Abstract
The ecophysiological linkage of leaf phosphorus (P) to photosynthetic capacity (A max) and to the A max–nitrogen relation remains poorly understood. To address this issue we compiled published and unpublished field data for mass-based A max, nitrogen (N) and P (n = 517 observations) from 314 species at 42 sites in 14 countries. Data were from four biomes: arctic, cold temperate, subtropical (including Mediterranean), and tropical. We asked whether plants with low P levels have low A max, a shallower slope of the A max–N relationship, and whether these patterns have a geographic signature. On average, leaf P was substantially lower in the two warmer than in the two colder biomes, with the reverse true for N:P ratios. The evidence indicates that the response of A max to leaf N is constrained by low leaf P. Using a full factorial model for all data, A max was related to leaf N, but not to leaf P on its own, with a significant leaf N × leaf P interaction indicating that the response of A max to N increased with increasing leaf P. This was also found in analyses using one value per species per site, or by comparing only angiosperms or only woody plants. Additionally, the slope of the A max–N relationship was higher in the colder arctic and temperate than warmer tropical and subtropical biomes. Sorting data into low, medium, and high leaf P groupings also showed that the A max–N slope increases with leaf P. These analyses support claims that in P-limited ecosystems the A max–N relationship may be constrained by low P, and are consistent with laboratory studies that show P-deficient plants have limited ribulose-1,5-bisphosphate regeneration, a likely mechanism for the P influence upon the A max–N relation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that leaf photosynthetic capacity (A max) is often related to leaf nitrogen (N) concentration because of the role that N-rich compounds, particularly ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), play in the biochemical fixation of carbon dioxide (Field and Mooney 1986). Among disparate species within or among communities, ecosystems, and biomes, there are generally consistent correlations of A max and leaf N when both are expressed on a mass basis (e.g., Reich et al. 1997; Wright et al. 2004). Phosphorus (P) is a plant nutrient essential to a variety of plant functions and is a major component of nucleic acids, sugar phosphates, ATP, and phospholipids, all of which play important roles in photosynthesis (Bielski 1973). Compared to N, both the broad interspecific patterns of correlation of P with A max and the underlying mechanisms remain both less studied and less well understood (Cordell et al. 2001; Whitehead et al. 2005).
Within a species, the lack of both N and P typically limit plant growth, primarily by restricting leaf area development and secondarily by reducing photosynthetic rate. The effect on photosynthesis is secondary because effects of low N or P availability on growth restrict C uptake and therefore reduce the dilution of N or P concentration that would otherwise occur (Marschner 1995). Variation in A max, N, and P among species in native habitats, however, results from both phenotypic and genotypic differences (Cordell et al. 2001; Richardson et al. 2004). Moreover, given strong tendencies for leaf N and P to co-vary in nature (Sterner and Elser 2002; Reich and Oleksyn 2004), it is difficult to separate their roles in explaining broad patterns of variation in A max.
Low leaf P is thought to limit A max through several different mechanisms. Experimental work with P-deficient plants has shown reductions in ribulose-1,5-bisphosphate (RuBP) regeneration (Jacob and Lawlor 1992; Rao and Terry 1995; Campbell and Sage 2006), carboxylation activity (Loustau et al. 1999), light use efficiency (Conroy et al. 1986), and stomatal conductance (Jacob and Lawlor 1991; Thomas et al. 2006). It has also been shown that P supply influences partitioning of N, including to Rubisco (Warren and Adams 2002; Warren et al. 2005). The importance of P for RuBP regeneration and N partitioning, coupled with the importance of Rubisco (and N concentrations) for A max, suggests there might be interactive effects of N and P availability on A max, as noted by Whitehead et al. (2005).
Reports of low A max for plants with low leaf P growing on low-P soils (e.g., Reich et al. 1994; Raaimakers et al. 1995; Wright et al. 2004; Whitehead et al. 2005; Denton et al. 2007), weaker A max–N relations for plants growing in soils considered more P- than N-limited (e.g., Reich and Schoettle 1988; Reich et al. 1994), and photosynthetic stimulation by P fertilization on P-limited soils (Cordell et al. 2001) suggest that the mechanisms found in the experimental studies may be widely applicable under more natural conditions. However, although it has been widely presumed and amply demonstrated that P limitation is more common in certain biomes and regions than others (e.g., Walker and Syers 1976), the empirical basis for characterizing leaf P levels and associated photosynthetic performance is weak (but see Cordell et al. 2001; Richardson et al. 2004; Whitehead et al. 2005). Moreover, we lack a comprehensive evaluation of whether A max is broadly associated with leaf P, and of whether the relationship of A max to leaf N is influenced by variation in leaf P. To address these issues we compiled published and unpublished data where measures of A max, N, and P were obtained from field-grown plants. These species and sites span a wide range, from arctic tundra, to cold temperate forests, to tropical rainforests. Thus, the species and sites span much of the gradient of global biogeochemistry, including the contrasts between tropical and cold temperate biomes that are characterized by differing N and P availability (Walker and Syers 1976; Vitousek 1984) and contrasting average leaf P concentrations and N:P ratios (Reich and Oleksyn 2004). We use these data to address the following questions: is leaf P correlated with A max, both when variation in leaf N is also considered and when it is not; does leaf P level influence the relation of A max to variation in leaf N; is there a biogeographic signature to these patterns?
Methods
Data were obtained from published literature and unpublished data of the authors. The criteria used were that measurements were from unfertilized field-grown plants. The total data set comprised 517 observations from 314 species at 42 sites in 14 countries, predominantly (approx. 99%) native species in naturally regenerated, unmanaged ecosystems (Appendices 1, 2 in the Electronic Supplementary Material). This compilation includes, but expands upon, the 212 observations of A max, N, and P included in Wright et al. (2004). As in prior studies (e.g., Reich et al. 1997; Wright et al. 2004) A max is defined as the typical maximum photosynthetic rate observed under optimal conditions in the field. This has been shown to be similar to A max measured under controlled laboratory conditions (Ellsworth and Reich 1992). The average number of observations at each site = 12.3 [±2.5 standard error (SE); range 1–85), and the average number of species studied at each site = 8.3 (±1.4, range 1–44). The number of observations from individual species ranged from 1 to 17, with the large majority of species represented by one (253 species) or two (35 species) observations. To check for undue influence of species with multiple observations, we conducted all analyses using species averages (by site) as well as using all observations. In cases where multiple observations of all three metrics were obtained for a species at a site (such as for multiple individuals, or in campaigns in different seasons or years), all observations were included in the database.
Based on climate, geography, and vegetation type, we divided the species and sites into four biome classes: arctic-boreal (arctic hereafter), temperate, Mediterranean/subtropical/warm temperate (subtropical hereafter), and tropical. Tropical sites were in Venezuela, Panama, Australia (Northern Territory), Hawaii, Guyana, Cameroon, Indonesia, and Costa Rica (in order from most to least observations); the vast majority were lowland sites with high mean annual temperature (MAT) (mean MAT among sites 24°C). The subtropical sites included studies in Australia (New South Wales), USA (California, Florida), and Spain (MAT range among sites 15–20°C). Temperate sites (MAT range among sites 5–9°C) included locations in Poland, Canada (southern Ontario), USA (several), and Germany. The arctic-boreal sites were in USA (mostly Alaska), Sweden, and Svalbard archipelago in the Arctic Ocean, all with MAT < –2°C.
Data analyses included simple and multiple regression (JMP ver. 5.1; SAS Institute, Cary, NC). The leaf traits were approximately log-normally distributed and thus were log10 transformed prior to analyses to approximate normality and minimize the heterogeneity of residuals. All subsequent references to A max, N, and P and their relations are on a log10 basis. Although N and P were correlated, the correlation (for all observations) was intermediate (R 2 = 0.40), and in a full factorial model including N, P and their interaction, the output showed modest collinearity (variance inflation factor <2). Thus, results of multiple regression analyses provide meaningful insight into the individual and interactive effects of leaf N and P on A max. Because A max is considered to be functionally dependent upon leaf N and leaf P, there is a strong causal element in the bivariate relations; hence, slopes of these are reported as standard least squares regressions.
Results
On average, leaf P was substantially lower and N:P ratios much higher in the two warmer biome groups than in the two colder groups (Table 1). Analysis of variance (ANOVA) tests (Tukey’s HSD) showed that the two warmest groups did not differ significantly (P > 0.20) from each other, nor did the two coldest groups, but both warmer groups differed significantly (P < 0.05) from both colder groups in both metrics. Based on their similar means and distributions of leaf P, leaf N, and leaf N:P ratios, as well as the Tukey’s tests, for certain analyses we also lumped the two warmer, low-latitude groups into one labeled “warm tropical” and the two colder, higher latitude groups into one labeled “cold temperate”.
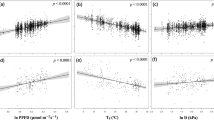
Considering all observations, A max was significantly (P < 0.0001) positively correlated with either leaf N (R 2 = 0.49) or leaf P (R 2 = 0.16). A full factorial multiple regression model including all observations (P < 0.0001, R 2 = 0.52) showed that A max was positively related to leaf N (P < 0.0001) but not to leaf P (P = 0.11) as main effects, and that there was a significant leaf N × P interaction (P < 0.0001) (Table 2). The positivity of the interaction indicates that as leaf P increases, there are increasingly positive responses of A max to rising leaf N, and vice versa. This can be demonstrated by comparing the A max–N relations for three groups obtained arbitrarily by dividing all observations in thirds according to the lowest, middle, and highest leaf P values (Fig. 1). The slope of A max–N is highest for the high leaf P group and lower for the low leaf P group. There was a significant interaction (P < 0.0001) of leaf N × “leaf P group”, demonstrating that the A max–N slope differs among groups; the relation for the low P group was significantly different (P < 0.01) than that for either the middle P or high P groups (which did not differ from each other). There was also a significant main effect of N (P < 0.0001) and no main effect of P group (P = 0.61).
Scatterplot of the relationship of leaf photosynthetic capacity (A max) versus N by three P groups. The groups were divided arbitrarily into the lowest, middle, and highest thirds according to leaf P level. All relationships were significant (P < 0.0001). The slopes, intercepts, and R 2 of the relationships were 1.509, 0.028, and 0.57 for the highest P group (n = 172); 1.378, 0.020, and 0.41 for the middle P group (n = 172); 0.868, 0.822, and 0.28 for the lowest P group (n = 173). The slopes for the low P group were significantly (P < 0.01) different than those for the other two groups
Hence, although the added predictive power of including P in A max–N models is very modest (it added 3% in this analysis), it is still a highly significant effect with considerable power to leverage changes in A max across varying leaf N levels. To illustrate the size of this effect, one can compare the slopes calculated for the species split into the low, medium, and high P groups (Fig. 1). Considering a threefold increase in leaf N, from 10 to 30 mg/g, A max increased 5.2-, 4.5-, and 2.6-fold in the high, medium and low P groups, respectively (i.e., A max increased twice as much in the high P group as in the low P group, over this shift in N).
When site was added as a random factor to the model predicting A max as a function of leaf N, leaf P and their interaction, site was significant (P < 0.0001), but the effects were otherwise similar (Table 3). The main effect of N and the N × P interaction remained significant (P < 0.0001), and leaf P became significant (P = 0.027) (full model R 2 = 0.68, P < 0.0001). Additionally, the results of the full model (including the regression coefficient for the N × P interaction terms) that included all observations were similar to analyses of various alternative data sets, including (1) using one value per species-site combination (n = 326), (2) comparing only angiosperms (n = 488), (3) considering only woody plants (n = 471), or (4) examining data from only the two warmer biomes (n = 410). In each case, leaf N and the interaction of N × P were significant (<0.0001) terms in the multiple regression model, with A max as the dependent variable, but leaf P was not (P > 0.10). Thus, these results are consistent regardless of how data were arranged.
Comparison of A max–N relations for observations divided into two or four biome groups leads to similar conclusions (Fig. 2). The four biome groups differ in leaf P and leaf N:P ratios (Table 1), with the two warmer groups having relatively low leaf P and high leaf N:P ratios compared to the two colder biomes. The slope of A max–N was highest in the group with the lowest N:P ratio (arctic), followed in descending order by the biome groups with increasing N:P ratios (temperate, tropical, subtropical), with the slope differences significant (P = 0.05) between the temperate and subtropical groups (Table 4). As the two warmer and two colder biomes represent pairs with similar P and N:P ratios (and given the small sample size for arctic plants), we also compared the A max–N relations after lumping observations into just two groups, the “warm tropical” and “cold temperate” groups. The analysis showed significant differences in slope for these two groups (P = 0.03). The slope of A max–N was higher for observations from the colder biomes (with lower N:P) than for those from the warmer biomes—the former relationship (n = 110, R 2 = 0.56) had a slope = 1.52, whereas the latter (n = 407, R 2 = 0.44) had a slope = 1.18.
Scatterplot of the relationship of A max versus leaf N by four biome groups. Details of the statistical relationships are shown in Table 4
When we subsequently ran the full multiple regression model (for N, P and their interaction) separately for these two groups, we found that for the “cold temperate” group there were significant positive effects of N (<0.0001) and P (=0.0026), and no significant interaction (full model R 2 = 0.60, n = 110), and for the “warm tropical” group there was significance for N (<0.0001) but not for P (>0.05), and there was significant interaction (<0.0001) (full model R 2 = 0.52, n = 407).
To test whether differences in the A max–N slopes seen in Fig. 1 were due to differences among biomes rather than simply due to difference in N:P stoichiometry relevant to A max, we also assessed A max–N relations for just the warm biome data set broken into three arbitrary P groups. The results were similar as those for the full data set: the slope is steepest in the group with highest leaf P values and lowest in the group with lowest P values (and the interaction of leaf N concentration by leaf P group was significant, P = 0.0002). Thus, the dependence of A max–N on leaf P is strongly detected in the “warm tropical” group but not the “cold biome” groups.
Discussion
The results show that the slope of the relationship between A max and leaf N is sensitive to the foliar P concentration, with low P values associated with a shallower slope of A max:N. As such, these results are consistent with what is known about photosynthetic physiology. Responsiveness to variations in leaf N should be muted by impacts of low P on either RuBP regeneration (Jacob and Lawlor 1992; Rao and Terry 1995; Loustau et al. 1999; Campbell and Sage 2006) or stomatal conductance (Jacob and Lawlor 1991; Thomas et al. 2006). Thus, despite the opportunity for evolutionary adaptation that might create a diversity of A max–N–P relations and thereby eliminate any predictable influence of leaf P on the A max–N relation, this signal was detectable. This observation suggests a strong and fundamental control on photosynthetic performance by compounds containing these two elements, as seen across both natural soil and fertilizer gradients (Reich and Schoettle 1988; Cordell et al. 2001; Whitehead et al. 2005). Moreover, the differences in A max–N–P relations noted among biomes are consistent with the long-held hypothesis that differences on average in N and P among biomes are a function of soil substrate age (e.g., time since last glaciation), with the result that high-latitude biomes tend to be N-limited and older subtropical and tropical soils either P-limited or N and P co-limited (Walker and Syers 1976; Vitousek 1984; Reich and Oleksyn 2004).
However, it is at least theoretically possible that these divergent biogeographic patterns arise, at least in part, from evolutionary (genotypic) differences among species adapted to different levels of N and P nutrition rather than from a direct coupled physiology–biogeochemistry-based mechanism. Perhaps because of widespread P limitations, species in chronically P-deficient habitats could have been selected to alter photosynthetic properties so as to have more modest increases in A max with increasing leaf N than those undergoing selection in biomes and/or microsites with higher P availability. This could arise theoretically if the increased carbon supply from higher A max placed plants at increased P limitation, whereas in areas with abundant P, the stoichiometry of future growth was such that sufficient N and P would be available to build new tissues with rising carbon supply. Such a response could work in a complementary manner to the direct physiological mechanism. However, one can argue that under such a scenario, plants should use any extra carbon to obtain more soil P (e.g., via mycorrhizae) until the point where the stoichiometric imbalance was eliminated. In any case, there is no evidence available to support this argument and no known mechanism, leaving us to conclude that the majority of A max–N–P differences among biomes are driven by nutrient supply and not by evolved differences between temperate and tropical taxa.
However, there were differences among biomes that suggest (at least hypothetically) either the potential for some degree of differential adaptation to differing N and P supply or a thermal influence on A max–N–P interactions. In biomes or groups that tend to have low P, A max was often higher at a given low N value, despite lower leaf P and lower leaf P:N levels (i.e., the A max–N lines crossed, with P-deficient taxa having low A max at high N but slightly higher A max at low N, compared to P-rich taxa). One could argue that this would be adaptive for plants characterized by low concentrations of both leaf P and leaf N, but the “warm tropical” group on average has lower leaf P but not lower leaf N than the “cold temperate” species in this compilation (Table 1). It is possible, although conjecture, that A max–N–P relations are influenced by ambient temperatures, as the groups with low P tend to be those found in warmer average conditions as well as warmer growing season conditions. If biochemical reactions are less frequently limited by low temperatures in warmer than cooler sites or biomes, which, not surprisingly, seems likely, this could influence the A max–N relationship at a given P level. In other words, higher temperatures could hypothetically compensate for low P in warm biomes, resulting in higher A max at any given low N and low P value than might occur in a cooler biome. Unfortunately not enough is known yet about possible thermal influence on photosynthesis–nutrient relations to say much more at this point in time.
In summary, the results of this study help us to better characterize the influence of P on the frequently observed interspecific A max–N relationship (e.g., Field and Mooney 1986; Wright et al. 2004). In particular, the main finding of our study—a shallower slope of A max to N at low leaf P values—appears to be influenced by both narrow and broad geographic differences in soil and plant P, consistent with prior research (Walker and Syers 1976; Reich and Schoettle 1988; Cordell et al. 2001; Richardson et al. 2004; Whitehead et al. 2005). Thus, the parsimonious conclusion is that observed patterns of A max–N–P are largely driven by soil substrate-related differences in N and P supply and, hence, in leaf N and P, among biomes. The analyses done here suggest that it is important to learn more about the physiology of P effects on A–N relationships (or N effects on A–P relationships) for modeling carbon and biogeochemical fluxes and vegetation–climate interactions, especially for regions where low P supply may play a role in limiting plant and ecosystem function.
References
Bielski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252
Campbell CD, Sage RF (2006) Interactions between the effects of atmospheric CO2 content and P nutrition on photosynthesis in white lupin (Lupinus albus L.). Plant Cell Environ 29:844–853
Conroy JP, Smillie RM, Küppers M, Bevege DI, Barlow EW (1986) Chlorophyll a fluorescence and photosynthetic and growth response of Pinus radiata to phosphorus deficiency, drought stress and high CO2. Plant Physiol 81:423–429
Cordell S, Goldstein G, Meinzer FC, Vitousek PM (2001) Regulation of leaf life-span and nutrient-use efficiency of Metrosideros polymorpha trees at two extremes of a long chronosequence in Hawaii. Oecologia 127:198–206
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ 30:1557–1565
Ellsworth DS, Reich PB (1992) Leaf mass per area, nitrogen content and photosynthetic carbon gain in Acer saccharum seedlings in contrasting forest light environments. Funct Ecol 6:423–435
Field C, Mooney HA (1986) The photosynthesis–nitrogen relationship in wild plants. In: Givnish T (ed) On the economy of plant form and function. Cambridge University Press, London, pp 25–55
Jacob J, Lawlor DW (1991) Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. J Exp Bot 42:1003–1011
Jacob J, Lawlor DW (1992) Dependence of photosynthesis of sunflower and maize leaves on phosphate supply, ribulose-1,5-bisphosphate carboxylase/oxygenase activity, and ribulose-1,5-bisphosphate pool size. Plant Physiol 98:801–807
Loustau D, Ben Brahim M, Gaudillére J-P, Dreyer E (1999) Photosynthetic responses to phosphorus nutrition in two-year-old maritime pine seedlings. Tree Physiol 19:707–715
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Raaimakers D, Boot RGA, Dijkstra R, Pot S, Pons T (1995) Photosynthetic rates in relation to leaf phosphorus content in pioneer versus climax tropical rainforest trees. Oecologia 102:120–125
Rao IM, Terry N (1995) Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet. IV. Changes with time following increased supply of phosphate to low-phosphate plants. Plant Physiol 107:1313–1321
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA 101:11001–11006
Reich PB, Schoettle AW (1988) Role of phosphorus and nitrogen in photosynthetic and whole plant carbon gain and nutrient use efficiency in eastern white pine. Oecologia 77:25–33
Reich PB, Walters MB, Ellsworth DS, Uhl C (1994) Photosynthesis–nitrogen relations in Amazonian tree species. I. Patterns among species and communities. Oecologia 97:62–72
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Richardson SJ, Peltzer DA, Allen RB, McGlone MS, Parfitt RL (2004) Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia 139:267–276
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Thomas DS, Montagu KD, Conroy JP (2006) Leaf inorganic phosphorus as a potential indicator of phosphorus status, photosynthesis and growth of Eucalyptus grandis seedlings. For Ecol Manage 223:267–274
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Warren CR, Adams MA (2002) Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol 22:11–19
Warren CR, McGrath JF, Adams MA (2005) Differential effects of N, P and K on photosynthesis and partitioning of N in Pinus pinaster needles. Ann For Sci 62:1–8
Whitehead D, Boelman NT, Turnbull MH, Griffin KL, Tissue DT, Barbour MM, Hunt JE, Richardson SJ, Peltzer DA (2005) Photosynthesis and reflectance indices for rainforest species in ecosystems undergoing progression and retrogression along a soil fertility chronosequence in New Zealand. Oecologia 144:233–244
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Acknowledgments
We thank the U.S. National Science Foundation Long-Term Ecological Research program (DEB 0080382, 0620652) and the Australian Research Council’s ARC–NZ Research Network for Vegetation Function for financial and intellectual support. All of the research reported herein was conducted in compliance with the laws of the relevant countries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Frederick C. Meinzer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reich, P.B., Oleksyn, J. & Wright, I.J. Leaf phosphorus influences the photosynthesis–nitrogen relation: a cross-biome analysis of 314 species. Oecologia 160, 207–212 (2009). https://doi.org/10.1007/s00442-009-1291-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1291-3