Abstract
Elevated atmospheric CO2 concentration increases the performance of invasive plants relative to natives when grown in monoculture, but it is unclear how that will affect the relative competitive abilities per se of invasive and native grasses grown together. We tested competitive outcomes for four native and four invasive perennial C3 and C4 grasses under ambient (390 ppm) and elevated (700 or 1000 ppm) CO2 concentrations in the greenhouse with non-limiting water and nutrients. We predicted that elevated CO2 would increase the competitive suppression of native grasses by invasive grasses. To test this, we determined the relative interaction intensity of biomass allocation for natives grown alone vs. those grown in native–invasive species pairs. We also measured photosynthetic traits that contribute to plant invasiveness and may be affected by elevated CO2 concentrations for species pairs in mixture to determine native–invasive relative performance. We found no effect of CO2 for the aboveground biomass and tiller production measures of interaction intensity or for relative performance for most of the measured photosynthetic traits. In competition, the invaders nearly always outperform natives in biomass and tiller production, regardless of CO2 level. The results suggest that increasing CO2 concentration alone has little effect on grass competitive outcomes under controlled conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fluctuating resource hypothesis suggests that high resource availability benefits the growth and fitness of invasive plants over those of non-invasive plants, favouring invasive plants in competitive conditions (Sher and Hyatt 1999; Davis et al. 2000; Blumenthal 2006). Elevated atmospheric carbon dioxide concentration (hereafter elevated CO2) is a source of carbon fertilization for plants and may thus favour invasive over non-invasive plant species due to traits that allow rapid carbon assimilation and use. Indeed, two meta-analyses involving individual plants or single species grown alone seem to support the idea that elevated CO2 increases the performance of invasive plants more than non-invasive plants (Sorte et al. 2013; Liu et al. 2017). However, there are caveats to interpret these analyses. In both analyses, “performance” is an amalgam of fitness, growth, and physiological trait measurements, and it is likely inappropriate to combine these very different types of measures because there is no reason to assume that they respond in the same way to elevated CO2 (Hillebrand and Gurevitch 2016). The overall effect sizes also combined both plants and arthropods in one case, and photosynthetic pathways (C3, C4) and plant growth forms (e.g., woody, forb, grass) in both cases, and there is also no reason to expect that these disparate groups respond similarly to elevated CO2. Partitioning these effect sizes could reveal differential responses of plant functional groups to elevated CO2 (e.g., Wand et al. 1999; Hager et al. 2016a; He et al. 2018), as strong responses in one group may outweigh smaller, zero, or negative responses in other groups. Regardless, experimental evidence cautions against extrapolating from individual responses to community responses to elevated CO2 because plant–plant interactions such as competition can affect community outcomes (e.g., Ackerly and Bazzaz 1995; Navas 1998; Poorter and Navas 2003; Brooker 2006; Larson et al. 2018). Different species than would be predicted based on their response to CO2 in isolation may become dominant in a community, as demonstrated for native plants interacting in (relatively depauperate) experimental communities (Navas 1998). Thus, comparisons of individual species responses to elevated CO2 may not be the best predictors of invasion-related competitive outcomes in future climates (Dukes and Mooney 1999; Larson et al. 2018).
Grasses are often very successful invaders (Linder et al. 2018) and can strongly alter ecosystem processes such as fire regimes (Linder et al. 2018; Fusco et al. 2019), herbivory (Linder et al. 2018), carbon cycling and moisture regimes (Koteen et al. 2011), and growing season phenology (Wilsey et al. 2018). Differential effects of elevated CO2 on the competitive abilities of invasive and non-invasive grasses could thus accelerate invasion and exacerbate its effects. In contrast to studies of species-level responses, however, elevated CO2 is not generally observed to promote invasive grasses in experimental grassland communities. For example, elevated CO2 did not favour an invasive annual C3 grass in semiarid mixed-grass prairie (Blumenthal et al. 2016) relative to other species in the community. It had no effect on invader relative abundance in experimental pots containing multiple invasive and native grass species (Tooth and Leishman 2014; Manea and Leishman 2014), and it did not influence the success of an invasive grass in a potted mixture of four native grasses (Manea et al. 2016). However, it may be difficult to control for confounding effects such as increased water use efficiency with elevated CO2 in the field (Owensby et al. 1993; Blumenthal et al. 2013; Holohan et al. 2019), and it can be difficult to distinguish effects of elevated CO2 from competition because of pre-existing species differences such as size and developmental seasonality in natural communities (Owensby et al. 1993).
Studies we could find that directly examine whether elevated CO2 increases the competitive advantage of invasive vs. native grasses also indicate that it may not favour invasive grasses (Hely and Roxburgh 2005; Manea and Leishman 2011; Larson et al. 2018). In two examples, elevated CO2 somewhat alleviated competition for a native perennial C3 grass competing with an invasive annual C3 grass (Hely and Roxburgh 2005; Larson et al. 2018). In addition, it had no overall effect on the relative competitive ability of six native–invasive perennial C4 grass pairs (data from Manea and Leishman 2011 reanalysed for grasses only, paired t test native ambient vs. elevated P = 0.48). The small number of studies and differences in lifespan and photosynthetic pathways make it difficult to draw general conclusions about grass competitive responses to elevated CO2.
Here, we add to the literature examining CO2 effects on grass competitive interactions by experimentally testing the effect of elevated CO2 on competitive outcomes for co-occurring invasive and native perennial grasses differing in invasive status and photosynthetic pathway. Direct measures of plant competitive ability involve two facets of competition: the suppressive (or facilitative) effect of neighbouring competitors on the performance of an individual or species of interest, and the competitive response performance of the species of interest in the presence of neighbours (Goldberg 1990; Goldberg and Landa 1991). We focused on the competitive effects of invasive grasses on native grasses and the competitive responses of the native grasses. We hypothesized that elevated CO2 would favour invasive over native grasses. We predicted that elevated CO2, relative to ambient conditions, would increase the competitive suppression of native grasses by invasive grasses, with a smaller effect on C3 than C4 natives due to a carbon fertilization effect. Additionally, we predicted that competitive outcomes would be related to differences in morphological and physiological traits associated with invasiveness that may also respond to changes in atmospheric CO2 concentration, including biomass allocation to leaves, photosynthetic rate, and leaf area per unit leaf mass (specific leaf area) (Dukes and Mooney 1999; Pritchard et al. 1999; Urban 2003; Pyšek and Richardson 2007; van Kleunen et al. 2010; Ordonez et al. 2010; Manea and Leishman 2011; Dusenge et al. 2019).
Methods
The experiment was conducted in the E. C. Bovey greenhouse at the University of Guelph, Canada, in nine CO2-controlled plexiglass closed-top chambers arranged in a 3 × 3 grid. Growth chambers were constructed and operated according to the description of Grodzinski et al. (1999). Each chamber received one of three CO2 concentrations within the range of projected increases by 2100 (IPCC 2013): ambient (390 ppm) or elevated (700 or 1000 ppm). CO2 concentrations, relative humidity (~ 40%), and temperature (23 °C) were maintained by an Argus Greenhouse Control System (Argus, Surrey, Canada). The nine chambers were arranged in three blocks according to a light gradient in the greenhouse, with one chamber of each CO2 concentration per block. Metal halide lights (approximately 150 μmol/m2 s) supplied supplementary lighting when natural light was < 600 μmol/m2 s on a 16:8 h light/dark cycle.
We used four (two C3, two C4) native-noninvasive and four (two C3, two C4) introduced-invasive grass species for the experiment (Table 1). All species distributions overlap in large areas of North America, although Cenchrus ciliaris currently tends to occur more southerly (US Department of Agriculture Plants Database https://plants.usda.gov). Grass seeds were germinated on filter paper in Petri dishes in early July, and germinants were transferred directly to PVC pots in the growth chambers to eliminate priority and acclimation effects. Pots were 7.6 cm diameter × 45.7 cm height to allow space for root growth and contained Sunshine Mix #1 (Canadian Sphagnum peat moss, coarse perlite, and dolomitic limestone; Sun Gro, Agawam, USA). One individual of each native species was planted into eight pots per chamber: four of these pots were monocultures and four pots were also planted with one of the four invasive species. Thus, there were 16 monoculture pots (four of each native species) and 16 mixture pots (all possible native-invasive pairs) randomly distributed in each chamber. Invasive species monocultures could not be planted because of space constraints. Pots were watered to field capacity as needed (usually every other day), alternating between deionized water and fertilizer water (1.25 g/L N–P–K, 20–8-20).
After 12 weeks of growth at the respective CO2 concentrations, photosynthesis and stomatal conductance were measured on the uppermost fully expanded leaf of each individual in the mixtures. All pots received deionized water on the morning when photosynthesis was measured. Blocks were measured on consecutive days because of time and daylight constraints. Measurements were made between 9:00 a.m. and 4:00 p.m. using a portable infrared gas analyzer (LI-6400 Portable Photosynthesis System; LI-COR, Lincoln, Nebraska) under quantum flux of 1600 μmol/m2 s (LI-6400-02B red/blue LED #670) and CO2 concentration set to the same level as the chamber in which the measured plant was grown, with flow rate of 500 μmol/m2 s, block temperature of 23 °C, and relative humidity of ~ 35–40%. The leaf was acclimated in the leaf chamber until readings stabilized (total coefficient of variation < 1%), after which the automatic data logger was initiated to record one measurement every 20 s for 2 min, for a total of six measurements per leaf. The leaf chamber was then opened, and the corresponding leaf portion was marked, excised from the plant, and measured to calculate its area as a trapezoid. The segment was then dried to constant mass at 55 °C, and its weight was used to calculate specific leaf area (SLA; leaf area/leaf dry mass). Leaf cuticle impressions were taken from the top (adaxial) and bottom (abaxial) surfaces of the leaf blade adjacent to the section in the leaf chamber using clear nail varnish, and the numbers of stomata and epidermal cells were counted under a light microscope for three fields of view and averaged. Photosynthesis and conductance measurements were recalculated in the LI-COR system using the measured leaf area and the species’ mean stomatal ratio, and the set of measurements was averaged for each leaf.
After 14 weeks of growth, plants were harvested individually, the numbers of tillers and inflorescences were counted, and the biomass was separated into inflorescence, leaf, stem, and root. Roots were washed thoroughly to remove soil; however, roots of plants grown in mixture could not be separated into their respective individuals and remained pooled. All biomass was dried to constant mass at 55 °C and weighed.
Estimation of plant interactions
We used relative interaction intensity (RII) to examine competitive outcomes for native grasses and a relative performance index (RPI) to examine the relative growth performance of native vs. invasive grasses in mixture. We examined the effect of competition on native species growth using RII based on a plant’s performance in mixture (i.e., in competition) relative to that when grown alone. Each native grass individual in mixture was randomly paired with an individual that was grown alone, and their biomass or tiller data were used to calculate RII = (performance in mixture − performance alone)/(performance in mixture + performance alone) (Armas et al. 2004). Positive values of RII indicate facilitation, and negative values indicate competition. RII was calculated for stem, leaf, and total shoot (leaf, stem, and reproductive) biomass and number of tillers.
Similarly, we examined the RPI of native and invasive pairs grown in mixture, where RPI = (performance of native − performance of invasive)/(performance of native + performance of invasive). Positive values of RPI indicate that the measured variable was greater for native individuals than for their invasive competitor, whereas negative values of RPI indicate the opposite. This measure is analogous to effect size in providing an index of the difference between two “treatments” (here, native and invasive) and accounts for the lack of independence between species pairs within pots. RPI was calculated for stem biomass, leaf biomass, total shoot (leaf, stem, and reproductive) biomass, number of tillers, photosynthesis and conductance rates, specific leaf area, and stomatal density on the leaf top and bottom.
Statistical analysis
Data were analysed using split-plot ANOVAs with restricted maximum likelihood estimation in JMP 13 (SAS Institute, Cary, NC, USA). The whole-plot factor of CO2 was tested using the adjusted CO2 × block error term, and the remaining sub-plot factors were tested using adjusted residual error. Block was treated as a random effect (Newman et al. 1997). We examined the effects of CO2, native competitive response, and invasive competitive effect on RII measures; CO2, native species identity, and neighbouring invasive species identity on absolute root biomass; and CO2, native species, and invasive species on RPI measures. All fixed-effects interactions were included in the analyses. Significant effects were further examined using post hoc Tukey tests. Contrasts were used to compare differences between C3 and C4 species within CO2 levels; however, results were often confounded by species-specific differences within photosynthetic pathway and are reported in Table S1 (see Supporting Information). All variables met statistical assumptions without transformation.
For reproductive output, only three native (Elymus virginicus, Andropogon gerardii, and Panicum virgatum), and one invasive (Cenchrus ciliaris) species produced inflorescences, and in some cases, only one individual of a species flowered in a given CO2 level. Therefore, we lacked the replicates necessary for full statistical comparisons of RII and RPI. For the three native species, we used generalized linear models in JMP 13 (SAS Institute, Cary, North Carolina, USA) to examine selected effects where replicates allowed. Best-fitting models were determined using the AICc and a measure of overdispersion (Pearson Chi-square/degrees of freedom), and the results are reported in Fig. S2 (see Supporting Information). Our curated raw experimental data are archived at Scholar’s Portal Dataverse (Hager et al. 2020).
Results
Relative interaction intensity
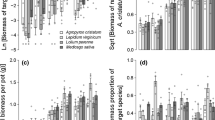
There were no significant effects of CO2 or its interactions on aboveground RII measures (see Table 2 for all test statistics, degrees of freedom, and significance values). However, there were significant effects of native response and invasive effect on RII for stem, leaf, and total shoot biomass and number of tillers (Table 2). The productivity of all native species was suppressed by invasive competitors (RII < 0). Native C4 species were consistently poorer response competitors than native C3 species for all aboveground measures of biomass (Fig. 1a; see Fig. S3 in Supporting Information for stem and leaf biomass). In contrast, the tiller production RII response of C3 Koeleria macrantha and C4 Panicum virgatum to invasive competitors was poorer than that of C4 A. gerardii (Fig. 1b). All invasive species had suppressive effects on their native competitors except for C4 Miscanthus sinensis, which had little effect on native aboveground biomass and tiller production (Fig. 1c, d).
The effect of a, b native competitive response and c, d invasive competitive effect on native species relative interaction intensity (RII; native species grown in competition with an invasive species vs. native species grown alone) for aboveground production measures (mean ± SE). Different lowercase letters indicate significant differences within panels based on post hoc Tukey tests (P < 0.05). See Table 1 for species name abbreviations
Root biomass
There was a significant main effect of CO2 and two-way interaction of CO2 × invasive neighbour on absolute total root biomass (Table 3). Root biomass increased significantly with increasing CO2 (main effect, figure not shown), and the effect of CO2 depended on the neighbouring invasive species identity (Fig. 2a). Root biomass was significantly greater in mixtures with C4 C. ciliaris than when native species grew alone at all CO2 levels, with other mixtures intermediate (Fig. 2a). Only the root biomass of natives grown alone did not increase with increasing CO2 (Fig. 2a). There were also significant effects of native species, neighbouring invasive species, and their two-way interaction on total root biomass (Table 3). Within native species, root biomass was significantly greater in pots with C. ciliaris than for natives alone (all species) and natives with C4 M. sinensis (except C3 E. virginicus; Fig. 2b). Overall, root biomass was significantly greater for C4 than C3 native species pairs, with the exception of mixtures grown with M. sinensis, which were only greater than natives grown alone (not shown).
The effect of CO2 and a species mixture and b native and neighbouring invasive species identity on total root biomass (g, mean ± SE). Native and invasive species’ roots could not be separated in mixtures. See Table 1 for species name abbreviations
Relative performance index
Aboveground biomass and tiller production
There were no significant effects of CO2 or its interactions on RPI for any aboveground growth measure, indicating that increasing CO2 did not alter the mean growth differences between native and invasive species. However, there were significant effects of native species, invasive species, and their interaction on stem, leaf, and total shoot biomass and number of tillers produced (Table 4). Generally, RPIs were negative, indicating that native species usually produced less biomass and fewer tillers than the invasive species with which they competed (Fig. 3a, b; see Fig. S3 in Supporting Information for stem and leaf biomass). In a few instances, natives performed better than or similar to invasives in mixture, particularly C3 E. virginicus (except for total shoot biomass compared to C4 C. ciliaris and C3 Phalaris arundinacea). Performance of natives relative to M. sinensis in mixtures was variable: natives often had similar or greater biomass and tillers than M. sinensis except for K. macrantha, which had lower shoot biomass, and A. gerardii, which had fewer tillers.
The effect of native and neighbouring invasive species identity on native species relative performance index (RPI, native species vs. invasive species, grown in competition) for a total shoot biomass and b number of tillers (mean ± SE). Different lowercase letters indicate significant differences within panels based on post hoc Tukey tests (P < 0.05). See Table 1 for species name abbreviations
Photosynthesis and leaf traits
There were no significant effects of CO2 or its interactions on RPI for photosynthesis or conductance, but there were significant main effects of native and invasive species identity (Table 4). Native C3 species had positive RPI for photosynthesis (Fig. 4a) and conductance rates (see Fig. S3 in Supporting Information), indicating generally higher photosynthetic rates than those of the invasive species with which they competed. Conversely, native C4 species had somewhat lower photosynthetic rates than their invasive competitor pairs, and the difference between RPI for native C3 and C4 species was significant (Fig. 4a). The photosynthetic advantage of native C3 species and disadvantage of native C4 species tended to appear at elevated CO2, as indicated by the CO2 × native species interaction (P < 0.08), which is slightly below the threshold for statistical significance (Fig. S4 [see Supporting Information]). Natives had greater photosynthesis rates than their invasive competitor pairs when grown with M. sinensis, C. ciliaris, and P. arundinacea, but lower photosynthesis compared to invasive Bromus inermis (Fig. 4b). RPIs for conductance rates followed a similar pattern (see Fig. S3 in Supporting Information).
The effect of a native and b invasive species identity on relative performance index (RPI, native species vs. invasive species, grown in competition) for photosynthetic rate (mean ± SE). RPI > 0 indicates that native species performed better than invasive species; RPI < 0 indicates that native species performed worse than invasive species. Different lowercase letters indicate significant differences within panels based on post hoc Tukey tests (P < 0.05). See Table 1 for species name abbreviations
There was a significant effect of CO2 × native species identity and a marginally significant main effect of CO2 on the RPI for SLA (Table 4). There was a trend for native species except E. virginicus to have higher RPI for SLA at elevated than at ambient CO2, although most within-species differences were not significant (Fig. 5a; see Fig. S3 in Supporting Information for CO2 main effect). There was also a significant effect of native × invasive species identity on RPI for SLA (Table 4). Native–invasive competitor pairs varied idiosyncratically in SLA, although natives usually had similar or lower SLA than their invasive competitor (Fig. 5b).
The effect of CO2 and a native identity and b native and invasive species identity on relative performance index (RPI) for specific leaf area (mean ± SE). Different lowercase letters indicate significant differences within panels based on post hoc Tukey tests (P < 0.05); bars without letters are not significantly different from any of the combinations indicated. See Table 1 for species name abbreviations
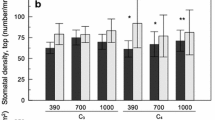
Native A. gerardii was excluded from the analysis of adaxial (leaf top) stomatal density because it lacks stomata on the upper leaf surface. There were significant effects of CO2 × native species and CO2 × native × invasive species on RPI for adaxial stomatal density (Table 4). RPI was ≥ 0 for most of the three-way treatment combinations, indicating that the three native species generally had similar or greater adaxial stomatal density than their invasive competitor pairs, but there was no consistent pattern to the differences in RPI among species pairs or CO2 levels (Fig. 6a–c). Only E. virginicus differed among CO2 levels, with generally greater RPI than its competitor pairs at 1000 than 400 ppm. There was also a significant effect of invasive species on RPI for adaxial stomatal density (Table 4), with RPI highest for native species compared to their C4 M. sinensis competitor pair, followed by C3 B. inermis, C4 C. ciliaris, and C3 P. arundinacea.
a–c The effect of CO2 × native species × invasive species identity on relative performance index (RPI) for stomatal density on the adaxial (top) leaf surface. d, e The effect of native and invasive species identity on RPI for stomatal density on the abaxial (bottom) leaf surface (mean ± SE). For the bottom panels, different lowercase letters indicate significant differences within panels based on post hoc Tukey tests (P < 0.05). See Table 1 for species name abbreviations
There were no significant effects of CO2 or its interactions on abaxial (leaf underside) stomatal density; only the main effects of native and invasive species identity were significant (Table 4). C3 E. virginicus and C4 P. virgatum had fewer stomata on the abaxial surface than their invasive competitors, whereas A. gerardii had more stomata (Fig. 6d). Natives had more abaxial stomata than their C3 B. inermis invasive competitor pair, and fewer abaxial stomata than their other competitor pairs (Fig. 6d).
Discussion
We found no indication that elevated CO2, at levels up to the highest projected to occur by 2100, alters the strength of aboveground competitive interactions for native C3 and C4 perennial grass seedlings competing with invasive C3 and C4 perennial grass seedlings under non-limiting water and soil nutrients. Our results corroborate those of previous competition-focused experiments that found little or no advantage of elevated CO2 on invasive grass success over native grasses (Hely and Roxburgh 2005; Manea and Leishman 2011; Larson et al. 2018). A similar lack of CO2 effect on “competition” outcomes (usually measured as relative abundance or biomass of invasives and natives) has been found for other functional group comparisons of invaders and native species mixes from serpentine grassland (Dukes 2002), riparian areas (Bradford et al. 2007), and savanna (Tooth and Leishman 2014), although the strength of competitive interactions, which requires monoculture treatments, is often not measured. In contrast, there are examples of woody invaders preferentially enhanced by elevated CO2 in various ecosystems (Ziska and George 2004). Thus, although invasive species are generally good competitors, they do not necessarily benefit from elevated CO2 in the absence of other global change factors (Poorter and Navas 2003).
Elevated CO2 can also result in shifts in biomass allocation to stems, leaves, and roots (Reekie 1996; Dukes 2000; Urban 2003), and subsequent competition for light can increase allocation to leaf production (Reynolds 1996). For example, Reekie (1996) found that tree seedlings shifted biomass allocation under elevated CO2 when grown in competition but not when grown alone. In contrast, He et al. (2018) found no shift in biomass allocation under elevated CO2 for an invasive forb competing with two congeners. We found no differences in patterns of stem and leaf competitive response or relative performance with elevated CO2. Although we could not separate root biomass in mixtures, it increased in response to elevated CO2 whereas root biomass of natives grown alone did not, suggesting that either only invasive root biomass responded to elevated CO2 or there was a synergistic response in species mixtures. If the former is the case, invasive C. ciliaris was by far the greatest contributor to root mass, which may explain its wide success in arid and semiarid regions (Marshall et al. 2012).
The competitive responses of native grasses to invasives were species specific, rather than dependent on CO2 level. Similar results have been found previously for six pairs of competing native and invasive C4 grasses (Manea and Leishman 2011) and two native C4 grasses competing with a C3 invasive annual forb (Khan et al. 2015). However, our measured responses also differed depending on which variable was measured (biomass vs. tiller production), as was also noted by Dukes et al. (2011) for shoot growth vs. biomass of the invasive forb yellow starthistle (Centaurea solstitialis) competing with the native annual grass Avena barbata. The aboveground biomass of the C4 natives was more suppressed by competition than that of C3 natives, but the response of tiller production followed a different pattern. It is likely that species are more constrained in their ability to alter resource allocation to tiller production than to biomass (e.g., Cheplick 2003; Aspinwall et al. 2017). The two C4 grasses produced fewer tillers than the C3s (2.5–13 times fewer when alone; 2.3–18 times fewer when in competition), and A. gerardii produced on average 1–2 tillers and P. virgatum 3–6 tillers, so they would have little capacity to respond to competition by decreasing tiller production. It may also be useful to measure plant height or tiller lengths in future experiments, particularly when species may be competing for light.
The competitive effects of invasive grass species on native grasses were also species specific, rather than related to CO2 level. All but M. sinensis had strong suppressive effects on biomass and smaller effects on tiller numbers. M. sinensis produced the fewest tillers among the invasive species (six on average), potentially contributed little to total root biomass compared to when natives were grown alone, and had virtually no effect on the native grasses in competition. Although established Miscanthus spp. populations are strongly rhizomatous and appear to exclude most other vegetation (Quinn et al. 2010; Hager et al. 2015b), M. sinensis seedling establishment and growth can be limited by light availability and competition (West et al. 2014; Hager et al. 2015a). Our experiment was designed to minimize priority effects by beginning with simultaneously germinated seeds. Given that competitive outcomes can depend on the relative developmental stages of the co-competitors (Hager 2004; Wang et al. 2010), results may differ for native seedlings germinating in established swards of invasive grasses or vice versa.
It is difficult to determine lifetime plant fitness (e.g., Louda and Potvin 1995; Swope and Parker 2010), particularly for clonal plants such as grasses, which could be an important factor in scaling to community outcomes in the field. Three of our four native species produced inflorescences in mixture during the 14-week experiment, although fewer individuals flowered than when grown alone. In contrast, only one of the invasive species flowered, and it also (potentially) contributed the greatest amounts to root biomass and produced the greatest total shoot mass (data not shown) of the four invasive species (C. ciliaris). The potential for CO2- and competition-induced lifetime fitness shifts to allow coexistence of native and invasive grasses might also depend on the ability of seedlings to establish and grow within existing or disturbed vegetation. These processes could be examined in future using demographic models (Williams et al. 2007; Carrara et al. 2015; but see Aschehoug and Callaway 2015).
Although we cannot exclude the possibility of pot size effects (volume of 2.1 L) in our experiment, we do not think the lack of CO2 effect was due to serious carbohydrate sink restrictions (Arp 1991; Poorter et al. 2012; Dusenge et al. 2019) for several reasons. We found little evidence of photosynthetic CO2 acclimation at 7 and 14 weeks in a previous experiment that included five of the species tested here (E. virginicus, A. gerardii, P. virgatum, B. inermis, and M. sinensis), with time differences only for M. sinensis at 390 and 700 ppm and E. virginicus at 1000 ppm [contrasts tested using data from Hager et al. (2016a, b)]. In addition, we provided ample water and nutrient solution to minimize soil depletion and used white pots to minimize elevation of root temperatures (Poorter et al. 2012). Although lateral root space may have been limited, we wanted to ensure below- and aboveground interaction between the species pairs, and pots still had uncolonized soil at the bottom at harvest. Finally, total root biomass increased with elevated CO2 for mixtures, even though it did not for natives grown alone. Nevertheless, caution should be made in scaling these results to outcomes in the field, where elevated CO2 influences and interacts with various environmental factors (Hager and Newman 2019) such as water stress (Blumenthal et al. 2013; Holohan et al. 2019) and nitrogen limitation (Brooker 2006; Dieleman et al. 2012), which can alter competitive outcomes.
Certain morphological and physiological traits are highly correlated with plant invasiveness. For example, invasive plants are often taller, grow faster, and have higher SLA, photosynthetic rates, leaf allocation, and water and nutrient use efficiencies than non-invasive plants (Pyšek and Richardson 2007; van Kleunen et al. 2010; Ordonez et al. 2010), and many of these traits also respond to changes in CO2. However, although the invasive grasses produced more biomass and tillers than the natives in most comparisons and had strong suppressive effects (except for M. sinensis), these patterns were not reflected in invasion-related photosynthetic traits. Although photosynthesis and conductance rates of native grasses were higher than (C3s) or similar to (C4s) those of their invasive competitors, they did not lead to greater biomass production for the natives. Only the invasive B. inermis had higher photosynthetic and conductance rates than the natives. These results suggest that other traits such as higher early relative growth rate allowing resource pre-emption (Reynolds 1996; Reichmann et al. 2016), more efficient use of nutrients (Lambers et al. 1998; Reichmann et al. 2016), and lower tissue construction costs (Nagel et al. 2004) may have contributed to the invasive success of these grasses.
The SLA of invasive grasses was generally greater than or similar to that of natives, which agrees with the general pattern observed for invasive species (Pyšek and Richardson 2007; van Kleunen et al. 2010; Ordonez et al. 2010). However, there was a trend for the SLA of native and invasive species to converge with increasing CO2. Indeed, the marginally significant main effect of CO2 for SLA indicates that the invasive advantage of greater SLA at ambient CO2 disappeared at 1000 ppm CO2. Reichmann et al. (2016) found that SLA was higher for an invasive C4 grass than for three native C4 grasses but converged as plants became larger and leaves required more structural support, which could also have occurred with our grasses.
Finally, stomatal density varies in response to changes in atmospheric CO2 concentration, with decreases in density and increases in pore size with increasing CO2 (Franks and Beerling 2009; but see Woodward and Kelly 1995; Tipping and Murray 1999), which decreases conductance, all else being equal (Franks and Beerling 2009). However, the proportion of stomata on each leaf surface is highly constrained by selective pressures (Muir 2015) and leaf vascular anatomy (Franks and Beerling 2009). We found that stomatal density was generally greater on the leaf top and lower on the leaf bottom for the native grasses compared to their invasive competitors, with some exceptions. One hypothesis for stomatal distribution is that minimizing stomatal density on the upper leaf surface could reduce the risk of infection by foliar pathogens (Muir 2015), which might indicate that invasive plants can minimize interactions with natural enemies via their stomatal distribution (Hager et al. 2016a). This idea remains to be explored.
Because of space constraints, we were unable to include monocultures of the invasive species, which would have allowed us to determine their RII in the presence of the native grasses. Rather, we chose to maximize the numbers of grass species investigated. Information on the competitive response of invasives could be useful in determining the role of photosynthetic pathway in the invasives’ ability to be successful under competition and provide information on the ability of these invasive grasses to establish in the presence of native competitors. It could also answer questions about the apparent lack of competitive effect of M. sinensis seedlings. Future experiments could examine these questions, as well as interactions with other global change factors.
References
Ackerly DD, Bazzaz FA (1995) Plant growth and reproduction along CO2 gradients: non-linear responses and implications for community change. Glob Change Biol 1:199–207. https://doi.org/10.1111/j.1365-2486.1995.tb00021.x
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686. https://doi.org/10.1890/03-0650
Arp WJ (1991) Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14:869–875. https://doi.org/10.1111/j.1365-3040.1991.tb01450.x
Aschehoug ET, Callaway RM (2015) Diversity increases indirect interactions, attenuates the intensity of competition, and promotes coexistence. Am Nat 186:452–459. https://doi.org/10.1086/682901
Aspinwall MJ, Fay PA, Hawkes CV, Lowry DB, Khasanova A, Bonnette J, Whitaker BK, Johnson N, Juenger TE (2017) Intraspecific variation in precipitation responses of a widespread C-4 grass depends on site water limitation. J Plant Ecol 10:310–321. https://doi.org/10.1093/jpe/rtw040
Blumenthal DM (2006) Interactions between resource availability and enemy release in plant invasion. Ecol Lett 9:887–895. https://doi.org/10.1111/j.1461-0248.2006.00934.x
Blumenthal DM, Resco V, Morgan JA, Williams DG, LeCain DR, Hardy EM, Pendall E, Bladyka E (2013) Invasive forb benefits from water savings by native plants and carbon fertilization under elevated CO2 and warming. New Phytol 200:1156–1165. https://doi.org/10.1111/nph.12459
Blumenthal DM, Kray JA, Ortmans W, Ziska LH, Pendall E (2016) Cheatgrass is favored by warming but not CO2 enrichment in a semi-arid grassland. Glob Change Biol 22:3026–3038. https://doi.org/10.1111/gcb.13278
Bradford MA, Schumacher HB, Catovsky S, Eggers T, Newingtion JE, Tordoff GM (2007) Impacts of invasive plant species on riparian plant assemblages: interactions with elevated atmospheric carbon dioxide and nitrogen deposition. Oecologia 152:791–803. https://doi.org/10.1007/s0042-007-0697-z
Brooker RW (2006) Plant–plant interactions and environmental change. New Phytol 171:271–284. https://doi.org/10.1111/j.1469-8137.2006.01752.x
Carrara F, Giometto A, Seymour M, Rinaldo A, Altermatt F (2015) Inferring species interactions in ecological communities: a comparison of methods at different levels of complexity. Methods Ecol Evol 6:895–906. https://doi.org/10.1111/2041-210X.12363
Cheplick GP (2003) Evolutionary significance of genotypic variation in developmental reaction norms for a perennial grass under competitive stress. Evol Ecol 17:175–196. https://doi.org/10.1023/A:1023057024776
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534. https://doi.org/10.1046/j.1365-2745.2000.00473.x
Dieleman WIJ, Vicca S, Dijkstra FA, Hagedorn F, Hovenden MJ, Larsen KS, Morgan JA, Volder A, Beier C, Dukes JS, King J, Leuzinger S, Linder S, Luo Y, Oren R, De Angelis P, Tingey D, Hoosbeek MR, Janssens IA (2012) Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob Change Biol 18:2681–2693. https://doi.org/10.1111/j.1365-2486.2012.02745.x
Dukes JS (2000) Will the increasing atmospheric CO2 concentration affect the success of invasive species? In: Mooney HA, Hobbs RJ (eds) Invasive species in a changing world. Island Press, Washington, DC, pp 95–113
Dukes JS (2002) Comparison of the effect of elevated CO2 on an invasive species (Centaurea solstitialis) in monoculture and community settings. Plant Ecol 160:225–234. https://doi.org/10.1023/A:1015813919850
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139. https://doi.org/10.1016/S0169-5347(98)01554-7
Dukes JS, Chiariello NR, Loarie SR, Field CB (2011) Strong response of an invasive plant species (Centaurea solstitialis L.) to global environmental changes. Ecol Appl 21:1887–1894. https://doi.org/10.1890/11-0111.1
Dusenge ME, Duarte AG, Way DA (2019) Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol 221:32–49. https://doi.org/10.1111/nph.15283
Franks PJ, Beerling DJ (2009) Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci USA 106:10343–10347. https://doi.org/10.1073/pnas.0904209106
Fusco EJ, Finn JT, Balch JK, Nagy RC, Bradley BA (2019) Invasive grasses increase fire occurrence and frequency across US ecoregions. Proc Natl Acad Sci USA 116:23594–23599. https://doi.org/10.1073/pnas.1908253116
Goldberg DE (1990) Components of resource competition in plant communities. In: Grace JB, Tilman D (eds) Perspectives on plant competition. Academic Press, San Diego, pp 27–49
Goldberg DE, Landa K (1991) Competitive effect and response: hierarchies and correlated traits in the early stages of competition. J Ecol 79:1013–1030. https://doi.org/10.2307/2261095
Grass Phylogeny Working Group II (2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol 193:304–312. https://doi.org/10.1111/j.1469-8137.2011.03972.x
Grodzinski B, Schmidt JM, Watts B, Taylor J, Bates S, Dixon MA, Staines H (1999) Regulating plant/insect interactions using CO2 enrichment in model ecosystems. Adv Space Res 24:281–291. https://doi.org/10.1016/S0273-1177(99)00315-4
Hager HA (2004) Competitive effect versus competitive response of invasive and native wetland plant species. Oecologia 139:140–149. https://doi.org/10.1007/s00442-004-1494-6
Hager HA, Newman JA (2019) Methodology I: detecting and predicting grassland change. In: Gibson DJ, Newman JA (eds) Grasslands and climate change. Cambridge University Press, Cambridge, pp 19–39
Hager HA, Quinn LD, Barney JN, Voigt TB, Newman JA (2015a) Germination and establishment of bioenergy grasses outside cultivation: a multi-region seed addition experiment. Plant Ecol 216:1385–1399. https://doi.org/10.1007/s11258-015-0516-2
Hager HA, Rupert R, Quinn LD, Newman JA (2015b) Escaped Miscanthus sacchariflorus reduces the richness and diversity of vegetation and the soil seed bank. Biol Invasions 17:1833–1847. https://doi.org/10.1007/s10530-014-0839-2
Hager HA, Ryan GD, Kovacs HM, Newman JA (2016a) Effects of elevated CO2 on photosynthetic traits of native and invasive C3 and C4 grasses. BMC Ecol 16:28. https://doi.org/10.1186/s12898-016-0082-z
Hager HA, Ryan GD, Kovacs HM, Newman JA (2016b) Effects of elevated CO2 on photosynthetic traits of native and invasive C3 and C4 grasses 2012 to 2013 [South-central Ontario, Canada]. Sch Portal Dataverse. https://doi.org/10.5887/AERDR/10864/TZBTY
Hager HA, Ryan GD, Newman JA (2020) Effects of elevated CO2 on competition between native and invasive grasses. Scholars Portal Dataverse. https://doi.org/10.5683/SP2/GJ94P8
He L, Kong J, Li G, Meng G, Chen K (2018) Similar responses in morphology, growth, biomass allocation, and photosynthesis in invasive Wedelia trilobata and native congeners to CO2 enrichment. Plant Ecol 219:145–157. https://doi.org/10.1007/s11258-017-0784-0
Hely SEL, Roxburgh SH (2005) The interactive effects of elevated CO2, temperature and initial size on growth and competition between a native C3 and an invasive C3 grass. Plant Ecol 177:85–98. https://doi.org/10.1007/s11258-005-2247-2
Hillebrand H, Gurevitch J (2016) Meta‐analysis and systematic reviews in ecology. In: eLS. Wiley. https://doi.org/10.1002/9780470015902.a0003272.pub2
Holohan AD, Müller C, McElwain J (2019) Heritable changes in physiological gas exchange traits in response to long-term, moderate free-air carbon dioxide enrichment. Front Plant Sci 10:1210. https://doi.org/10.3389/fpls.2019.01210
IPCC (Intergovernmental Panel on Climate Change) (2013) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Khan N, George D, Shabbir A, Hanif Z, Adkins SW (2015) Rising CO2 can alter fodder-weed interactions and suppression of Parthenium hysterophorus. Weed Res 55:113–117. https://doi.org/10.1111/wre.12127
Koteen LE, Baldocchi DD, Harte J (2011) Invasion of non-native grasses causes a drop in soil carbon storage in California grasslands. Environ Res Lett 6:044001. https://doi.org/10.1088/1748-9326/6/4/044001
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, New York
Larson CD, Lehnhoff EA, Noffsinger C, Rew LJ (2018) Competition between cheatgrass and bluebunch wheatgrass is altered by temperature, resource availability, and atmospheric CO2 concentration. Oecologia 186:855–868. https://doi.org/10.1007/s00442-017-4046-6
Linder HP, Lehmann CER, Archibald S, Osborne CP, Richardson DM (2018) Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biol Rev 93:1125–1144. https://doi.org/10.1111/brv.12388
Liu Y, Oduor AMO, Zhang Z, Manea A, Tooth IM, Leishman MR, Xu X, Van Kleunen M (2017) Do invasive alien plants benefit more from global environmental change than native plants? Glob Change Biol 23:3363–3370. https://doi.org/10.1111/gcb.13579
Louda SM, Potvin MA (1995) Effect of inflorescence-feeding insects on the demography and lifetime fitness of a native plant. Ecology 76:229–245. https://doi.org/10.2307/1940645
Manea A, Leishman MR (2011) Competitive interactions between native and invasive exotic plant species are altered under elevated carbon dioxide. Oecologia 165:735–744. https://doi.org/10.1007/s00442-010-1765-3
Manea A, Leishman MR (2014) Leaf area index drives soil water availability and extreme drought-related mortality under elevated CO2 in a temperate grassland model system. PLoS ONE 9:e91046. https://doi.org/10.1371/journal.pone.0091046
Manea A, Sloane DR, Leishman MR (2016) Reductions in native grass biomass associated with drought facilitates the invasion of an exotic grass into a model grassland system. Oecologia 181:175–183. https://doi.org/10.1007/s00442-016-3553-1
Marshall VM, Lewis MM, Ostendorf B (2012) Buffel grass (Cenchrus ciliaris) as an invader and threat to biodiversity in arid environments: a review. J Arid Environ 78:1–12. https://doi.org/10.1016/j.jaridenv.2011.11.005
Muir CD (2015) Making pore choices: repeated regime shifts in stomatal ratio. Proc R Soc B 282:20151498. https://doi.org/10.1098/rspb.2015.1498
Nagel JM, Huxman TE, Griffin KL, Smith SD (2004) Co-2 enrichment reduces the energetic cost of biomass construction in an invasive desert grass. Ecology 85:100–106. https://doi.org/10.1890/02-3005
Navas ML (1998) Individual species performance and response of multispecific communities to elevated CO2: a review. Funct Ecol 12:721–727. https://doi.org/10.1046/j.1365-2435.1998.00260.x
Newman J, Bergelson J, Grafen A (1997) Blocking factors and hypothesis tests in ecology: is your statistics text wrong? Ecology 78:1312–1320. https://doi.org/10.1890/0012-9658(1997)078[1312:BFAHTI]2.0.CO;2
Ordonez A, Wright IJ, Olff H (2010) Functional differences between native and alien species: a global-scale comparison. Funct Ecol 24:1353–1361. https://doi.org/10.1111/j.1365-2435.2010.01739.x
Owensby CE, Coyne PI, Ham JM, Auen LM, Knapp AK (1993) Biomass production in a tallgrass prairie ecosystem exposed to ambient and elevated CO2. Ecol Appl 3:644–653. https://doi.org/10.2307/1942097
Poorter H, Navas ML (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157:175–198. https://doi.org/10.1046/j.1469-8137.2003.00680.x
Poorter H, Bühler J, van Dusschoten D, Climent J, Postma JA (2012) Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Funct Plant Biol 39:839–850. https://doi.org/10.1071/FP12049
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Glob Change Biol 5:807–837. https://doi.org/10.1046/j.1365-2486.1999.00268.x
Pyšek P, Richardson DM (2007) Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W (ed) Biological invasions. Springer, Berlin, pp 97–125
Quinn LD, Allen DJ, Stewart JR (2010) Invasiveness potential of Miscanthus sinensis: implications for bioenergy production in the United States. Glob Change Biol Bioenergy 2:310–320. https://doi.org/10.1111/j.1757-1707.2010.01062.x
Reekie EG (1996) The effect of elevated CO2 on developmental processes and its implications for plant–plant interactions. In: Körner C, Bazzaz FA (eds) Carbon dioxide, populations, and communities. Academic Press, San Diego, pp 333–346
Reichmann LG, Schwinning S, Polley HW, Fay PA (2016) Traits of an invasive grass conferring an early growth advantage over native grasses. J Plant Ecol 9:672–681. https://doi.org/10.1093/jpe/rtw014
Reynolds HL (1996) Effects of elevated CO2 on plants grown in competition. In: Körner C, Bazzaz FA (eds) Carbon dioxide, populations, and communities. Academic Press, San Diego, California, pp 273–286
Sher AA, Hyatt LA (1999) The disturbed resource-flux invasion matrix: a new framework for patterns of plant invasion. Biol Invasions 1:107–114. https://doi.org/10.1023/A:1010050420466
Sorte CJB, Ibanez I, Blumenthal DM, Molinari NA, Miller LP, Grosholz ED, Diez JM, D’Antonio CM, Olden JD, Jones SJ, Dukes JS (2013) Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol Lett 16:261–270. https://doi.org/10.1111/ele.12017
Sun W, Ubierna N, Ma J-Y, Walker BJ, Kramer DM, Cousins AB (2014) The coordination of C4 photosynthesis and the CO2-concentrating mechanism in maize and Miscanthus × giganteus in response to transient changes in light quality. Plant Physiol 164:1283–1292. https://doi.org/10.1104/pp.113.224683
Swope SM, Parker IM (2010) Trait-mediated interactions and lifetime fitness of the invasive plant Centaurea solstitialis. Ecology 91:2284–2293. https://doi.org/10.1890/09-0855.1
Taylor SH, Hulme SP, Rees M, Ripley BS, Woodward FI, Osborne CP (2010) Ecophysiological traits in C-3 and C-4 grasses: a phylogenetically controlled screening experiment. New Phytol 185:780–791. https://doi.org/10.1111/j.1469-8137.2009.03102.x
Tipping C, Murray DR (1999) Effects of elevated atmospheric CO2 concentration on leaf anatomy and morphology in Panicum species representing different photosynthetic modes. Int J Plant Sci 160:1063–1073. https://doi.org/10.1086/314201
Tooth IM, Leishman MR (2014) Elevated carbon dioxide and fire reduce biomass of native grass species when grown in competition with invasive exotic grasses in a savanna experimental system. Biol Invasions 16:257–268. https://doi.org/10.1007/s10530-013-0448-5
Urban O (2003) Physiological impacts of elevated CO2 concentration ranging from molecular to whole plant responses. Photosynthetica 41:9–20. https://doi.org/10.1023/A:1025891825050
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. https://doi.org/10.1111/j.1461-0248.2009.01418.x
Wand SJE, Midgley GF, Jones MH, Curtis PS (1999) Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Glob Change Biol 5:723–741. https://doi.org/10.1046/j.1365-2486.1999.00265.x
Wang P, Stieglitz T, Zhou DW, Cahill JF Jr (2010) Are competitive effect and response two sides of the same coin, or fundamentally different? Funct Ecol 24:196–207. https://doi.org/10.1111/j.1365-2435.2009.01612.x
West NM, Matlaga DP, Davis AS (2014) Quantifying targets to manage invasion risk: light gradients dominate the early regeneration niche of naturalized and pre-commercial Miscanthus populations. Biol Invasions 16:1991–2001. https://doi.org/10.1007/s10530-014-0643-z
Williams AL, Wills KE, Janes JK, Schoor JKV, Newton PCD, Hovenden MJ (2007) Warming and free-air CO2 enrichment alter demographics in four co-occurring grassland species. New Phytol 176:365–374. https://doi.org/10.1111/j.1469-8137.2007.02170.x
Wilsey BJ, Martin LM, Kaul AD (2018) Phenology differences between native and novel exotic-dominated grasslands rival the effects of climate change. J Appl Ecol 55:863–873. https://doi.org/10.1111/1365-2664.12971
Woodward FI, Kelly CK (1995) The influence of CO2 concentration on stomatal density. New Phytol 131:311–327. https://doi.org/10.1111/j.1469-8137.1995.tb03067.x
Ziska LH, George K (2004) Rising carbon dioxide and invasive, noxious plants: potential threats and consequences. World Resour Rev 16:427–447
Acknowledgements
We thank A. Patchett, K. Shukla, M. Melkic, K. Bolton, S. McGee, E. Staples, and J. Dale for help with the experiment; Y. Zheng, E. Lyons, H. Earl, and G. Otis for providing equipment; R. Dutton for managing the greenhouse, W. McCloskey for providing buffelgrass seed, C. Breton for data archiving assistance, and two anonymous reviewers for comments. This research was funded by Mitacs (HAH); Ontario Ministry of Agriculture, Food and Rural Affairs (all authors); Natural Sciences and Engineering Research Council of Canada (JAN); and J.D. Webster Postdoctoral Fellowship (GDR).
Author information
Authors and Affiliations
Contributions
HAH and GDR designed and executed the study. HAH analysed the data and wrote the manuscript. JAN advised on experimental design and statistics. HAH, GDR, and JAN edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Yanjie Liu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hager, H.A., Ryan, G.D. & Newman, J.A. Effects of elevated CO2 on competition between native and invasive grasses. Oecologia 192, 1099–1110 (2020). https://doi.org/10.1007/s00442-020-04636-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04636-6