Abstract
This study analyzed the roles of puerarin and LncRNA ANRIL in myocardial ischemia–reperfusion injury. Hypoxia/reperfusion (H/R) model was established with H9C2 cells. Effects of puerarin of gradient concentrations on cardiomyocytes at different time points of hypoxia and reoxygenation were detected by quantitative real-time polymerase chain reaction (qRT-PCR), cell counting kit-8 (CCK-8), and microscope observation. Effects of puerarin on cardiomyocyte viability, ANRIL expression, contents of lactate dehydrogenase (LDH) and malondialdehyde (MDA), apoptosis, and expressions of autophagy-related genes after H/R injury were determined by CCK-8, quantitative real-time polymerase chain reaction (qRT-PCR), ELISA, flow cytometry, and Western blot, respectively. After cell transfection, the effects of overexpressed and knockdown of ANRIL on cardiomyocytes and H/R-injured cardiomyocytes were examined by rescue experiments. The ischemia–reperfusion (I/R)-injured rat model was established to examine the protective effect of puerarin in vivo. Prolonged hypoxia downregulated ANRIL expression in cardiomyocytes and reduced cardiomyocyte viability. Prolonged reoxygenation increased apoptosis. Both cardiomyocyte viability and ANRIL expression showed a dose-dependent relationship with puerarin. Puerarin reversed the effects of H/R injury on cardiomyocyte viability, ANRIL expression, contents of LDH and MDA, apoptosis, and expressions of autophagy-related genes. Overexpression and knockdown of ANRIL regulated the functions of cardiomyocytes and the expressions of autophagy-related genes. Puerarin reversed the effects of knockdown of ANRIL on H/R-injured cells. The results of In vivo experiments confirmed that puerarin protected myocardial tissues by up-regulating ANRIL and inhibiting autophagy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary heart diseases as common cardiovascular diseases show an increasing rate of mortality (Dalen et al. 2014; Sriharibabu 2016). During the progression of coronary heart disease, long-term and complete lumen occlusion could be caused by atheromatous plaque rupture and bleeding, intravascular thrombosis, etc., resulting in the interruption of blood supply to the myocardium, thus leading to myocardial ischemia and necrosis (Di Angelantonio et al. 2011; Wirtz and von Kanel 2017). Therefore, a timely and effective restoration of blood flow perfusion is a fundamental method for preventing and treating myocardial ischemic injury (Wirtz and von Kanel 2017). To meet such a treatment purpose, reperfusion methods such as thrombolytic therapy, percutaneous coronary intervention, and coronary artery bypass grafting are widely used in clinical treatment (Erdmann 2017). However, after a short period of interruption of myocardial blood supply, restoring blood supply could cause more severe damage to the original ischemic myocardium, which is known as myocardial ischemia reperfusion injury (MIRI) (Binder et al. 2015; Hausenloy and Yellon 2013). MIRI-induced cardiac insufficiency, arrhythmia, and even sudden death have aroused widespread concern among researchers.
Puerarin is a flavonoid glycoside extracted from the root of the leguminous plant Pueraria lobata and is one of the main active ingredients of Radix Puerariae Lobatae (Zhang 2019). Modern pharmacological research showed that puerarin has the effects of improving blood circulation, antiarrhythmia, reducing blood pressure, lowering blood lipids and blood sugar, protecting nerves, anti-tumor, and anti-oxidation (Zhou et al. 2014). Tan et al. (2017) emphasized that puerarin can overcome vascular insulin resistance in patients with salt-sensitive hypertension and protect the cardiovascular system, thus can be used for blood pressure lowering treatment; Kong et al. (2019) found that puerarin exhibited a time- and dose-dependent distribution in the hippocampus of rats with acute cerebral ischemia, which provided a dose and time basis for puerarin in the treatment of stroke; Liu et al. (2017) demonstrated that puerarin may be a novel drug for breast cancer treatment because it can inhibit the metastasis and adhesion of breast cancer cells by blocking the NF-κB and Erk signaling pathways; Wang et al. (2018) indicated that puerarin promotes cell survival and lowers blood glucose in patients with type 1 diabetes by inhibiting oxidative stress in MIN6 cells. Puerarin has been widely used in the clinical treatment of cardiovascular and cerebrovascular diseases, liver injury, diabetes, and tumor diseases (Zhou et al. 2014).
ANRIL is a long-chain non-coding RNA (LncRNA) transcribed from the INK4B-ARF-INK4A gene cluster on human chromosome 9p21 and is mainly located in the nucleus (Holdt and Teupser 2018). INK4b, INK4a, and ARF are three important tumor suppressor genes encoded in this gene cluster, and ANRIL involved in the epigenetic regulation of the INK4B-ARF-INK4A gene cluster has also been confirmed to be abnormally expressed in a variety of tumors (Guil et al. 2012; Yu et al. 2008). In addition, the polymorphism of the ANRIL gene is also related to the risk of developing atherosclerosis and diabetes (Kong et al. 2018). Many researchers have reported on the mechanisms of ANRIL on interfering with diseases, including the regulation of apoptosis-related genes and vascular endothelial growth factor (VEGF), the regulation of TGF-β/Smad and AMPK/SIRT1 signaling pathways, and specific binding to polycombs (Sun et al. 2018; Thomas et al. 2017; Yap et al. 2010).
Though both puerarin and ANRIL could regulate cardiovascular disease, their regulatory mechanisms during the progression of myocardial ischemia–reperfusion injury remained unclear. Whether a regulatory relationship exists between puerarin and ANRIL has not been reported. This study analyzed the regulatory mechanisms of puerarin and lncRNA ANRIL and the regulatory relationship between them in myocardial ischemia–reperfusion injury, hoping to provide novel therapeutic targets for the treatment of myocardial ischemia–reperfusion injuries.
Materials and methods
Ethics statement
The animal experiments involved in this study were approved by the Committee of Experimental Animals of The First Affiliated Hospital of Henan Science and Technology University (No. 20181031XNK). All operations in animals were conducted in consideration of animal welfare, and the suffering of laboratory animals was minimized. The experiments were performed at The First Affiliated Hospital of Henan Science and Technology University, and the animals were bred under stable conditions of at 22 ± 1 °C in humidity of 55 ± 5% under 12-h light/dark cycle. The housed animals had free access to food and water. Twelve male SD rats weighing 220 ± 5 g and aged 6 weeks were provided by Jiangsu ALF Biotechnology Co., Ltd. (China).
H9C2 cardiomyocyte culture and model construction
H9C2 cells (American Type Culture Collection (ATCC)® CRL-1446 ™, USA) from Rattus norvegicus were cultured in an MCO-5 M/18 M incubator (SANYO, Japan) in complete media containing Dulbecco’s modified Eagle’s medium (DMEM, ATCC® 30–2002 ™, USA) and 10% fetal bovine serum (FBS) (100064-FBS, RWD, China) with 5% CO2 at 37 °C. The well-grown H9C2 cells were then transferred into sugar-free medium (11,966,025, Gibco, USA) to a hypoxia/anaerobic workstation Concept 400 (Ruskinn, UK) for establishing a hypoxia/reoxygenation (H/R) injury cell model and simulate myocardial ischemia–reperfusion (I/R) injury. We set different hypoxia and reoxygenation time for H9C2 cells; specifically, the hypoxia time was set as 1 h. 2 h, 3 h, and 4 h, while the reoxygenation time was set for 3 h; when the hypoxia time was set as 3 h, the reoxygenation time was set as 1 h, 2 h, and 3 h. After reoxygenation, the cell culture medium was replaced with a complete medium. An inverted phase contrast microscope (TS2, Nilkon, Japan) was used to observe the growth of the cell model.
CCK-8
Cardiomyocytes were seeded into a 96-well plate at a density of 2 × 104 cells/well and cultured in an incubator with 5% CO2 (MCO-5 M/18 M, SANYO, Japan) at 37 °C for 24 h. About 10 μL of cell counting kit-8 (CCK-8) solution (Cat. No.: HY-K0301) purchased from MedChemExpress (USA) was carefully added to each well without generation of bubble. Next, the plate was incubated for 24 h under the same conditions as above. The mixture was gently mixed to ensure homogeneous distribution of color. Finally, a microplate reader (PR 3100 TSC, Bio-Rad, USA) was used to measure the absorbance (OD value) at 450 nm.
Puerarin treatment
To examine the protective effect of Puerarin on H9C2 cells injured by I/R, we used Puerarin (P816258, Macklin, China) of gradient concentrations (50 μM, 100 μM, 200 μM, 400 μM, 800 μM) to pretreat H9C2 cardiomyocytes for 24 h. According to the results of CCK-8, cardiomyocytes treated with 200 μM or 400 μM Puerarin for 24 h were selected to be used in subsequent studies.
RNA isolation and qRT-PCR
According to the manufacturer’s instructions, TRIzol ™ Reagent (1 mL, Catalog Number 15596018, Invitrogen, USA) and Trichloromethane (200 μL, GS016, Guangzhou Chemical Regent Factory, China) were employed to separate myocyte suspensions or myocardial tissue homogenate into a transparent RNA-containing water layer, a middle layer, and a red lower organic layer. Next, isopropyl alcohol (500 μL, M23307, MERYER, China) was added to precipitate RNA, and the RNA precipitate was washed with ethanol (75%, 80,176,961, HUSHI, China). The RNA concentration was measured, and then the RNA was reverse-transcribed into cDNA using the Titan One Tube RT-PCR Kit (11939823001, Roche, Swit). Next, diluted cDNA (2 μL/well), DEPC water (6 μL/well), diluted primers (2 μL/well), and PowerUp™ SYBR™ Green Master Mix (10 μL/well, A25780, ThermoFisher, USA) were added to the 96-well plate, which was then placed in the ThermoFisher QuantStudio 3 real-time PCR system under the following conditions: pre-denaturation at 95 °C for 10 min, denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, for a total of 40 cycles. GAPDH and β-actin were internal references, and the 2−ΔΔCT method was adopted to quantify the mRNA expressions of genes (Schmittgen and Livak 2008). Primer sequences were listed as follows: LncRNA ANRIL-F, 5′-TGCTCTATCCGCCAATCAGG-3′, and LncRNA ANRIL-R, 5′-GGGCCTCAGTGGCACATACC-3′; light chain 3B (LC3B)-F, 5′-GTTAAGCCCCTACCAAGGCA-3′, and LC3B-R, 5′-AGGGACTGTTTCCAGGGACT-3′; Beclin-1-F, 5′-GAATGGAGGGGTCTAAGGCG-3′, and Beclin-1-R, 5′-CTTCCTCCTGGCTCTCTCCT-3′; and β-actin-F, 5′-ACCCACACTGTGCCCATCTAC-3′ and β-actin-R, 5′-TCGGTGAGGATCTTCATGAGGTA-3’.
Detection of LDH and MDA
Changes in the contents of lactate dehydrogenase (LDH) and malondialdehyde (MDA) were detected using an LDH activity detection kit (BC0685, Beijing Solarbio Technology Co., Ltd.) and a MDA activity detection kit (BC0025, Beijing Solarbio Technology Co., Ltd.), respectively. After cardiomyocytes (5 × 106 cells) or myocardial tissues (0.1 g) were sonicated, 1 mL of LDH (or MDA) extract was added to sample extracts for testing, according to the instructions of the kits. Finally, a Bio-Rad microplate reader (PR 3100 TSC, USA) was used to measure the OD value, which was used to further calculate the changes in the contents of LDH or MDA. Absorbance at 450 nm, 532 nm, and 600 nm was measured for MDA, while absorbance at 450 nm was measured for LDH.
Cardiomyocyte apoptosis
Cardiomyocyte apoptosis was detected by CytoFLEX flow cytometry (Backman, USA). Cardiomyocytes pre-digested with 0.25% trypsin (T1426, Sigma-Aldrich, USA) for 24 h were made into cell suspensions and fixed with 70% ethanol for 24 h at 4 °C. Then, Annexin V-FITC reagent (5 μL, AP101-100-AVF, MultiSciences, China) and propidium iodide reagent (PI, 10 μL, S19136, Yuanye, China) were used to stain the cells for 20 min. Next, the stained cells were placed in CytoFLEX flow cytometer (BECKMAN COULTER, USA) to analyze the effects of different conditions on H9C2 cell apoptosis. In the graph of flow cytometry, the apoptosis rate is the sum of early (right lower quadrant) and late apoptosis rate (right upper quadrant).
Western blot
The expression level of protein is generally compared and analyzed by Western blot (Hirano 2012). After being sonicated, the proteins of H9C2 cells and rat myocardial tissues were obtained using RIPA buffer (CW2334S, CWBIO, China), and the protein concentrations were determined using the BCA Protein Assay Kit (M052460, MREDA, China). Then the proteins (50 μg) were subject to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred to a methanol-immersed PVDF membrane (3,010,040,001, Roche, Swit). The membrane was then blocked with 5% skimmed milk in an incubation box for 2 h. After the blocking solution was washed off, the membrane was incubated with the following primary antibodies at 4 °C overnight: light chain 3 (LC3)-I (ab48394, 19 kD, 1: 500, Abcam, UK), LC3-II (ab48394, 17 kD, 1: 1000, Abcam, UK), Beclin 1 (ab62557, 52KD, 1: 2000, Abcam, UK), and β-actin (ab8226, 42kD, 1: 5000, Abcam, UK). After the primary antibodies were washed off the next day, the membrane was further incubated with Goat Anti-Rabbit IgG H and L horseradish peroxidase antibodies (ab205718, 1: 5000, Abcam, UK) for 1.5 h. After the incubation, the membrane was covered with Immobilon ECL Ultra Western HRP Substrate (WBULS0500, Millipore, USA) for 1 min, and then GelDoc XR (Bio-rad, USA) and ImageJ v1.8.0 (NIH, Bethesda, USA) were used to analyze the gray value of gene expression. β-actin was used as an internal reference for protein analysis.
Transfection
Transfections of overexpressed LncRNA ANRIL and knockdown of LncRNA ANRIL (siANRIL) were performed using pcDNA ™ 3.1 (+) vector (VT1001, YouBio, China) and pSingle-tTS-shRNA vector (VT2205, YouBio, China), respectively. The target sequence for siANRIL was 5′-TTGGAAAACAAGCGAAATTAAAC-3′. The pre-digested H9C2 cells were transfected with the plasmids using Lipofectamine™ 2000 kit (CAT. NO. 11668-019, Invitrogen, USA), according to the instructions. About 50 μL of Opti-MEM™ I reduced Serum Medium (31,985,070, Gibco, USA) was diluted first with 1 μg of overexpression or knockdown plasmid and then with 3 μL of Lipofectamine™ 2000 reagent for 20 min. The above mixture was then added into the cardiomyocytes and incubated at 37 °C for 24 h. Finally, the transfection efficiency was determined by qRT-PCR.
Animal model of myocardial I/R injury
The method of establishing the myocardial I/R injury animal model and the evaluation criteria were based on previous research reports. The method of ligating the left anterior descending coronary artery (LAD) was employed (Reichert et al. 2017). After 3 days of adaptive breeding, 12 SD rats were randomly divided into 4 groups (3 rats/group) according to body weight as follows: sham operation group (Sham), ischemia reperfusion (I/R) model + saline group (I/R), I/R model + Puerarin group (I/R + Pue), and Puerarin group (Pue). Urantan (5%, CE5151, Coolaber, China) at a dose of 1.2 g/kg was used to anesthetize the rats to minimize the pain. The anesthetized rats were fixed on a console with their chests fully exposed. VentStar small animal ventilator (R415, RWD, China) was used to control the rats’ breathing (the breathing frequency was 70 times/min; the tidal volume was 8 ml/kg, and the breathing ratio was 1: 2) during the operation. The LAD was surrounded and ligated using a 6–0 silk suture slipknot when it was fully exposed. After 30 min, the silk suture was released, and the chest cavities of the experimental rats were closed layer by layer. Next, penicillin G potassium (8 IU/rat, P8010-1MU, Solarbio, China) was intraperitoneally injected into the rats to prevent wound infection. For rats in the Sham and Pue groups, the silk suture simply passed through the coronary arteries without ligation. After a 20-min ischemia in the LAD, rats in the Sham group and the I/R group were injected with saline (50 mg/kg) via tail vein, and rats in the I/R + Pue group and the Pue group were injected with puerarin (50 mg/kg) in the same way. The occurrence of arrhythmia in the rats was continuously monitored from the electrocardiogram (FX-8322, FUKUDA SANGYO, Japan) during the surgery and was also used as the evaluation standard of I/R injury after the experiment. Twenty-four hours after model preparation, all the rats were anesthetized again and heart tissues of the rats were removed with pentobarbital sodium (50 mg/kg, P3761-25G, Sigma-Aldrich, USA).
CK activity
After sonication, the myocardial tissue homogenate was diluted using a rat creatine kinase ELISA kit (MM-20460R2, MEIMIAN, China) according to the instructions. An enzyme-labeled reagent and a chromogenic solution were then added to the samples to be tested at 37 °C for 30 min. After adding a stop solution into the tissues for 10 min, the Bio-Rad microplate reader was used to determine the OD value of the myocardial tissue homogenate of each group at 450 nm.
HE staining
Hematoxylin–eosin (HE) staining was performed for observing the pathological damage of myocardial tissue. The collected heart tissues were fixed in 4% paraformaldehyde, dehydrated by gradient alcohol, embedded into paraffin, and finally cut into sections. The sections were then deparaffinized with xylene and hydrated with gradient alcohol, then stained with hematoxylin (N19007, mlbio, China) for 5 min and dyed with an eosin staining solution (ZLI-9644, ZSGB-BIO, China) for 5 min at room temperature. After another round of dehydration, pathological changes of the myocardial tissues in the stained sections were observed under a TS2 microscope (Nilkon, Japan) at × 200 magnification.
TUNEL staining
The apoptosis of myocardial tissue were analyzed by TdT-mediated dUTP Nick-End Labeling (TUNEL) staining (11,684,817,910, Roche, Swiss). The deparaffinized sections were soaked with Proteinase K working solution in the kit for 20 min. Then, the TUNEL reaction mixture consisted of 50 μL of TdT reagent and 450 μL of dUTP reagent. The sections containing the TUNEL reaction mixture (50 μL) were covered with coverslips and reacted in a dark wet box for 1 h at 37 °C. Next, 50 μL of converter-POD was added dropwise to the sections and reacted in a wet box for 30 min at 37 °C. After that, 50 μL of DAB substrate was added dropwise to the sectioned tissues for 10 min. After rinsing in PBS, the sections were dehydrated with gradient alcohol, cleared with xylene, and sealed with neutral gum. Finally, apoptotic cells in the dried sections were observed under a TS2 microscope at 200 × magnification.
Statistical analysis
Differences between two groups were analyzed by Student’s two-tailed t-test, and differences between more than two groups were analyzed by one-way ANOVA using SPSS 23.0 software (IL, USA). P < 0.05 was considered as statistically significant.
Results
Effect of different hypoxia and reoxygenation time on ANRIL content and cell growth of H/R injury myocardial cell model
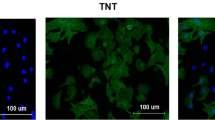
To construct a H/R injury myocardial cell model, we examined the changes in H9C2 cells at hypoxia time of 1 h, 2 h, 3 h, and 4 h and a reoxygenation time of 3 h. As the hypoxia time was prolonged, the mRNA level of ANRIL in cardiomyocytes was downregulated (Fig. 1a; P < 0.05). When the hypoxia time reached 2 h, the cell viability decreased gradually in a time-dependent manner (Fig. 1b; P < 0.01). Different reoxygenation times also had different effects on the apoptosis of cardiomyocytes. As shown in Fig. 1c, the cells in the Control group grew well with fusiform cell morphology and smooth cell membranes. However, as the reoxygenation time reached 3 h, the number of viable cells was gradually decreased, the field of vision was round, and the number of floating apoptotic cells was gradually increased. Based on the above results, we finally determined that 3 h of hypoxia and 3 h of reoxygenation as the preparation conditions for building the H/R injury myocardial cell model in this study.
Effects of hypoxia/reoxygenation (H/R) injury and gradient concentration of puerarin on the expression of LncRNA ANRIL and cell growth in H9C2 cells. a The effects of 1 h, 2 h, 3 h, and 4 h hypoxia with 3 h reoxygenation on ANRIL expression in H9C2 cells were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). GAPDH was an internal reference. b The effects of 1 h, 2 h, 3 h, and 4 h hypoxia with 3 h reoxygenation on the viability of H9C2 cells were detected by Cell Counting Kit-8 (CCK-8). c The effects of 3 h hypoxia and 1 h, 2 h, and 3 h reoxygenation on cell apoptosis were observed with a microscope (magnification, × 200). d The effect of gradient concentrations of puerarin (50 μM, 100 μM, 200 μM, 400 μM, 800 μM) on the viability of cardiomyocytes was detected by CCK-8. e The effect of gradient concentrations of puerarin (50 μM, 100 μM, 200 μM, 400 μM, 800 μM) on the expression of ANRIL in cardiomyocytes was analyzed by qRT-PCR. GAPDHwas an internal reference. f Effect of puerarin and H/R injury on the viability of H9C2 cells. g Effect of puerarin and H/R injury on ANRIL expression in H9C2 cells. All the experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs Control; ##P < 0.01, ###P < 0.001 vs H/R; ^^P < 0.01, ^^^P < 0.001 vs 200 μM of Puerarin (Pue 200); &&P < 0.01 vs 400 μM of Puerarin (Pue 400)
Effect of gradient concentrations of puerarin on cardiomyocyte viability and ANRIL expression
We also analyzed the effects of gradient concentrations of puerarin on cardiomyocytes viability and ANRIL expression. The results showed that after the puerarin concentration reached 100 μM, as the puerarin concentration increased, the expression of ANRIL in cardiomyocytes was significantly upregulated (Fig. 1e; P < 0.01). Moreover, viability of cardiomyocytes was also increased, but the difference was not statistically significant (Fig. 1d). Based on the above results, we finally selected 200 μM and 400 μM puerarin for 24 h as the intervention conditions for subsequent studies.
Puerarin reversed the effects of H/R injury on cardiomyocyte viability, ANRIL expression, contents of LDH and MDA, apoptosis, and expressions of autophagy-related genes
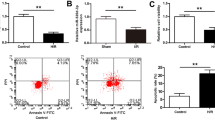
We investigated the effects of puerarin and H/R injury on the basic physiological functions of H9C2 cells and the expressions of ANRIL and autophagy-related genes. The data demonstrated that compared with the Control group, H/R injury significantly inhibited the viability of H9C2 cells (Fig. 1f; P < 0.001) and ANRIL expression (Fig. 1g; P < 0.001), but promoted the contents of LDH (Fig. 2a; P < 0.001) and MDA (Fig. 2b; P < 0.001) and apoptosis (Fig. 2c–d; P < 0.001). In addition, puerarin pretreatment significantly reversed the effect of H/R on the H9C2 cells, including that puerarin pretreatment significantly reduced the increase of apoptosis rate, H/R-caused LDH and MDA, and the decreased viability and ANRIL expression of H/R-stimulated H9C2 cells (Figs. 1f- 2d; P < 0.001). Moreover, the mRNA levels of LC3B and Beclin-1 in H9C2 cells were upregulated after H/R treatment (Fig. 2e; P < 0.01). It was found that H/R injury upregulated the levels of LC3-II and Beclin-1, yet downregulated the level LC3-I, thus increasing the ratio of LC3-II/LC3-I (Fig. 2f–h; P < 0.001).
Puerarin reversed the effects of H/R injury on contents of lactate dehydrogenase (LDH) and malondialdehyde (MDA), apoptosis, and expressions of autophagy-related genes in H9C2 cells. a Effects of puerarin and H/R injury on contents of LDH in H9C2 cells. b Effects of puerarin and H/R injury on contents of MDA in H9C2 cells. c–d Effects of puerarin and H/R injury on H9C2 cell apoptosis. e Effects of puerarin and H/R injury on the mRNA levels of light chain 3B (LC3B) and Beclin-1 in H9C2 cells. β-actin was an internal reference. f–g Effects of puerarin and H/R injury on the protein levels of LC3-I, LC3-II, and Beclin-1 in H9C2 cells. β-actin was an internal reference. h Effects of puerarin and H/R injury on the ratio of LC3-II/LC3-I in H9C2 cells. All the experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs H/R; ^P < 0.05, ^^^P < 0.001 vs Pue 200; &P < 0.05, &&&P < 0.001 vs Pue 400
Puerarin produced a completely opposite effect to H/R injury, specifically, upregulated ANRIL expression and reduced the contents of LDH and MDA and apoptosis (Figs. 1f-2d; P < 0.05). The mRNA level of Beclin-1 and the protein levels of LC3-II and Beclin-1 in cardiomyocytes were downregulated after puerarin treatment (Fig. 2e–g; P < 0.01), while the protein expression of LC3-I was upregulated (Fig. 2f–g; P < 0.001); as a result, the ratio of LC3-II/LC3-I was reduced (Fig. 2h; P < 0.001). However, puerarin significantly affected cardiomyocyte viability and mRNA level of LC3B (Figs. 1f and 2e).
It was found that both puerarin at 200 μM and 400 μM rescued the effects of H/R injury on viability, ANRIL expression, contents of LDH and MDA, apoptosis, and expressions of autophagy-related genes of cardiomyocytes (Figs. 1f–g and 2; P < 0.05).
The effects of overexpressed and knockdown of ANRIL (siANRIL) on ANRIL expression, cell viability, and expressions of autophagy-related genes of cardiomyocytes
To analyze the effect of ANRIL on cardiomyocytes, we transfected overexpressed ANRIL and siANRIL into H9C2 cells, respectively. Figure 3 a showed the transfection efficiency detected by qRT-PCR. It could be found that ANRIL expression was significantly promoted by overexpressed ANRIL, whereas it was downregulated after siANRIL treatment (Fig. 3a; P < 0.001). Compared with the Control group (NC), knocking down ANRIL inhibited H9C2 cell viability, which, however, was promoted by overexpressed ANRIL (Fig. 3b; P < 0.01). The mRNA levels of LC3B and Beclin-1 and the protein levels of LC3-II and Beclin-1 were upregulated in cardiomyocytes after siANRIL treatment (Fig. 3c–e; P < 0.05), whereas the protein expression of LC3-I was downregulated (Fig. 3d–e; P < 0.001), and thus, the ratio of LC3-II/LC3-I was increased (Fig. 3f; P < 0.001). Overexpression of ANRIL produced the opposite effect to knockdown of ANRIL (Fig. 3c–f; P < 0.05).
Effects of overexpressed and knocking down ANRIL on ANRIL expression, cell viability and expressions of autophagy-related genes of H/R injured cardiomyocytes. a The transfection rates of ANRIL overexpressed and knocking down were detected by qRT-PCR. GAPDH was an internal reference. b Effect of overexpression and knockdown of ANRIL on the viability of H9C2 cells. c Effect of overexpressed and knocking down ANRIL on the mRNA levels of LC3B and Beclin-1 in H9C2 cells. β-actin was an internal reference. d–e Effect of overexpressed and knocking down ANRIL on the protein levels of LC3-I, LC3-II, and Beclin-1 in H9C2 cells. β-actin was an internal reference. f Effect of overexpressed and knocking down ANRIL on the ratio of LC3-II/LC3-I in H9C2 cells. All the experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs Negative Control (NC)
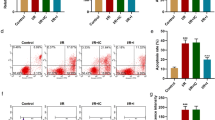
Effect of puerarin and siANRIL on cell viability, ANRIL expression, apoptosis, and expressions of autophagy-related genes in H/R-injured cardiomyocytes
We used 400 μM puerarin and siANRIL to process H/R-injured cardiomyocytes for analyzing the interaction between puerarin and ANRIL in regulating the basic physiological functions of cardiomyocytes and autophagy-related genes. The results of CCK-8 assay, qRT-PCR, and flow cytometry showed that siANRIL inhibited the viability of H/R-injured cardiomyocytes (Fig. 4a; P < 0.01) and ANRIL expression (Fig. 4b; P < 0.001), and significantly promoted H9C2 cell apoptosis (Fig. 4c–d; P < 0.05). The mRNA levels of LC3B and Beclin-1 and the protein levels of LC3-II and Beclin-1 were upregulated in H/R-injured H9C2 cells after siANRIL treatment (Fig. 4e–g; P < 0.05), whereas the protein expression of LC3-Iwas downregulated (Fig. 4f–g; P < 0.001), and thus, the ratio of LC3-II/LC3-I was increased (Fig. 4h; P < 0.001). However, the effect of siANRIL on H/R-injured H9C2 cells was partially reversed by 400 μM puerarin (Fig. 4a–h; P < 0.05).
Effect of puerarin and knockdown ANRIL on H/R-injured cardiomyocytes on cell viability, ANRIL expression, apoptosis, and expressions of autophagy-related genes. a Effect of puerarin and knocking down ANRIL on viability of H/R-injured H9C2 cells. b Effect of puerarin and knocking down ANRIL on ANRIL expression in H/R-injured H9C2 cells. c–d Effect of puerarin and knocking down ANRIL on apoptosis of H/R-injured H9C2 cells. e Effect of puerarin and knocking down ANRIL on the mRNA levels of LC3B and Beclin-1 in H/R-injured H9C2 cells. β-actin was an internal reference. f–g Effect of puerarin and knocking down ANRIL on the protein levels of LC3-I, LC3-II, and Beclin-1 in H/R-injured H9C2 cells. β-actin was an internal reference. h Effect of puerarin and knocking down ANRIL on the ratio of LC3-II/LC3-I in H/R-injured H9C2 cells. All the experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs Negative Control (NC); ^P < 0.05, ^^P < 0.01, ^^^P < 0.001 vs H/R + NC; &P < 0.05, &&P < 0.01, &&&P < 0.001 vs H/R + knockdown ANRIL; ##P < 0.01, ###P < 0.001 vs H/R + Pue 400 + NC
Puerarin reversed the effects of I/R injury on injury index, CK activity, LDH content, ANRIL expression, tissue damage, apoptosis, and expressions of autophagy-related genes in rats
We verified the effect of puerarin by building an I/R injury rat model. The results showed that the injury index, creatine kinase (CK) activity, and LDH content of the rats in the I/R group were significantly increased compared with the Sham group (Fig. 5a–c; P < 0.001), while these indexes were downregulated after puerarin treatment (Fig. 5a–c; P < 0.001). Moreover, the expression of ANRIL in I/R-injured rats was distinctly downregulated, whereas it was upregulated after puerarin treatment (Fig. 5d; P < 0.001). The HE and TUNEL staining results were presented in Fig. 5 e, f. The size and arrangement of cardiomyocytes in the Sham group were normal. In the I/R group, the cardiomyocytescells were swollen and deformed, and abnormally arranged, with a large number of inflammatory cells infiltrated; also, there was a large area of positive expression of apoptosis in the myocardial tissues. After treatment with puerarin, the arrangement of cardiomyocytes in the rats gradually recovered, the number of infiltrated inflammatory cells was significantly reduced, and the area of positive expression of apoptosis in the myocardial tissues was significantly downregulated. The expressions of LC3-II and Beclin-1 were upregulated after I/R injury treatment (Fig. 5g–h; P < 0.001), whereas the protein expression of LC3-I was downregulated (Fig. 5g–h; P < 0.001), and therefore, the ratio of LC3-II/LC3-I was increased (Fig. 5i; P < 0.001). However, puerarin reversed the above changes of gene expressions (Fig. 5g–i; P < 0.001).
The injury index, CK activity, contents of LDH, ANRIL expression, tissue damage, apoptosis, and expressions of autophagy-related genes in rats. a Effect of puerarin on injury index of I/R-injured rats. b Effect of puerarin on CK activity in I/R-injured rats. c Effect of puerarin on contents of LDH in I/R-injured rats. d Effect of puerarin on ANRIL expression in the myocardial tissues of I/R-injured rats. GAPDH was an internal reference. e Pathological changes of myocardial tissue were observed by hematoxylin–eosin (HE) staining (magnification, × 200). f The effect of puerarin on the apoptosis of myocardial tissues in I/R-injured rats was analyzed by TdT-mediated dUTP Nick-End Labeling (TUNEL) staining (magnification, × 200). g–h Effect of puerarin on the expressions of LC3-I, LC3-II, and Beclin-1 in myocardial tissues of I/R-injured rats. β-actin was an internal reference. i Effect of puerarin on the ratio of LC3-II/LC3-I in myocardial tissues of I/R-injured rats. All the experiments were performed in triplicate. **P < 0.01, ***P < 0.001 vs Sham; ^^^P < 0.001 vs I/R; ##P < 0.01, ###P < 0.001 vs Pue
Discussion
Myocardial ischemia–reperfusion injury was proposed by Jennings in 1960 (Jennings et al. 1960). Studies have shown that myocardial ischemia itself is not the main factor causing damage to tissue cells but aggravates the damage caused by recovery of blood reperfusion (Hearse 1977). With the continuous development of blood revascularization technology in clinical treatment, the occurrence of myocardial ischemia–reperfusion injury has gradually increased. How to reduce myocardial ischemia–reperfusion injury and protect myocardial cells to the greatest extent has become the goal of many researchers (Papageorgiou et al. 2018). Establishment of a reliable model of myocardial ischemia–reperfusion injury with the study of myocardial ischemia and hypoxia at the cellular level has become an important direction of studying myocardial ischemia–reperfusion injury in recent years (Taylor and Pouyssegur 2007); at the same time, such a study approach could exclude the effects of systemic nerve-humoral interactions and local different types of cell interactions (Taylor and Pouyssegur 2007). In this study, an I/R injury cardiomyocyte model was used to simulate the in vitro environment of myocardial ischemia–reperfusion injury. According to the effect of different hypoxia and reoxygenation time on the growth status of cardiomyocytes, 3 h of hypoxia and 3 h of reoxygenation were selected as the modeling conditions. A rat model of myocardial ischemia–reperfusion injury had been successfully established in in vivo experiments by classic LAD method (Reichert et al. 2017) to further verify the results of the in vitro experiments.
Our results suggested that puerarin had a significant protective effect on H/R-injured cardiomyocytes. It was found that puerarin promoted cardiomyocyte viability, inhibited cardiomyocyte apoptosis, reduced the contents of LDH and MDA, and reduced myocardial damage through regulating autophagy-related genes and inhibiting cardiomyocyte autophagy. The cardiovascular protective effects of puerarin have been extensively studied. For example, in angiotensin II-induced hypertension model rats, puerarin reduces systolic blood pressure to protect vascular endothelial function (Li et al. 2017); in isoproterenol (ISO)-induced myocardial infarction mice, puerarin inhibits myocardial infarction by regulating the PPAR-Υ/NF-κB pathway (Li et al. 2018); puerarin also exerts the cardioprotective effect through affecting sodium–potassium pump and calcium ion channels to improve myocardial fibers, thereby regulating the PI3K/Akt, NF-κB, and Caspases signaling pathways to reduce cardiovascular damage caused by inflammation, oxidative stress and apoptosis (Wei 2015). Tang et al. (2017) obtained a similar result to our study that puerarin can attenuate I/R injury by inhibiting autophagy in the Akt pathway.
Autophagy is a conservative biological process that degrades cytoplasmic components in lysosomes formed during biological evolution (Lockshin and Zakeri 2004). Under physiological conditions, autophagy repairs cells by degrading intracellular dysfunction and aging organelles to maintain normal life activities of the body. At the same time, excessive or insufficient autophagy can also cause diseases (Lockshin and Zakeri 2004). Beclin1, LC3-I, and LC3-II are important autophagy-related genes (Lockshin and Zakeri 2004). Recent studies have showed that autophagy contributes to the development of I/R. Valentim reported that downregulation of Beclin1 expression in cardiomyocytes reduces I/R-induced autophagy and increases cell survival (Valentim et al. 2006); Gurusamy also found that the expressions of autophagy-specific proteins (LC3-II and Beclin-1) were upregulated in the myocardial tissues of I/R rats (Gurusamy et al. 2009). The results obtained in this study were consistent with those of previous studies. We found that the expressions of LC3-II and Beclin-1 in H/R-injured H9C2 cells were upregulated, whereas the expression of LC3-I was inhibited, indicating that H/R injury promoted autophagy of cardiomyocytes. However, research has reported that autophagy plays a beneficial role in I/R. For example, Hamacher-Brady et al. (2007) found that in I/R cardiomyocytes, enhanced autophagy can reduce Bnip3-mediated cell death; Ma et al. (2015) revealed that activating AMPK can induce cell autophagy and exert cardioprotection against I/R. These findings indicated that autophagy has a dual regulatory effect in I/R, and understanding the clear role of autophagy in diseases offers a more comprehensive view to the development of therapeutic drugs.
ANRIL, which has genetic susceptibility to atherosclerotic diseases such as coronary heart disease and abdominal aortic aneurysm (Helgadottir et al. 2008), can interfere with the development of cardiovascular disease through a variety of mechanisms. In this study, knocking down ANRIL reduced myocardial cell viability and exacerbated H/R injury by promoting autophagy. Zeng et al. (2019) also proved that ANRIL can upregulate beclin-1 expression by regulating miR-99a and miR-449a, and it promotes cell autophagy. This study also explored the interaction of puerarin and ANRIL in H/R-injured cardiomyocytes, and the results indicated that puerarin can upregulate the expression of ANRIL in H/R-injured cardiomyocytes and attenuate the damage caused by knockdown of ANRIL. These findings have not been reported in previous studies.
In summary, puerarin had a protective effect on cardiomyocytes, and reduced myocardial injury caused by myocardial ischemia–reperfusion by up-regulating ANRIL and inhibiting cardiomyocyte autophagy.
References
Binder A, Ali A, Chawla R, Aziz HA, Abbate A, Jovin IS (2015) Myocardial protection from ischemia-reperfusion injury post coronary revascularization Expert review of cardiovascular therapy 13:1045–1057. https://doi.org/10.1586/14779072.2015.1070669
Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS (2014) The epidemic of the 20(th) century: coronary heart disease The American journal of medicine 127:807–812. https://doi.org/10.1016/j.amjmed.2014.04.015
Di Angelantonio E, Thompson A, Wensley F, Danesh J (2011) Coronary heart disease IARC scientific publications:363–386
Erdmann E (2017) [Coronary heart disease: advances in diagnostics and therapy] Deutsche medizinische Wochenschrift (1946) 142:1565. https://doi.org/10.1055/s-0042-112806
Guil S et al (2012) Intronic RNAs mediate EZH2 regulation of epigenetic targets Nature structural & molecular biology 19:664–670. https://doi.org/10.1038/nsmb.2315
Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK (2009) Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein Journal of cellular and molecular medicine 13:373–387. https://doi.org/10.1111/j.1582-4934.2008.00495.x
Hamacher-Brady A et al (2007) Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy Cell death and differentiation 14:146–157. https://doi.org/10.1038/sj.cdd.4401936
Hausenloy DJ, Yellon DM (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Investig 123:92–100. https://doi.org/10.1172/jci62874
Hearse DJ (1977) Reperfusion of the ischemic myocardium. J Mol Cell Cardiol 9:605–616. https://doi.org/10.1016/s0022-2828(77)80357-x
Helgadottir A et al (2008) The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm Nature genetics 40:217–224. https://doi.org/10.1038/ng.72
Hirano S (2012) Western blot analysis Methods in molecular biology (Clifton, NJ) 926:87–97. https://doi.org/10.1007/978-1-62703-002-1_6
Holdt LM, Teupser D (2018) Long noncoding RNA ANRIL: Lnc-ing genetic variation at the chromosome 9p21 Locus to Molecular Mechanisms of Atherosclerosis Frontiers in cardiovascular medicine 5:145. https://doi.org/10.3389/fcvm.2018.00145
Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H (1960) Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 70:68–78
Kong H et al (2019) Distribution kinetics of puerarin in rat hippocampus after acute local cerebral ischemia. J Pharm Biomed Anal 164:196–201. https://doi.org/10.1016/j.jpba.2018.10.038
Kong Y, Hsieh CH, Alonso LC (2018) ANRIL: A lncRNA at the CDKN2A/B locus with roles in cancer and metabolic disease Frontiers in endocrinology 9:405. https://doi.org/10.3389/fendo.2018.00405
Li X, Lin Y, Zhou H, Li Y, Wang A, Wang H, Zhou MS (2017) Puerarin protects against endothelial dysfunction and end-organ damage in Ang II-induced hypertension Clinical and experimental hypertension (New York, NY : 1993) 39:58–64. https://doi.org/10.1080/10641963.2016.1200603
Li X et al (2018) Cardioprotective effects of Puerarin-V on isoproterenol-induced myocardial infarction mice is associated with regulation of PPAR-Upsilon/NF-kappaB pathway molecules (Basel, Switzerland) 23. https://doi.org/10.3390/molecules23123322
Liu X, Zhao W, Wang W, Lin S, Yang L (2017) Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-kappaB and Erk pathway Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 92:429–436. https://doi.org/10.1016/j.biopha.2017.05.102
Lockshin RA, Zakeri Z (2004) Apoptosis, autophagy, and more The international journal of biochemistry & cell biology 36:2405–2419. https://doi.org/10.1016/j.biocel.2004.04.011
Ma S, Wang Y, Chen Y, Cao F (2015) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochem Biophys Acta 1852:271–276. https://doi.org/10.1016/j.bbadis.2014.05.010
Papageorgiou N, Briasoulis A, Tousoulis D (2018) Ischemia-reperfusion injury: complex pathophysiology with elusive treatment Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese 59:329–330. https://doi.org/10.1016/j.hjc.2018.11.002
Reichert K, Colantuono B, McCormack I, Rodrigues F, Pavlov V, Abid MR (2017) Murine left anterior descending (LAD) coronary artery ligation: an improved and simplified model for myocardial infarction Journal of visualized experiments : JoVE https://doi.org/10.3791/55353
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method Nature protocols 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Sriharibabu M (2016) Changing trends in the prevalence of coronary heart disease Indian heart journal 68:445–446. https://doi.org/10.1016/j.ihj.2016.04.008
Sun LY et al (2018) LncRNA ANRIL regulates AML development through modulating the glucose metabolism pathway of AdipoR1/AMPK/SIRT1 Molecular cancer 17:127. https://doi.org/10.1186/s12943-018-0879-9
Tan C, Wang A, Liu C, Li Y, Shi Y, Zhou MS (2017) Puerarin improves vascular insulin resistance and cardiovascular remodeling in salt-sensitive hypertension The American journal of. Chinese medicine 45:1169–1184. https://doi.org/10.1142/s0192415x17500641
Tang H, Song X, Ling Y, Wang X, Yang P, Luo T, Chen A (2017) Puerarin attenuates myocardial hypoxia/reoxygenation injury by inhibiting autophagy via the Akt signaling pathway Molecular medicine reports 15:3747–3754. https://doi.org/10.3892/mmr.2017.6424
Taylor CT, Pouyssegur J (2007) Oxygen, hypoxia, and stress Annals of the New York Academy of Sciences 1113:87–94. https://doi.org/10.1196/annals.1391.004
Thomas AA, Feng B, Chakrabarti S (2017) ANRIL: a regulator of VEGF in diabetic retinopathy Investigative ophthalmology & visual science 58:470–480. https://doi.org/10.1167/iovs.16-20569
Valentim L et al (2006) Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury Journal of molecular and cellular cardiology 40:846–852. https://doi.org/10.1016/j.yjmcc.2006.03.428
Wang T et al (2018) Puerarin promotes MIN6 cell survival by reducing cellular reactive oxygen species Molecular medicine reports 17:7281–7286. https://doi.org/10.3892/mmr.2018.8731
Wei SY (2015) [Progress on cardiovascular protections and mechanism research of puerarin] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 40:2278–2284
Wirtz PH, von Kanel R (2017) Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep 19:111. https://doi.org/10.1007/s11886-017-0919-x
Yap KL et al (2010) Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a Molecular cell 38:662–674. https://doi.org/10.1016/j.molcel.2010.03.021
Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, Cui H (2008) Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA Nature 451:202–206. https://doi.org/10.1038/nature06468
Zeng R, Song XJ, Liu CW, Ye W (2019) LncRNA ANRIL promotes angiogenesis and thrombosis by modulating microRNA-99a and microRNA-449a in the autophagy pathway American journal of translational research 11:7441–7448
Zhang L (2019) Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine Drug delivery 26:860–869. https://doi.org/10.1080/10717544.2019.1660732
Zhou YX, Zhang H, Peng C (2014) Puerarin: a review of pharmacological effects Phytotherapy research : PTR 28:961–975. https://doi.org/10.1002/ptr.5083
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The animal experiments involved in this study were approved by the Committee of Experimental Animals of The First Affiliated Hospital of Henan Science and Technology University (No. 20181031XNK).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, Y., Wang, H., Wang, Y. et al. Puerarin protects cardiomyocytes from ischemia–reperfusion injury by upregulating LncRNA ANRIL and inhibiting autophagy. Cell Tissue Res 385, 739–751 (2021). https://doi.org/10.1007/s00441-021-03463-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03463-2