Abstract
Ant-dispersed plants usually produce seeds with appendages (elaiosomes) as reward for ants. Plants that produce high-quality elaiosomes benefit because ants preferentially disperse their diaspores. We therefore hypothesized that seeds and elaiosomes differ in chemical composition in ways that make elaiosomes of high nutritional quality for ants, capable of providing essential dietary components that explain the increased fitness and higher gyne production documented for colonies with elaiosome consumption. To test the hypothesis we analysed the content and composition of lipids, amino acids, soluble carbohydrates, proteins and starch in seeds and elaiosomes of 15 central European ant-dispersed plants. After separating the different fractions, total lipids were determined gravimetrically, fatty acids and soluble carbohydrates were detected by gas chromatography (GC) and GC–mass spectrometry, free amino acids by an amino acid analyser while starch and protein were analysed photometrically. Seeds accumulated high molecular weight compounds such as proteins and starch, whereas elaiosomes accumulated more easily digestible low molecular weight compounds such as amino acids and monosaccharides. Analysis of similarities and similarity percentages analysis demonstrated that the composition of fatty acids, free amino acids and carbohydrates differed markedly between elaiosomes and seeds. The most important difference was in total amino acid content, which was on average 7.5 times higher in elaiosomes than in seeds. The difference was especially marked for the nitrogen-rich amino acid histidine. The availability of essential nutrients and, in some species, the higher nitrogen content in elaiosomes suggest that their nutritional value for larvae plays a key role in this interaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mature plants overcome their limited mobility in numerous ways by using animals as vectors for offspring dispersal. The overwhelming majority of seeds or fruits (hereafter “diaspores”) are transported by vertebrates, whereas invertebrates allegedly “play only an anecdotal role as seed-dispersers” (Herrera 2002). A big exception, of course, are ants. Myrmecochory, the dispersal of seeds and fruits by ants, occurs worldwide in over 3,000 plant species from more than 80 plant families (Beattie and Hughes 2002); a huge variety of ant species disperse seeds, but their numbers have not yet been estimated. Ant dispersal is regarded as a mutualistic relationship beneficial for plants and ants. Key structures in the interrelationship are various types of elaiosomes, fleshy, usually lipid-rich and edible appendages of myrmecochorous diaspores. Elaiosomes are considered to be adaptations to promote dispersal and/or burial of diaspores by ants, as they are usually carried into the nest, where the elaiosomes are removed and eaten. The seed is then discarded either within the nest or nearby (Hughes and Westoby 1990). However, elaiosomes of different plant species are not equally attractive to ants (Sernander 1906; Bresinsky 1963). Differences in diaspore mass (Oostermeijer 1989; Hughes and Westoby 1992; Mark and Olesen 1996; Gómez et al. 2005), morphology of elaiosomes (Lanza et al. 1992), and spatial distribution and density of diaspores (Smith et al. 1989; Hughes and Westoby 1990) are important factors influencing removal rates and subsequent dispersal success. Because the principal benefit of diaspore transport for ants is the food reward represented by the elaiosome, the nutritional quality of the latter should also affect its attractiveness. Carroll and Janzen (1973) suggested that elaiosomes represent “from a foraging standpoint simply a dead insect analogue”. This assertion is strengthened by the fact that the fatty acid composition of elaiosomes in some Australian species is more similar to insect hemolymph than to that of the respective seeds (Hughes et al. 1994). One study pointed out that in addition to lipids, other compounds may be important, and that elaiosomes may contain nutrients particularly important for ants (Brew et al. 1989). Elaiosomes are frequently fed to the larvae (Fischer et al. 2005), thus enhancing colony reproduction, fitness (Gammans et al. 2005) and gyne production (Bono and Heithaus 2002), since diploid larvae that had consumed more elaiosomes tended to develop into gynes while others developed into workers. This indicates a high nutritional value of elaiosomes.
Due to the prevalence of the hypothesis that particular lipids or fatty acids, above all, 1,2-diolein and 1,3-diolein (Marshall et al. 1979; Skidmore and Heithaus 1988; Brew et al. 1989), act as stimulating substances to trigger seed-carrying behaviour, most studies have focused only on the lipid fraction of the appendages of myrmecochorous fruits and seeds (Soukup and Holman 1987; Kusmenoglu et al. 1989; Lanza et al. 1992; Hughes et al. 1994; Morrone et al. 2000). Aside from fatty acids, several amino acids (O’Dowd and Hay 1980; Brew et al. 1989), proteins (Lanza et al. 1992), carbohydrates and vitamins (Bresinsky 1963) are reported to be constituents of elaiosomes, and these substances may also play a major role. The nutritional quality of elaiosomes has only received little attention so far.
As previous studies have shown that fitness and gyne production in colonies with access to elaiosomes were increased (Bono and Heithaus 2002; Gammans et al. 2005), we here explore whether elaiosome constituents provide essential dietary components that could explain this phenomenon (and thus make myrmecochorous diaspores highly attractive for ants, increasing plant fitness). For this purpose, we analysed and compared the nutritional quality, as well as the content and composition of fatty acids, amino acids, proteins, soluble carbohydrates and starch in seeds and their respective elaiosomes from 15 common myrmecochorous species of central Europe.
Materials and methods
Plant material
Mature diaspores of 15 myrmecochorous plant species from seven families were investigated: Asarum europaeum (Aristolochiaceae); Helleborus niger, Helleborus dumetorum, Hepatica nobilis (Ranunculaceae); Chelidonium majus (Papaveraceae); Corydalis cava, Corydalis solida (Fumariaceae); Pulmonaria officinalis, Symphytum officinale, Borago officinalis (Boraginaceae); Knautia dipsacifolia, Knautia arvensis, Knautia drymeia (Dipsacaceae); Leucojum vernum, and Galanthus nivalis (Amaryllidaceae). The diaspores usually consisted of seeds with attached elaiosomes except for Boraginaceae, Dipsacaceae and Hepatica nobilis, where the diaspore is a fruit with elaiosome.
Diaspores were collected in the surroundings of Vienna and the Botanical Garden of the University of Vienna, between the months of April and August, from 1999 to 2001. Voucher specimens of the plants are deposited in the Herbarium of the Faculty Centre of Botany at the University of Vienna. Directly after collection, diaspores were subjected to a microwave treatment for 1 min to deactivate enzymes, dried for 24 h at 60°C and stored in a dark and dry place over silica gel until further use.
Prior to extraction, elaiosomes were detached from the rest of the diaspores using forceps, scalpel and latex gloves to avoid possible contamination. Although in some species (see above) the diaspore was not a seed plus elaiosome but a fruit with elaiosome, the term “seed” will be used in the following both for seeds and for fruits with an artificially detached elaiosome, and the term “diaspore” will refer both to seeds and fruits with the elaiosome still attached. Three replicates were analysed for all species, except for the elaiosomes of A. europaeum (n = 1; except for protein with n = 2) and C. solida (n = 1).
Extraction and chemical analysis of seeds and elaiosomes
Both elaiosomes and seeds were ground to a fine powder with a mortar and pestle under liquid nitrogen and re-dried for 20 min under reduced pressure in a vacuum concentrator. Cyclohexane (1 ml) was added to 50 mg of dried elaiosomes and to approximately 100 mg of dried seeds, respectively. Samples were then extracted at room temperature by continuously shaking for 5 min. After centrifugation (10 min, 7,000 r.p.m.), the supernatant was collected and the pellet re-extracted 3 times using 1 ml cyclohexane as above. The combined supernatants were evaporated under a stream of nitrogen and further dried under reduced pressure in a vacuum concentrator. The residue (=lipophilic fraction) was weighed and stored in liquid nitrogen at −20°C until further analysis. This fraction mainly consisted of lipids, but may also contain minor amounts of phospholipids and other lipophilic substances.
The residual pellet from the cyclohexane extraction was dried in vacuo and extracted with 1 ml of 30% (v/v) ethanol at 60°C for 30 min to analyse soluble carbohydrates and amino acids. The remaining pellet was washed twice with 30% ethanol and once with absolute ethanol, dried and extracted with 2% (w/v) sodium dodecyl sulphate for 30 min at 60°C for the determination of soluble protein following Peterson (1977) with bovine serum albumin as a standard.
Soluble carbohydrates were analysed by gas chromatography (GC) and GC–mass spectrometry (GC–MS) of their trimethylsilyl esters (Peterbauer et al. 1998) and free amino acids were analysed by a ninhydrin-based amino acid analyser (Fischer et al. 2002). Starch was digested enzymatically and determined as glucose (Wanek et al. 2001).
Fatty acid methyl esters were prepared from the lipid fraction by dissolving the lipid sample in methanol to a final concentration of 5 mg ml−1 and then adding 5 ml methanol–acetylchloride (50:1, v/v) (Welz et al. 1990). The mixture was gently shaken for 45 min at 25°C, and the reaction was stopped by adding 2.5 ml of 6% aqueous potassium carbonate solution. The esters were extracted with 0.3 ml cyclohexane. The fatty acid methyl esters were separated by GC (Perkin Elmer Autosystem, Rodgau-Jügesheim, Germany) on a PE225 column (30 m × 0.32 mm internal diameter, 0.25-μm film thickness; Perkin Elmer) with helium as carrier gas and temperatures between 70 and 205°C at a rate of 7°C min−1 to 165°C followed by a rate of 2°C min−1. The GC was coupled to a Turbomass Quadrupole mass spectrometer: electron energy 70 eV, filament emission 200 μA, ion source 180°C, transfer line 250°C (Perkin Elmer Turbomass). The identities of the fatty acid methyl esters were assigned by comparison of MS spectra and retention times to a fatty acid methyl ester standard mixture (Supelco, Deisenhofen, Germany) and to a mass spectral database (Wiley Registry of Mass Spectral Data, 6th edition and/or NIST/EPA/NIH Mass Spectral Library 1.5a).
To determine carbon and nitrogen contents, weighed aliquots (1–2 mg) of dried, ground seeds and elaiosomes were placed into tin capsules and analysed by an elemental analyser (EA 1110; CE Instruments, Milan) as described in Fischer et al. (2003).
All results remained non-normally distributed even after transformation. The proportions of lipids, amino acids, proteins and carbohydrates between seeds and elaiosomes of each species were compared using Wilcoxon’s matched pairs tests (Statistica 7.1; StatSoft, USA). A distance matrix was created based on Bray–Curtis dissimilarities (Bray and Curtis 1957) and tested for differences between groups (elaiosomes and seeds) by a one-way analysis of similarities (ANOSIM) in Primer 5 for Windows (Plymouth Routines in Multivariate Ecological Research; Primer-E, UK). The results were plotted with a non-metric multi-dimensional scaling (MDS) in Statistica 7.1 (StatSoft). Similarity percentages (SIMPER) in Primer-E were used to compare the overall percentage contribution each component made to the dissimilarity between groups (elaiosomes and seeds) in the ANOSIM analysis.
Results
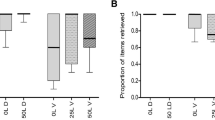
Significant differences in the quantitative pattern of nutrients were found between seeds and elaiosomes. While elaiosomes had higher free amino acid concentrations, seeds accumulated more proteins (Fig. 1; Table 1). The lipid content was not significantly different between elaiosomes and seeds though some species accumulated considerably more lipids in their elaiosomes than in their seeds (e.g. K. arvensis with 386.5 ± 4.7 mg g−1 dry mass (DM) in elaiosomes compared with 183.9 ± 4.7 mg g−1 DM in seeds). In four species (A. europaeum, H. dumetorum, H. niger, L. vernum), the lipid content of the elaiosome was less than 5% of DM. On a family basis, no difference in the lipid content between elaiosomes and seeds was found in Fumariaceae, Boraginaceae and Amaryllidaceae, while two families (Ranunculaceae and Papaveraceae) had a lower lipid content in elaiosomes, and Dipsacaceae had a higher lipid content in elaiosomes than in seeds (see Table 1 in the Electronic Supplementary Material). ANOSIM analyses of amino acid, fatty acid and carbohydrate composition of elaiosomes and seeds gave a global r > 0.3% (significance level 0.1%), which means that the elaiosomes of the 15 different species from seven different families are more similar to each other than each is to the seeds of the same species. The components that contributed >5% to the dissimilarity (SIMPER analysis) in the fatty acid composition of seeds and elaiosomes were: oleic acid (C18:1), linoleic acid (C18:2), palmitic acid (C16:0), γ-linolenic acid (C18:3n6), capric acid (C10:0), and linolenic acid (C18:3n3) (Table 2). The polyunsaturated fatty acid linoleic acid was quantitatively dominant in seeds with the exception of Dipsacaceae (capric acid) and the genus Helleborus (γ-linolenic acid). Capric acid and linolenic acid were frequently found in seeds but were not detected in elaiosomes (Table 1). Besides the widespread, dominant linoleic acid, seeds exhibited a complex taxon-specific fatty acid pattern. With the exception of Fumariaceae, each of the analysed families accumulated a distinct fatty acid which was not detectable in other families, e.g. cis-11,14-eicosatrienoic acid in the genus Helleborus, linolenic acid in the Boraginaceae, caprylic and capric acid in the Dipsacaceae, and cis-11,14-eicosatrienoic and cis-13,16-docosadienoic acid in the Amaryllidaceae.
Chemical composition of 15 central European myrmecochorous seeds and their detached elaiosomes. Contents of lipid, amino acids, protein, soluble carbohydrates (including glycerol and myo-inositol) and starch are indicated in mg g−1 dry mass (DM). Bars signify means + 1 SE. *P < 0.05, ns not significant (Wilcoxon test)
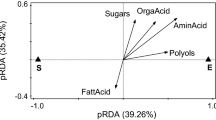
In contrast, elaiosomes had a much more homogeneous fatty acid composition, with oleic acid as the main component (except for two Knautia species) and palmitic, linoleic and γ-linolenic acid as further major fatty acids, independent of the systematic position of the plants (Table 1). This is also obvious in the two-dimensional scaling plot derived from the similarity analysis (Fig. 2). While all elaiosomes grouped together (with the exception of L. vernum), seeds were grouped according to their phylogenetic relationship.
Non-parametric multidimensional scaling of the profiles of fatty acids, amino acids and carbohydrates of seeds and elaiosomes based on the Bray–Curtis similarity index. The 15 plant species show significant differences in the composition of the seeds and appendant elaiosomes. Open circles represent elaiosomes, black circles seeds. Ae Asarum europaeum, Bo B. officinalis, Cc Corydalis cava, Cm Chelidonium majus, Gn G. nivalis, Cs Corydalis solida, Hd Helleborus dumetorum, Hn H. niger, Hen Hepatica nobilis, Ka Knautia arvensis, Kd K. dipsacifolia, Kdr K. drymeia, Lv L. vernum, Po P. officinalis, So S. officinale
Elaiosomes accumulated more amino acids than the respective seeds in all species but one, the only exception being H. dumetorum with 4.9 ± 0.4 mg g−1 DM in elaiosomes and 12.9 ± 0.1 mg g−1 DM in seeds (Table 1). Elaiosomes of S. officinale and H. niger had amino acid concentrations above 20% of DM compared to 0.3 and 1% in seeds of the same species (see Table 2 in the Electronic Supplementary Material). Detectable amino acids with concentrations above 5% of total amino acids in at least one species are listed in Table 1. All other detectable amino acids (i.e. phosphoserine, taurine, phosphoethanolamine, asparagine acid, sarcosine, α-aminoadipine acid, citrulline, α-aminobutyric acid, cysteine, methionine, cystathione, β-alanine, ornithine, lysine, 1-methylhistidine) were found only in some species and always in quantities less than 5% of total amino acids. Methionine was found in traces in elaiosomes of S. officinale, B. officinalis, K. dipsacifolia, L. vernum and G. nivalis (data not shown). Seeds accumulated mainly glutamic acid, glutamine and asparagine, while elaiosomes accumulated preferably glutamine, asparagine, proline, histidine, arginine, and, in contrast to seeds, only small amounts of glutamic acid (Tables 1, 2). Both seeds and elaiosomes of Amaryllidaceae accumulated mainly the nitrogen-rich amino acid arginine, which consists of 31% nitrogen per molecular weight. The elaiosomes of Dipsacaceae also had a high content of arginine, as well as nitrogen-rich histidine. The amount of amino acid-derived nitrogen was significantly higher in elaiosomes than in seeds (P = 0.0012, Wilcoxon test). Elaiosomes contained on average 11.38 μg nitrogen mg−1 DW in amino acids, seeds only 1.65 μg.
While amino acid content was higher in elaiosomes, protein content was higher in seeds (Table 1), with the exception of C. majus and B. officinalis. The highest protein content of seeds was measured in P. officinalis (16% of dry mass) and the highest protein content of elaiosomes in B. officinalis with 7% of DM.
No general pattern in the composition of elaiosomes and seeds could be detected in the total concentration of soluble carbohydrates and the polyols glycerol and myo-inositol (Table 1). Some families accumulated more carbohydrates in seeds, others in elaiosomes. Elaiosomes of C. majus and both Amaryllidaceae species accumulated carbohydrates to concentrations above 10% of DM. A general trend was noticed in the sugar composition. Seeds accumulated mainly sucrose while elaiosomes showed a more diverse pattern, accumulating fructose, glucose, myo-inositol and trehalose in addition to sucrose (Tables 1, 2; Fig. 2). A. europaeum and C. majus showed deviant patterns, both in seeds (by accumulating mainly glucose and glycerol, respectively) and in elaiosomes (by accumulating mainly myo-inositol and an unknown carbohydrate, respectively), C. cava differed by its high amount of glucose in seeds and trehalose in elaiosomes (see Table 3 in the Electronic Supplementary Material).
Starch content was highest in seeds of Amaryllidaceae and Ranunculaceae, but never exceeded 10% of DM. In elaiosomes, starch was negligible, amounting to less than 1% of DM in all cases (Fig. 1, Table 1).
Neither the overall nitrogen nor the carbon content differed significantly between seeds and elaiosomes (Wilcoxon test). Based on DM, seeds and elaiosomes had a carbon content of 51.9 ± 1.2% and 51.3 ± 2.0% (n = 15, mean ± SE) and a mean nitrogen content of 3.2 ± 0.2% and 3.0 ± 0.3% (n = 15, mean ± SE), respectively. There was no significant difference between the mean carbon/nitrogen ratio of seeds (16.7 ± 0.8, n = 15) and elaisosomes (20.1 ± 2.4, n = 15) (P = 0.910, Wilcoxon test).
Discussion
In this study, the overall chemical composition of elaiosomes and their respective seeds from different families was compared for the first time. In eight of the 15 investigated species, the elaiosomes are ontogenetically derived from seed tissue, and there would be reason to expect that their elaiosome composition should be similar to the average chemical composition of ground-up seeds. However, we consistently found striking differences in the composition and nutritional quality between elaiosomes and seeds. The elaiosome composition of plant species across different families tended to be independent of phylogenetic relationship (Fig. 2). In particular, the composition of fatty acids and amino acids, as well as the total amino acid (P = 0.0008, Wilcoxon test) and protein content (P = 0.019, Wilcoxon test), differed markedly between seeds and elaiosomes. Palmitic, palmitoleic and oleic acid were significantly higher in elaiosomes, the oleic acid content showing particularly striking differences, being on average 2.6 times higher in elaiosomes than in seeds of the species investigated. High oleic acid content is typical for most elaiosomes investigated so far (Soukup and Holman 1987; Lanza et al. 1992; Hughes et al. 1994; Gammans et al. 2005; Boulay et al. 2006). In only a few species is the oleic acid content reported to be more or less equal in seeds and elaiosomes (e.g. in North American Trillium, see Table 1 in Hughes et al. 1994). Oleic acid is the most abundant fatty acid in plant and animal tissue and the biosynthetic precursor of linoleic and linolenic acids (Christie 2005), both essential nutrients that cannot be synthesized by Hymenoptera (Hagen et al. 1984; Barbehenn et al. 1999). Two possible explanations may account for the high oleic acid content of elaiosomes. First, and this has been the most generally accepted explanation, oleic acid may be the major behaviour-releasing agent, perhaps in the form of diacylglycerol (1,2 or 1,3-diolein) as suggested by Marshall et al. (1979), Skidmore and Heithaus (1988), and Brew et al. (1989). In our opinion, however, the releasing agent may also be triacylglycerol (triolein). Triolein has been identified as the major brood-tending pheromone (Bigley and Vinson 1975) in Solenopsis invicta and could function in “brood mimicry”, forcing the ants to carry the brood-dummy (=diaspore) “back” to the nest. Indeed, Brew et al. (1989) stated in their study on elaiosomes of Acacia myrtifolia and Tetratheca stenocarpa that pieces of pith treated with triolein were removed at rates comparable to intact elaiosomes, and Boulay et al. (2006) found no difference between the removal rate of filter papers soaked with triglycerides and those soaked with diglycerides from extracts of Helleborus foetidus elaiosomes. As Wilson et al. (1958) pointed out, oleic acid seems to be a major chemical inducer for necrophoric behaviour in ants, and it seems to release a variety of other reactions also, independent of its configuration. Plants may be mimicking this chemical stimulus to their own advantage.
A second explanation is that the high oleic acid content may be of particular nutritional importance for ants. Diolein is reported to be the main constituent in insect hemolymph (Thompson 1973) and very often occurs in the 1,2 configuration (Beenakkers et al. 1985). This supports the hypothesis that “elaiosomes are insect analogues” (Carroll and Janzen 1973; Hughes et al. 1994) which in its core focuses on the similarity between the nutritional value of insect prey and that of elaiosomes. As the major hemolymph constituent, oleic acid may be easy to metabolize; studies have shown that the incorporation of oleic acid in larval stages is more rapid than with other fatty acids (Municio et al. 1975). The importance of elaiosomes for larvae is underlined by the combined concentrations of linoleic acid and linolenic acid, which ranged between 10 and 52% of the total fatty acids detected (Table 1). Both are essential for animals, including insects (Hagen et al. 1984; Barbehenn et al. 1999). Linoleic acid is necessary for successful pupal eclosion in some insects (Dadd 1973), and Hymenoptera need linolenic acid for the proper formation of the adult insect during metamorphosis (Canavoso et al. 2001).
The major difference between elaiosomes and seeds, however, is the total amino acid content, which in elaiosomes averages nearly 60 mg g−1 DM more than in seeds (=7.5 times higher). Some nitrogen-rich amino acids were found in higher concentrations in elaiosomes than in seeds, e.g. glutamine (carbon/nitrogen ratio 2.5) with 14.5% (±2.8) of total amino acids in seeds, and 16.7% (±3.2) in elaiosomes, histidine (carbon/nitrogen ratio 2.0) with 1.4% (±0.3) of total amino acids in seeds and 7.2% (±1.6) in elaiosomes, or arginine (carbon/nitrogen ratio 1.5) with 9.5% (±2.9) in seeds and 11.3 (±2.4) in elaiosomes. In few plant species, e.g. G. nivalis, the nitrogen content of elaiosomes and seeds was more or less equal, but in other species it was much higher in the elaiosome. In C. cava, for example, the nitrogen content in elaiosomes was 4.42% (SD ± 0.13) of total dry mass (DM) compared to 3.05% (SD ± 0.23) in seeds, amounting to a difference of 44.9% more nitrogen per gram DM in elaiosomes. Available nitrogen is the central limiting feature for growth, development and fecundity of insects (Hagen et al. 1984). Its presence in elaiosomes may explain why they are mainly fed to larvae (Fischer et al. 2005). It explains further the 48% increase in larval weight in laboratory colonies fed with elaiosomes (Gammans et al. 2005) and the shift in the sex ratio in favour of gynes (Morales and Heithaus 1998; Bono and Heithaus 2002).
In addition to providing nitrogen, several amino acids found in elaiosomes fulfil other functions in insects, such as in the metabolism of muscles (arginine; Dadd 1973), or the formation of chitin fibres (tyrosine; Baker et al. 1978). Proline, which was present in a much higher concentration in elaiosome tissues than in seeds, is an especially important amino acid for many insects, since its serves as a source of metabolic energy (Carter et al. 2006) and is suggested to play a crucial role in cold tolerance (Story and Storey 1992; Misener et al. 2001). Since insects can store amino acids, elaiosomes which provide proline may be important for the overwintering of the colony.
Moreover, trehalose, the main disaccharide in insect hemolymph (Wyatt 1967), was found in elaiosomes of some species in considerably higher amount than in seeds.
In this study we could demonstrate for the first time, that elaiosomes of the plant species investigated here exhibit a certain pattern: they accumulated more easily digestible low molecular weight compounds such as amino acids and monosaccharides (Tables 1, 2), whereas seeds accumulated high molecular weight compounds such as proteins and starch. Until now it was not known, that the total amino acid content is on average 7.5 times higher in elaiosomes than in seeds. We have further shown, that the chemical composition of seeds tends to be family specific while the elaiosome composition of plant species across different families tends to be independent of the phylogenetic relationship (Fig. 2). The information about the chemical composition of elaiosomes is important for the design of future experiments to test the selective mechanism responsible. Future experiments are needed to test whether ants prefer elaiosomes (or other foods) with lots of amino acids (rather than proteins) and monosaccharides (rather than starch), so that plants that produce elaiosomes which are better adapted to the ants’ requirement achieve higher fitness (through better dispersal benefits) than plants that produce less-adapted elaiosomes.
References
Baker HG, Opler PA, Baker I (1978) A comparison of the amino acid complements of floral and extrafloral nectars. Bot Gaz 139:322–332
Barbehenn RV, Reese JC, Hagens KS (1999) The food of insects. In: Huffaker CB, Gutierrez AP (eds) Ecological entomology, 2nd edn. Wiley, New York, pp 83–121
Beattie AJ, Hughes L (2002) Ant–plant interactions. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions. An evolutionary approach. Blackwell, Oxford, pp 211–235
Beenakkers AMT, Van der Horst DJ, Van Marrewijk WJA (1985) Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res 24:19–67
Bigley WS, Vinson SB (1975) Characterization of a brood pheromone isolated from the sexual brood of the imported fire ant, Solenopsis invicta. Ann Entomol Soc Am 68:301–304
Bono JM, Heithaus ER (2002) Sex ratios and the distribution of elaiosomes in colonies of the ant, Aphaenogaster rudis. Insectes Soc 49:320–325
Boulay R, Coll-Toledano J, Cerdá X (2006) Geographic variations in Helleborus foetidus elaiosome lipid composition: implications for dispersal by ants. Chemoecology 16:1–7
Bray RJ, Curtis JI (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Bresinsky A (1963) Bau, Entwicklungsgeschichte und Inhaltsstoffe der Elaiosomen. Bibl Bot 126:1–54
Brew GR, O’Dowd DJ, Rae JD (1989) Seed dispersal by ants: behaviour releasing compounds in elaiosomes. Oecologia 80:490–497
Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Annu Rev Nutr 21:23–46
Carroll CR, Janzen DH (1973) Ecology of foraging by ants. Annu Rev Ecol Syst 4:231–258
Carter C, Shafir S, Yehonatan L, Palmer RG, Thornburg R (2006) A novel role for proline in plant floral nectars. Naturwissenschaften 93:72–79
Christie WW (2005) The lipid library. http://www.lipidlibrary.co.uk
Dadd RH (1973) Insect nutrition: current developments and metabolic implications. Annu Rev Entomol 18:381–429
Fischer RC, Richter A, Wanek W, Mayer V (2002) Plants feed ants: food bodies of myrmecophytic Piper and their significance for the interaction with Pheidole bicornis ants. Oecologia 133:186–192
Fischer RC, Wanek W, Richter A, Mayer V (2003) Do ants feed plants? A 15N labelling study of nitrogen fluxes from ants to plants in the mutualism of Pheidole and Piper. J Ecol 91:126–134
Fischer RC, Oelzant SM, Wanek W, Mayer V (2005) The fate of Corydalis cava elaiosomes within an ant colony of Myrmica rubra: elaiosomes are preferentially fed to larvae. Insectes Soc 52:55–62
Gammans N, Bullock JM, Schonrogge K (2005) Ant benefits in a seed dispersal mutualism. Oecologia 146:43–49
Gómez C, Espadaler X, Bas JM (2005) Ant behaviour and seed morphology: a missing link of myrmecochory. Oecologia 146:244–246
Hagen KS, Dadd RH, Reese J (1984) The food of insects. In: Huffaker CB, Rabb RL (eds) Ecological entomology. Wiley, New York, pp 79–111
Herrera CM (2002) Seed dispersal by vertebrates. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions. An evolutionary approach. Blackwell, Oxford, pp 185–208
Hughes L, Westoby M (1990) Removal rates of seeds adapted for dispersal by ants. Ecology 71:138–148
Hughes L, Westoby M (1992) Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73:1300–1312
Hughes L, Westoby M, Jurado E (1994) Convergence of elaiosomes and insect prey: evidence from ant foraging behaviour and fatty acid composition. Funct Ecol 8:358–365
Kusmenoglu S, Roockwood LL, Gretz MR (1989) Fatty acids and diacylglycerols from elaiosomes of some ant-dispersed seeds. Phytochemistry 28:2601–2602
Lanza J, Schmitt MA, Awad AB (1992) Comparative chemistry of elaiosomes of 3 species of Trillium. J Chem Ecol 18:209–221
Mark S, Olesen JM (1996) Importance of elaiosome size to removal of ant-dispersed seeds. Oecologia 107:95–101
Marshall DL, Beattie AJ, Bollenbacher WE (1979) Evidence for diglycerides as attractants in an ant–seed interaction. J Chem Ecol 5:335–344
Misener SR, Chen C-P, Walker VK (2001) Cold tolerance and proline metabolic gene expression in Drosophila melanogaster. J Insect Physiol 47:393–400
Morales MA, Heithaus ER (1998) Food from seed-dispersal mutualism shifts sex ratios in colonies of the ant Aphaenogaster rudis. Ecology 79:734–739
Morrone O, Vega AS, Maier M (2000) Elaiosomes in Urochloa paucispicata (Poaceae: Panicoideae: Paniceae): anatomy and chemical composition. Flora 195:303–310
Municio AA, Odriozola JM, Pérez-Albarsanz A (1975) Biochemistry of development in insects. Eur J Biochem 60:123–128
O’Dowd DJ, Hay E (1980) Mutualism between harvester ants and a desert ephemeral: seed escape from rodents. Ecology 61:531–540
Oostermeijer JGB (1989) Myrmecochory in Polygala vulgaris L., Luzula campestris (L.) DC. and Viola curtisii Forster in a Dutch dune area. Oecologia 78:302–311
Peterbauer T, Puschenreiter M, Richter A (1998) Metabolism of galactosylononitol in seeds of Vigna umbellata. Plant Cell Physiol 39:334–341
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Sernander R (1906) Entwurf einer Monographie der europaeischen Myrmekochoren. Kungl Svenska Vetenskapsakadem Handl 41:1–407
Skidmore BA, Heithaus ER (1988) Lipid cues for seed-carrying by ants in Hepatica americana. J Chem Ecol 14:2185–2196
Smith BH, Forman PD, Boyd AE (1989) Spatial patterns of seed dispersal and predation of two myrmecochorous forest herbs. Ecology 70:1649–1656
Soukup VG, Holman RT (1987) Fatty acids of seeds of North American pedicillate Trillium species. Phytochemistry 26:1015–1018
Story KB, Storey JM (1992) Biochemical adaptations for winter survival in insects, 1. In: Steponkus PL (ed) Advances in low temperature biology. JAI, London, pp 101–140
Thompson SN (1973) A review and comparative characterization of the fatty acid composition of seven insect orders. Comp Biochem Physiol 45B:467–482
Wanek W, Heintel S, Richter A (2001) Preparation of starch and other carbon fractions from higher plant leaves for stable carbon isotope analysis. Rapid Commun Mass Spectrom 15:1136–1140
Welz W, Sattler W, Leis H-J, Malle E (1990) Rapid analysis of non-esterified fatty acids as methyl esters from different biological specimens by gas chromatography after one-step esterification. J Chromatogr 526:319–329
Wilson EO, Durlach NJ, Roth LM (1958) Chemical releasers of necrophoric behaviour in ants. Psyche 65:108–114
Wyatt GR (1967) The biochemistry of sugars and polysaccharides in insects. Adv Insect Physiol 4:287–360
Acknowledgements
We thank Michaela Manhart for assistance in extraction of seeds and elaiosomes. Special thanks are expressed to Christian Schulze for help with the statistical analysis. The comments of Doyle McKey and an anonymous reviewer greatly improved the manuscript. This work was supported by a grant of the Austrian Science Foundation (FWF, project P-13720-Bio) to V. M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Julia Koricheva.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Fischer, R.C., Richter, A., Hadacek, F. et al. Chemical differences between seeds and elaiosomes indicate an adaptation to nutritional needs of ants. Oecologia 155, 539–547 (2008). https://doi.org/10.1007/s00442-007-0931-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0931-8