels because they generally have growth and allocation traits that allow rapid carbon capture (Rejmanek et al. 2005; Grotkopp and Rejmanek 2007; Leishman et al. 2007, 2010). The calculated measure of competitive ability that we used (CRCI) is based on the competitive response of the target plant, but also incorporates the competitive effect of the neighbor plants. We did not find a difference in competitive response of the invasive exotics between CO2 treatments, or between native and invasive exotics in either CO2 treatment, in contrast to our expectations. However, we did find a difference in the competitive response of native species between CO2 treatments which may be due to either trait differences of the target native species or to trait differences of the neighbour invasive exotics under the CO2 treatments, or a combination of the two. However, the pair-wise comparisons of trait values we used were based on the target plants only, and so we were unable to assess whether differences in traits of the invasive exotic neighbours contributed to the reduced competitive response of natives under elevated CO2. Native species had lower SLA and LAR values under elevated compared with ambient CO2. Reductions in both these traits would result in reduced carbon capture and hence reduced biomass, seen in this study as reduced competitive response. Previous studies that have assessed what traits contribute to greater biomass of exotic species under elevated compared to ambient CO2 have found higher growth rate (Sasek and Strain 1988, 1991; Smith et al. 2000; Dukes 2002; Ziska 2002; Belote et al. 2003), larger leaf area (Sasek and Strain 1988, 1991; Ziska et al. 2004; Ziska et al. 2005, 2007), higher net assimilation rate (Sasek and Strain 1988) and longer stems (Hattenschwiler and Korner 2003) to be important traits.
Interestingly, we found that, irrespective of plant type, traits that were associated with competitive superiority at both ambient and elevated CO2 were LWR, LAR, RGR and NAR. LWR and LAR were significantly smaller in the more competitive plants while RGR and NAR were significantly larger. This suggests that, in the conditions of this experiment (high light, soil water and nutrient availability), plants with relatively smaller allocation to leaves and high NAR can achieve high relative growth rates and hence larger biomass, conferring a competitive advantage. A high RGR allowing rapid biomass accumulation is often associated with a superior competitive ability (Sasek and Strain 1988, 1991; Smith et al. 2000; Dukes 2002; Ziska 2002; Belote et al. 2003). These results suggest that, in general, traits associated with growth and allocation can enable predictions of outcomes of competition under particular environmental conditions.
Plant species’ response to elevated CO2 has been shown in numerous studies to be constrained by resource availability (Poorter et al. 1996; Oren et al. 2001; Reich et al. 2006). Thus, when soil resources such as nutrients or moisture are limiting, plants may be unable to take advantage of the increased CO2 concentration. In this study, the natural soil of the Cumberland Plain Woodland made up a large component of the soil mixture that was used. This is a shale-derived soil and is consequently relatively fertile (Little 2003). We also provided slow release fertilizer to maintain soil fertility throughout the experiment and to ensure that plants received sufficient soil moisture. Previous studies have shown that invasive exotic species tend to have traits that enable rapid growth in non-limiting environments (Grotkopp and Rejmanek 2007; Leishman et al. 2010) but that water availability does not affect relative success of native and invasive exotics (Baruch and Jackson 2005; Leishman and Thomson 2005; Coleman and Levine 2007). By providing non-limiting soil resources in this experimental system, we have provided optimal conditions for the invasive exotic plants to take advantage of additional CO2 and to increase their relative competitive ability. Thus, the conclusions from our study should be applied tentatively to environments where soil resources are limiting.
The nature of pair-wise experimental designs means that they may be influenced by the selection of species. We chose to reduce variation by controlling for photosynthetic pathway, growth form and life history within species-pairs. However, it may be these differences in plant characteristics that contribute to invasion success in exotic species (Vila and Weiner 2004). Further experimental work could examine competitive interactions under ambient and elevated CO2 concentrations for species of contrasting growth form, physiology or allocation traits. Different results for species-pairs could also be due to seed mass contrasts within each species-pair as larger-seeded species have been shown to be more competitive (Eriksson 1997; Turnbull et al. 1999; Leishman 2001; Susko and Cavers 2008). Of the 14 species-pairs in this study, there were large (>5 times) differences in seed mass within 4 pairs (Table 1). Within these four pairs, the species with the larger seed size was more competitive. However, in these four pairs, the invasive exotic species had larger seed mass in two cases and the native species had larger seed mass in the other two cases. Thus, we do not think that consistent seed mass differences between native and invasive exotic species within species-pairs were responsible for the overall result.
Large-scale mesocosm and FACE experiments are now considered essential to understand community-level responses to elevated CO2 (Vila et al. 2007). These experiments provide data on a range of community responses that can best be understood when all components of the system are included. However, we argue that there is still a need for glasshouse experiments to advance our understanding of the mechanisms and processes that underpin these community-level responses (Vila and Weiner 2004). This study has illustrated the role that competitive interactions may have in determining community-level outcomes under future CO2 conditions. It has shown that the relative advantage of competitively superior invasive exotic plants compared to native neighbors may increase under elevated CO2. This knowledge is important to help mitigate the future impact of invasive exotic plants under higher atmospheric CO2 levels.
References
Bando K (2006) The roles of competition and disturbance in a marine invasion. Biol Invasions 8:755–763
Barney J, Whitlow T, DiTommaso A (2009) Evolution of an invasive phenotype: shift to belowground dominance and enhanced competitive ability in the introduced range. Plant Ecol 202:275–284
Baruch Z, Jackson RB (2005) Responses of tropical native and invader C4 grasses to water stress, clipping and increased atmospheric CO2 concentration. Oecologia 145:522–532
Beck KG et al (2009) Invasive species defined in a policy context: recommendations from the Federal Invasive Species Advisory Committee. Invasive Plant Sci Manage 1:414–421
Belote RT, Weltzin JF, Norby RJ (2003) Response of an understory plant community to elevated [CO2] depends on differential responses of dominant invasive species and is mediated by soil water availability. New Phytol 161:827–835
Bradford MA, Schumacher HB, Catovsky S, Eggers T, Newingtion JE, Tordoff GM (2007) Impacts of invasive plant species on riparian plant assemblages: interactions with elevated atmospheric carbon dioxide and nitrogen deposition. Oecologia 152:791–803
Burns JH (2006) Relatedness and environment affect traits associated with invasive and non-invasive introduced Commelinaceae. Ecol Appl 16:1367–1376
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Coleman H, Levine J (2007) Mechanisms underlying the impacts of exotic annual grasses in a coastal California meadow. Biol Invasions 9:65–71
Corbin JD, D’Antonio CM (2004) Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology 85:1273–1283
Dassonville N, Vanderhoeven S, Vanparys V, Hayez M, Gruber W, Meerts P (2008) Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 157:131–140
Dukes JS (2000) Will increasing atmospheric CO2 concentration affect the success of invasive species. Island Press, Washington DC
Dukes JS (2002) Comparison of the effect of elevated CO2 on an invasive species (Centaurea solstitialis) in monoculture and community settings. Plant Ecol 160:225–234
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139
Eriksson O (1997) Colonization dynamics and relative abundance of three plant species (Antennaria dioica, Hieradum pilosella and Hypochoeris maculata) in dry semi-natural grasslands. Ecography 20:559–568
Ewing K (2002) Effects of initial site treatments on early growth and three-year survival of Idaho Fescue. Restor Ecol 10:282–288
Goldberg DE, Rajaniemi T, Gurevitch J, Stewart-Oaten A (1999) Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology 80:1118–1131
Grace JB (1995) On the measurement of plant competition intensity. Ecology 76:305–308
Grotkopp E, Rejmanek M (2007) High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. Am J Bot 94:526–532
Grotkopp E, Rejmanek M, Rost TL (2002) Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat 159:396
Groves RH, Austin MP, Kaye PE (2003) Competition between Australian native and introduced grasses along a nutrient gradient. Austral Ecol 28:491–498
Hager H (2004) Competitive effect versus competitive response of invasive and native wetland plant species. Oecologia 139:140–149
Hamilton JG, Holzapfel C, Mahall BE (1999) Coexistence and interference between a native perennial grass and non-native annual grasses in California. Oecologia 121:518–526
Hattenschwiler S, Korner C (2003) Does elevated CO2 facilitate naturalization of the non-indigenous Prunus laurocerasus in Swiss temperate forests? Funct Ecol 17:778–785
Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS (2008) Five potential consequences of climate change for invasive species. Conserv Biol 22:534–543
Huxman T, Smith S (2001) Photosynthesis in an invasive grass and native forb at elevated CO2 during an El Niño year in the Mojave Desert. Oecologia 128:193–201
LaDeau SL, Clark JS (2001) Rising CO2 levels and the fecundity of forest trees. Science 292:95–98
Leishman MR (2001) Does the seed size/number trade-off model determine plant community structure? An assessment of the model mechanisms and their generality. Oikos 93:294–302
Leishman MR, Thomson VP (2005) Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. J Ecol 93:38–49
Leishman MR, Haslehurst T, Ares A, Baruch Z (2007) Leaf trait relationships of native and invasive plants: community and global-scale comparisons. New Phytol 176:635–643
Leishman MR, Thomson VP, Cooke J (2010) Native and exotic invasive plants have fundamentally similar carbon capture strategies. J Ecol 98:28–42
Levine JM, Vila M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond B 270:775–781
Li Y, Xiao Y, Wang C, Li X (2008) Growth characteristics and relative competitive capacity of Plantago virginica and P. asiatica. Chin J Ecol 27:514–518
Little D (2003) Bringing back the bush to western Sydney. Department of infrastructure, planning and natural resources, Parramatta
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences and control. Ecol Appl 10:689–710
Markham JH, Chanway CP (1996) Measuring plant neighbor effects. Funct Ecol 10:548–549
Maron JL, Connors PG (1996) A native nitrogen-fixing shrub facilitates weed invasion. Oecologia 105:302–312
McPeek TM, Wang X (2007) Reproduction of Dandelion (Taraxacum officinale) in a higher CO2 environment. Weed Sci 55:334–340
Miller AL, Duncan RP (2004) The impact of exotic weed competition on a rare New Zealand outcrop herb, Pachycladon cheesemanni (Brassicaceae). N Z J Ecol 28:113–124
Minitab (2007) Minitab 15: statistical software. State College, PA, United States
Nagel JM, Huxman TE, Griffin KL, Smith SD (2004) CO2 enrichment reduces the energetic cost of biomass construction in an invasive desert grass. Ecology 85:100–106
Oksanen L, Sammul M, Magi M (2006) On the indices of plant–plant competition and their pitfalls. Oikos 112:149–155
Oren R et al (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469–472
Pfeifer-Meister L, Cole E, Roy B, Bridgham S (2008) Abiotic constraints on the competitive ability of exotic and native grasses in a Pacific Northwest prairie. Oecologia 155:357–366
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Poorter H, Navas M-L (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157:175–198
Poorter H, Roumet C, Campbell BD (1996) Interspecific variation in the growth response of plants to elevated CO2: a search for functional types. In: Korner C, Bazzaz FA (eds) Carbon dioxide, populations and communities. Academic, San Diego, pp 375–412
Reich PB et al (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Rejmanek M, Richardson DM, Higgins SI, Pitcairn MJ, Grotkopp E (2005) Ecology of invasive plants: state of the art. Island Press, Washington DC
Rhazi M, Grillas P, Rhazi L, Charpentier A, Médail F (2009) Competition in microcosm between a clonal plant species (Bolboschoenusmaritimus) and a rare quillwort (Isoetessetacea) from Mediterranean temporary pools of southern France. Hydrobiologia 634:115–124
Roura-Pascual N et al (2009) Ecology and management of alien plant invasions in South African fynbos: accommodating key complexities in objective decision making. Biol Conserv 142:1595–1604
Sasek TW, Strain BR (1988) Effects of carbon dioxide enrichment on the growth and morphology of Kudzu (Pueraria lobata). Weed Sci 36:28–36
Sasek TW, Strain BR (1991) Effects of CO2 enrichment on the growth and morphology of a native and an introduced honeysuckle vine. Am J Bot 78:69–75
Smith MD, Knapp AK (2001) Physiological and morphological traits of exotic, invasive exotic and native plant species in tallgrass prairie. Int J Plant Sci 162:785–792
Smith SD, Strain BR, Sharkey TD (1987) Effects of CO2 enrichment on four Great Basin grasses. Funct Ecol 1:139–143
Smith SD et al (2000) Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 408:79–82
Song L, Wu J, Li C, Li F, Peng S, Chen B (2009) Different responses of invasive and native species to elevated CO2 concentration. Acta Oecol 35:128–135
Suding KN, LeJeune KD, Seastedt TR (2004) Competitive impacts and responses of an invasive weed: dependencies on nitrogen and phosphorus availability. Oecologia 141:526–535
Susko DJ, Cavers PB (2008) Seed size effects and competitive ability in Thlaspi arvense L. (Brassicaceae). Botany 86:259–267
Tangley L (2001) Greenhouse effects: high CO2 levels may give fast-growing trees an edge. Science 292:36–37
Taylor K, Potvin C (1997) Understanding the long-term effect of CO2 enrichment on a pasture: the importance of disturbance. Can J Bot 75:1621–1627
Turnbull LA, Rees M, Crawley MJ (1999) Seed mass and the competition/colonization trade-off: a sowing experiment. J Ecol 87:899–912
van der Wal R, Truscott A-M, Pearce ISK, Cole L, Harris MP, Wanless S (2008) Multiple anthropogenic changes cause biodiversity loss through plant invasion. Glob Chang Biol 14:1428–1436
Vila M, Weiner J (2004) Are invasive plant species better competitors than native plant species? Evidence from pair-wise experiments. Oikos 105:229–238
Vila M, Gómez A, Maron J (2003) Are alien plants more competitive than their native conspecifics? A test using Hypericum perforatum L. Oecologia 137:211–215
Vila M, Corbin JD, Dukes JS, Pino J, Smith SD (2007) Linking plant invasions to global environmental change. In: Canadell JJ, Pataki DE, Pitelka LF (eds) Terrestrial ecosystems in a changing world. The IGBP series. Springer, Berlin
Wang Q et al (2006) Effects of growing conditions on the growth of and interactions between salt marsh plants: implications for invasibility of habitats. Biol Invasions 8:1547–1560
Werner C, Zumkier U, Beyschlag W, Maguas C (2010) High competitiveness of a resource demanding invasive acacia under low resource supply. Plant Ecol 206:83–96
White VA, Holt JS (2005) Competition of artichoke thistle (Cynara cardunculus) with native and exotic grassland species. Weed Sci 53:826–833
IPCC (2007) Climate change 2007: a physical science basis. In: Solomon S et al. (eds) Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change, Cambridge, Uniting Kingdom and New York, USA
Ziska LH (2002) Influence of rising atmospheric CO2 since 1900 on early growth and photosynthetic response of a noxious invasive weed, Canada thistle (Cirsium arvense). Funct Plant Biol 29:1387–1392
Ziska LH (2003) Evaluation of the growth response of six invasive species to past, present and future atmospheric carbon dioxide. J Exp Bot 54:395–404
Ziska LH, Faulkner S, Lydon J (2004) Changes in biomass and root:shoot ratio of field-grown Canada thistle (Cirsium arvense), a noxious, invasive weed, with elevated CO2: implications for control with glyphosate. Weed Sci 52:584–588
Ziska LH, Reeves JB, Blank B (2005) The impact of recent increases in atmospheric CO2 on biomass production and vegetative retention of Cheatgrass (Bromus tectorum): implications for fire disturbance. Glob Chang Biol 11:1325–1332
Ziska LH, Sicher RC, George K, Mohan JE (2007) Rising atmospheric carbon dioxide and potential impacts on the growth and toxicity of poison ivy (Toxicodendron radicans). Weed Sci 55:288–292
Acknowledgments
We gratefully acknowledge the Plant Invasion and Restoration Ecology Laboratory (PIREL) of Macquarie University for their input throughout the experiment, Muhammad Masood for assistance in the glasshouses and Francesca Manea for helping with harvesting. This research was funded by an Australian Research Council Linkage grant (LP0776758) to M.L. Two anonymous reviewers and Paul Downey provided constructive comments on earlier versions of the manuscript. The experiments conducted complied with all current laws and regulations of Australia, where they were conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alice Winn.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manea, A., Leishman, M.R. Competitive interactions between native and invasive exotic plant species are altered under elevated carbon dioxide. Oecologia 165, 735–744 (2011). https://doi.org/10.1007/s00442-010-1765-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1765-3