Abstract
Larvae of the leaf beetle Chrysomela lapponica obtain salicyl glucosides (SGs) from the host plant to produce a defensive secretion with salicylaldehyde. In northern Russia, larvae and pupae experience high parasitism by the phorid fly Megaselia opacicornis and tachinid fly Cleonice nitidiuscula. We compared the suitability of the SG-rich Salix borealis and SG-poor S. caprea and S. phylicifolia to Ch. lapponica and tested whether enemy pressure on Ch. lapponica varies among host species that differ in SG content. In the laboratory, survival of Ch. lapponica larvae was higher on S. borealis than on S. caprea and S. phylicifolia, while adult body mass was higher on S. borealis and S. caprea than on S. phylicifolia. In the field, parasitism by both M. opacicornis and Cl. nitidiuscula was greater on beetles from S. borealis than from the SG-poor S. caprea or S. phylicifolia. In a laboratory choice test, the pupal parasitoid M. opacicornis laid similar numbers of eggs on beetles reared on SG-rich and SG-poor willows, suggesting that the host plant-derived defence is not effective against this parasitoid. In a field enemy-exclusion experiment, beetle survival was greatly enhanced by the exclusion of enemies, but survival rates did not differ between S. borealis and S. caprea, although larvae developed faster on S. borealis. On the other hand, parasitism and predation were observed more often on S. borealis than on S. caprea. Thus, beetle larvae perform better but also suffer higher predation and parasitism on S. borealis than on SG-poor willows. Ch. lapponica does not appear to obtain enemy-free space by feeding on SG-rich willow species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbivorous insects face two substantial challenges: feeding on a resource that is often nutritionally poor or even toxic, and being exposed to a wide array of natural enemies. Ecologists have proposed that these challenges can be linked if herbivore success on a host plant depends on its risk of predation or parasitism on that host (Price et al. 1980; Jeffries and Lawton 1984; Berdegue et al. 1996; Dicke and van Poecke 2002). Numerous recent studies have revealed that plants attacked by herbivores produce substances that attract natural enemies (Turlings et al. 1990; Vet and Dicke 1992; Paré et al. 1999). However, fewer studies have determined whether host plant species affect the risk of predation or parasitism for herbivores (Ohsaki and Sato 1990; Rank et al. 1998; Gratton and Welter 1999; Ballabeni et al. 2001). This is unfortunate, because natural enemies can impose a significant selective force affecting host plant suitability (Feder 1995; Yamaga and Ohgushi 1999), and may ultimately play an important role in the evolution of host plant choice (Price et al. 1980; Bernays and Graham 1988; Thompson 1988b; Stamp 2001).
The interaction between willows, leaf beetles (Chrysomela spp. and Phratora vitellinae L.), and their natural enemies presents an excellent system to determine how natural enemies affect host plant suitability to herbivores. Larvae of these beetles convert phenolic glucosides from their host plants into a larval defensive secretion that consists mostly of salicylaldehyde (Pasteels et al. 1983a; Hilker and Schulz 1994; Köpf et al. 1998). Beetles feeding on hosts poor in salicyl glucosides (SGs) produce little defensive secretion and it does not contain salicylaldehyde (Rowell-Rahier and Pasteels 1982; Smiley et al. 1985; Gross and Hilker 1995; Rank et al. 1998). The salicylaldehyde secretion protects beetle larvae from some generalist predators (Wallace and Blum 1969; Pasteels et al. 1983b; Palokangas and Neuvonen 1992; Lundvall et al. 1998), and attracts some specialist predators (Rank 1994; Rank et al. 1996; Köpf et al. 1997; Gross 2001), but effects of the secretion on parasitoids have never been studied.
It is possible that search behaviour of parasitoids is affected by the host plant-derived secretion produced by beetle larvae. On the other hand, parasitoid performance, and survival may also be affected by the quality of the herbivore's host plant (Campbell and Duffy 1979; Blumberg 1991; Turlings and Benrey 1998; Kruse and Raffa 1999). Healthier and larger herbivores that develop on high-quality host plants may provide parasitoids with better nutrition (Bourchier 1991; Jervis and Copland 1996; Benrey et al. 1998), or may have better physiological resistance to parasitoids, expressed as enhanced ability to encapsulate the parasitoid before it causes substantial damage to host tissue (Salt 1963; Van den Bosch 1964; Blumberg 1997). The relationship between host plant quality and parasitoid attack must be examined empirically to determine which of these two outcomes apply to a particular plant-herbivore-parasitoid interaction.
In Finnish Lapland and adjacent north-west Russia, populations of the leaf beetle Chrysomela lapponica L. are particularly dense where Salix borealis (Fries.) Nasar. is abundant (Zvereva et al. 1995a, 1995b, 1997; Gross 2001). This willow species contains high amounts (up to 15% of dry weight) of the SGs (Julkunen-Tiitto 1989b; Kolehmainen et al. 1995) that are used by Ch. lapponica larvae as precursors of salicylaldehyde (Gross and Hilker 1995). Although Ch. lapponica prefers S. borealis to other local willows (Zvereva et al. 1995a), some beetle larvae are found on SG-poor willow species. This naturally broad herbivore diet made it possible to quantify host-plant effects on parasitism by two principal parasitoids, the scuttle fly Megaselia opacicornis Schmitz and tachinid fly Cleonice nitidiuscula (Zett.) on Ch. lapponica, which together cause up to 80% mortality in Russian populations of Ch. lapponica (Zvereva and Kozlov 2000). Close relatives of these parasitoids are known to cause high levels of mortality on chrysomelids in other regions (Kanervo 1946; Devantoy 1948; Cox 1994; Baur and Rank 1996; Rank et al. 1996), but no previous studies have investigated the relationship between host plant chemistry, beetle survival and parasitoid success.
The aim of the present study is to test the hypothesis that leaf beetle larvae feeding on the SG-rich S. borealis obtain "enemy-free space" (EFS) via reduced parasitism on this host plant due to more efficient chemical defence. We will also evaluate the alternative hypothesis that parasitoids should favour beetles on the host on which herbivores perform most effectively. We asked four questions:
-
1.
First, how does larval survival and body size of Ch. lapponica vary in the absence of natural enemies among three host plant species that differ substantially in SG chemistry?
-
2.
Second, does the herbivore's host plant species affect parasitism by M. opacicornis or Cl. nitidiuscula?
-
3.
Third, does parasitoid performance relate to herbivore performance on a given host plant?
-
4.
Finally, does survival of Ch. lapponica differ between the SG-rich host, Salix borealis and an alternative SG-poor host, Salix caprea, in the presence and absence of natural enemies? This question will be addressed by comparing larval field survival on both willow hosts in a enemy-exclusion experiment and by quantifying natural predation and parasitism rates.
Materials and methods
Natural history and study system
When disturbed, larvae of Ch. lapponica release droplets of defensive secretion from nine pairs of eversible glands on the dorsal side of the thorax and abdomen. A major component of this secretion consists of salicylaldehyde derived from host plant SGs; when Ch. lapponica feeds on a SG-poor host plant, the defensive secretion is autogenous and it does not contain salicylaldehyde (Hilker and Schulz 1994; Gross and Hilker 1995; Schulz et al. 1997). When larvae complete development, they cease feeding and attach themselves to leaves by the tip of the abdomen. Defensive glands remain active during this immobile stage, called prepupa. Pupation occurs on the upper side of host plant leaves at the beginning of August. In the Kola Peninsula Ch. lapponica feeds on seven willow species, but S. borealis is preferred over the others (Zvereva et al. 1995a).
Megaselia opacicornis is a small (2–3 mm) parasitoid of chrysomelid pupae abundant in north-west Russia (Disney et al. 2001), where it causes up to 35% mortality of Ch. lapponica (Zvereva et al. 1995b; Zvereva and Kozlov 2000). This parasitoid was previously referred to as M. rubricornis (Zvereva et al. 1995b, 1997). The tachnid Cleonice nitidiuscula (body length 5 mm) causes up to 66% mortality of Ch. lapponica (Zvereva et al. 1995b, 1997; Richter and Zvereva 1996).
Study site and plants
This study was conducted in 1994, 2000 and 2001 in the Monchegorsk region on the Kola Peninsula (NW Russia) in sites situated along the Murmansk–St. Petersburg road. Three willows abundant in the Kola Peninsula were chosen: the SG-rich S. borealis and two SG-poor species, Salix caprea L. and Salix phylicifolia L. (Tahvanainen et al. 1985b; Julkunen-Tiitto 1989a, 1989a).
We used pairs or triplets of medium-sized plants (1–1.5 m high) as blocks, which allowed us to statistically separate host species differences from random spatial variation in survival and parasitism rate (Rank 1994). Within each block, maximum distance between plants was 5 m, and blocks were separated by at least 500 m. In 1994, we selected 15 S. borealis/S. caprea and eight S. borealis/S. phylicifolia pairs. In 2000, we chose four triplets of S. borealis/S. caprea /S. phylicifolia. In 2001, we selected 12 S. borealis/S. caprea and eight S. borealis/S. phylicifolia pairs. We used a subset of plants chosen in a given year for experiments described below.
Assessment of plant quality to Ch. lapponica
To assess host plant quality to Ch. lapponica, we collected beetle eggs in the field in June 2001 and reared larvae on each host in the laboratory. We placed seven newly hatched larvae onto a fresh willow leaf in a vial and reared them at room temperature under natural light. Leaves were changed every second day. We quantified larval survival to pupation.
We compared body mass of adults that emerged from field-collected pupae in 1994. We collected five pupae per plant and weighed each adult beetle to the nearest 0.1 mg immediately after eclosion from the pupa. Plant averages were calculated for body mass before statistical analysis.
In June 2001 we added Ch. lapponica egg clutches to each experimental plant. Before adding clutches, we removed naturally occurring eggs and larvae and continued to remove new ones every 5 days throughout the summer. At the time of mass pupation in late July, we collected all prepupae, brought them to the laboratory, and counted the number of survivors. When three or fewer larvae survived to pupation, the plant pair was deleted from analysis. The Ch. lapponica prepupae collected in this experiment were also used to assess parasitism by M. opacicornis and Cl. nitidiuscula.
Assessment of parasitism by the scuttle fly M. opacicornis
To assess levels of parasitism by M. opacicornis, we examined beetles that developed naturally on their host plants (summer 2000) or had been experimentally placed on plants (summer 2001). At mass pupation we collected beetle pupae and used a dissecting microscope to count the number of M. opacicornis eggs laid on each pupa or on the leaf within 5 mm of the pupa (103 pupae in 2000 and 958 in 2001). We kept pupae at room temperature until parasitoids pupated, dissected beetle pupae to determine cause of death and calculated the proportion of pupae parasitized by M. opacicornis. Beetle pupae that had been consumed by sucking arthropod predators, parasitized by Cl. nitidiuscula or died for unknown reasons were excluded from analysis.
To study host preference of M. opacicornis in the laboratory, we reared beetles from first instar to just before pupation on three willow species (five plants per species), and obtained adult M. opacicornis by aspirator at the field site. We placed two beetle prepupae reared on S. borealis and two reared on a SG-poor willow into one of two 1-l jars containing 20–50 M. opacicornis individuals. When we observed multiple M. opacicornis eggs on at least one beetle prepupa (usually after 2–10 h), we counted number of parasitoid eggs per pupa. Five preference trials were conducted for each S. borealis (BOR) vs. S. caprea (CAP) and S. borealis (BOR) vs. S. phylicifolia (PHY) comparison.
Assessment of parasitism by the tachinid Cl. nitidiuscula
To quantify the proportion of Ch. lapponica pupae that were parasitized by Cl. nitidiuscula, we collected 1,287 pupae in 1994 and 958 pupae in 2001 using the same willows as those used for studies of M. opacicornis parasitism. We kept pupae in the laboratory until parasitoid emergence and pupation and determined parasitism rate for each plant. We weighed each Cl. nitidiuscula pupa and calculated mean parasitoid body mass per host plant in 1994. When no beetles were parasitized on a plant, the plant pair was omitted from analysis.
To determine the relationship between plant nutritive quality, parasitoid attack and parasitoid body mass, we collected Ch. lapponica pupae from S. borealis individuals in ten study sites (five plants per site) in 1994. Pupae were kept in the laboratory until adult beetles emerged or parasitoids pupated. For each plant, we determined the proportion of pupae that were parasitized, weighed all Cl. nitidiuscula pupae, and weighed five beetles that had emerged from unparasitized pupae.
Field enemy-exclusion experiment
During summer 2000, we compared number of survivors on a control branch exposed to natural enemies to survival on a branch enclosed in a mesh bag. We chose five study sites located, on average, 5 km apart, and selected five S. borealis plants and five S. caprea plants growing within 20 m of each other within each site. On 3 July 2000, we removed naturally occurring beetle eggs and larvae from host plants and added 15 newly hatched Ch. lapponica larvae to two branches on each plant: one branch control (exposed) and one branch enclosed to a mesh bag. After 21 days, we collected surviving individuals, determined life stage, and kept them in the laboratory until any parasitoids emerged. To determine average life stage per branch, we multiplied the number of individuals in each stage by a value (1=larva or prepupa, 2=pupa, 3=eclosed adult), summed these values, and divided the sum by the total. We also calculated proportion survival (day 0 to day 21). We quantified mortality from parasitoids by dividing number of parasitized beetles by initial larval number.
Statistical analysis
We analysed Ch. lapponica survival, body mass, abundance, and parasitism by both parasitoids by mixed model ANOVA, with SG rank (BOR=SG-rich, CAP and PHY=SG-poor) and species pair (BOR vs. CAP, BOR vs. PHY) as fixed effects. Plant pair within species pair was considered a nested random effect. The SG rank factor tested for differences between the SG-rich S. borealis and the two SG-poor species. The species pair factor tested for differences among species pairs and the interaction tested whether the difference between SG-rich and SG-poor host depended on species pair. Finally, the plant pair factor determined whether significant spatial variation existed, as described in Rank (1994).
Laboratory choice tests with M. opacicornis were analysed using mixed-model ANOVA, with each choice trial as a random nested factor in each species pair. We calculated plant averages for number of scuttle fly pupae per beetle pupa before statistical analysis. Preliminary analysis using trial duration as a covariate revealed that it had no effect on significance values, so we left trial duration out of the final model.
To analyse average beetle life stage in the enemy-exclusion experiment, we used three-way factorial ANOVA with host species, exclosure treatment, and site as grouping factors and all interactions. One site was removed from this analysis because there were no survivors on S. caprea, which would have resulted in a missing cell in the ANOVA. To analyse proportion survival, we calculated site averages and conducted ANOVA, with host species, exclosure treatment and site as grouping factors and all possible two-way interactions. (Fractional dfs are reported in the denominator of some F-tests because JMP employs the Satterthwaite approximation when cell sample sizes are not completely balanced in the ANOVA). Parasitism included many zeroes because no parasitoids were found in some branches. To avoid violating ANOVA assumptions in the analysis of parasitism, we determined whether parasitism had occurred on a given plant and then analysed parasitism and predation by contingency table analysis. We excluded 17 plants from analysis because the mesh bag was damaged, predators had accidentally entered a bag, or non-experimental larvae of similar size had wandered onto the branch.
Results
Assessment of plant quality to Ch. lapponica
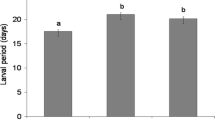
In the laboratory, larval survival on the SG-rich S. borealis was significantly greater than on the SG-poor S. caprea or S. phylicifolia (Table 1, Fig. 1A). Similarly, end of season number of survived individuals (of larvae that had been experimentally placed on willows) was significantly greater on S. borealis than on S. caprea or S. phylicifolia (Fig. 1C, Table 1), suggesting strongly that survival was greatest on S. borealis.
Performance of Chrysomela lapponica on three willow species: Salix borealis (BOR), S. caprea (CAP), and S. phylicifolia (PHY). Larval survival (pupation rate) in the laboratory (2001) (A); mass of newly emerged beetles from field collected pupae (1994) (B); number of prepupae that remained from three experimentally added egg batches per plant (2001) (C). Data shown are least-squares means of plant species pairs (±SE). Numbers of replicates for the 1994 and 2001 experiments are described in the Study sites and plants section
Mean body mass of newly emerged beetles reared from field-collected pupae was greater on S. borealis and S. caprea than on S. phylicifolia (Fig. 1B). The difference between SG-rich and SG-poor hosts varied among species pairs (Table 1).
Assessment of parasitism by M. opacicornis
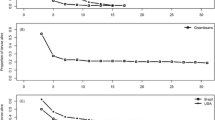
Megaselia opacicornis usually attacked beetle pupae at higher rates on S. borealis than on S. caprea (Fig. 2A, C). In 2001, parasitism rates were significantly higher on S. borealis than on other willows (Table 1). Parasitoid egg number per host tended to be lower on SG-poor willows (Fig. 2B, D), but no significant difference among host species was observed in 2000 (F 2,5=0.80, P=0.49).
Parasitism by Megaselia opacicornis (proportion of hosts with parasitoid eggs) and number of eggs per parasitized host on different willow species in field experiments of 2000 (A, B) and 2001 (C, D). Data reported are least-squares means (±SE) of each plant species (A, B) or plant species pair (C, D). Numbers of replicates are described in the Study sites and plants section. For abbreviations, see Fig. 1
In the laboratory choice test, M. opacicornis females laid a similar number of eggs on host prepupae reared on high- and low-SG willow species (SG-rank effect, F 1,8=1.2, P=0.31). There was no difference among species pairs (F 1,8=1.4, P=0.29) or among trials (F 8,8=1.6, P=0.27) in M. opacicornis egg number.
Assessment of parasitism by the tachinid Cl. nitidiuscula
Parasitism by Cl. nitidiuscula was significantly higher on S. borealis than on SG-poor willows (Fig. 3A, Table 1). Cl. nitidiuscula pupal mass tended to be higher on S. borealis than SG-poor hosts, especially on S. phylicifolia (Fig. 3B), but this was only marginally significant (Table 1).
Chrysomela lapponica parasitism by Cleonice nitidiuscula on different willow species (A) and mass of tachinid pupae (B) on hosts collected from three willow species. Data shown are least-squares means of plant species pairs (±SE). Numbers of replicates are described in the Study sites and plants section. For abbreviations, see Fig. 1
Plant specific body mass of adult beetles hatched from unparasitized pupae on S. borealis was related to the Cl. nitidiuscula parasitism rate (y=0.51x+1.86, r=0.54, n=47, P=0.001) and Cl. nitidiuscula pupal mass (y=2.13x−24.6, r=0.33, n=50, P=0.037). There was no significant relationship between parasitism rate and Cl. nitidiuscula body mass (y=0.91x+9.02, r=0.13, n=47, P=0.39).
Field enemy-exclusion experiment
Chrysomela lapponica larvae developed more rapidly on S. borealis than on S. caprea (F 1,3.4=7.5, P=0.06). This difference was more pronounced when enemies were excluded (host species by treatment interaction, F 1,3.8=9.8, P=0.037; Table 2, Fig. 4A). By the last day of the experiment, most individuals had pupated or emerged from pupae on both hosts (Table 2). Beetle survival was similar on both hosts in the presence of predators, although it tended to be lower on S. borealis than on S. caprea when enemies were excluded (Table 3, Fig. 4B). Finally, on open branches, parasitism occurred significantly more often on Ch. lapponica feeding on S. borealis (12 of 23 plants) than on S. caprea (three of 20 plants, Fisher's exact test, P=0.023). On average, 12% of beetles that had died from natural enemy attack on S. borealis were parasitized, compared to 1% for beetles on S. caprea. Predation on beetle pupae also tended to be observed more often on S. borealis (ten of 23 plants) than on S. caprea (four of 20 plants, Fisher's exact test, P=0.060).
Effects of willow species on average larval life stage (A) and survival (B) of Chrysomela lapponica exposed to or excluded from enemies in nature. Data shown are least squares means (±SE). For abbreviations, see Fig. 1
Discussion
Larval survival of Ch. lapponica was greater (in the laboratory) and development was faster (in the field) on SG-rich S. borealis than on SG-poor willow species. This result is consistent with studies of the SG-using leaf beetle Phratora vitellinae (Denno et al. 1990; Rank et al. 1998) and some Chrysomela species (Burkot and Benjamin 1979; Horton 1989; Topp et al. 1989; Rank 1994). Like other SG-using leaf beetles (Rowell-Rahier and Pasteels 1982; Denno et al. 1990; Rank 1992; Kolehmainen et al. 1995; Rank et al. 1998), Ch. lapponica prefers the SG-rich willow (S. borealis) against SG-poor willows (S. caprea and S. phylicifolia) in the field and laboratory (Zvereva et al. 1995b). Nevertheless, Ch. lapponica often use SG-poor hosts in nature and larval performance is sometimes just as high on SG-poor hosts as on SG-rich ones (Zvereva et al. 1995b). This lack of correspondence between host plant preference and larval performance has been found in a large number of plant herbivore interactions (Thompson 1988a), and the "enemy free space" (EFS) hypothesis was proposed, in part, to provide a general explanation for this phenomenon (Bernays 1988). A number of researchers have suggested that the EFS hypothesis explains preference/performance relationships in SG-using leaf beetles (Rowell-Rahier and Pasteels 1982; Smiley et al. 1985; Denno et al. 1990).
Predators respond in very different ways to the salicylaldehyde secretion found in larvae of SG-using leaf beetles. While some generalist predators, including birds, ants, and ladybird beetles, are repelled by the secretion (Wallace and Blum 1969; Pasteels et al. 1983b; Denno et al. 1990; Palokangas and Neuvonen 1992; Lundvall et al. 1998), generalist bugs that prey on SG-using leaf beetles in nature do not appear to be repelled by it (Rank et al. 1998; Gross 2001). Several species of predators specialize on SG-using leaf beetles or related beetles that produce autogenous (rather than host plant-derived) larval secretions (Rank and Smiley 1994; Rank et al. 1996; Sears et al. 2001). Some specialist predators use the beetle secretion as an olfactory attractant and feeding stimulant (Köpf et al. 1997; Gross 2001).
Attack rates of both parasitoids were not reduced on Ch. lapponica feeding on the SG-rich host plant, indicating that salicylaldehyde secretion does not repel either parasitoid species. Results of the laboratory choice test indicated that the amount and composition of the defensive secretion does not affect the attractiveness of the host for M. opacicornis. However, M. opacicornis field parasitism and egg number per host were greatest on beetle pupae on the SG-rich S. borealis, indicating that these parasitoids tend to aggregate on this willow species. Earlier studies have demonstrated positive spatial density-dependence in this host-parasitoid system (Zvereva and Kozlov 2000). The high beetle density on S. borealis may explain the high M. opacicornis parasitism rates on beetles feeding on this host plant. Parasitism by the tachinid fly Cl. nitidiuscula, which was also highest on S. borealis, was not density dependent (Zvereva and Kozlov 2000), suggesting that factors other than host density govern prey choice in this fly.
Our results indicate that the quality of the host plant to the herbivore affects larval performance of the parasitoid Cl. nitidiuscula. This was evident from the positive relationship between body mass of beetles reared on individual plants of S. borealis and pupal mass of Cl. nitidiuscula individuals collected from beetles reared on the same plants. We also found that host plant species differences in beetle body mass correspond to differences in Cl. nitidiuscula pupal mass. Numerous examples have demonstrated a positive correlation between the quality of insect hosts to parasitoids, host selection by parasitoids, and parasitoid fitness (Charnov and Skinner 1984; Sequeira and Mackauer 1992; Petitt and Wietlisbach 1995; Wen et al. 1995). Our results agree with studies, which found that parasitoid performance was related to herbivore performance on different host plant species (Bourchier 1991; English-Loeb et al. 1993; Godfray 1994; Benrey et al. 1998). Our data indicate that parasitism is most successful on host plants that provide Ch. lapponica with maximum nutritional quality, but not reduced on host plants that maximize the amount of salicylaldehyde defensive secretion.
The enemy-exclusion experiment allowed us to determine whether beetles obtain EFS under natural conditions from natural predators and parasitoids, according to the three criteria proposed Berdegue et al. (1996). As noted by Stamp (2001), few studies have met all three criteria. First, we demonstrate that natural enemies affect leaf beetle fitness, because survival of beetles exposed to enemies was much lower than survival of beetles when enemies were excluded. The second criterion for demonstrating EFS is that prey fitness in the "alternative" habitat or host (the EFS habitat), in the presence of enemies, is greater than fitness in the original (unprotected) habitat in the presence of enemies. In our study, mortality of Ch. lapponica due to parasitoids was higher on the SG-rich host than on SG-poor willows (Figs. 2, 3; Table 2), and survival of Ch. lapponica exposed to enemies was similar on SG-rich and SG-poor willows (Fig. 4B). Because survival was not greater on the SG-rich host in the presence of enemies, our data are not consistent with the second criterion. The third criterion is that prey fitness in the EFS habitat should be lower in the absence of enemies than fitness in the unprotected habitat. This criterion is based on the idea that demonstrating EFS requires that enemy protection incurs a cost, which is outweighed by the benefit of EFS. In our case, larval performance and survival tended to be greater on S. borealis than on S. caprea, suggesting that no such cost for host specialization exists for beetles living on S. borealis. Therefore we conclude that this plant-herbivore-enemy interaction does not fit the EFS hypothesis.
Our enemy-exclusion experiment takes into account larval and pupal mortality from all natural enemies, but not egg mortality. On average within a generation, three-quarters of beetle mortality occurs during the larval and pupal stage (Zvereva and Kozlov 2000), suggesting that the experiments reported here have targeted the most important life cycle stages in order to understand effects of enemies on host plant suitability. Earlier studies showed that egg parasitism in our populations of Ch. lapponica was extremely low, and the principal egg predator was the hoverfly Parasyrphus nigritarsis, which caused up to 55.3% egg mortality (Zvereva and Kozlov 2000). The biology of this hoverfly is very similar to that of Nearctic P. melanderi (Rank et al. 1996; Köpf et al. 1997; Gross 2001), which laid more eggs on leaf beetle batches oviposited on SG-rich willows than on SG-poor willows (Rank and Smiley 1994). Therefore it is likely that in our system egg mortality from natural enemies, similarly to mortality from parasitoids, is higher on SG-rich S. borealis than on other hosts.
Our results with Ch. lapponica differ from those obtained by Denno et al. (1990), who found support for EFS in a study of the SG-using leaf beetle Phratora vitellinae in Sweden. Denno et al. (1990) showed that P. vitellinae obtained EFS from the ladybird beetle predator Adalia bipunctata when feeding on a SG-rich willow, S. fragilis. A similarity between our study and Denno et al.'s (1990) work is that larval survival was greater in the laboratory (in the absence of enemies) on the SG-rich host than on the SG-poor ones. A difference between the studies is that our experiments were conducted in the field on native host plants, with beetles exposed to naturally occurring enemies, while Denno et al.'s (1990) study was conducted in the laboratory, using non-native host plants and a predator that is not known to feed on SG-using leaf beetles in nature (Kanervo 1939, 1946; Zvereva and Kozlov 2000). Our results are consistent with other studies that indicate that SG-using leaf beetles do not obtain EFS on naturally occurring hosts under field conditions (Rank 1994; Rank et al. 1996, 1998).
The EFS hypothesis suggests that insect herbivores may escape generalist enemies by narrowing their host plant range (Bernays 1988; Stamp 2001). However, specialist parasitoids may provide a selective pressure for a broader host plant range for host insects (Lawton 1986; Gratton and Welter 1999). In line with these studies, our results indicated that the host plant-derived chemical defence did not provide Ch. lapponica with EFS from parasitoids, and therefore appears not to be a selective force leading to specialization of Ch. lapponica on SG-rich willows. On the other hand, Ch. lapponica may obtain EFS from parasitoids on SG-poor willow species, as indicated by lower parasitism rates on them. Thus, a selection pressure imposed by parasitoids may favour an expansion of the breadth of diet of Ch. lapponica to include SG-poor willow species. Similarly, Gross (2001) suggested that escape from specialist predators has a significant impact on a host plant shift from willow to birch in some populations of Ch. lapponica. The low performance of Ch. lapponica Cl. nitidiuscula on SG-poor willows (in our study) and birch (Gross 2001) in the absence of enemies demonstrates the costs of such host range expansion.
Some studies of plant-herbivore-enemy interactions reveal little evidence for EFS (Keese 1997; Williams et al. 2001), while others provide evidence for it (Feder 1995; Yamaga and Ohgushi 1999; Gruenhagen and Perring 2001; Stamp 2001; Oppenheim and Gould 2002). We suspect that evolutionary history of the predator-prey (or parasitoid-prey) relationship strongly affects the role of natural enemies in determining suitability of the host plant to the herbivore. In the case of SG-using leaf beetles, phylogenetic studies indicate that use of SGs for defensive secretions in leaf beetles is not associated with a narrow diet (Köpf et al. 1998; Termonia et al. 2001). The results presented here are therefore consistent with phylogenetic and ecological evidence indicating that natural enemies are not responsible for host specialization in SG-using leaf beetles (Rank et al. 1996; Köpf et al. 1997).
References
Ballabeni P, Wlodarczyk M, Rahier M (2001) Does enemy-free space for eggs contribute to a leaf beetle's oviposition preference for nutritionally inferior host plant? Funct Ecol 15:318–324
Baur R, Rank NE (1996) Influence of host quality and natural enemies on the life history of the alder leaf beetles Agelastica alni and Linaeidea aenea. In: Jolivet PH, Cox ML (eds) Chrysomelidae biology, vol 2. Ecological studies. SPB, Amsterdam, pp 173–194
Benrey B, Callejas A, Rios L, Oyama K, Denno RF (1998) The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biol Contr 11:130–140
Berdegue M, Trumble JT, Hare JD, Redak RA (1996) Is it enemy-free space? The evidence for terrestrial insects and freshwater arthropods. Ecol Entomol 21:203–217.
Bernays EA (1988) Host specificity in phytophagous insects: selection pressure from generalist predators. Entomol Exp Appl 49:131–140
Bernays E, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Blumberg D (1991) Seasonal variations in the encapsulation of eggs of the encyrtid parasitoid Metaphycus stanleyi by the pyriform scale, Protopulvinaria pyriformis. Entomol Exp Appl 58:231–237
Blumberg D (1997) Parasitoid encapsulation as a defence mechanism in the coccoidea (Homoptera) and its importance in biological control. Biol Contr 8:225–236
Bourchier RS (1991) Growth and development of Compsilura concinnata (Meigan) (Diptera: Tachinidae) parasitizing gypsy moth larvae feeding on tannin diets. Can Entomol 123:1047–1056
Burkot TR, Benjamin DM (1979) The biology and ecology of the cottonwood leaf beetle, Chrysomela scripta (Coleoptera: Chrysomelidae), on tissue cultured hybrid Aigeiros (Populus × euramericana) subclones in Wisconsin. Can Entomol 111:551–556
Campbell CAM, Duffy SS (1979) Tomatine and parasitic wasps: potential incompatibility of plant antibiosis with biological control. Science 205:700–702
Charnov EL, Skinner SW (1984) Evolution of host selection and clutch size in parasitoid wasps. Fla Entomol 67:5–21
Cox ML (1994) The Hymenoptera and Diptera parasitoids of Chrysomelidae. In: Jolivet PH, Cox ML, Petitpierre E (eds) Novel aspects of the biology of Chrysomelidae. Kluwer, Dordrecht, pp 419–467
Denno RF, Larsson S, Olmstead KL (1990) Role of enemy-free space and plant quality in host-plant selection by willow beetles. Ecology 71:124–137
Devantoy J (1948) Les Prédateurs et les parasites de la chrysomèle du peuplier. Feuille Nat 3:85–89
Dicke M, van Poecke MP (2002) Signalling in plant–insect interactions: signal transduction in direct and indirect plant defence. In: Scheel D, Wasternack C (eds) Plant signal transduction. Oxford University Press, New York, pp 289–316
Disney RHL, Zvereva EL, Mostovski MB (2001) A scuttle fly (Diptera: Phoridae) parasitizing a beetle (Coleoptera: Chrysomelidae) in Russia. Entomol Fenn 12:59–63
English-Loeb GM, Brody AK, Karban R (1993) Host-plant-mediated interactions between a generalist foliavore and its tachinid parasitoid. J Anim Ecol 62:465–471
Feder JL (1995) The effects of parasitoids on sympatric host races of Rhagoletis pomonella (Diptera: Tephritidae). Ecology 76:801–813
Godfray HCJ (1994) Parasitoids. Behavioural and evolutionary ecology. Princeton University Press, Princeton, N.J.
Gratton C, Welter SC (1999) Does "enemy-free space" exist? Experimental host shifts of an herbivorous fly. Ecology 80:773–785
Gross J (2001) On the evolution of host plant specialization in leaf beetles (Coleoptera: Chrysomelina). Inaugural-dissertation zur Erlangung des Doktorgrades. Institut für Biology — Angewandte Zoologie/Ökologie der Tiere, Freien Universität Berlin, Logos, Berlin
Gross J, Hilker M (1995) Chemoecological studies of the exocrine glandular larval secretion of two chrysomelid species (Coleoptera): Phaedon cochleariae and Chrysomela lapponica. Chemoecology 5/6:185–189
Gruenhagen NM, Perring TM (2001) Plant influences on silverleaf whitefly oviposition and development and the potential for enemy-free space. Entomol Exp Appl 99:387–391
Hilker M, Schulz S (1994) Composition of larval secretion of Chrysomela lapponica (Coleoptera, Chrysomelidae) and its dependence on host plant. J Chem Ecol 20:1075–1093
Horton DR (1989) Performance of a willow-feeding beetle, Chrysomela knabi Brown, as affected by host species and dietary moisture. Can Entomol 121:777–780
Jeffries MJ, Lawton J (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Jervis MA, Copland MJV (1996) The life cycle. In: Jervis M, Kidd N (eds) Insect natural enemies: practical approaches to their study and evaluation. Chapman and Hall, London, pp 63–161
Julkunen-Tiitto R (1989a) Distribution of certain phenolics in Salix species (Salicaceae). Univ Joensuu Publ Sci 15:1–19
Julkunen-Tiitto R (1989b) Phenolic constituents of Salix: a chemotaxonomic survey of further Finnish species. Phytochemistry 28:2115–2125
Kanervo V (1939) Beobachtungen und Versuche zur Ermittlung der Nahrung einiger Coccinelliden (Col.). Ann Entomol Fenn 5:89–110
Kanervo V (1946) Tutkimuksia lepän lehtikuoriaisen, Melasoma aenea L. (Col., Chrysomelidae), luontaisista vihollisista.. Ann Zool Soc Zool Bot Fenn Vanamo 12:1–202
Keese MC (1997) Does escape to enemy-free space explain host specialization in two closely related leaf-feeding beetles (Coleoptera: Chrysomelidae)? Oecologia 112:81–86
Kolehmainen J, Julkunen-Tiitto R, Roininen H, Tahvanainen J (1995) Phenolic glucosides as feeding cues for willow–feeding leaf beetles. Entomol Exp Appl 74:235–243
Köpf A, Rank N, Roininen H, Tahvanainen J (1997) Defensive larval secretions of leaf beetles attract a specialist predator Parasyrphus nigritarsis. Ecol Entomol 22:176–183
Köpf A, Rank NE, Roininen H, Julkunen-Tiitto R, Pasteels JM, Tahvanainen J (1998) Phylogeny and the evolution of host plant use and sequestration in the willow leaf beetle genus Phratora (Coleoptera: Chrysomelidae). Evolution 52:517–528
Kruse JJ, Raffa KF (1999) Effect of food plant switching by a herbivore on its parasitoid: Cotesia melanoscela development in Lymantria dispar exposed to reciprocal dietary crosses. Ecol Entomol 24:37–45
Lawton JH (1986) The effect of parasitoids on phytophagous insect communities. In: Waage J, Greathead D (eds) Insect parasitoids. Academic Press, London, pp 265–289
Lundvall P, Neuvonen S, Halonen M (1998) Interspecific differences in the susceptibility of adult leaf beetles (Coleoptera: Chrysomelidae) to predation by willow warbles (Phylloscopus trochilus). Rep Kevo Subarct Res Stn 22:19–24
Ohsaki N, Sato Y (1990) Avoidance mechanisms of three Pieris butterfly species against the parasitoid wasp Apanteles glomeratus. Ecol Entomol 15:169–176
Oppenheim AJ, Gould F (2002) Behavioral adaptations increase the value of enemy free space for Heliothis subflexa, a specialist herbivore. Evolution 56:679–689
Palokangas P, Neuvonen S (1992) Differences between species and instars of leaf beetles in the probability to be preyed on. Ann Zool Fenn 29:273–278
Paré PW, Lewis WJ, Tumlinson JH (1999) Induced plant volatiles: biochemistry and effects on parasitoids. In: Agrawal AA, Tuzun S, Bent E (eds) Induced plant defenses against pathogens and herbivores. APS, St. Paul, Minn., pp 167–180
Pasteels JM, Gregoire JC, Rowell–Rahier M (1983a) The chemical ecology of defense in arthropods. Annu Rev Entomol 28:263–289
Pasteels JM, Rowell-Rahier M, Braekman JC, Dupont A (1983b) Salicin from host plant as precursor of salicylaldehyde in defensive secretion of chrysomeline larvae. Physiol Entomol 8:307–314
Petitt FL, Wietlisbach DO (1995) Effects of host instar and size on parasitization efficiency and life history parameters of Opius dissitus. Entomol Exp Appl 66:227–236
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Rank NE (1992) Host plant preference based on salicylate chemistry in a willow leaf beetle (Chrysomela aeneicollis). Oecologia 90:95–101
Rank NE (1994) Host plant effects on larval survival in a salicin–using leaf beetle Chrysomela aeneicollis (Coleoptera: Chrysomelidae). Oecologia 97:342–353
Rank NE, Smiley JT (1994) Host-plant effects on Parasyrphus melanderi Curran (Diptera: Syrphidae) feeding on a willow leaf beetle Chrysomela aeneicollis Schaeffer (Coleoptera: Chrysomelidae). Ecol Entomol 19:31–38
Rank NE, Smiley JT, Köpf A (1996) Natural enemies and host plant relationships for chrysomeline leaf beetles feeding on Salicaceae. In: Jolivet PH, Cox ML (eds) Chrysomelidae biology, vol 2: Ecological studies. SPB, Amsterdam, pp 147–171
Rank NE, Köpf A, Julkunen-Tiitto R, Tahvanainen J (1998) Host preference and larval performance of the salicylate-using leaf beetle Phratora vitellinae. Ecology 79:618–631
Richter VA, Zvereva EL (1996) The tachinid species Cleonice nitidiuscula Zetterstedt new for fauna of Murmansk Province (Diptera: Tachinidae). Zoosyst Ross 6:202
Rowell-Rahier M, Pasteels JM (1982) The significance of salicin for a Salix-feeder, Phratora (Phyllodecta) vitellinae. In: Visser JH, Minks AK (eds) Proceedings of the 5th International Symposium on Insect-Plant Relationships. Pudoc, Wageningen, pp 73–79
Salt G (1963) The defense reactions of insects to metazoan parasites. Parasitology 53:527–642
Schulz S, Gross J, Hilker M (1997) Origin of defensive secretion of the leaf beetle Chrysomela lapponica. Tetrahedron 53:9203–9212
Sears ALW, Smiley JT, Hilker M, Muller F, Rank NE (2001) Nesting behavior and prey use in two geographically separated populations of the specialist wasp Symmorphus cristatus (Vespidae: Eumeninae). Am Midl Nat 145:233–246
Sequeira R, Mackauer M (1992) Nutritional ecology of an insect host–parasitoid association: the pea-aphid-Aphidius ervi system. Ecology 73:183–189
Smiley JT, Horn JH, Rank NE (1985) Ecological effects of salicin at three trophic levels: new problems from old adaptations. Science 229:649–651
Stamp N (2001) Enemy-free space via host plant chemistry and dispersion: assessing the influence of tri-trophic interactions. Oecologia 128:153–163
Tahvanainen J, Julkunen-Tiitto R, Kettunen J (1985b) Phenolic glycosides govern the food selection pattern of willow feeding leaf beetles. Oecologia 67:52–56
Termonia A, Hsiao TH, Pasteels JM, Milinkovitch MC (2001) Feeding specialization and host-derived chemical defense in Chrysomeline leaf beetles did not lead to an evolutionary dead end. Proc Natl Acad Sci USA 98:3909–3914
Thompson JN (1988a) Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl 47:3–14
Thompson JN (1988b) Evolutionary genetics of oviposition preference in swallowtail butterflies. Evolution 42:1223–1234
Topp W, Beracz P, Zimmerman K (1989) Distribution pattern, fecundity, development, and survival of Melasoma vigintipunctata (Scop.) (Coleoptera: Chrysomelidae). Entomography 6:355–371
Turlings TCJ, Benrey B (1998) Effects of plant metabolites on behavior and development of parasitic wasps. Ecoscience 5:1–13
Turlings TCJ, Tumlinson JH, Lewis WJ, Vet LEM (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253
Van den Bosch R (1964) Encapsulation of eggs of Bathyplectes curculionis (Thompson) (Hymenoptera: Ichneumonidae) in larvae of Hypera brunneipennis (Boheman) and Hypera postica (Gyllenhal) (Coleoptera, Curculionidae). J Insect Pathol 6:343–367
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Wallace JB, Blum MS (1969) Refined defensive mechanisms in Chrysomela scripta. Ann Entomol Soc Am 62:503–506
Wen B, Weaver DK, Brower JH (1995) Size preference and sex ration for Pteromalus cerealellae (Hymenoptera: Pteromalidae). Environ Entomol 24:1160–1166
Williams IS, Jones TH, Hartley SE (2001) The role of resources and natural enemies in determining the distribution of an insect herbivore population. Ecol Entomol 26:204–211
Yamaga Y, Ohgushi T (1999) Preference–performance linkage in a herbivorous lady beetle: Consequences of variability of natural enemies. Oecologia 119:183–190
Zvereva EL, Kozlov MV (2000) Effects of air pollution on natural enemies of the leaf beetle Melasoma lapponica. J Appl Ecol 37:298–308
Zvereva EL, Kozlov MV, Neuvonen S (1995a) Decrease of feeding niche breadth of Melasoma lapponica (Coleoptera: Chrysomelidae) with increase of pollution. Oecologia 104:323–329
Zvereva EL, Kozlov MV, Neuvonen S (1995b) Population density and performance of Melasoma lapponica (Coleoptera: Chrysomelidae) in surroundings of a smelter complex. Environ Entomol 24:707–715
Zvereva EL, Kozlov MV, Haukioja E (1997) Population dynamics of a herbivore in an industrially modified landscape: case study with Melasoma lapponica (Coleoptera: Chrysomelidae). Acta Phytopathol Entomol Hung 32:251–258
Acknowledgements
We thank V. Zverev for assistance in the fieldwork and R. H. L. Disney and V. Richter for the discussions on parasitoid biology. We are very grateful to E. Dahlhoff, M. Kozlov, S. Neuvonen, H. Roininen, J. Tahvanainen and three anonymous reviewers for valuable comments on earlier drafts of the manuscripts. This study was financially supported by the Maj and Tor Nessling Foundation and the Turku University Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zvereva, E.L., Rank, N.E. Host plant effects on parasitoid attack on the leaf beetle Chrysomela lapponica . Oecologia 135, 258–267 (2003). https://doi.org/10.1007/s00442-003-1184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1184-9