Abstract
The recent decline of Lepidoptera species strongly correlates with the increasing intensification of agriculture in Western and Central Europe. However, the effects of changed host-plant quality through agricultural fertilization on this insect group remain largely unexplored. For this reason, we tested the response of six common butterfly and moth species to host-plant fertilization using fertilizer quantities usually applied in agriculture. The larvae of the study species Coenonympha pamphilus, Lycaena phlaeas, Lycaena tityrus, Pararge aegeria, Rivula sericealis and Timandra comae were distributed according to a split-brood design to three host-plant treatments comprising one control treatment without fertilization and two fertilization treatments with an input of 150 and 300 kg N ha−1 year−1, respectively. In L. tityrus, we used two additional fertilization treatments with an input of 30 and 90 kg N ha−1 year−1, respectively. Fertilization increased the nitrogen concentration of both host-plant species, Rumex acetosella and Poa pratensis, and decreased the survival of larvae in all six Lepidoptera species by at least one-third, without clear differences between sorrel- and grass-feeding species. The declining survival rate in all species contradicts the well-accepted nitrogen-limitation hypothesis, which predicts a positive response in species performance to dietary nitrogen content. In contrast, this study presents the first evidence that current fertilization quantities in agriculture exceed the physiological tolerance of common Lepidoptera species. Our results suggest that (1) the negative effect of plant fertilization on Lepidoptera has previously been underestimated and (2) that it contributes to the range-wide decline of Lepidoptera.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The recent decline of Lepidoptera species strongly correlates with the increasing intensification of agriculture in Western and Central Europe (Maes and van Dyck 2001; Conrad et al. 2006; van Dyck et al. 2009; van Swaay et al. 2015). Agricultural intensification implies manifold impacts on the landscape and its biodiversity such as through the application of fertilizer, habitat fragmentation, higher mowing frequencies and livestock densities (Tilman et al. 2001; Tscharntke et al. 2005; Manning et al. 2015). Although several studies highlight the decline of Lepidoptera (Maes and van Dyck 2001; Thomas et al. 2004; Conrad et al. 2006), the number of experimental investigations trying to pinpoint the specific reasons for these negative population trends in response to agricultural intensification is rather negligible (but see Klop et al. 2015). With increasing knowledge about meta-population dynamics (Dover and Settele 2009) and the habitat requirements of adult and immature stages (Dennis et al. 2006; García-Barros and Fartmann 2009), it became clear that increasing fragmentation and too-high mowing frequencies in addition to agricultural abandonment contribute to the decline of Lepidoptera (Löffler et al. 2013; van Swaay et al. 2015; Bruppacher et al. 2016). However, similar investigations on the effect of agricultural fertilization on non-pest Lepidoptera species are almost completely missing. This is remarkable since nitrogen enrichment has been assumed as one reason for the large-scale decline of Lepidoptera for more than one decade (Öckinger et al. 2006; van Dyck et al. 2009).
Agricultural fertilization, as one of the most important indicators of agricultural land-use intensity (Herzog et al. 2006), not only influences the plant species composition of grasslands (Stevens et al. 2004; Socher et al. 2013) but also causes substantial changes in the tissue chemistry and, thus, the quality of the host plants (Prudic et al. 2005; Nijssen et al. 2017). To our knowledge, so far only two studies investigating Lepidoptera (Prudic et al. 2005; Kurze et al. 2017) linked their fertilization treatments to quantities commonly used in agriculture. Prudic et al. (2005) focused on the response of the American butterfly Junonia coenia to Plantago lanceolata fertilized with about 90 kg N ha−1 year−1, whereas Kurze et al. (2017) examined the response of Aglais io and Aglais urticae to Urtica dioica plants receiving either 150 and 300 kg N ha−1 year−1. In both studies, fertilization changed the host-plant quality. However, the survival rate of the larvae remained nearly constant in J. coenia (Prudic et al. 2005) and increased in both Aglais species (Kurze et al. 2017). In contrast, other studies orientated their fertilization treatments, if any (Fischer and Fiedler 2000; Mevi-Schütz et al. 2003; Chen et al. 2004), to atmospheric nitrogen deposition (Throop and Lerdau 2004; Klop et al. 2015), but not to agricultural fertilization. Thus, it is largely unknown how changes in host-plant quality through fertilizer quantities now regularly used in agriculture affect Lepidoptera. However, increasing nitrogen enrichment due to atmospheric nitrogen deposition, the enormous application of fertilizer in agriculture (Ellenberg and Leuschner 2010; Liu et al. 2015; WallisDeVries and Bobbink 2017) and the ongoing decline of Lepidoptera (van Swaay et al. 2015) reinforce the significance of studying this relationship.
The nitrogen requirements of herbivorous insects override the nitrogen concentration of the plant tissue (Mattson 1980; White 1993). This mismatch rests upon the different stoichiometry of the two species groups (Mattson 1980; White 1993). The low nitrogen concentrations of plant tissues provide an inadequate environment for herbivorous insects; most plant tissues are primarily composed of carbon compounds, whereas insects use nitrogen-based proteins as tissue building blocks (Mattson 1980). Due to its limited availability, nitrogen is considered to be the most important nutrient for herbivorous insects and the best indicator of host-plant quality (White 1993; Throop and Lerdau 2004). Several studies support this assumption, presenting empirical evidence that a higher dietary nitrogen content enhances the performance of Lepidoptera species (e.g. Slansky and Feeny 1977; Myers and Post 1981; Chen et al. 2008; Klop et al. 2015). This better performance is reflected through beneficial shifts in several fitness-related traits, such as a shorter larval time, higher pupal weight, higher fecundity and/or longer forewings due to a higher metabolic efficiency (Slansky and Feeny 1977; Myers and Post 1981; Chen et al. 2004; Klop et al. 2015). All these trait shifts decrease the risk of an individual dying before reproduction and increase reproductive potential (Loader and Damman 1991; Awmack and Leather 2002). Furthermore, the nitrogen content of the host plant determines the survival of the larvae, which show increased mortality rates on host plants with low nitrogen concentrations (Myers and Post 1981; Chen et al. 2004). The positive relationship between the nitrogen content of the host plant and the performance of herbivorous insects has been summarized by White (1993) as the nitrogen-limitation hypothesis.
In contrast to the nitrogen-limitation hypothesis, the theory of ecological stoichiometry predicts that the optimal performance of every species depends on a certain concentration of every nutrient, concerning macronutrients such as nitrogen as well as micronutrients (Sterner and Elser 2002). Hence, too high and too low nutrient concentrations reduce the performance of the species, either due to a scarcity of essential nutrients or excessive demands on regulation mechanisms (Sterner and Elser 2002; Raubenheimer et al. 2005; Boersma and Elser 2006). Hump-shaped relationships between species performance and the dietary nitrogen content indeed have been verified in a few Lepidoptera species (Brewer et al. 1985; Sarfraz et al. 2009; Han et al. 2014).
Furthermore, the strong support of the nitrogen-limitation hypothesis for Lepidoptera rests mainly upon fertilization experiments with pest species (e.g. Tabashnik 1982; Wheeler and Halpern 1999; Chen et al. 2008; Han et al. 2014), whereas non-pest species were largely ignored (but see Bink and Siepel 1996; Fischer and Fiedler 2000; Prudic et al. 2005; Klop et al. 2015; Kurze et al. 2017). Most interesting though is the negative response of non-pest Lepidoptera to fertilized host plants (Fischer and Fiedler 2000). The mortality of L. tityrus individuals that fed on fertilized compared to unfertilized host plants approximately doubled (Fischer and Fiedler 2000). This suggests that the two groups respond differently to fertilized plants and undermines the general applicability of the nitrogen-limitation hypothesis (Fischer and Fiedler 2000). However, the studies on non-pest Lepidoptera remain too anecdotal to explicitly evaluate whether this species group responds more sensitively to fertilized plants, and whether their response differs from the predictions of the nitrogen-limitation hypothesis.
This study provides the first comprehensive test for the response of common non-pest Lepidoptera species to plants receiving fertilizer quantities commonly used in agriculture. With Coenonympha pamphilus, Lycaena phlaeas, Lycaena tityrus and Pararge aegeria, we considered four butterfly species, and with Rivula sericealis and Timandra comae, two moth species, preferring either species of Polygonaceae or Poaceae as host plants. The rearing of the Lepidoptera species and host plants was spatially separated and the larvae were fed with randomly collected leave samples. This experimental design allows the response of the Lepidoptera species to be exclusively attributed to host-plant quality and excludes other effects of fertilization such as a higher biomass production of fertilized plants as well as further interactions with other biotic and abiotic factors. Based on the conflicting assumptions about the response of Lepidoptera species to fertilized host plants, we hypothesized a decreasing or increasing survival rate of the larvae feeding on fertilized compared with unfertilized plants in a dose-dependent manner. Besides the standardized fertilization procedure, we controlled the plant quality by measurement of the nitrogen concentration and C:N ratio of the leaves, expecting higher nitrogen concentrations and lower C:N ratios with increasing fertilizer quantities.
Materials and methods
Study species
In Germany, all six study Lepidoptera species, C. pamphilus, L. phlaeas, L. tityrus, P. aegeria, R. sericealis and T. comae, are common, widespread and considered not threatened (Ebert and Rennwald 1991; Ebert 1997, 2001; Reinhardt and Bolz 2011). They are habitat generalists rather than specialists, inhabiting a wide variety of different habitats at oligotrophic to, at most, slightly eutrophic soils. The five species of open habitats, C. pamphilus, L. phlaeas, L. tityrus, R. sericealis and T. comae, colonize different types of grassland, wastelands, embankments, clearings or quarries and tolerate various land-use intensities and moisture conditions. In contrast, P. aegeria is a species of semi-open and woodland habitats such as edges of coniferous and deciduous forests, clearings, glades or gardens. In Germany, all six species are at least bivoltine and hibernate as larva and, in P. aegeria, also as pupa.
The larvae of L. phlaeas, L. tityrus and T. comae use Polygonaceae as host plants. While both Lycaena species monophagously feed on Rumex plants with a strong preference for R. acetosa and R. acetosella (Ebert and Rennwald 1991; Ebert 2001; Bräu et al. 2013), preferences for a certain host-plant species within the Polygonaceae are unknown for T. comae (Ebert 2001). These three Lepidoptera species were thus reared with R. acetosella in the fertilization experiment (hereafter referred to as sorrel-feeding species).
In contrast, C. pamphilus, P. aegeria and R. sericealis are grass-feeding species using different Poaceae genera such as Poa or Festuca as host plants (Ebert and Rennwald 1991; Ebert 1997). All three species were reared with Poa pratensis in the fertilization experiment.
In Europe, both the herb R. acetosella and the grass P. pratensis have a widespread occurrence in open habitats (Grime et al. 2007). R. acetosella mainly grows on dry, non-calcareous and infertile soils (Grime et al. 2007; Stopps et al. 2011). P. pratensis exploits both fertile and infertile moist or dry soils (Grime et al. 2007).
Host-plant treatments
Typical fertilization levels in permanent grasslands in modern-day Western European agriculture range between zero to over 500 kg N ha−1 year−1 (Herzog et al. 2006; Kleijn et al. 2009). For this reason, we used three host-plant treatments for all Lepidoptera species, including a control group without fertilization and two fertilization treatments with an input of 150 kg N ha−1 year−1 (hereafter referred to as N150) and 300 kg N ha−1 year−1 (N300). Due to the high abundance of L. tityrus in 2015, we were able to extend the fertilization experiment with this species by two lower fertilization treatments with an input of 30 kg N ha−1 year−1 (N30) and 90 kg N ha−1 year−1 (N90). Fertilization took place with the common fertilizer ammonium nitrate sulphate, including 26% ammonium nitrate to ensure a fast and sufficient nitrogen uptake by the plants (Karmoker et al. 1991; Salvagiotti et al. 2009). For application, this fertilizer was dissolved in water.
Host plants were sown (seeds from commercial supplier Templiner Kräutergarten) in pots filled with sand (about 90%) and a thin layer of garden soil at the bottom in the autumn preceding the appropriate experiment. All potted plants grew under field conditions but received water in ample supply depending on their requirements, evenly distributed among all treatments. Pots were rearranged once a week. The whole fertilizer quantity calculated on the basis of the pot areas was given to the host plants in different portions, which were synchronized with the life cycle of the Lepidoptera species: When the neonates of a certain Lepidoptera species hatched, the host plants got 5% of the whole fertilizer amount. A few days passed until the transfer of the larvae to the treatments, when the plants received the first of five further portions, which contained altogether 95% (5 × 19%) of the whole amount. After the larvae finished three-quarters of their development, the plants got the last 19% portion.
Ten random samples of leaf cuttings of R. acetosella from each experimental treatment with L. tityrus (five treatments) and T. comae (three treatments) in 2015 and three random samples of P. pratensis (three treatments) were gathered five days after the plants received the last fertilizer portion. The leaf cuttings were air dried, ground to a homogeneous powder and analysed with the C/N Analyzer vario EL III (company Elementar) to determine the nitrogen (%N) and carbon (%C) concentration as well as the C:N ratio in reference to the dry weight.
Rearing experiment
To obtain the larvae of all study species for the fertilization experiments, at least eight females from each species were caught during spring and summer of 2014 and 2015 in different regions across the German Federal State of Saxony (Table 1).
Females of all Lepidoptera were kept individually in cages for oviposition (30 × 30 × 30 cm or 23 × 16 × 17 cm in smaller species). These cages contained cuttings of unfertilized host plants and cotton balls prepared with sugar solution and stood outside except in the case of rain. Every 3 days the eggs were collected and separated female specifically in ventilated plastic boxes (10 × 10 × 6 cm). The boxes with the eggs and neonates were checked daily to ensure a sufficient humidity and an ample supply of fresh leaves of unfertilized host plants. For reasons of feasibility and to prevent biased results of the fertilization experiments due to the randomly occurring food refusal and death of neonates, the larvae had to reach a species-specific size before their transfer to the host-plant treatments: 3 mm in both Lycaena species (stage L3), 5 mm in T. comae (loss of cross bands), 7 mm in C. pamphilus and P. aegeria (stage L2) and 2 mm in R. sericealis.
The larvae of each female were, according to a split-brood design, randomly and evenly distributed to the three or five host-plant treatments. Only the low offspring number of some L. tityrus females in 2015 prevented this approach. However, the results between the dataset containing all females or only female lineages with a full split-brood design did not differ significantly (results not shown). Siblings, which simultaneously reached the appropriate size for the transfer to the treatments, were reared treatment specifically in small groups in ventilated plastic boxes (10 × 10 × 6 cm). Individual rearing was not feasible due to the high number of individuals. The synchronous development of the larvae of both Lycaena species in 2014 and of R. sericealis allowed their distribution to the host-plant treatments in less than 5 days. In all other species, the variable development of the individuals resulted in a more staggered distribution of the larvae to the host-plant treatments, which was considered in the statistical analysis. All rearing boxes contained moistened filter paper at the bottom to ensure a sufficient humidity and fresh randomly chosen leaf cuttings of the specific host-plant treatment in ample supply. Depending on the food and humidity requirements of the larvae, the boxes were monitored at least every second day including a rearrangement of the randomized position of the boxes. The rearing conditions of the larvae met the diurnal and seasonal variation of temperature. Only the P. aegeria larvae were reared under controlled conditions with an average temperature of 22 °C to ensure the complete development of this last (third) generation within the season. After the experiments, surviving individuals were released into their respective habitats.
For each species, we calculated the survival rate for the offspring of each female as the proportion of individuals surviving until pupation compared with the number of larvae distributed to the treatments. Unexpectedly, in 2015 the larvae of L. tityrus, R. sericealis and T. comae were highly sensitive to the unfavourable weather conditions and interrupted their food intake to hibernate as larvae. To reduce stress for the individuals, the experiments were stopped for each larva after the same time period: 60 days in L. tityrus, 30 days in R. sericealis and 40 days in T. comae. In these species, the survival rate represented the proportion of surviving versus dead larvae after that time. Also in C. pamphilus we counted the number of surviving larvae after 40 days of larval development.
Statistical analysis
Differences in the nitrogen concentration and C:N ratio of R. acetosella between the three (as host plant of T. comae) or five treatments (as host plant of L. tityrus) and of P. pratensis between two treatments (CONTROL vs. N150/300) were checked with linear models (LM). To test the effect of the host-plant treatments on the survival of the Lepidoptera species, we fitted species-specific binomial generalized mixed-effect models (GLMM) with Laplace approximation with the package lme4 (Bates et al. 2015). Female identity and habitat were implemented as random factors in these models to account for the genetic similarity of siblings and for population effects. Due to the variable development of the larvae in L. tityrus (2015), T. comae, C. pamphilus and P. aegeria, we considered the distribution date of the larvae to the treatments as an additional categorical random factor. In L. tityrus (2015) and T. comae, it was also necessary to include an individual observer-level to correct for over-dispersion (Bolker et al. 2009). We calculated the significance of the treatment as a single fixed factor in each species-specific GLMM with a Wald χ2 test on the full model (Bolker et al. 2009).
The determination of marginal R2 (\( R^{ 2}_{\text{m}} \)) defined as the variance explained by the fixed factors alone and conditional R2 (\( R^{ 2}_{\text{c}} \)) as the variance explained by both the fixed and random factors (Nakagawa and Schielzeth 2012) took place with the package MuMIn (Barton 2016). However, an observer-level causes misleading high \( R^{ 2}_{\text{c}} \) of the model, because the observer effect represents a nuisance parameter with low biological importance (Harrison 2014). The differences in the survival rate between the three or five treatments in each species were checked with Bonferroni t tests to prevent excessive type I error in pairwise comparisons [package lsmeans (Lenth 2016)].
All statistical analyses were calculated with R 3.4.1 (R Core Team 2017).
Results
Fertilization nearly doubled the nitrogen concentration in the fertilized R. acetosella plants compared to the control group (Fig. 1). Accordingly, the C:N ratio was nearly twice as high in the control compared with the fertilized plants. Within the fertilization treatments, differences in the nitrogen concentration and C:N ratio were rather small and often not significant. However, a tenfold increase of the fertilizer quantity (N30 to N300) increased the nitrogen concentration and decreased the C:N ratio by a third. In P. pratensis, the nitrogen concentration increased by approximately a third and the C:N ratio declined by the same magnitude in the fertilization treatments (N150/300) (Fig. 2).
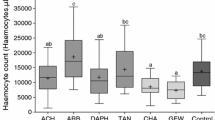
Nitrogen concentration (a) and C:N ratio (b) of Rumex acetosella depending on the treatment as host plant for Lycaena tityrus (five treatments) and Timandra comae (three treatments). Data are expressed as mean (± SE). Linear Model (LM) was used to analyse the treatment effect. Different letters above error bars indicate significant differences between treatments (Bonferroni t tests as post hoc test, P < 0.05). CONTROL = control group; N30, N90, N150 and N300, respectively = fertilization treatment with 30, 90, 150 and 300 kg N ha−1 year−1, respectively. LM statistics: host plants of L. tityrus a nitrogen concentration, F4, 45 = 63.86, P < 0.001, R2 = 0.84; b C:N ratio, F4, 45 = 120.3, P < 0.001, R2 = 0.91; host plants of T. comae (a) nitrogen concentration, F2, 27 = 139.09, P < 0.001, R2 = 0.91; b C:N ratio, F2, 27 = 84.86, P < 0.001, R2 = 0.85
Nitrogen concentration (a) and C:N ratio (b) of Poa pratensis (two treatments). Data are expressed as mean (± SE). LM was used to analyse the treatment effect. Different letters above error bars indicate significant differences between treatments (Bonferroni t tests as post hoc test, P < 0.05). CONTROL, control group; N150/N300, fertilization treatment with 150 and 300 kg N ha−1 year−1. LM statistics: a nitrogen concentration, F1, 7 = 4.86, P = 0.06, R2 = 0.33; b C:N ratio, F1, 7 = 7.24, P < 0.05, R2 = 0.44
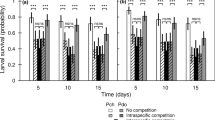
Host-plant fertilization significantly decreased the survival rate of the larvae in all six Lepidoptera species (Figs. 3 and 4). In 2014, the survival rate of both Lycaena species decreased in a dose-dependent manner with increasing fertilization of R. acetosella (Fig. 3a). The survival rate of both species in the N300 treatment was nearly 50% lower than in the control group. Also, the survival rate of L. tityrus in 2015 was almost 40% higher in the control than in the three highest fertilization treatments (N90, N150, N300) (Fig. 4). Only a decline of one-third between the lowest fertilization treatment (N30) and the control group did not reach significance. Between the four fertilization treatments, significant changes in the survival rate of L. tityrus were missing with two exceptions: between the N30 and N90 treatments, the survival rate dropped by about 15%, and the ten-fold increase of the fertilizer quantity (N30 to N300) reduced the survival rate by 20%. In T. comae both fertilization treatments lowered the survival rate by more than two-thirds compared with the control group, pointing out a more sensitive response from this moth compared with both Lycaena species (Fig. 3a).
Survival rate of sorrel-feeding species (Lycaena phlaeas, L. tityrus [2014] and Timandra comae) (a) and grass-feeding species (Coenonympha pamphilus, Pararge aegeria and Rivula sericealis) (b) depending on the treatment. Data are expressed as mean (± SE). GLMM were used to analyse the treatment effect (see Table 2). Different letters above error bars indicate significant differences between treatments (Bonferroni t tests as post hoc test, P < 0.05). CONTROL, control group; N150 and N300, respectively, fertilization treatment with 150 and 300 kg N ha−1 year−1, respectively
Survival rate of Lycaena tityrus (2015) depending on the treatment. Data are expressed as mean (± SE). GLMM were used to analyse the treatment effect (see Table 2). Different letters above error bars indicate significant differences between treatments (Bonferroni t tests as post hoc test, P < 0.05). CONTROL, control group; N30, N90, N150 and N300, respectively, fertilization treatment with 30, 90, 150 and 300 kg N ha−1 year−1, respectively
The survival rate of C. pamphilus and P. aegeria declined by about one-third in both fertilization treatments compared with the control group, representing a smaller decrease of survival in these grass-feeding species compared with the sorrel-feeding species (Fig. 3b). However, in P. aegeria some larvae reached pupation, whereas in C. pamphilus no larvae feeding on fertilized plants survived longer than 45 days or reached pupation. Among all the grass-feeding species, R. sericealis was the most sensitive. In this moth, the survival rate declined by half between the control and the N150 treatment and about 80% between the control and the N300 treatment.
Discussion
The fertilization-induced shifts in host-plant quality decreased the survival rate in all six Lepidoptera species, i.e. the higher the nitrogen concentration in the host plants (lower C:N ratios, respectively), the lower the survival rate of the Lepidoptera species.
The decline of the C:N ratio due to an increase in the nitrogen concentration of the fertilized plants compared to the control group is in accordance with previous findings on other plant species (e.g. Schädler et al. 2007; Chen et al. 2008; Han et al. 2014). In particular, the increasing nitrogen concentration in R. acetosella across the five treatments reflects a saturation curve, which results from the non-linear nitrogen uptake from the soil (Melzer et al. 1984). However, the plants receiving the highest fertilizer amount (N300) started to suffer from this treatment, resulting in an increased dying off of individuals at the end of the experiment. The host-plant quality of R. acetosella and P. pratensis across all treatments was nevertheless still within the range occurring in nature. Nitrogen content of grasses naturally varies between 0.5 and 7.0%N (Joern and Behmer 1997), whereas R. acetosella is characterized by C:N ratios between 8.0 and 12.5 (Rose 2010), though individuals growing on nutrient-poor soils can have nitrogen contents of only 1.56%N (Vick and Young 2011). This wide range indicates that R. acetosella did not suffer from nitrogen limitation in our experiments and, furthermore, the fertilized plants match the plant quality typically occurring in nature. In conclusion, the fertilization experiment covered the middle range of food quality in both plant species without reaching excessively high or low nitrogen concentrations. Accordingly, Lepidoptera species frequently have to cope with these host-plant qualities in their habitats.
The declining survival rate of the larvae feeding on fertilized plants in all six Lepidoptera species challenges the broad evidence (Slansky and Feeny 1977; Myers and Post 1981; Chen et al. 2004; Klop et al. 2015) and well-accepted nitrogen-limitation hypothesis (White 1993) predicting a positive relationship between the nitrogen content of the diet and the performance of herbivorous insects. Most importantly, survival rates of about 80% in the control treatment in both moth and Lycaena species (2014) indicate that the unfertilized plants almost optimally fulfil the requirements of the species. Higher nitrogen concentrations did not improve the survival rate, but rather decreased the performance of these species. This observation is also supported by the sensitive response of L. tityrus to the lower fertilization treatments N30 and N90. Since these comparatively low fertilizer quantities also reduce the survival rate of the larvae (however, not significantly in the N30 treatment), a positive response of L. tityrus to fertilized plants is very unlikely. The exclusively negative response of the six Lepidoptera species to the fertilized plants distinguish the present results from a few previous studies that found with hump-shaped relationships at least a restricted positive response of Lepidoptera species to increased nitrogen contents in their host plants (Brewer et al. 1985; Sarfraz et al. 2009; Han et al. 2014). Indeed, we do not present the first negative responses of non-pest Lepidoptera species to fertilized plants, but previous studies with negative responses did not analyse the host-plant quality (Turlure et al. 2013) or the fertilizer input of the host plants (Fischer and Fiedler 2000). Hence, to date, an association between negative responses of Lepidoptera species and certain host-plant qualities or nitrogen availabilities under natural conditions has not been identified. The present results thus suggest for the first time that conventional fertilizer inputs to permanent grasslands exceed the physiological tolerance of common Lepidoptera species with tremendous negative effects on the survival of their larvae.
The consistent negative response of all six common Lepidoptera and especially the response of both Lycaena species fit to the findings of Fischer and Fiedler (2000). They focused on the response of three female lineages of the alpine subspecies L. tityrus subalpinus to fertilized plants of Rumex acetosa. The survival rate of the individuals feeding on fertilized plants (average C:N ratio 7.2) decreased to 33% compared with 73% in the control group (Fischer and Fiedler 2000). These concordant responses suggest that the detrimental bottom-up effect of agricultural fertilization on widespread Lepidoptera species is underestimated and more common than apparent from the literature. In recent decades, fertilization experiments with Lepidoptera were mainly restricted to two main topics. On the one hand, several studies investigate pest species (e.g. Slansky and Feeny 1977; Wheeler and Halpern 1999; Chen et al. 2008). However, these species benefit from increased nitrogen concentrations in their diet, even if they reach similar concentrations as in the present experiment (Slansky and Feeny 1977; Wheeler and Halpern 1999; Chen et al. 2008). In turn, if these species suffer from human-induced quality changes in their host plants, they never became pests. On the other hand, studies considering non-pest butterflies mainly focus on host-plant quality changes caused by drought or enhanced CO2 concentrations (Goverde and Erhardt 2003; Mevi-Schütz et al. 2003; Klop et al. 2015). In this context, non-pest butterflies also respond positively to higher nitrogen concentrations in their host plants (Goverde and Erhardt 2003; Mevi-Schütz et al. 2003; Klop et al. 2015). However, these quality shifts are rather negligible compared to changes caused by agricultural fertilization. In studies with a positive response of grass-feeding butterflies, the C:N ratio of grasses ranged between 60 and 25 (e.g. Goverde and Erhardt 2003; Mevi-Schütz et al. 2003). In contrast, the agriculturally fertilized grasses in the present experiment were characterized by C:N ratios around 10. To date, no study has considered this wide range of host-plant qualities. A joint consideration of all these results may refer, in accordance with the theory of ecological stoichiometry (Sterner and Elser 2002), to a hump-shaped relationship between the performance of non-pest Lepidoptera species and the nitrogen content of their host plants across the whole range from nitrogen scarcity to excess. The nitrogen-limitation hypothesis, thus, would describe only the first, positive part of the response curve. To explicitly identify the kind of the whole relationship, future studies should investigate the performance of Lepidoptera species feeding on host plants growing under a wide range of nitrogen availabilities. The present findings though already undermine the general applicability of the nitrogen-limitation hypothesis as explanation for the response of Lepidoptera species to fertilized plants.
Apart from the declining survival rate in all six species, their response to the fertilized plants varied in a species-specific manner, but without a clear difference in the sensitivity of sorrel- and grass-feeding Lepidoptera. In particular, both moth species feeding on the two different host plants showed a near-similar response compared with the butterflies more sensitive response. Considering the different secondary chemistry of the two plant families with almost negligible concentrations of toxic chemicals in grasses (Tscharntke and Greiler 1995) and oxalate, a potentially deleterious organic acid in R. acetosella (Hatcher et al. 1997; Stopps et al. 2011), this result is remarkable. Thus, grass-feeding species are not necessarily less vulnerable to host-plant quality changes as it has been assumed by previous studies (Klop et al. 2015).
In conclusion, with the consideration of six Lepidoptera species feeding on two different host-plant families, this study goes beyond previous anecdotal and rather descriptive investigations (cf. Nijssen et al. 2017) and substantially improves our understanding about the response of widespread non-pest Lepidoptera to enrichment of their host plants with nitrogen. We provide the first evidence that under an experimental setup nitrogen enrichment in plants due to agricultural fertilization goes beyond the physiological tolerance of common Lepidoptera species with tremendous effects on the survival of the larvae. Thus, host-plant quality changes due to agricultural fertilization or atmospheric nitrogen deposition might substantially contribute to the range-wide decline of Lepidoptera species in Western and Central Europe. Investigating the extent of this effect, i.e. which species are affected by these quality changes, which fertilizer quantities provoke an increase in mortality and how under natural conditions higher nitrogen contents in the host plants interact with other factors influencing the performance of Lepidoptera species, is important for the development of appropriate future mitigation and conservation measures (cf. Nijssen et al. 2017).
References
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844. https://doi.org/10.1146/annurev.ento.47.091201.145300
Barton K (2016) MuMIn: multi-model inference. R package version 1.15.6. http://CRAN.R-project.org/package=MuMIn. Accessed 29 July 2017
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bink FA, Siepel H (1996) Nitrogen and phosphorus in Molinia caerulea (Gramineae) and its impact on the larval development in the butterfly-species Lasiommata megera (Lepidoptera: Satyridae). Entomol Gen. 20:271–280. https://doi.org/10.1127/entom.gen/20/1996/271
Boersma M, Elser JJ (2006) Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology 87:1325–1330. https://doi.org/10.1890/0012-9658(2006)87[1325:tmoagt]2.0.co;2
Bolker BM, Brooks ME, Clark CJ, Gaenge SW, Poulsen JR, Stevens MHH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Bräu M, Bolz R, Kolbeck H, Nummer A, Voith J, Wolf W (2013) Tagfalter in Bayern. Eugen Ulmer, Stuttgart
Brewer JW, Capinera JL, Deshon RE, Walmsley ML (1985) Influence of foliar nitrogen levels on survival, development, and reproduction of western spruce budworm, Choristoneura occidentals (Lepidoptera: Tortricidae). Can Entomol 117:23–32. https://doi.org/10.4039/ent11723-1
Bruppacher L, Pellet J, Arlettaz R, Humbert J (2016) Simple modifications of mowing regime promote butterflies in extensively managed meadows: evidence from field-scale experiments. Biol Conserv 196:196–202. https://doi.org/10.1016/j.biocon.2016.02.018
Chen Y, Lin L, Wang C, Yeh C, Hwang S (2004) Response of two Pieris (Lepidoptera: Pieridae) species to fertilization of a host plant. Zool Stud 43:778–786
Chen Y, Ruberson JR, Olson DM (2008) Nitrogen fertilization rate affects feeding, larval performance, and oviposition preference of the beet armyworm, Spodoptera exigua, on cotton. Entomol Exp Appl 126:244–255. https://doi.org/10.1111/j.1570-7458.2007.00662.x
Conrad KF, Warren MS, Fox R, Parsons MS, Woiwod IP (2006) Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol Conserv 132:279–291. https://doi.org/10.1016/j.biocon.2006.04.020
Dennis RLH, Shreeve TG, van Dyck H (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15:1943–1966. https://doi.org/10.1007/s10531-005-4314-3
Dover JW, Settele J (2009) The influences of landscape structure on butterfly distribution and movement: a review. J Insect Conserv 13:3–27. https://doi.org/10.1007/s10841-008-9135-8
Ebert G (ed) (1997) Die Schmetterlinge Baden-Württembergs. Band 5: Nachtfalter III. Eugen Ulmer, Stuttgart
Ebert G (ed) (2001) Die Schmetterlinge Baden-Württembergs. Band 8: Nachtfalter VI. Eugen Ulmer, Stuttgart
Ebert G, Rennwald E (1991) Die Schmetterlinge Baden-Württembergs. Band 1: Tagfalter I. Eugen Ulmer, Stuttgart
Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht, 6th edn. Eugen Ulmer, Stuttgart
Fischer K, Fiedler K (2000) Response of the copper butterfly Lycaena tityrus to increased leaf nitrogen in natural food plants: evidence against the nitrogen limitation hypothesis. Oecologia 124:235–241. https://doi.org/10.1007/s004420000365
García-Barros E, Fartmann T (2009) Butterfly oviposition: sites, behaviour and modes. In: Settele J, Shreeve TG, Konvička M, van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 29–42
Goverde M, Erhardt A (2003) Effects of elevated CO2 on development and larval food-plant preference in the butterfly Coenonympha pamphilus (Lepidoptera, Satyridae). Glob Change Biol 9:74–83. https://doi.org/10.1046/j.1365-2486.2003.00520.x
Grime JP, Hodgson JG, Hunt R (2007) Comparative plant ecology, 2nd edn. Castlepoint Press, Dalbeattie
Han P, Lavoir A, Le Bot J, Amiens-Desneux E, Desneux N (2014) Nitrogen and water availability to tomato plants triggers bottom-up effects on the leafminer Tuta absoluta. Sci. Rep. 4:4455. https://doi.org/10.1038/srep04455
Harrison XA (2014) Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2:e616. https://doi.org/10.7717/peerj.616
Hatcher PE, Paul ND, Ayres PG, Whittaker JB (1997) The effect of nitrogen fertilization and rust fungus infection, singly and combined, on the leaf chemical composition of Rumex obtusifolius. Funct Ecol 11:545–553. https://doi.org/10.1046/j.1365-2435.1997.00123.x
Herzog F, Steiner B, Bailey D, Baudry J, Billeter R, Bukacek R, De Blust G, De Cock R, Dirksen J, Dormann CF, De Filippi R, Frossard E, Liira J, Schmidt T, Stöckli R, Thenail C, van Wingerden W, Bugter R (2006) Assessing the intensity of temperate European agriculture at the landscape scale. Eur J Agron 24:165–181. https://doi.org/10.1016/j.eja.2005.07.006
Joern A, Behmer ST (1997) Importance of dietary nitrogen and carbohydrates to survival, growth, and reproduction in adults of the grasshopper Ageneotettix deorum (Orthoptera: Acrididae). Oecologia 112:201–208. https://doi.org/10.1007/s004420050301
Karmoker JL, Clarkson DT, Saker LR, Rooney JM, Purves JV (1991) Sulphate deprivation depresses the transport of nitrogen to the xylem and the hydraulic conductivity of barley (Hordeum vulgare L.) roots. Planta 185:269–278. https://doi.org/10.1007/bf00194070
Kleijn D, Kohler F, Báldi A, Batáry P, Concepción ED, Clough Y, Díaz M, Gabriel D, Holzschuh A, Knop E, Kovács A, Marshall EJP, Tscharntke T, Verhulst J (2009) On the relationship between farmland biodiversity and land-use intensity in Europe. Proc R Soc B 276:903–909. https://doi.org/10.1098/rspb.2008.1509
Klop E, Omon B, WallisDeVries MF (2015) Impact of nitrogen deposition on larval habitats: the case of the Wall Brown butterfly Lasiommata megera. J Insect Conserv 19:393–402. https://doi.org/10.1007/s10841-014-9748-z
Kurze S, Heinken T, Fartmann T (2017) Nitrogen enrichment of host plants has mostly beneficial effects on the life history traits of nettle-feeding butterflies. Acta Oecol 85:157–164. https://doi.org/10.1016/j.actao.2017.11.005
Lenth RV (2016) Least-squares means: the r package lsmeans. J Stat Softw 69:1–33
Liu Y, Pan X, Li J (2015) A 1961–2010 record of fertilizer use, pesticide application and cereal yields: a review. Agron Sustain Dev 35:83–93. https://doi.org/10.1007/s13593-014-0259-9
Loader C, Damman H (1991) Nitrogen content of food plants and vulnerability of Pieris rapae to natural enemies. Ecology 72:1586–1590. https://doi.org/10.2307/1940958
Löffler F, Stuhldreher G, Fartmann T (2013) How much care does a shrub-feeding hairstreak butterfly, Satyrium spini (Lepidoptera: Lycaenidae), need in calcareous grasslands? Eur J Entomol 110:145–152. https://doi.org/10.14411/eje.2013.020
Maes D, van Dyck H (2001) Butterfly diversity loss in Flanders (north Belgium): Europe’s worst case scenario? Biol Conserv 99:263–276. https://doi.org/10.1016/s0006-3207(00)00182-8
Manning P, Gossner MM, Bossdorf O, Allan E, Zhang Y, Prati D, Blüthgen N, Boch S, Böhm S, Börschig C, Hölzel N, Jung K, Klaus VH, Klein AM, Kleinebecker T, Krauss J, Lange M, Müller J, Pašalić E, Socher SA, Tschapka M, Türke M, Weiner C, Werner M, Gockel S, Hemp A, Renner SC, Wells K, Buscot F, Kalko EKV, Linsenmair KE, Weisser WW, Fischer M (2015) Grassland management intensification weakens the associations among the diversities of multiple plant and animal taxa. Ecology 96:1492–1501. https://doi.org/10.1890/14-1307.1
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Evol Syst 11:119–161. https://doi.org/10.1146/annurev.es.11.110180.001003
Melzer A, Gebauer G, Rehder H (1984) Nitrate content and nitrate reductase activity in Rumex obtusifolius L. II. Responses to nitrate starvation and nitrogen fertilization. Oecologia 63:380–385. https://doi.org/10.1007/bf00390669
Mevi-Schütz J, Goverde M, Erhardt A (2003) Effects of fertilization and elevated CO2 on larval food and butterfly nectar amino acid preference in Coenonympha pamphilus. Behav Ecol Sociobiol 54:36–43. https://doi.org/10.1007/s00265-003-0601-8
Myers JH, Post BJ (1981) Plant nitrogen and fluctuations of insect populations: a test with the cinnabar moth-tansy ragwort system. Oecologia 48:151–156. https://doi.org/10.1007/bf00347957
Nakagawa S, Schielzeth H (2012) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Nijssen ME, WallisDeVries MF, Siepel H (2017) Pathways for the effects of increased nitrogen deposition on fauna. Biol Conserv 212:423–431. https://doi.org/10.1016/j.biocon.2017.02.022
Öckinger E, Hammarstedt O, Nilsson SG, Smith HG (2006) The relationship between local extinctions of grassland butterflies and increased soil nitrogen levels. Biol Conserv 128:564–573. https://doi.org/10.1016/j.biocon.2005.10.024
Prudic KL, Oliver JC, Bowers MD (2005) Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143:578–587. https://doi.org/10.1007/s00442-005-0008-5
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accessed 29 July 2017
Raubenheimer D, Lee KP, Simpson SJ (2005) Does Bertrand’s rule apply to macronutrients? Proc R Soc B 272:2429–2434. https://doi.org/10.1098/rspb.2005.3271
Reinhardt R, Bolz R (2011) Rote Liste und Gesamtartenliste der Tagfalter (Rhopalocera) (Lepidoptera: Papilionoidea et Hesperioidea) Deutschlands. In: Binot-Hafke M, Balzer S, Becker N, Gruttke H, Haupt H, Hofbauer N, Ludwig G, Matzke-Hajek G, Strauch M (eds) Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 3: Wirbellose Tiere (Teil 1). Naturschutz und Biologische Vielfalt 70. Bonn, Bad Godesberg, pp 165–194
Rose S (2010) Generalist vs. Specialist? Egg laying and food plant preferences in two related lycaenid butterflies. Diploma Thesis. Institute of Landscape Ecology, Westphalian Wilhelms-University, Münster, Germany
Salvagiotti F, Castellarín JM, Miralles DJ, Pedrol HM (2009) Sulfur fertilization improves nitrogen use efficiency in wheat by increasing nitrogen uptake. Field Crop Res 113:170–177. https://doi.org/10.1016/j.fcr.2009.05.003
Sarfraz RM, Dosdall LM, Keddie AB (2009) Bottom-up effects of host plant nutritional quality on Plutella xylostella (Lepidoptera: Plutellidae) and top-down effects of herbivore attack on plant compensatory ability. Eur J Entomol 106:583–594. https://doi.org/10.14411/eje.2009.073
Schädler M, Roeder M, Brandl R, Matthies D (2007) Interacting effects of elevated CO2, nutrient availability and plant species on a generalist invertebrate herbivore. Glob Change Biol 13:1005–1015. https://doi.org/10.1111/j.1365-2486.2007.01319.x
Slansky F, Feeny P (1977) Stabilization of the rate of nitrogen accumulation by larvae of the cabbage butterfly on wild and cultivated food plants. Ecol Monogr 47:209–228. https://doi.org/10.2307/1942617
Socher SA, Prati D, Boch S, Müller J, Baumbach H, Gockel S, Hemp A, Schöning I, Wells K, Buscot F, Kalko EKV, Linsenmair KE, Schulze E, Weisser WW, Fischer M (2013) Interacting effects of fertilization, mowing and grazing on plant species diversity of 1500 grasslands in Germany differ between regions. Basic Appl Ecol 14:126–136. https://doi.org/10.1016/j.baae.2012.12.003
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879. https://doi.org/10.1126/science.1094678
Stopps GJ, White SN, Clements DR, Upadhyaya MK (2011) The biology of Canadian weeds. 149. Rumex acetosella L. Can J Plant Sci 91:1037–1052. https://doi.org/10.4141/cjps2011-042
Tabashnik BE (1982) Responses of pest and non-pest Colias butterfly larvae to intraspecific variation in leaf nitrogen and water content. Oecologia 55:389–394. https://doi.org/10.1007/bf00376927
Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Fox R, Clarke RT, Lawton JH (2004) Comparative losses of British butterflies, birds, and plants and global extinction crisis. Science 303:1879–1881. https://doi.org/10.1126/science.1095046
Throop HL, Lerdau MT (2004) Effects of nitrogen deposition on insect herbivory: implications for community and ecosystem processes. Ecosystems 7:109–133. https://doi.org/10.1007/s10021-003-0225-x
Tilman D, Fargione J, Wolff B, Ḋ’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284. https://doi.org/10.1126/science.1057544
Tscharntke T, Greiler H (1995) Insect communities, grasses, and grasslands. Annu Rev Entomol 40:535–558. https://doi.org/10.1146/annurev.en.40.010195.002535
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol Lett 8:857–874. https://doi.org/10.1111/j.1461-0248.2005.00782.x
Turlure C, Radchuk V, Baguette M, Meijrink M, van den Burg A, WallisDeVries M, van Duinen G (2013) Plant quality and local adaptation undermine relocation in a bog specialist butterfly. Ecol Evol 3:244–254. https://doi.org/10.1002/ece3.427
van Dyck H, van Strien AJ, Maes D, van Swaay CAM (2009) Declines in common, widespread butterflies in a landscape under intense human use. Conserv Biol 23:957–965. https://doi.org/10.1111/j.1523-1739.2009.01175.x
van Swaay CAM, van Strien AJ, Aghababyan K, Åström S, Botham M, Brereton T, Chambers P, Collins S, Domènech Ferrés M, Escobés R, Feldmann R, Fernández-García JM, Fontaine B, Goloshchapova S, Gracianteparaluceta A, Harpke A, Heliölä J, Khanamirian G, Julliard R, Kühn E, Lang A, Leopold P, Loos J, Maes D, Mestdagh X, Monasterio Y, Munguira ML, Murray T, Musche M, Õunap E, Pettersson LB, Popoff S, Prokofev I, Roth T, Roy D, Settele J, Stefanescu C, Švitra G, Teixeira SM, Tiitsaar A, Verovnik R, Warren MS (2015) The European Butterfly Indicator for Grassland species 1990–2013. Report VS2015.009, De Vlinderstichting, Wageningen
Vick JK, Young DR (2011) Spatial variation in environment and physiological strategies for forb distribution on coastal dunes. J Coastal Res 27:1113–1121. https://doi.org/10.2112/jcoastres-d-10-00156.1
WallisDeVries M, Bobbink R (2017) Nitrogen deposition impacts on biodiversity in terrestrial ecosystems: mechanisms and perspectives for restoration. Biol Conserv 212:387–389. https://doi.org/10.1016/j.biocon.2017.01.017
Wheeler GS, Halpern MD (1999) Compensatory responses of Samea multiplicalis larvae when fed leaves of different fertilization levels of the aquatic weed Pistia stratiotes. Entomol Exp Appl 92:205–216. https://doi.org/10.1046/j.1570-7458.1999.00539.x
White TCR (1993) The inadequate environment—nitrogen and the abundance of animal. Springer, Berlin, Heidelberg
Acknowledgements
We are grateful to the Kurze family (Dresden) and Tommy Kästner (Dresden) for contributing to the capture of females of different species for the experiments. C:N analyses were carried out by Antje Möhlmeyer (Osnabrück). Moreover, we would like to thank two anonymous reviewers for helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Contributions
SK, TF and TH designed the experiments. SK conducted the experiments, analysed the data and wrote the article. TF and TH made substantial contributions to the manuscript, revising and commenting on subsequent drafts.
Corresponding author
Additional information
Communicated by Klaus Fischer.
Rights and permissions
About this article
Cite this article
Kurze, S., Heinken, T. & Fartmann, T. Nitrogen enrichment in host plants increases the mortality of common Lepidoptera species. Oecologia 188, 1227–1237 (2018). https://doi.org/10.1007/s00442-018-4266-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4266-4