Abstract
We examined 15 traits in leaves and stems related to leaf C economy and water use for 32 co-existing angiosperms at ridge sites with shallow soil in the Bonin Islands. Across species, stem density was positively correlated to leaf mass per area (LMA), leaf lifespan (LLS), and total phenolics and condensed tannins per unit leaf N (N-based), and negatively correlated to leaf osmotic potential and saturated water content in leaves. LMA and LLS were negatively correlated to photosynthetic parameters, such as area-, mass-, and N-based assimilation rates. Although stem density and leaf osmotic potential were not associated with photosynthetic parameters, they were associated with some parameters of the leaf C economy, such as LMA and LLS. In the principal component (PCA) analysis, the first three axes accounted for 74.4% of total variation. Axis 1, which explained 41.8% of the total variation, was well associated with parameters for leaf C and N economy. Similarly, axis 2, which explained 22.3% of the total variation, was associated with parameters for water use. Axis 3, which explained 10.3% of the total variation, was associated with chemical defense within leaves. Axes 1 and 2 separated functional types relatively well, i.e., creeping trees, ruderal trees, other woody plants, C3 shrubs and forbs, palms, and CAM plants, indicating that plant functional types were characterized by similar attributes of traits related to leaf C and N economy and water use. In addition, when the plot was extended by two unrelated traits, leaf mass-based assimilation rates and stem density, it also separated these functional types. These data indicate that differences in the functional types with contrasting plant strategies can be attributed to functional integration among leaf C economy, hydraulics, and leaf longevity, and that both leaf mass-based assimilation rates and stem density are key factors reflecting the different functions of plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant species often exhibit substantial variation in leaf and stem traits even within sites, and can be classified into different functional types that share a similar attribute of particular traits (Grime 1977; Leishman and Westoby 1992; Craine et al. 2001; Westoby et al. 2002; Díaz et al. 2004). The functional diversity of plant species can be understood through identifying why a similar attribute of traits is favored and understanding how the sets of attributes link with plant strategy (Grime et al. 1997; Ackerly 2004; McGill et al. 2006; Westoby and Wright 2006; Wright et al. 2006). When two or more plant traits are well correlated across species, the continuous traits with a wide spectrum can reflect a fundamental trade-off (e.g., Reich et al. 2003). A well-known example is leaf lifespan. Leaf lifespan is positively correlated to leaf mass per area, and negatively correlated to mass-based photosynthetic rates and N contents across a wide array of biome and angiosperm taxa (Reich et al. 1994, 1997; Wright et al. 2004). At one end of the leaf-lifespan spectrum, long-lived leaves retain nutrients for a long period of time (e.g., Chapin 1980; Aerts 1995), but they must invest a large amount of C resource for defense against physical damage and herbivory (e.g., Coley 1988). At the opposite end of the spectrum, short-lived leaves achieve high productivity, but they must retrieve the cost of leaf construction for a short period of time.
Leaf longevity is associated with leaf gas exchange, and leaf gas exchange is regulated by plant hydraulic conductance. Hydraulic conductance in the roots controls leaf osmotic potentials and plant morphology, such as the ratio of leaf area to fine-root area (Shimizu et al. 2005), and twig hydraulic conductance regulates stomatal conductance and thus the rate of photosynthesis (Uemura et al. 2004). Wood density, in addition to being positively associated with mechanical strength of stems (Givnish 1995), is negatively associated with hydraulic conductivity in twigs (Santiago et al. 2004), percent loss of twig hydraulic conductivity under drought conditions (Hacke et al. 2001; Sperry 2003), and daily minimum water potentials in leaves (Ackerly 2004; Bucci et al. 2004; Santiago et al. 2004). Leaf C economy and whole-plant hydraulic system are thus coordinated with each other, and the coordination may be associated with life history features of a plant species. However, it is still unclear how leaf and stem traits related to C, nutrients, and water use are coordinated across species (Wright et al. 2006).
In this study, we examined relationships between 15 traits of leaves and stems related to leaf C economy and water use across 32 co-existing angiosperms in sub-tropical oceanic islands, Japan. Good correlations (high r 2-values) between two traits suggest that functional integration can exist. On the other hand, weak correlations (low r 2-values) are also of particular interest because a matrix extended by unrelated traits can define niche partitioning with different functional strategies (Ackerly 2004). Our primary objective was to examine the extent to which leaf and stem traits related to leaf C economy and water use can explain the variation in functional types. To do this, we: (1) constructed a matrix of correlations among all examined variables to determine a possible set of the leaf and stem attributes, and (2) performed a principal component analysis (PCA) to reveal how such sets were correlated within given functional types.
Materials and methods
Study sites and plant species
The Bonin Islands are small oceanic islands that formed as a result of volcanic activity during the Tertiary period. The study was conducted on Chichi-jima island (27°04′N, 142°13′E), one of the Bonin islands, located in the subtropical North Pacific Ocean about 1,000 km south of Tokyo. Chichi-jima island has an maximum elevation of 317.9 m above sea level and an area of 24 km2. For the period from 1969 to 2005, the mean annual temperature was 23.1°C with mean temperatures of 27.6°C in the hottest month (July) and 17.7°C in the coolest month (February) (Chichi-jima Meteorological Observatory, the Meteorological Agency of Japan). The year-to-year variation in annual precipitation was relatively large. The mean annual precipitation was 1,277 mm with a minimum of 750 mm in 1971 and a maximum of 1,857 mm in 1989. The soil (Chromic Cambisols) on Chichi-jima island is sandy, acidic, and of volcanic origin. The nutrient contents in the soil are low, and the pH values are 4.6–5.3 (Morita 1981).
A large part of the islands is covered by broad-leaved evergreen forests and, like the Galapagos and Hawaiian Islands, they have a rich flora with a high degree of endemism (70% for tree species; Kobayashi 1978). The ridge sites on the islands are characterized by relatively shallow soil (<30 cm thick) and can favor dwarf plants with sclerophyllous and xerophitic leaves (Shimizu and Tabata 1991). Although the top canopy reaches a height of 15 m at valley sites with deep soil, the top canopy at the ridge sites is only 2–3 m high. The forest canopies are well closed even at the ridge sites, but the bedrock is exposed in several places. Almost all creeping trees, shrubs, and forbs with low plant heights (<50 cm plant heights) are found at the crack or edge of the bedrock. The co-occurring plants exhibit substantial interspecific variations in leaf lifespan (LLS) (Shimizu 1983), daily leaf water potentials (Mishio 1992), and photosynthetic rates (Yamashita et al. 2000; Ishida et al. 2001). The leaf water potentials in the dark just before dawn are notably low, e.g., −0.9 MPa in Dodonaea viscose (a creeping tree) (Mishio 1992). Thus severe drought seems to occur frequently, especially in summer. The forest vegetation has been occasionally damaged by strong wind and salt spray caused by typhoons, but no conspicuous damage due to typhoons was found during this study (2000–2005). Forest fires do not occur on the islands.
We selected 32 angiosperm species representing 24 families (19 orders) at the ridge sites (Table 1). All angiosperms usually found at the ridge sites in the islands were included, although we could not treat some species with low population density due to conservation of plant species. The plant species were divided into seven discrete functional types: (1) trees (except for creeping and ruderal trees) (18 species), (2) creeping trees (three species), (3) ruderal trees (four species), (4) climber (two species), (5) C3 shrub (one species) and forbs (two species), (6) CAM (one species), and (7) palm (one species) (Table 1). Santalum boninense and Korthalsella japonica (order Santales) are hemiparasite trees attached to the stem and the root systems of a host tree, respectively. Although all 32 species are relatively drought adapted, Trema orientalis and Fagara boninensis cannot live at sites with extremely shallow soils. Of the 32 species, seven are alien to the Bonin Islands.
Examination of plant traits
Fifteen leaf and stem traits related to leaf morphology, leaf gas exchange, and water use were examined (Table 2). For four species, we were unable to collect data on some of the traits. For example, leaf area and LLS could not be determined in K. japonica because it is leafless (due to degeneration) and in Casuarina equisetifolia that has needle-like leaves only. We collected young, fully expanded sun leaves with no or little sign of herbivory or pathogen damage, during the productive season (Cornelissen et al. 2003). Except for LLS, plant traits were examined during the summer season.
Leaf lifespan
LLS (L leaf) was examined from 2000 to 2005. More than seven sunlit twigs or shoots from three individual plants were selected for each species. Shoots or individuals that died during the examination period were replaced with new shoots or individuals. In June and December of each year, some nodes of each shoot were marked with water- and photo-proof maker pens (PC-3M; Mitsubishi Pencil, Tokyo) and the number of leaves produced during the preceding 6 months and number of attached leaves were counted for each shoot. LLS was defined as: L leaf (year) = (the number of attached leaves/the number of newly produced leaves for a year).
Leaf gas exchange, N, and morphology
Light-saturated net assimilation rate and leaf conductance were measured in seven sunlit, mature leaves for at least four individuals. The measurements were done in the morning (8–11 a.m) to avoid the midday depression (Ishida et al. 2001) with an LI-6400 portable photosynthesis open system (LI-COR, Lincoln, Neb.). Measurement of H2O and CO2 concentrations in the gas stream within the system is based on infrared absorption. The CO2 concentration of the inlet gas stream was 370 μmol mol−1 and the photon flux density supplied by red–blue LED lamps was 2,000 μmol m−2 s−1 at the leaf surface. After the measurement, the area of individual leaves excluding petioles was determined with a LI-3000A portable area meter (LI-COR). Where leaves were smaller than the LI-COR chamber (3 cm in length and 2 cm in width), the lamina inside the chamber was cut and then the area was determined. The examined leaves were dried at 70°C for at least 3 days and weighed to determine leaf mass per area (LMA, M leaf). Area-based net assimilation rates (A a) and mass-based net assimilation rates (A m) were calculated with M leaf. N content of leaves (green stems in the case of K. japonica) was determined with an NC-800 N-C analyzer (Sumigraph, Sumitomo-Kagaku, Osaka). Mass-based N concentrations (N m) and net assimilation rates per unit N (N-based) (A N; photosynthetic N-use efficiency) were calculated with LMA. δ13C ratios were determined with a MAT 525 stable isotope ratio mass spectrometer (Finnigan-MAT, Bremen, Germany). The δ13C values of dried leaves (green stems in the case of K. japonica) were calculated with reference to the Peedee Belemnite (PDB) standard as follows: δ13C (‰) = [(R sample–R PDB)/R PDB]1000, where R sample and R PDB are the 13C/12C ratio in the samples and the PDB standard (R PDB = 0.0112372), respectively. The leaf thickness was examined in five sun leaves from each of five individual plants. Immediately after leaf collection, the thickness of the lamina between thick veins was measured using a digital micrometer (CLM1-15QM; Mitsutoyo, Kanagawa, Japan) with a measuring force of 0.5 N.
In the present study, leaf area may have been underestimated because of the curvature of leaf surface (thick laminae were often not truly flat), or overestimated because of the edge effect in small leaves (Wilson et al. 1999). Also, leaf thickness can change with the measuring force of the digital micrometer. Although measuring errors were included in the present study, most errors would have been smaller than the species-to-species variations.
To calculate N resorption rates (N r) during leaf senescence, dead leaves were sampled from plants just before leaf fall. In K. japonica (a stem hemiparasite), no dead parts were found. The dead-leaf samples were dried at 70°C for at least 3 days before determination of N contents. N resorption rate (%) was defined as [(N leaf − N litter)/N leaf]100, where N leaf and N litter are the mean values of dry-mass-based N contents in green and dead leaves, respectively.
Leaf water relations
The osmotic potentials and water contents in water-saturated leaves (green stems in the case of K. japonica) were examined in seven shoots from at least three individuals. Stems were recut under water immediately after sampling. To rehydrate to saturation, the collected shoots were covered with plastic bags and their cut ends kept under water for more than 7 h in dark conditions at room temperature. Leaves in which water was observed to infiltrate into lamina were excluded. After determination of fresh mass, the leaves were dried at 70°C for at least 3 days and weighed again. The saturated water content (W sat %) was defined as [(fresh mass–dry mass)/dry mass]100. Some leaf samples were frozen and melted to break cell walls. Osmotic potentials at full turgor were determined at room temperature (20°C) with extract from these samples, using a VAPRO 5520 Osmometer (Wescor, Utah).
Leaf total phenolics and condensed tannins
Phenolics and tannins possibly have defensive functions against herbivores (cf. Coley 1988) and play regulatory roles in nutrient cycling within an ecosystem (cf. Hättenschwiler and Vitousek 2000). The concentration of total phenolics and condensed tannins within leaves were determined in at least eight leaves (green stems in the case of K. japonica) from four individuals. The leaf samples were dried at room temperature (25°C) with an FDC-830 vacuum drier (EYELA, Tokyo). The concentrations of total phenolics and condensed tannins were determined by the Folin–Ciocalteu method (Julkunen-Tiitto 1985) and the proanthocyanidin method (Bate-Smith 1977), respectively, and were expressed on an N basis (g g−1 N) (Cunningham et al. 1999).
Stem or wood density
Stem or wood density (D stem) was measured for three individuals for each species. We collected wood cores in trees with an increment borer, and the cores were taken at heights of <0.5 m. In the case of forbs or vines, we sampled parts of the stems with a pair of shears. Fresh volumes were determined with a measuring cylinder with a narrow diameter or calculated from the length and diameter of a wood core. The samples were oven-dried at 70°C for at least 3 days and weighed. D stem was calculated as the ratio of dry mass to fresh volume.
Statistical analysis
A non-parametric Spearman rank correlation between the pairs of all traits (Table 1) was analyzed with StatView (version 4.5J; ABACUS Concept, USA). The PCA was conducted with R (version 2.2.0; Development Core Team, Austria). For the PCA, we used the mean values in each species, but four species (C. equisetifolia, Schima mertensiana, Psidium cattleianum, K. japonica) that have an incomplete data set were excluded from the analysis. It is known that plant traits often vary with season and this can affect the results of the PCA (Craine et al. 2002), but most of our measurements were made only in the summer.
Results
A matrix of rank correlation and coefficients of determination (r 2) between all possible pairing of the 15 examined traits is shown in Table 3. Of a total of 105 possible pairings, 58 were significantly correlated (P < 0.05). The pairing of A m and A N had the largest coefficient of determination (r 2 = 0.863). Across species, A m, A N, and N m were positively correlated to each other, and each of these three traits was negatively correlated to LMA and LLS. Mass-based traits in relation to leaf gas exchange (A m, A N, and N m) were positively correlated to area-based traits (A a and G a). Parameters of photosynthetic traits (A a, A m, and A N) were not correlated to traits related to water relations (ψo, W sat, and D stem), except that A m was positively related to W sat. High δ13C (i.e., less negative δ13C) was associated with high LMA, leaf thickness, and LLS, and with low N m, A m, A a, A N, and G a.
Within parameters of water relations, W sat was negatively correlated to D stem, indicating that plants with succulent leaves favor succulent stems. High ψo (i.e., less negative leaf osmotic potential) was linked with high W sat and N m, low LMA and D stem, and short LLS. Although the parameters of water relations (ψo and D stem) were not correlated to photosynthetic parameters (A m, A N, and N m), they were associated with some parameters of leaf C economy (LAM and LLS).
LLS was associated with some photosynthetic and hydraulic parameters. LLS was positively correlated to LMA, leaf thickness, and D stem and negatively correlated to ψo, W sat, N m, A m, A N, and A a. N resoption rate was not correlated with any variables, indicating that it is determined independently from the other examined traits. Although the leguminous trees, Acacia confusa and Leucaena glauca, showed higher N m (3.12 ± 0.22 mol kg−1, mean ± 1 SD) than did the other plants (1.07 ± 0.33 mol kg−1, mean ± 1 SD), the value of N resoption rate in the leguminous trees (57 ± 17%, mean ± 1 SD) was equivalent to that in the other plants (51 ± 16%, mean ± 1 SD). LLS was not associated with N-based total phenolic and condensed tannin contents. N-based total phenolics was positively correlated to LMA and N-based condensed tannins, and negatively correlated to W sat, N m, A m, and A a. N-based condensed tannins was positively correlated to D stem, and negatively correlated to W sat and N m.
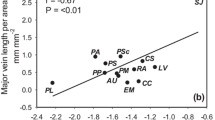
In a PCA of the 15 traits in 28 species, the first three axes accounted for 74.4% of the total variation. Axis 1 explained 41.8%, axis 2 explained 22.3%, and axis 3 explained 10.3% of the total variation. The results for axes 1, 2, and 3 for each species are shown in Table 1, and the correlations (r) with axes 1, 2, and 3 for each plant trait are shown in Table 2. N resorption rate showed the lowest contribution among the traits. Functional types grouped separately along axes 1 and 2, except that the climbers were positioned within the group of the trees (Fig. 1b).
Principal component analysis for (a) 15 traits and (b) 28 plant species. Species codes and the value on each axis are shown in Table 1. Trait codes and the correlations between axes and traits are shown in Table 2. Filled circles represent the trees (except for non-creeping and non-ruderal trees), open squares represent the creeping trees, filled squares represent the ruderal trees, filled triangles represent the C3 shrub and forbs, open triangles represent the climbers, open circles represent the palm, filled diamonds represent CAM, and asterisks represent alien plant species in the Bonin Islands
Axis 1 was positively correlated to LMA and LLS, and negatively correlated to A m, A N, and N m (P < 0.001) (Table 2), representing a contrast between “tough leaves” and “productive leaves” (Fig. 1a). Axis 1 was thus associated with different strategies for leaf C economy and nutrient use. The ruderal trees and the shrub and forbs are located at more negative positions along axis 1, which are associated with high photosynthetic capacity, fast leaf turnover, and low LMA. In contrast, the trees are located at more positive positions along axis 1, which are associated with lower photosynthetic capacity, slower leaf turnover, and higher LMA.
Axis 2 was positively correlated to D stem and LLS, and negatively correlated to ψo, W sat, δ13C, and leaf thickness (P < 0.001) (Table 2), representing a contrast between “hard tissue” and “watery tissue” (Fig. 1a). Axis 2 was thus associated with different strategies for water use. Bryophyllum pinnatum (Crassulaceae) exhibited an extremely negative position along axis 2 (Fig. 1b), because of their extreme leaf traits, i.e., CAM metabolism (−22.2‰ in δ13C) and succulency (11.4 g g−1 in W sat, 1,586 μm in leaf thickness). Axis 2 significantly separated the trees and the creeping trees from the shrub and forbs (Table 4).
Axis 3 was positively correlated with N-based total phenolics and condensed tannins (P < 0.001) (Table 2), and therefore can be considered as an axis of the secondary metabolic compounds that possibly determine defense against herbivory and decomposability of leaves. The ruderal trees had negative values, and the creeping trees had positive values along axis 3 (Table 4).
The results of the PCA analysis showed that the different functional types took different positions along the axes of leaf C economy and nutrient use (axis 1) and water use (axis 2). However, axes 1 and 2 could not separate climbers from trees (Fig. 1). A m and D stem were not correlated with each other (Table 3), and the vectors for A m and D stem were obliquely angled (Fig. 1a). As a result, the matrix of two unrelated traits, A m and D stem, separated the functional types well (Fig. 2). This matrix enhances the results of the PCA analysis, and shows that A m and D stem can be represented as key traits of leaf C economy and water use, respectively. The ruderal trees and the shrubs and forbs showed high A m (>100 nmol g−1 s−1), and were separated by a value of 0.35 g m−3 in D stem. The trees, the creeping trees, and the climbers showed low A m (<100 nmol g−1 s−1), and the climbers and the trees and the creeping trees groups were separated by a value of 0.55 g m−3 in D stem. A m at a given D stem was higher in the creeping trees than in the trees.
Species plot of mass-based net photosynthetic rate (A m) and stem or wood density (D stem). See Fig. 1 for explanation of symbols
Discussion
The PCA analysis showed that axis 1, which was related to leaf C and N economy, and axis 2, which was related to water use, explained 41.8 and 22.3% of the total variation, respectively. Axes 1 and 2 separated plant functional types relatively well, indicating that plant functional types were characterized by a similar attribute of particular traits related to leaf C and N economy and water use.
The first PCA axis reflects a fundamental trade-off between rapid acquisition and conservation of nutrient resource. The ruderal trees and the shrub and forbs with more negative values of axis 1 exhibited high N m, A m, A a, A N, and G a, low LMA, and short LLS, indicating a non-conservative nutrient use. In contrast, the trees with more positive values of axis 1 exhibited the opposite properties, indicating a conservative nutrient use strategy. It has been known that high LMA needs high C investment for leaf construction (Garnier and Laurent 1994; Garnier et al. 1997; Wilson et al. 1999), and imposes high resistance to CO2 diffusion within the leaf (Vitousek et al. 1990; Kogami et al. 2001), lowering photosynthetic capacity. However, it contributes to the notable toughness of the canopy leaves which allows them a long LLS and long resident time of nutrients within the plant body (Chabot and Hicks 1982; Escudero et al. 1992; Aerts 1995; Eamus and Prichard 1998; Ishida et al. 2006).
The second most important axis (axis 2) in the PCA was associated with water use. The trees and the creeping trees exhibited more positive values of axis 2 than did the ruderal trees and the shrubs and forbs. Our results show a negative correlation between D stem and ψO, and the trees and creeping trees had high D stem and low ψO. This is consistent with other studies, which have shown negative relationships between wood density and daily minimum leaf water potentials (Meinzer 2003; Ackerly 2004; Bucci et al. 2004; Santiago et al. 2004), because low osmotic potentials contribute to the maintenance of turgor under low leaf water potentials (Turner and Jones 1980). Thus, the trees and the creeping trees with high D stem and low ψO can have more negative leaf water potentials in the daytime, i.e., stronger force to pull water up from the soil. The high D stem will contribute to the avoidance of xylem conduit implosion under low leaf or stem water potentials (Hacke et al. 2001). It has been known that drought-induced xylem cavitation in the absence of freezing and thawing is not strongly related to the diameters of xylem conduits (Hacke and Sperry 2001), and wider conduits can lead to high hydraulic conductivity in stems as according to Poiseuille’s Law. Among the examined woody plants, the tree species with higher photosynthetic capacity exhibited wider xylem conduits in stems, but the ratio of total conduit area to sap wood area was not correlated to photosynthetic capacity (data not shown). This suggests that the canopy leaves with high gas exchange rates favor wide conduits in stem xylems.
D stem was negatively correlated to ψO across species (Table 3). Nevertheless, the hemiparasitic species (S. boninense and K. japonica) had more negative ψO at a given D stem than did the other species (data not shown). The low ψO values in hemiparasites allow them to endure more negative minimum leaf water potentials (i.e., larger water potential gradients), in order to extract sap and its solutes from the host plants (Schulze and Ehleringer 1984).
The ruderal trees and the shrubs exhibited low D stem (0.469 ± 0.136 g m−3, mean ± 1 SD), short LLS (0.5 ± 0.2 year, mean ± 1 SD), and low LMA (84 ± 23 g m−2, mean ± 1 SD), reflecting an opportunistic life strategy. In spite of the combination of low D stem, short LLS, and low LMA across species (Table 3), the palm had a low D stem (0.187 g m−3), a long LLS (1.3 years), and a high LMA (209 g m−2). Because monocotyledons cannot produce secondary vascular tissue in stems, the xylem in palms seems to dysfunction easily. Tomlinson et al. (2001) suggested that palm plants either prevent embolism in the xylem vessels or refill the embolized vessels to maintain stem function for a long time. However, in palm plants, it is still unclear how hydraulic function in stem xylem is maintained for a long time.
Axis 3 was positively correlated to N-based total phenolics and condensed tannins. These attributes were associated with anti-herbivory mechanisms (Cunningham et al. 1999) or decomposability (Hättenschwiler and Vitousek 2000). The association between LLS and total phenolic and condensed tannin contents is not general among biomes. Across 41 rainforest trees, LLS was positively correlated to mass-based condensed tannin contents (Coley 1988). Across seven winter-deciduous trees in Japan, LLS was positively correlated to mass-based total phenolic contents, but it was not correlated to mass-based condensed tannin contents (Matsuki and Koike 2006). In the present study, LMA was positively correlated to N-based condensed tannins, but LLS was not correlated to either N-based condensed tannins or total phenolics (Table 3) nor to mass-based condensed tannins or total phenolics (data not shown). Our results show that N-based condensed tannins were positively correlated to D stem; the combination may result in low decomposition rates of plants, and subsequently, in slow nutrient recycling within an ecosystem (Hättenschwiler and Vitousek 2000).
Our primary aim was to examine the extent to which the variation of leaf and stem traits that are related to leaf C economy and water use can explain variation in plant functional types. A closed correlation between D stem and ψo was found, and these water relation parameters were not correlated to leaf gas exchange parameters, such as A a, A m, A N, and G a (Table 3). However, D stem and ψowere associated with parameters of the leaf C economy, such as LMA and LLS, which were closely associated with leaf gas exchange parameters. This is probably due to the negative correlation of ψo and W sat or N m. Although we have accumulated much knowledge of correlations between leaf gas exchange and leaf longevity (e.g., Reich et al. 1997; Wright et al. 2004), we still only poorly understand whether such complex coordination between hydraulic properties and leaf form, or leaf longevity, or leaf gas exchange is found across a wide array of biome or angiosperm taxa. Wright et al. (2006) showed that woody plants with relatively low D stem developed more leaf area per unit shoot mass. This seems to be consistent with our result, in which LMA was positively correlated to D stem (Table 3), because the low LMA of plants with low D stem can lead to a large individual leaf area, which, in turn, leads to high leaf area per unit shoot mass. Our data indicate that different functional types have different sets of attributes of traits related to photosynthesis and water use, and both A m and D stem are key factors reflecting the different functions of plant species. Such functional integration will be a key aspect not only of biodiversity, but also of ecosystem functioning, such as productivity and nutrient and water cycling.
References
Ackerly DD (2004) Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol Monogr 74:25–44
Aerts R (1995) The advantages of being evergreen. Trends Ecol Evol 10:402–407
Bate-Smith EC (1977) Astringent tannins of Acer species. Phytochemistry 16:1421–1426
Bucci SJ, Goldstein G, Meinzer FC, Scholz FG, Franco AC, Bustamante M (2004) Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiol 24:891–899
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Annu Rev Ecol Syst 13:229–259
Chapin III FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Coley PD (1988) Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 74:531–536
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, Steege ter H, Morgan HD, Heijden van der MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS III (2001) The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 93:274–285
Craine JM, Tilman D, Wedin D, Reich P, Tjoelker M, Knops J (2002) Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct Ecol 16:563–574
Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecology 69:569–588
Díaz S, Hodgson JG, Thompson K, Cabido M, Cornelissen JHC, Jalili A, Montserrat-Martí G, Grime JP, Zarrinkamar F, Asri Y, Band SR, Basconcelo S, Castro-Diez P, Funes G, Hamzehee B, Khoshnevi M, Pérez-Harguindeguy N, Pérez-Rontomé MC, Shirvany FA, Vendramini F, Yazdani S, Abbas-Azimi R, Bogaard A, Boustani S, Charles M, Dehghan M, de Torres-Espuny L, Falczuk V, Guerrero-Campo J, Hynd A, Jones G, Kowsary E, Kazemi-Saeed F, Maestro-Martínez M, Romo-Díez A, Shaw S, Siavash B, Villar-Salvador P, Zak MR (2004) The plant traits that drive ecosystems: evidence from three continents. J Veg Sci 15:295–304
Eamus D, Prichard H (1998) A cost-benefit analysis of leaves of four Australian savanna species. Tree Physiol 18:537–545
Escudero A, del Arco JM, Sanz IC, Ayala J (1992) Effects of leaf longevity and retranslocation efficiency on the retention time of nutrients in the leaf biomass of different woody species. Oecologia 90:80–87
Garnier E, Laurent G (1994) Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytol 128:725–736
Garnier E, Cordonnier P, Guillerm J-L, Sonié L (1997) Specific leaf area and leaf nitrogen concentration in annual and perennial grass species growing in Mediterranean old-fields. Oecologia 111:490–498
Givnish TJ (1995) Plant stems: biomechanical adaptation for energy capture and influence on species distributions. In: Gartner BL (ed) Plant stems. Physiology and functional morphology. Academic Press, California, pp 3–49
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Grime JP, Thompson K, Hunt R, Hodgson JG, Cornelissen JHC, Rorison IH, Hendry GAF, Ashenden TW, Askew AP, Band SR, Booth RE, Bossard CC, Campbell BD, Cooper JEL, Davison AW, Gupta PL, Hall W, Hand DW, Hannah MA, Hillier SH, Hodkinson DJ, Jalili A, Liu Z, Mackey JML, Matthews N, Mowforth MA, Neal AM, Reader RJ, Reiling K, Ross-Fraser W, Spencer RE, Sutton F, Tasker DE, Thorpe PC, Whitehouse J (1997) Integrated screening validates primary axes of specialisation in plants. Oikos 79:259–281
Hacke UG, Sperry JS (2001) Functional and ecological xylem anatomy. Perspect Plant Ecol Evol Syst 4:97–15
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243
Ishida A, Nakano T, Uemura A, Yamashita N, Tanabe H, Koike N (2001) Light-use properties in two sun-adapted shrubs with contrasting canopy structures. Tree Physiol 21:497–504
Ishida A, Diloksumpun S, Ladpala P, Staporn D, Panuthai S, Gamo M, Yazaki K, Ishizuka M, Puangchit L (2006) Contrasting seasonal leaf habits of canopy trees between tropical dry-deciduous and evergreen forests in Thailand. Tree Physiol 26:643–656
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217
Kobayashi S (1978) A list of the vascular plants occurring in the Ogasawara (Bonin) Islands (in Japanese with English abstract). Ogasawara Res 1–2:1–33
Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T (2001) CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum leaves from low and high altitudes. Plant Cell Environ 24:529–538
Leishman MR, Westoby M (1992) Classifying plants into groups on the basis of associations of individual traits––evidence from Australian semi-arid woodlands. J Ecol 80:417–424
Matsuki S, Koike T (2006) Comparison of leaf life span, photosynthesis and defensive traits across seven species of deciduous broad-leaf tree seedlings. Ann Bot 97:813–817
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185
Meinzer FC (2003) Functional convergence in plant responses to the environment. Oecologia 134:1–11
Mishio M (1992) Adaptations to drought in five woody species co-occurring on shallow-soil ridges. Aust J Plant Physiol 19:539–553
Morita Y (1981) The dark-red forest soils of the Ogasawara Islands (I) Site condition, and morphological, mechanical and chemical properties (in Japanese with English abstract). J Jpn For Soc 63:1–7
Reich PB, Walters MB, Ellsworth DS, Uhl C (1994) Photosynthesis-nitrogen relations in Amazonian tree species. I. Patterns among species and communities. Oecologia 97:62–72
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164(Suppl):S143–S164
Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T (2004) Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140:543–550
Schulze E-D, Ehleringer JR (1984) The effect of nitrogen supply on growth and water-use efficiency of xylem-tapping mistletoes. Planta 162:268–275
Shimizu M, Ishida A, Hogetsu T (2005) Root hydraulic conductivity and whole-plant water balance in tropical saplings following a shade-to-sun transfer. Oecologia 143:189–197
Shimizu Y (1983) Phenological studies of the subtropical broad-leaved evergreen forests at Chichijima island in the Bonin (Ogasawara) Islands. Jpn J Ecol 33:135–147
Shimizu Y, Tabata H (1991) Forest structures, composition, and distribution on a Pacific island, with reference to ecological release and speciation. Pacific Sci 45:28–49
Sperry JS (2003) Evolution of water transport and xylem structure. Int J Plant Sci 164:S115–S127
Tomlinson PB, Fisher JB, Spangler RE, Richer RA (2001) Stem vascular architecture in the rattan palm Calamus (Arecaceae-Calamoideae-Calaminae). Am J Bot 88:797–809
Turner NC, Jones MM (1980) Turgor maintenance by osmotic adjustment: a review and evaluation. In: Turner NC, Kramer PJ (eds) Adaptation of plants to water and high temperature stress. Wiley, New York, pp 87–103
Uemura A, Ishida A, Tobias DJ, Koike N, Matsumoto Y (2004) Linkage between seasonal gas exchange and hydraulic acclimation in the top canopy leaves of Fagus trees in a mesic forest in Japan. Trees 18:452–459
Vitousek PM, Field CB, Matson PA (1990) Variation in foliar δ13C in Hawaiian Metrosideros polymorpha: a case of internal resistance? Oecologia 84:362–370
Westoby M, Wright IJ (2006) Land-plant ecology on the basis of functional traits. Trends Ecol Evol 21:261–268
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159
Wilson PJ, Thompson K, Hodgson JG (1999) Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol 143:155–162
Wright IJ, Falster DS, Pickup M, Westoby M (2006) Cross-species patterns in the coordination between leaf and stem traits, and their implications for plant hydraulics. Physiol Plant 127:114–456
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Yamashita N, Ishida A, Kushima H, Tanaka N (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125:412–419
Acknowledgments
This study was supported by grants-in-aid (14360091, 18255011, 18658067) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We wish to thank Drs H. Ikeda, Y. Yasumura, and N. M. Watanabe for helpful suggestions and Prof. N. Kachi, Dr Y. Yamamura, and Ms Y. Abe for helpful support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kouki Hikosaka.
Rights and permissions
About this article
Cite this article
Ishida, A., Nakano, T., Yazaki, K. et al. Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. Oecologia 156, 193–202 (2008). https://doi.org/10.1007/s00442-008-0965-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-0965-6