Abstract
Mesenchymal stromal cells (MSCs) are emerging as an ideal candidate for regenerative medicine. It is known that the culture conditions impact the cellular properties of MSCs and their therapeutic behavior. Moreover, maintenance of MSCs in low oxygen tension for a short duration has shown to be beneficial for MSCs as it is similar to that of their physiological niche. However, the precise mechanism through which hypoxia pre-conditioning affects MSCs is not clear yet. Thus, in this study, we have investigated the effect of hypoxia exposure (1% O2) on tissue-specific MSCs over a period of time under serum-free culture conditions and evaluated the changes in expression of immuno-modulatory molecules and exosome biogenesis and secretion markers. It was observed that all MSCs responded differentially towards hypoxia exposure as indicated by the expression of HIF-1α. Moreover, this short-term exposure did not induce any changes in MSCs cellular morphology, proliferation rate, and surface marker profiling. In addition, we observed an enhancement in the expression of immunomodulatory factors (HLA-G, PGE-2, and IDO) after hypoxia exposure of 12 to 24 h in all tissue-specific MSCs. Interestingly, we have also observed the upregulation in exosome secretion that was further corelated to the upregulation of expression of exosome biogenesis and secretion markers (ALIX, TSG101, RAB27a, RAB27b). Though there was a differential response of MSCs where WJ-MSCs and BM-MSCs showed upregulation of these markers at 6–12 h of hypoxia pre-conditioning, while AD-MSCs showed similar changes beyond 24 h of hypoxia exposure.
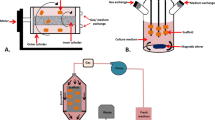
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stromal cells (MSCs) are multipotent cells that have unique properties of self-renewal and differentiation. They are distributed in many parts of the human body including the bone marrow (BM), adipose tissue (AD), dental pulp (DP), umbilical cord (UC), and placenta. All these tissues reside, particularly in a niche with low oxygen concentration ranging from 1 to 8% depending upon the location (Adolfsson et al. 2020). Thus, oxygen concentration is an important component of the stem cell niche, thereby maintaining the proliferation and plasticity of MSCs. Several studies have reported that hypoxia preconditioning enhances regenerative properties of MSCs (Kwon et al. 2017). In-depth analysis has shown that low O2 level suppresses the prolyl hydroxylation that leads to hypoxia inducible factors (HIF) accumulation and their nuclear translocation majorly, HIF-1α and HIF-2α. After nuclear translocation, it forms a heterodimer with HIF-1ß and binds to hypoxia response element (HRE) in the target gene involved in metabolism, angiogenesis, migration, and cell fate (Weidemann and Johnson 2008). However, in MSCs, they are responsible for transcription of genes involved in regulation of cellular metabolism thus maintaining the metabolic fate and multipotency of the cells. HIF-1 α specifically have been evaluated in different studies and have shown to be the master regulator during hypoxia exposure which initiates several other pathways for repair and regeneration (Dionigi et al. 2014). On the contrary, it has been observed that after isolation, these MSCs are usually cultured at four to ten times higher oxygen concentration in in vitro conditions. This alternation might affect their properties and therapeutic applications. Thus, it becomes crucial to mimic the natural microenvironment of these stem cells by maintaining in vitro low O2 tension that could provide results closer to what happens in in vivo.
There is significant evidence regarding the overexpression of HIF-1α and enhancing MSCs potential therapeutic role in various diseases like cardiovascular, wound healing, liver regeneration, and acute kidney injury (Shi et al. 2018; Gyöngyösi et al. 2010; Pacary et al. 2006). However, most of these studies are primarily on the bone marrow-derived MSCs (BM-MSCs). Limited studies have been reported to evaluate other MSC sources for the effect of hypoxia preconditioning and the associated mechanisms in much detail. Moreover, various reports have observed that hypoxic preconditioning influences MSCs properties such as immuno-modulatory potential and exosome secretion, but the degree and extent of hypoxia effect on these MSCs secretome have received little attention. Therefore, in this study, we have investigated the effect of hypoxia on MSCs stemness, proliferation, immunomodulation markers (indoleamine 2,3-dioxygenase (IDO), Prostaglandin E2 (PGE-2), human leukocyte antigen-G (HLA-G)), and exosome biogenesis and secretion markers (CD63, ALIX, TSG101, Rab27a/b). This study provides new evidences regarding the importance of low oxygen concentration culturing for enhanced properties of MSCs and its future applications.
Materials and methods
Isolation, expansion, and characterization of tissue-specific human mesenchymal stromal cells
Mesenchymal stromal cells used in this study were isolated from healthy donors with written consent after obtaining ethical clearance (Ref No. ICSCR/34/15(R)) from Institutional Committee for Stem Cell research (ICSCR), AIIMS, New Delhi. Mesenchymal stromal cells (MSCs) obtained from the bone marrow (BM-MSCs), Wharton’s jelly (WJ-MSCs), and adipose tissue (AD-MSCs) were used in this study. The bone marrow was collected from the patient’s donor (n = 3, 25–50 years, male) undergoing the routine medical test procedure in the Department of Hematology, AIIMS, New Delhi. Briefly, BM-MSCs were isolated and cultured as previously described (Rawat et al. 2018). Adipose tissue sample was collected from the patients (n = 3, 25–50 years, male) undergoing pre-scheduled surgical procedure in the Department of Pediatric Surgery, AIIMS, New Delhi. The sample was collected in a 5 ml transport vial containing 1 X PBS with 1% Penicillin (100U/ml), Streptomycin (100ug/ml) + Gentamycin (250ug/ml). The sample was washed extensively with 1X of PBS and explant culture methodology was proceeded as described in previously reported studies (Kakkar et al. 2019). For isolating Wharton’s jelly-derived MSCs, the umbilical cord (n = 3) was collected and processed within 24 h of normal or cesarean delivery of the donors (30–45 years, females) from the Department of Obstetrics and Gynecology, AIIMS, New Delhi. Briefly, the umbilical cord was collected in a 50 ml Scott bottle containing transport media. Upon arrival of sample, it was washed extensively with 1X PBS containing 1% Antibiotics. The cord was cut to expose the jelly part, chopped into a small piece using surgical blade (approx. ~ 1 mm), and was placed in a 35-mm culture dish kept undisturbed. The cultures were incubated overnight at 37 °C and 5% CO2 with 1 ml complete medium and changed every 3 to 4 days (Singh et al. 2020).
At 80% confluence, cells were harvested using 0.05% trypsin–EDTA (Invitrogen-Gibco) and transferred into a 60 mm culture dish. Cultures were monitored by phase-contrast microscopy (Olympus, Central Vally, PA) in order to evaluate the cell morphology and confluency.
Culture of MSCs in hypoxia
MSCs were cultured in normoxia (21% O2) and hypoxia (1% O2) culture conditions for different time points (6–48 h) For hypoxic condition, MSCs were exposed to 1% oxygen concentration using tri-gas incubator (ESCO, USA). At the end of each time period, conditioned media was collected for exosome isolation and cells were used for downstream experiments. Cell imaging was done at each time point using phase-contrast microscopy (Olympus, Central Vally, PA) in order to evaluate the cell morphology and confluency.
Population doubling time
MSCs for each source were seeded at a density of 50 × 103 cells per 35 mm petri dish (Becton Dickinson, USA) under normoxia (21%O2) and hypoxia (1%O2). After 72 h, MSCs were enumerated and assessed for viability using Trypan Blue dye exclusion assay. The PDT was obtained by the formula.
whereas T: time of harvestingTo: time of seedingN: number of cells harvestedNo: number of cells seeded
All experiments were performed in duplicates for each MSC donor (n = 3), for all tissue-specific MSCs.
Immunophenotyping
At passage 3, MSCs were characterized under normoxia and hypoxia using monoclonal antibodies specific for CD105-APC, CD73-PE, CD29-FITC, CD90-PerCp-Cy5.5, HLA-ABC-APC, HLA-DR-FITC, CD34/45-PE/FITC (BD Pharmingen, France). Acquisition and data analysis were performed using flow cytometry BD-LSR-II (BD Bioscience) and FACS Diva Software Version 6.2. For HLA-G flow cytometry studies, the mouse anti-HLA-G1/HLA-G5- MEMG/9 PE antibody (Exbio, Praha, Czech Republic) was used at 1:200 final concentration for 75,000 cells and incubated for 40 min at 4 °C in the dark, whereas intracellular staining of HIF-1α, cells were fixed using 2% Paraformaldehyde (PFA) at RT for 20 min. Cells were permeabilized using 0.05%Triton X-100 for 20 min at RT, followed by blocking step using 2% BSA for 20 min at RT. Anti-HIF-1α antibody (Thermo Fisher Scientific, USA) was used at 1:100 final concentration for 75,000 cells and incubated for 1 h 20 min at RT and secondary antibody used was anti-rabbit-AF488 at 1:500 for 40 min at RT in the dark. For analysis, isotype controls were included. All experiments were performed in duplicates for each MSC donor (n = 3), for all tissue-specific MSCs.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s protocol. Then, 2 μg of total RNA was reverse transcribed to cDNA (ABI, Invitrogen), which was then used as the template, and combined with standard SYBR Green (Kappa, USA) on the Real-Time PCR Detection System (Bio-Rad, USA). GAPDH served as the internal control and the data were analyzed using the 2-ΔΔCt method. All experiments for each time point were performed in duplicates for each MSC donor (n = 3), for all tissue-specific MSCs. List of primers is shown in Table S1.
ELISA for assessment of PGE-2
Tissue-specific MSCs were cultured for different time in hypoxia (0 h, 6 h, 12 h, 24 h, 48 h) and were seeded in 6 well plates at 50,000 cells/well and cultured for respective time and dose concentration in 2 ml medium without FBS. Culture supernatant was then collected and the concentration of PGE-2 determined by ELISA (Cayman, USA). All experiments for each time point were performed in duplicates for each MSC donor (n = 3) for all tissue-specific MSCs.
Determination of IDO activity
We evaluated the biological activity of IDO by measuring the level of kynurenine in supernatant collected from different preconditioned tissue-specific MSCs. We mixed 100 μl of collected supernatant with 50 ml of 30% trichloroacetic acid (Sigma, USA), vortexed and centrifuged at 8000 g for 5 min. Then, we transferred 75 μl of the supernatant with an equal volume of Ehrlich reagent (100 mg p-dimethyl benzaldehyde in 5 ml glacial acetic acid) (Sigma, USA) to a 96-well microtiter plate and recorded the absorbance at 490 nm. The supernatant collected from hypoxia preconditioned tissue-specific MSCs, whereas HeLa was used as a positive control. All experiments for each time point were performed in duplicates for each MSC donor (n = 3) for all tissue-specific MSCs.
Isolation of MSCs-derived exosomes
MSCs were cultured for 48 h in a serum-free media and conditioned media was collected for exosome isolation as previously described (Gupta et al. 2018). Briefly, the cellular debris was removed by centrifugation at 300 g for 10 min, followed by centrifugation at 10,000 g for 30 min to remove microvesicles. Conditioned media was loaded slowly over 30% sucrose solution forming a layer and centrifuged at 1,00,000 g, 4 °C for 90 min using Sorvall™ WX 90 + ultracentrifuge in swinging Bucket rotor (Thermo Scientific, USA). Supernatant was discarded and sucrose layer was resuspended in 1 × PBS. This was the ultracentrifuge at 1, 00,000 g at 4 °C for 90 min to pellet down the exosomes. After this, the exosomes were resuspended in 500 µl 1 × PBS and stored at − 80 °C for further use.
Characterization of exosomes
Nanoparticle tracking analysis
The exosomes were diluted (1:10) in 1 × PBS for nanoparticle tracking analysis (NTA) by NanoSight LM20 (Malvern Instruments Company, Nanosight, Malvern, United Kingdom). The Brownian motion of each particle was tracked between frames and the size was calculated using the Strokes-Einstein equation.
Transmission electron microscopy (TEM)
The exosome suspension was diluted at concentration of 1:1,000 in 1 × PBS and was placed on Formvar-carbon coated copper grids and allowed to adsorb for 5 min in a dry environment. The grids were washed in drops of 1 × PBS and stained with 2% phosphotungstic acid solution for 1 min. Thereafter, grids were air dried and were observed under the electron microscope (Tecnai, FEI, USA).
Western blotting
Exosomes were lysed in RIPA buffer, 1 mM PMSF, and protease inhibitors. Protein concentration of lysates was measured using bicinchoninic acid assay (BCA) protein assay kit (Pierce, USA). Twenty micrograms of exosomal lysate was subjected to 12.5% SDS-PAGE in non-reducing conditions for CD63 and reducing conditions for ALIX (Genetex,USA), Rab27a (Genetex, USA), Rab27b(Genetex, USA), HIF-1α (ThermoFisher, USA) and GAPDH (Genetex, USA), and transferred to a PVDF membrane (MDI) using wet transfer system (Biorad, USA). The blot was blocked in 5% Non-Fat Skimmed milk in 1 × TBS-T solution for 1 h followed by incubation in primary antibody overnight at 4 °C. The blot was then washed and incubated with respective HRP-conjugated secondary antibody and developed using ECL imager (Invitrogen, USA).
Statistics
All data analysis was done using the GraphPad Prism 5 software. p values were calculated using Student’s t test, two-way ANOVA, and post-test Tukey. All the data were expressed as mean ± SD for each experiment. For all analyses, differences with a p value < 0.05 were considered statistically significant..
Results
Characterization of tissue-specific MSCs under hypoxic and normoxia conditions
MSCs isolated from the bone marrow, adipose tissue, and Wharton’s jelly were characterized as per the International Society for Cellular Therapy (ISCT) guidelines. The cells were examined for their morphologies and no significant difference was observed between hypoxia and normoxia culture conditions up to 48 h. All tissue-specific MSCs showed fibroblast-like morphology with bipolar or multipolar elongated shape and had granularity around the nucleus (Fig. 1a). There was also no significant difference in the proliferation rate of MSCs when cultured in two different conditions. However, there were subtle variation observed amongst different tissue-specific MSCs wherein WJ-MSCs were found to have significantly lower PDT compared to AD-MSCs and BM-MSCs. Doubling time for WJ-MSCs was found to be 27 ± 2 h and AD-MSC and BM-MSCs had a PDT of 36 ± 4 h (Fig. 1b). Furthermore, the surface marker expression of common MSC markers was evaluated by flow cytometry for normoxia and hypoxia culture conditions up to 48 h. It was observed that despite being originated from different tissue sources, all the MSCs showed similar expression of characteristic stem cell-associated surface markers. All tissue-specific MSCs showed > 90% positivity for signature markers including CD90, CD105, CD29, CD73, and HLA-Class I, while negative for HLA-Class II and CD34/45 in BM-MSCs (Fig. 1c), AD-MSCs (Fig. 1d), and WJ-MSCs (Fig. 1e). Moreover, hypoxia preconditioning was also observed to induced mRNA levels of pluripotency/ stemness markers (KLF-4, OCT-4, and SOX2) in all tissue-specific MSCs. It was observed that the expression of these markers showed significant upregulation when exposed to hypoxia for different time points. Kruppel like factor 4 (KLF-4) expression in BM-MSC and AD-MSCs was of 5 ± threefold change while that of WJ-MSCs was of 2 ± 0.5-fold change at early time points of 6–12 h of hypoxia exposure (Fig. 1f). Octamer-binding transcription factor 4 (OCT-4) expression was observed to be gradually increasing to 4 ± 1 and 8 ± 1.2-fold change for BM-MSCs and AD-MSCs, respectively, when cultured for at least 48 h in hypoxia, whereas WJ-MSCs showed a variable fold change expression for OCT-4 during this culture duration (Fig. 1g). Notably, SRY-box 2 (SOX-2) expression was also enhanced after hypoxia exposure, though BM-MSCs and AD-MSCs had a constant expression (5 ± twofold change and 2 ± 0.5-fold change respectively) at all time points while WJ-MSCs showed an upregulation of 6 ± 1.5-fold change after 48 h of exposure (Fig. 1h).
Influence of hypoxia on basic characteristics of tissue-specific MSCs; a the pictographs represent the morphological features of BM-MSCs, AD-MSCs, and WJ-MSCs under different oxygen concentrations at 48 h; bar graph representing b population doubling time of tissue-specific MSCs and shows WJ-MSCs has least PDT under low oxygen concentration, c–e the surface marker profiling, f–h fold change for expression of stemness markers (KLF-4, OCT-4, and SOX-2), in all the tissue-specific MSCs under hypoxia culture condition at different time points. Data shown as mean ± SD; ***p < 0.001, **p < 0.01, *p < 0.05. Scale bar 100 μm
Hypoxia induces increase in expression of HIF-1α in tissue-specific MSCs
To optimize hypoxic induction for MSCs preconditioning, HIF-1α expression was analyzed at transcriptional and translational level. It was observed that HIF-1α expression was significantly upregulated after hypoxia exposure in all tissue-specific MSCs though with subtle variations. BM-MSCs and WJ-MSCs showed significant increase in HIF-1α mRNA expression by 12.97 ± twofold change and 11.38 ± 2.2-fold change at 6 h of hypoxia exposure respectively. This fold change was observed to significantly decrease by approx. ninefold change with further hypoxia exposure time points. In contrast, HIF-1α mRNA expression in AD-MSCs was found to increase to. 2.2 ± 0.2-fold change at 6 h and this change was maintained until 48 h. (Fig. 2a). Furthermore, the protein expression of HIF-1α was evaluated using flow cytometry where it was observed that WJ-MSCs and BM-MSCs showed an increase in HIF-1α expression from 6 h onwards, with the highest expression at 24 h. Furthermore, in AD-MSCs, this expression was maintained at all the time points of hypoxia exposure (Fig. 2b). Furthermore, HIF-1α expression was also evaluated through western blotting (Fig. 2c). The densitometric analysis revealed similar expression at all the time points from 6–48 h in BM-MSCs and AD-MSCs, while in WJ-MSCs, 12 h and 24 h showed the highest expression of HIF-1α.
Expression level of HIF-1α in tissue-specific MSCs after exposure to hypoxia at different time points; a the bar graph represents the fold change in mRNA expression of HIF-1α in tissue-specific MSCs; b the bar graph depicting the MFI of HIF-1α as evaluated by flow cytometry; c western blot images for the expression of HIF-1α in in tissue-specific MSCs; d densitometric analysis for HIF-1α protein expression using the AlphaEaseFC software (Version 4.0.0). Data shown as mean ± SD; ***p < 0.001, **p < 0.01, *p < 0.05
Hypoxia mediates differential expression of immuno-modulatory markers secreted by MSCs
MSCs possess wide immuno-modulatory properties that aid in its repair and regenerative process. This is achieved via either direct interaction with immune cells or by secretion of soluble factors. Thus, evaluating the changes in the expression of these factors in MSCs after hypoxia exposure is crucial. In this study, we have targeted three major immuno-modulatory molecules including HLA-G, PGE-2, and IDO. The qRT-PCR results showed that HLA-G expression was the highest in WJ-MSCs at 24 h with 10 ± twofold change and BM-MSCs showed an increase in fold change of 7 ± 2 at 6 h of hypoxia conditioning. Moreover, AD-MSCs was least responsive to hypoxia exposure as we could not observe any changes in mRNA expression of HLA-G (Fig. 3a). IDO expression was significantly increased in WJ-MSCs and BM-MSCs with a fold change of 5 ± 3 at 6 h whereas AD-MSCs again did not show any response to hypoxia for all the time points (Fig. 3b). PGE-2 gene expression was observed to be the highest in AD-MSCs (fold change of 55 ± 5) at 12 h followed by BM-MSCs that showed 20 ± twofold change at 24 h. In contrast to this, WJ-MSCs did not show any significant response (Fig. 3c).
Gene expression level of immunomodulatory molecule in tissue-specific MSCs; a the bar graph represents the expression of HLA-G at different time points after exposure to hypoxia, where WJ-MSCs and BM-MSCs show significant increase in fold change at 6 h followed by AD-MSCs. b The bar graph represents the expression of IDO, where WJ-MSCs and BM-MSCs show significant upregulation at 6 h followed by AD-MSCs. c The bar graph represents the expression of PGE-2, where AD-MSCs showed the significant increase in fold change at 12 h followed by BM-MSCs at 24 h, whereas WJ-MSCs did not show any response to hypoxia preconditioning. Data shown as mean ± SD; ***p < 0.001, **p < 0.01, *p < 0.05
Next, we have also evaluated the changes in protein expression of these immunomodulatory factors in all the tissue-specific MSCs. We have investigated the intracellular expression of HLA-G1/G5 by flow cytometry and observed that at 48 h of hypoxia exposure, the percent positivity of BM-MSCs for HLA-G1/G5 expression was 40 ± 5 percent, whereas the percent positivity of AD-MSCs and WJ-MSCs for HLA-G1/G5 expression was 25 ± 5 percent at 24 h of hypoxia exposure (Fig. 4a–c). Moreover, we have observed similar results by immunofluorescence assay in tissue-specific MSCs (Fig. S2). However, IDO activity was evaluated by measuring its byproduct kynurenine. Kynurenine production was first detectable at 6–12 h in BM-MSCs and continued to increase, demonstrating that IDO remains active during this extended course of hypoxia exposure, WJ-MSCs showed detectable range from 24 h onwards whereas AD-MSCs did not show any significant increase of IDO activity after exposure to different time points under hypoxia (Fig. 4d–f). Additionally, the secretion of soluble PGE-2 in the hypoxia-cultured tissue-specific MSCs was evaluated by ELISA, and it was observed that AD-MSCs and WJ-MSCs secreted 320 ± 10 pg/ml at 12 h, while BM-MSCs showed increased expression of 150 ± 20 pg/ml at 6 h and interestingly the expression decreased after 24 h (Fig. 4g–i). Altogether, our results at gene level and protein level correlate and signifie the influence of hypoxia on immunomodulatory factor secretion in tissue-specific MSCs.
Protein expression level of immunomodulatory molecule in tissue-specific MSCs; a the bar graph represents flow cytometry results for the expression of intracellular HLA-G1/G5 at different time points after exposure to hypoxia, where WJ-MSCs and BM-MSCs show significant increase at 24 h and 12 h followed by AD-MSCs at 24 h. b The bar graph represents the expression of IDO activity, where WJ-MSCs and BM-MSCs show significant upregulation at 24 h and 6 h respectively, AD-MSCs where it did not show any response to hypoxia. c The bar graph represents the concentration of PGE-2 assessed by ELISA, where AD-MSCs and BM-MSCs showed the significant increase in PGE-2 concentration at 6 h, whereas WJ-MSCs showed any highest response to hypoxia preconditioning at 12 h. Data shown as mean ± SD; ***p < 0.001, **p < 0.01, *p < 0.05
Hypoxia preconditioning affects exosome secretion by MSCs
Previous studies have reported that hypoxia culture conditions enhance exosome secretion by MSCs. Thus, to evaluate the effect of hypoxia on MSCs exosome secretion profile, we have first measured the exosome-specific marker CD63 expression in MSCs by qRT-PCR and flow cytometry. It was observed that there was a significant increase in CD63 mRNA expression (greater than 20 ± threefold change) for BM-MSCs and WJ-MSCs after 6 h of hypoxia exposure while AD-MSCs was found to be least responsive (Fig. 5a). In contrast to this, the expression CD 63 protein was found to increase after 6 h of hypoxia exposure in all tissue-specific MSCs with the highest expression within 12 to 24 h of exposure (Fig. 5b). Furthermore, the exosomes isolated at 24 h from all tissue-specific MSC conditioned media for both hypoxia and normoxia culture conditions were characterized for its number, size, and morphology. NTA was used to generate size density histograms for tissue-specific MSCs-derived exosomes obtained from two different culture conditions. It was observed that all tissue-specific MSC cultured either in hypoxia or normoxia secreted exosomes with size measurements of diameter 30 to 150 nm. However, hypoxia preconditioning significantly enhanced the exosome secretion by all tissue-specific MSCs (Fig. 5c). The percentage increase in exosome number secreted by MSCs after preconditioning with hypoxia was 30%, 29%, and 24.7% for BM-MSCs, AD-MSCs, and WJ-MSCs respectively. Moreover, it was also observed that these exosomes obtained from MSC preconditioned with hypoxia and normoxia exhibited other common characteristics including cup-shaped morphology (Fig. 5d). They also showed similar expressions of exosomal specific markers including CD63 and ALIX (Fig. 5e).
Effect of hypoxia preconditioning on expression of exosome-specific marker CD 63 in tissue-specific MSCs. a The line diagram represents the CD63 mRNA expression at different time points after exposure to hypoxia. b The bar graph represents the variation in CD 63 positive cells percentage as evaluated by flow cytometry. c The bar graph representing the variation in concentration of exosomes secreted by tissue-specific MSCs cultured in hypoxia and normoxia as evaluated by NTA. d Representative TEM images for size of exosomes; f western blot images for the expression of ALIX and CD63 in exosomes isolated from tissue-specific MSCs. Data shown as mean ± SD; ***p < 0.001, **p < 0.01, *p < 0.05
Effect of hypoxia preconditioning on genes affecting exosome biogenesis and secretion by tissue-specific MSCs
To understand why exosome secretion was enhanced after hypoxia exposure, we analyzed the exosome biogenesis and secretion markers expression. Cellular exosome biogenesis and secretion involves several molecules which includes ALIX, TSG101, Rab27a, and Rab27b (Fig. 6a). Thus, next, we evaluated the effect of hypoxia on mRNA and protein expression for exosome biogenesis and secretion markers. At gene level, with respect to BM-MSCs, it was observed that at 6 h of hypoxia exposure with increase in expression of HIF-1α gene (fold-change12.97 ± 2), there was significant increase in gene expression of ALIX (fold-change11.29 ± 1.9), TSG101(fold-change 9.24 ± 2.3), Rab27a (fold-change 5.42 ± 1.5), and Rab27b (fold-change19.1 ± 2.1). However, there was significant decrease in fold change of HIF-1α expression after 12 h of hypoxia exposure. This was followed by a decrease in expression of ALIX (fold-change 3.15 ± 0.91), TSG101 (fold-change 1.16 ± 0.03), Rab27a (fold-change 0.97 ± 0.02), and Rab27b (fold-change 4.46 ± 1.3). Moreover, with further hypoxia exposure for 24 to 48 h, the fold change was maintained with slight variations for all the genes including HIF-1α (fold-change 2.49 ± 1.02), ALIX (fold-change 4.19 ± 1.21), TSG101(fold-change 1.17 ± 0.68), Rab27a (fold-change 2.24 ± 0.98), and Rab27b (fold-change 5.79 ± 0.36) (Fig. 6b). In contrast, AD-MSCs were observed to be least responsive to hypoxia as indicated by low expression of HIF-1α gene (fold change 2.2 ± 0.2). Similar results were observed for TSG 101 (fold change 1.59 ± 0.43) for all time points of hypoxia exposure. However, there was significant fold change increase in exosome secretion markers, Rab 27a (fold change 4.1 ± 1.23) and Rab 27b (fold change 69.78 ± 2.3) at 6 h of hypoxia exposure, and with further time points, a gradual decrease in expression of these markers was observed. Additionally, ALIX gene expression was also observed to increase from 2.4 ± 0.8-fold change at 6 h to 14.05 ± 2.6-fold change at 12 h, and this fold change was maintained until 48 h of hypoxia exposure (Fig. 6c). Furthermore, with respect to WJ-MSCs, at 6 h of hypoxia exposure, there was significant increase in mRNA expression of HIF-1α (fold change 11.38 ± 2.2), ALIX (fold change 2.42 ± 1.2), Rab 27a (fold change 2.72 ± 1.02), and Rab 27b (fold change 2.31 ± 0.02). However, the expression of this expression was observed to decrease with increase in time duration of hypoxia. Moreover, Rab 27b mRNA expression was found to increase significantly at 48 h of hypoxia exposure (fold change 8.78 ± 2.5) and no significant change was observed in expression of TSG101 during hypoxia exposure (Fig. 6d).
Hypoxia exposure upregulated the expression of exosome biogenesis and its secretion markers in tissue-specific MSCs. a Representative schematic diagram depicts the potential effect of hypoxia on exosome biogenesis and exosome secretion markers. Representative line graphs plots indicating the variation in mRNA expression of hypoxia inducing gene HIF-1α and other genes involved in biogenesis (ALIX and TSG101) and secretion (Rab27a/b) in b BM-MSCs, c AD-MSCs, and d WJ-MSCs
We have also evaluated the expression of biogenesis and secretion markers in tissue-specific MSCs through immunoblotting assays. GAPDH was used as a housekeeping gene. Densitometric analysis of CD63 revealed the increase in its expression after hypoxia exposure from 6 h onwards; however, most of the upregulation was observed at 24 h for all tissue-specific MSCs. Similar results were observed for ALIX, Rab27a, and Rab27b. It was observed that BM-MSC and WJ-MSCs showed enhanced expression at 12 h or 24 h while AD-MSCs showed enhanced expression beyond 24 h of hypoxia exposure (Fig. 7).
Western blot analysis validates the potential effect of hypoxia preconditioning for different time points in exosome biogenesis and secretion markers expression in a BM-MSCs, b AD-MSCs, and c WJ-MSCs. GAPDH was used as a housekeeping gene. Densitometric analysis was done using the AlphaEaseFC software (Version 4.0.0). Data from three donors and shown as mean ± SD; ***p < 0.001, **p < 0.01, *p < 0.05
Discussion
Mesenchymal stromal cells holds great potential to treat various diseases as evidenced by growing number of pre-clinical and clinical stage published studies. The properties of MSCs are affected by changing environmental conditions that may induce MSCs to adapt to specific functions. Oxygen tension is one such condition where it is known that MSCs reside in a low oxygen niche in in vivo; however, they are exposed to higher oxygen concentration when expanded in in vitro culture conditions. These altered culture conditions may influence MSC properties and their functional activity. Thus, accumulating evidence have shown that maintaining them in low oxygen tension may aid in maintaining their properties and enhance therapeutic potential. However, most of these studies have used MSCs from a single origin and evaluated them. Moreover, it is also known that all MSCs despite sharing similar characteristics including morphology and surface marker profiling, they show subtle variations such as difference in their secretome including immuno-modulatory factor secretion and exosome secretion (Alhattab et al. 2019; Ren et al. 2009; Paliwal et al. 2017). These variations correspond to the tissue of origin and understanding them in much details may aid in identifying alternative sources of MSCs for therapeutic potential.
Thus, in this study, we have made an attempt to understand the impact of hypoxia on tissue-specific MSCs isolated from different sources (adipose tissue, bone marrow, and Wharton’s jelly); different O2 concentrations have been reported previously, but many of these studies have identified 1% oxygen tension for enhancing MSCs functionality both in in vitro and in vivo experimental models (Kim et al. 2016; Antebi et al. 2018). Moreover, one of the important parameters to evaluate this hypoxia preconditioning is the changes in expression of HIF-α gene, a master regulator of the hypoxia response. This gene is usually ubiquitinated under normoxia culture condition and stabilized only after hypoxia exposure. Thus, the expression of this gene indicates the response of the cell towards hypoxia. Surprisingly, all the tissue-specific MSCs showed a significant increase in HIF-1α protein expression as early as at 6 h and it peaked at 12 to 24 h of hypoxia exposure. In contrast to this, there are several studies where HIF-1α expression was observed even after 24 h and up to 7 days after hypoxia preconditioning (Dionigi et al. 2014; Lee et al. 2017). Our results further indicated that hypoxia preconditioning for short duration of 48 h did not affect the spindle shaped cell morphology and immunophenotyping. Moreover, Choi et al. reported that hypoxia exposure for short duration showed a significant impact on proliferation and differentiation ability of adipose tissue derived MSCs (Choi et al. 2017). In contrast to this, we observed that hypoxia preconditioning did not modulate the population doubling time of tissue-specific MSCs. These discrepancies are likely due to the different protocols, culture media compositions, oxygen tension, and heterogeneity of the donors (Holzwarth et al. 2010). However, there were tissue-specific variations observed where the population doubling time of WJ-MSCs was lowered compared to AD-MSCs and BM-MSCs. These tissue-specific variations were similar to other reported studies (Kwon et al. 2016). Furthermore, interestingly, different time points of hypoxia exposure significantly increased the stemness marker expression by tissue-specific MSCs. This increase in stemness markers is known to inhibit senescence of MSCs (Zhou et al. 2020). Thus, hypoxia pre-conditioning may have beneficial effect over normoxia culture by maintaining MSCs properties including proliferative and pluripotency.
Hypoxia preconditioning has been shown to alter the MSCs secretome in various experimental models (Teixeira et al. 2015). This effect has crucial implication in tissue repair and regeneration by MSCs. Thus, we investigated the effect of low O2 tension on MSCs secretome including immuno-modulatory factors and exosome secretion. Increasing evidences have shown that MSCs exert immunosuppressive effect through secretion of soluble factors such as indoleamine 2,3-dioxygenase (IDO), Prostaglandin E2 (PGE-2), and human leukocyte antigen-G (HLA-G) (Gebler et al. 2012). This has made MSCs as an ideal cell source for cell-based therapy specifically where immunomodulation is required such as during cell or organ transplantation. Based on the importance of some of the immuno-modulatory molecules, we selected three important factors and evaluated their expression in hypoxic preconditioning. First molecule was indoleamine-2,3-dioxygenase (IDO), an enzyme that catalyzes the conversion of tryptophan to kynurenine in an interferon gamma-dependent manner. In turn, this kynurenine inhibits T-cell proliferation and induces the differentiation of regulatory T cells (T-reg) (Amobi et al. 2017; Wachowska et al. 2020). Interestingly, in our study, we have observed that IDO activity was enhanced after hypoxia exposure in all tissue-specific MSCs, though there was a subtle variation where hypoxia exposure time played a crucial role. BM-MSCs showed an upregulation in the IDO activity as early as 6 h of minimum exposure, and then, there was a decrease in the IDO activity for next 24 h, but after 48 h of exposure, we observed a significant increase in IDO activity. On the other hand, AD-MSCs and WJ-MSCs showed significant increase in IDO activity at 24 h of hypoxia exposure. Similar results have been reported previously where IDO expression has been shown to increase in MSCs after hypoxia exposure (Saparov et al. 2016). However, there are few contrasting studies where it was observed that IDO activity is preserved under hypoxic conditions (Schmidt et al. 2013, Roemeling-van Rhijn et al. 2013), but these studies have evaluated MSCs from a single source and not evaluated a broad range of time points. Next, we have also evaluated the expression of second potent immuno-modulator, PGE-2 which is a lipid mediator, synthesized from arachidonic acid via the actions of cyclooxygenase (COX) enzymes. In response to certain inflammatory milieu, MSCs secrete PGE-2 and regulate the activation of the immune cells and their maturation (Burnham et al. 2020). Similar to IDO, there have been many contrasting studies for expression of PGE-2 by MSCs; however, in our study, it was observed that all tissue-specific MSCs showed significant increase in PGE-2 expression at 6 to 12 h of minimum hypoxia exposure (Sareen et al. 2020). Furthermore, we investigated the expression of third molecule, HLA-G1/G5 protein, an important tolerogenic molecule playing an essential role in maternal–fetal tolerance, which constitutes the perfect example of successful physiological immunotolerance of semi-allografts (Ferreira et al. 2017; Wobma et al. 2018). We observed that BM-MSCs showed an upregulation in the expression of intracellular HLA-G1/G5 as early as 6 h of minimum exposure, and then, there was a decrease in this expression for next 24 h, but after 48 h of exposure, we observed a significant increase in expression of intracellular HLA-G1/G5. On the other hand, AD-MSCs and WJ-MSCs showed significant increase in HLA-G1/G5 expression at 24 h of hypoxia exposure. These results provided an insight into the optimum culture conditions for MSCs and the target immuno-modulatory molecule based on the specific requirements. By amending these conditions, MSCs-specific priming will further aid in their enhanced therapeutic role.
In addition, effect of hypoxia on secretion of exosomes was also evaluated. Exosomes secreted from MSCs are crucial mediators and messengers of paracrine signaling action and delivery (Elahi et al. 2020). They have the potential to modify the function of damaged cells via transfer of miRNAs, proteins, etc. (Li et al. 2018). Accumulating evidence suggests that MSC exosomes possess a superior safety profile, anti-inflammatory effects, and low immunogenicity. Due to small size, risk of vascular obstruction is reduced and the possibility of crossing blood brain barriers is enhanced (Aryani and Denecke 2016). Moreover, the surface of the exosomes could be modified and exploited into engineered exosomes, which can bind the specific target cells and escape immune response (Luan et al. 2017). Despite these advantages, widespread therapeutic use of exosomes is hampered by their limited cellular secretion. Thus, having the means for increasing exosome biogenesis and secretion will be highly important for development of exosome-based applications (Lee et al. 2020). Several studies have reported that low oxygen tension upregulates exosome secretion, specifically in cancer cells. Limited number of studies have investigated this approach in MSCs, and observed an increase in exosome secretion by them (Wang et al. 2020). However, the associated mechanisms are yet unknown. Thus, in this study, we have made an attempt to understand the impact of hypoxia (1% oxygen concentration) on MSCs and their derivative exosomes isolated from different tissue sources i.e., the adipose tissue, bone marrow, and Wharton’s jelly and compared with that of normoxia culture conditions. As reported in previous reports, we have observed that hypoxia enhances the exosome secretion in conditioned media by all tissue-specific MSCs (Panigrahi et al. 2018; Ferreira et al. 2018). To further gain insights into the potential mechanistic role for this enhanced secretion by MSCs, we choose certain markers that have been reported to play a crucial role in exosome biogenesis and secretion. Available studies have reported the role of endosomal sorting complex required for transport (ESCRT) dependent and independent pathways in exosome biogenesis regulation (Babst 2011, Tschuschke et al. 2020). ESCRT-dependent machinery includes four complexes (ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III) and the associated proteins (ALIX, VPS4, and VTA1). All these proteins are involved in orchestrating a discrete step for MVB formation and recognizing and escorting the targeting protein to the exosomes (Larios et al. 2020). Furthermore, ESCRT-independent mechanisms include ceramides and tetraspanin proteins (CD63, CD81, and CD9). These proteins are also responsible for sorting and loading various cargo molecules to the exosomes (Datta et al. 2018). However, both these pathways work synergistically and a heterogeneous population of exosomes is secreted by cells. Additionally, the RAB family of small GTPase proteins (Rab7, Rab27a, Rab27b, and Rab35) has been implicated in intracellular MVB trafficking to the plasma membrane for exosome release (Ruan et al. 2018; Rezaie et al. 2018). Thus, understanding the effect of hypoxia preconditioning on this regulatory machinery is crucial. Therefore, we choose four important molecules of this machinery as follows: ALIX, TSG 101, Rab27a, and Rab27b as important markers for further evaluation. It was observed that all tissue-specific MSC showed differential response for expression of these markers at transcription level; however, when evaluated for protein expression, we found that most of the marker’s expression peaked between 12 and 24 h of hypoxia exposure. During this period, HIF-1α expression was also elevated in all tissue-specific MSCs indicating a correlation between hypoxia and exosome biogenesis and secretion pathways. Our findings demonstrated that hypoxia preconditioning could be used as a strategy for the mass scale production of exosomes required for cell free therapeutic approach without altering basic properties of MSCs or exosomes.
To the best of our knowledge, this study is first of its kind providing encouraging insight into how tissue-specific MSCs responded towards the hypoxia preconditioning. Furthermore, 12 to 24 h exposure appears to be an ideal time point for the hypoxia preconditioning and enhancing MSCs therapeutic role. Additionally, these culture conditions are applicable to a wide range of MSCs sources, though we have focused only on the bone marrow, Wharton’s jelly, and adipose tissue in our study. Furthermore, it is important to gain insights into the cargo profiling of exosomes during hypoxia preconditioning as the exosome cargo packaging is dependent on the parent cell status and its environment. Thus, evaluating these changes is warranted for better utilizing the effect of these stimuli on immunomodulation, exosome secretion, and associated therapeutic effects.
Conclusion
We have shown that hypoxia preconditioning is an effective strategy for enhancing MSCs secretome thereby improving its immuno-modulatory properties and exosome secretion. This preconditioning did not alter MSCs basic characteristics but may modulate their therapeutic potential. Further investigation into the associated pathways for HIF-1α orchestrated modulation of these variations is warranted.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Adolfsson E, Helenius G, Friberg Ö, Samano N, Frøbert O, Johansson K (2020) Bone marrow-and adipose tissue-derived mesenchymal stem cells from donors with coronary artery disease; growth, yield, gene expression and the effect of oxygen concentration. Scand J Clin Lab Invest 80(4):318–326
Alhattab D, Jamali F, Ali D, Hammad H, Adwan S, Rahmeh R, Awidi A (2019) An insight into the whole transcriptome profile of four tissue-specific human mesenchymal stem cells. Regen Med 14(9):841–865
Amobi A, Qian F, Lugade AA, Odunsi K (2017) Tryptophan catabolism and cancer immunotherapy targeting IDO mediated immune suppression. Tumor Imm Microenviron Cancer Progress Cancer Ther 129–144
Antebi B, Rodriguez LA, Walker KP, Asher AM, Kamucheka RM, Alvarado L, Cancio LC (2018) Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther 9(1):1–15
Aryani A, Denecke B (2016) Exosomes as a nanodelivery system: a key to the future of neuromedicine?. Mol Neurobiol 53(2):818–834. https://doi.org/10.1007/s12035-014-9054-5
Babst M (2011) MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol 23(4):452–457. https://doi.org/10.1016/j.ceb.2011.04.008
Burnham AJ, Foppiani EM, Horwitz EM (2020) Key metabolic pathways in MSC-mediated immunomodulation: implications for the prophylaxis and treatment of graft versus host disease. Front Immunol 11
Choi JR, Yong KW, Wan Safwani WK (2017) Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cell Mol Life Sci 74(14):2587–2600
Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, Abdel-Mageed AB (2018) High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep 8(1):1–13
Dionigi B, Ahmed A, Pennington EC, Zurakowski D, Fauza DO (2014) A comparative analysis of human mesenchymal stem cell response to hypoxia in vitro: Implications to translational strategies. J Pediatr Surg 49(6):915–918
Elahi FM, Farwell DG, Nolta JA, Anderson JD (2020) Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 38(1):15–21
Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM (2018) Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol 9:2837. https://doi.org/10.3389/fimmu.2018.02837
Ferreira LM, Meissner TB, Tilburgs T, Strominger JL (2017) HLA-G: at the interface of maternal–fetal tolerance. Trends Immunol 38(4):272–286
Gebler A, Zabel O, Seliger B (2012) The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med 18(2):128–134
Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK, Mohanty S (2018) An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther 9(1):1–11
Gyöngyösi M, Hemetsberger R, Posa A, Charwat S, Pavo N, Petnehazy Ö, Maurer G (2010) Hypoxia-inducible factor 1-alpha release after intracoronary versus intramyocardial stem cell therapy in myocardial infarction. J Cardiovasc Transl Res 3(2):114–121
Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Müller I (2010) Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol 11(1):1–11
Kakkar A, Nandy SB, Gupta S, Bharagava B, Airan B, Mohanty S (2019) Adipose tissue derived mesenchymal stem cells are better respondents to TGFβ1 for in vitro generation of cardiomyocyte-like cells. Mol Cell Biochem 460(1):53–66
Kim DS, Ko YJ, Lee MW, Park HJ, Park YJ, Kim DI, Yoo KH (2016) Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress Chaperones 21(6):1089–1099
Kwon A, Kim Y, Kim M, Kim J, Choi H, Jekarl DW, Park IY (2016) Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep 6(1):1–11
Kwon SY, Chun SY, Ha YS, Kim DH, Kim J, Song PH, Kwon TG (2017) Hypoxia enhances cell properties of human mesenchymal stem cells. Tissue Eng Regenerat Med 14(5):595–604
Larios J, Mercier V, Roux A, Gruenberg J (2020) ALIX-and ESCRT-III–dependent sorting of tetraspanins to exosomes. J Cell Biol 219(3)
Lee JH, Ha DH, Go HK, Youn J, Kim HK, Jin RC, Yi YW (2020) Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int J Mol Sci 21(13):4774
Lee JH, Yoon YM, Lee SH (2017) Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt axis. Int J Mol Sci 18(6):1320
Li SP, Lin ZX, Jiang XY, Yu XY (2018) Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin 39(4):542–551
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38(6):754–763
Pacary E, Legros H, Valable S, Duchatelle P, Lecocq M, Petit E, Bernaudin M (2006) Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci 119(13):2667–2678
Paliwal S, Kakkar A, Sharma R, Airan B, Mohanty S (2017) Differential reduction of reactive oxygen species by human tissue-specific mesenchymal stem cells from different donors under oxidative stress. J Biosci 42(3):373–382
Panigrahi GK, Praharaj PP, Peak TC, Long J, Singh R, Rhim JS, Deep G (2018) Hypoxia-induced exosome secretion promotes survival of African-American and Caucasian prostate cancer cells. Sci Rep 8(1):1–13
Rawat S, Srivastava P, Prabha P, Gupta S, Kanga U, Mohanty S (2018) A comparative study on immunomodulatory potential of tissue specific hMSCs: Role of HLA-G
Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, Shi Y (2009) Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27(8):1954–1962
Rezaie J, Nejati V, Khaksar M, Oryan A, Aghamohamadzadeh N, Shariatzadeh MA, Mehranjani MS (2018) Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res 374(3):555–565
Roemeling-van Rhijn M, Mensah FK, Korevaar SS, Leijs MJ, van Osch GJ, Ijzermans JN, Betjes MG, Baan CC, Weimar W, Hoogduijn MJ (2013) Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front Immunol 4:203. https://doi.org/10.3389/fimmu.2013.00203
Ruan XF, Ju CW, Shen Y, Liu YT, Kim IM, Yu H, Tang Y (2018) Suxiao Jiuxin pill promotes exosome secretion from mouse cardiac mesenchymal stem cells in vitro. Acta Pharmacol Sin 39(4):569–578
Saparov A, Ogay V, Nurgozhin T, Jumabay M, Chen WC (2016) Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Int 2016:3924858. https://doi.org/10.1155/2016/3924858
Sareen N, Abu-El-Rub E, Ammar HI, Yan W, Sequiera GL, ShamsEldeen AM, Dhingra S (2020) Hypoxia-induced downregulation of cyclooxygenase 2 leads to the loss of immunoprivilege of allogeneic mesenchymal stem cells. FASEB J 34(11):15236–15251
Schmidt SK, Ebel S, Keil E, Woite C, Ernst JF, Benzin AE, Rupp J, Däubener W (2013) Regulation of IDO activity by oxygen supply: inhibitory effects on antimicrobial and immunoregulatory functions. PLoS One 8(5):e63301. https://doi.org/10.1371/journal.pone.0063301
Shi X, Zhang G, Sun H, Bai Y, Liu Y, Zhang W, Xiao H (2018) Effects of over-expression of HIF-1alpha in bone marrow-derived mesenchymal stem cells on traumatic brain injury. Eng Life Sci 18(6):401–407
Singh M, Vaishnav PK, Dinda AK, Mohanty S (2020) Evaluation of priming efficiency of forskolin in tissue-specific human mesenchymal stem cells into dopaminergic neurons: an in vitro comparative study. Cells 9(9):2058
Teixeira FG, Panchalingam KM, Anjo SI, Manadas B, Pereira R, Sousa N, Behie LA (2015) Do hypoxia/normoxia culturing conditions change the neuroregulatory profile of Wharton Jelly mesenchymal stem cell secretome?. Stem Cell Res Ther 6(1):1–14
Tschuschke M, Kocherova I, Bryja A, Mozdziak P, Angelova Volponi A, Janowicz K, Sibiak R, Piotrowska-Kempisty H, Iżycki D, Bukowska D, Antosik P, Shibli JA, Dyszkiewicz-Konwińska M, Kempisty B (2020) Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. J Clin Med 9(2):436. https://doi.org/10.3390/jcm9020436
Wachowska M, Stachura J, Tonecka K, Fidyt K, Braniewska A, Sas Z, Muchowicz A (2020) Inhibition of IDO leads to IL-6-dependent systemic inflammation in mice when combined with photodynamic therapy. Cancer Immunol Immunother 69(6):1101–1112
Wang J, Bonacquisti EE, Brown AD, Nguyen J (2020) Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells 9(3):660
Weidemann A, Johnson RS (2008) Biology of HIF-1 α. Cell Death Differ 15(4):621–627
Wobma HM, Kanai M, Ma SP, Shih Y, Li HW, Duran-Struuck R, Vunjak-Novakovic G (2018) Dual IFN-γ/hypoxia priming enhances immunosuppression of mesenchymal stromal cells through regulatory proteins and metabolic mechanisms. J Immunol Regenerat Med 1:45–56
Zhou X, Hong Y, Zhang H, Li X (2020) Mesenchymal stem cell senescence and rejuvenation: current status and challenges. Front Cell Developmen Biol 8
Funding
The work has been supported by the All-India Institute of Medical Sciences, New Delhi, and the BIRAC-PACE (BT/AIR0987/PACE-19/19), Department of Biotechnology, Government of India and DST (EMR/2017/000229).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by SG and SR. Experiments were performed by SG, SR, VK, and EPR. The first draft of the manuscript was written by SG and all authors read and approved the final manuscript. SR contributed to analysis of data, paper writing, and first revision. SM contributed to supervision of experiments and critical revision of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Mesenchymal stromal cells used in this study were isolated from healthy donors with written consent after obtaining ethical clearance (Ref No. ICSCR/34/15(R)) from Institutional Committee for Stem Cell research (ICSCR), AIIMS, New Delhi.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, S., Rawat, S., Krishnakumar, V. et al. Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res 388, 535–548 (2022). https://doi.org/10.1007/s00441-022-03615-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-022-03615-y