Abstract
Seed dispersal by ants (myrmecochory) is mediated by the presence of a lipid-rich appendage (elaiosome) on the seed that induces a variety of ants to collect the diaspores. When seeds mature or fall onto the ground, these ant species transport them to their nest. After eating the elaiosome, the seed is discarded in nest galleries or outside, in the midden or farther away, where seeds can potentially germinate. The final location of seeds with their elaiosomes removed was evaluated to assess the importance of possible handles (structures that ants can grasp to carry) in transporting ants during re-dispersal experiments of seeds from nests of six species of ants. The results indicate that seeds remained within the nest because the ants were not able to transport them out of the nest. As a consequence of the elaiosome being removed, small ant species could not take Euphorbia characias seeds out of their nests. Only large ant species could remove E. characias seeds from their nests. Attaching an artificial handle to E. characias seeds allowed small ant species to redistribute the seeds from their nests. On the other hand, Rhamnus alaternus seeds that have a natural handle after the elaiosome removal were removed from the nests by both groups of ant species. If a seed has an element that acts as a handle, it will eventually get taken out of the nest. The ants’ size and their mandible gap can determine the outcome of the interaction (i.e. the pattern of the final seed shadow) and as a consequence, could influence the events that take place after the dispersal process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For myrmecochorous plants, the final location of seeds depends, basically, on which ants interact with the seed. Myrmecochorous plants have seeds that have caruncles or arils that act as elaiosomes. When ants find a seed, in most cases it is transported to the nest. They usually do this by using the elaiosome as a handle (Byrne and Levey 1993). After eating the elaiosome, the seed may remain in the nest or be discarded on the midden or farther away. Thus, the pattern of final seed location is directly influenced by ant behaviour. The consequences of seed and seedling distribution in relation to density-dependent selection, the juvenile plants’ defences and predation are well known (e.g. Howe 1989).
There is also a well-documented body of evidence showing the advantages of seeds remaining in the nest, where they are protected against fire and/or predators (e.g. Christian and Stanton 2004). The presence of an elaiosome may be important for the transport of the seed to the nest, but its absence after seed manipulation implies that the shape of the seed, its size, or the presence of surfaces that can provide a new handle for the ants, may be key factors in the outcome of the interaction i.e. the final location of the seed.
Given the variation in final seed locations in myrmecochorous dispersal systems, we decided to evaluate how important the presence of possible handles in relation to the transport capacity of ants was to the final location of seeds.
Materials and methods
Seeds
The seeds used came from two myrmecochorous species: (a) Euphorbia characias L. The seeds have a caruncle that acts as an elaiosome and is used as a handle by ants. Without the elaiosome, the seed has a rounded shape withing that can act as a new handle. Mean seed dimensions without elaiosome are 2.82×2.18×1.92 mm (n=25). (b) Rhamnus alaternus L. The seeds have a deep longitudinal groove on one side filled with a soft, oily tissue (elaiosome). The dimensions of the R. alaternus seed are 4.21×2.71×1.69 mm (n=25), with a minimum section of 0.68 mm. in the groove that ants use as a handle when transporting seeds before and after elaiosome removal. (c) E. characias seeds with an artificial handle (a small expanded polystyrene fragment, 2×1×1 mm) glued to the opposite side of the elaiosome. The artificial handle did not affect the time the seeds spent on the soil until they were found and transported by ants to the nest (F 1,98=0.097, P=0.75; un-manipulated seed mean±SD: 3′22′′ ± 2′54′′; seed with the artificial handle: 2′55′′ ± 2′03′′; fifty replicates per treatment and data log transformed). The ant species that transported these seeds in the study plot were Pheidole pallidula (Nyl.), Tapinoma nigerrimum Nyl., Aphaenogaster senilis Mayr and Messor barbarus (L.).
Field trials
Prior to field trials all the elaiosomes were removed with forceps. A trial consisted of putting five seeds into the nest entrance one by one to avoid blocking the entrance and causing an artificial response. The number of seeds that were taken out of the nest by ant workers was noted until 1 h after the last seed was placed in the nest. Trials were performed with six soil nesting ant species. Three were small-sized ants: P. pallidula (worker length: 2.2–4.5 mm), Tapinoma nigerrimum (2.5–5.1 mm) and T. ruginode Stitz (3.2–4.0 mm). The other three ant species were bigger: A. senilis (6.4–7.7 mm), Formica rufibarbis Fabr. (4.5–7.5 mm) and Camponotus cruentatus (Latr.) (5.8–14.6 mm). After the trial, in some cases the entry zone of the nest was dug up to check that seeds had not been taken deeper into the galleries. For the bigger ant species (A. senilis, C. cruentatus) and in separate trials, we placed five seeds of E. characias seeds and five R. alaternus seeds in five nests. For the ant-seed pairing of F. rufibarbis–E. characias, the trial was carried out in ten nests. For small ant species we put five seeds of each type into ten nests of P. pallidula, ten nests of T. nigerrimum and five nests of T. ruginode. The number of seeds taken out of the nest by different ant species was compared with two two-way ANOVAs with Bonferroni correction. We began with ant species (six levels) and types of seed (two levels) as fixed effects, first considering the seeds of E. characias and R. alaternus seeds, and then analysing the interaction between the small ant species (three levels) and the three types of seeds.
The field tests were carried out in four natural and suburban areas in Spain with Mediterranean sclerophylle vegetation (Girona (41°24′N, 2°9′E), Can Llevallol (41°25′N, 2°6′E), Rubí (41°56′N, 2°50′E) and Sant Cugat (41°55′N, 2°51′E)) in April and May 2000.
Results
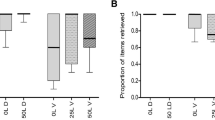
The number of E. characias seeds without elaiosome transported out of the nest by small ants was lower than in the other combinations (two-way ANOVA, P<0.001) (F 5,73=28.07 for ant species, F 1,73=85.46 for seed type, F 5,73=22.38 for interaction; Tukey post-hoc analysis, α=0.05) (Table 1).
Small ant species took out of the nest a similar number of E. characias seeds with the artificial handle and R. alaternus seeds but a much smaller number of the E. characias seeds without a handle. The small ant species used the soft polystyrene fragment as a new handle. Significant differences were found between ant species and the type of seeds (two-way ANOVA, F 2,66=6.91, P=0.0018 for ant species, F 2,66=107.89, P<0.001 for seed type and F 4,66=1.52, P=0.2 for interaction). In addition, P. pallidula transported more E. characias seeds out of the nest than T. nigerrimum.
Discussion
The shape and size of diaspores appeared to have a larger effect on the ant’s ability to carry the seed. If a seed has a handle, it eventually gets taken out of the nest (R. alaternus seeds). E. characias seeds—without an elaiosome—are rounded and do not have anything that can act as a handle. This absence is not important when the ant workers are big in relation to seed size. Although E. characias seeds do not have a handling point the big worker ants are capable of grasping them because their mandible gaps are larger than the minimum section of the seeds. This pattern cannot be applied to granivorous ants, which keep granaries in the nest.
These relationships are relative since a seed can be large for a given ant species and small for another ant species. Dillwinia juniperina seeds (4×2×1 mm) can be considered large compared to Pheidole sp1 workers (1–3 mm), who left the seeds in the nest, while these seeds are small compared to Rhytidoponera metallica (Smith) workers (5 mm) (Hughes and Westoby 1992) who took the seeds out of the nest (e.g. Gómez and Espadaler 1998).
Making a general conclusion about the final distribution of seeds is problematic. For myrmecochorous systems, it can be expected that the seed shadow will correlate strongly with the ant nest distribution. But the possibility and capacity of seed transport after the elaiosome has been removed and the morphological characteristics of the seed may alter the expected spatial pattern. Discarded seeds that are carried outside the ant nest often end up in the midden of an ant colony, or they are lost along ant trails (in a line) or at the borders of the territory (Gorb and Gorb 2003). In all these cases seed distribution is far from being random.
Documenting the causes of final seed location after the seed dispersal process can give an initial link between seed dispersal and plant demography.
References
Byrne MM, Levey DJ (1993) Removal of seeds from frugivore defecations by ants in a Costa Rican rain forest. Vegetatio 107/108:363–374
Christian CE, Stanton ML (2004) Cryptic consequences of a dispersal mutualism: seed burial, elaiosome removal, and seed-bank dynamics. Ecology 85:1101–1110
Gómez C, Espadaler X (1998) Aphaenogaster senilis Mayr (Hymenoptera, Formicidae): a possible parasite in the myrmecochory of Euphorbia characias. Sociobiology 32:441–450
Gorb EV, Gorb SN (2003) Seed dispersal by ants in a deciduous forest ecosystem: mechanisms, strategies, adaptations. Kluwer, Dordrecht
Horvitz CC, LeCorff J (1993) Spatial scale and dispersion pattern of ant- and bird-dispersed herbs in two tropicals lowland rain forests. Vegetatio 107/108:351–362
Howe HF (1989) Scatter- and clump-dispersal and seedling demography: hypothesis and implications. Oecologia 79:417–426
Hughes L, Westoby M (1992) Fate of seeds adapted for dispersal by ants in Australia sclerophyll vegetation. Ecology 73:1285–1299
Acknowledgements
We would like to thank Josep Morera and the Sangrà family for allowing us to work on their properties. We also thank David Casellas and Gemma Vila for field and laboratory assistance. E. Garcia-Berthou and two anonymous reviewers revised a previous draft of the manuscript. This study was funded by the Ministry of Education and Science of the Spanish Government (CGL2004-05240/BOS). The experiments comply with the current Spanish laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Koerner
Rights and permissions
About this article
Cite this article
Gómez, C., Espadaler, X. & Bas, J.M. Ant behaviour and seed morphology: a missing link of myrmecochory. Oecologia 146, 244–246 (2005). https://doi.org/10.1007/s00442-005-0200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0200-7