Abstract
Cigarette smoke is known to be a risk for the development of intrauterine growth restriction (IUGR). Our objective was to assess the effects of secondhand smoke (SHS) during pregnancy and to what extent it regulates the activation of mTOR family members and murine trophoblast invasion. Mice were treated to SHS for 4 days. Placental and fetal weights were recorded at the time of necropsy. Immunohistochemistry was used to determine the level of placental trophoblast invasion. Western blots were utilized to assess the activation of caspase 3, XIAP, mTOR, p70 and 4EBP1 in treated and control placental lysates. As compared to controls, treated animals showed: (1) decreased placental (1.4-fold) and fetal (2.3-fold) weights (p < 0.05); (2) decreased trophoblast invasion; (3) significantly decreased active caspase 3 (1.3-fold; p < 0.02) and increased active XIAP (3.6-fold; p < 0.05) in the placenta; and (4) a significant decrease in the activation of placental mTOR (2.1-fold; p < 0.05), p70 (1.9-fold; p < 0.05) and 4EBP1 (1.3-fold; p < 0.05). Confirmatory in vitro experiments revealed decreased trophoblast invasion when SW71 cells were treated with 0.5 or 1.0 % cigarette smoke extract (CSE). Similar to primary smoking, SHS may induce IUGR via decreased activation of the mTOR family of proteins in the placenta. Increased activation of the placental XIAP protein could be a survival mechanism for abnormal trophoblast cells during SHS exposure. Further, CSE reduced trophoblast invasion, suggesting a direct causative effect of smoke on susceptible trophoblast cells involved in IUGR progression. These results provide important insight into the physiological consequences of SHS exposure and smoke-mediated placental disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrauterine growth restriction (IUGR) is a significant complication of pregnancy that affects up to 10 % of all pregnancies and significantly increases risks of fetal and neonatal morbidity and mortality (Brar and Rutherford 1988; Gray et al. 1999; Pollack and Divon 1992). Complications observed in IUGR patients include perinatal hypoxia and asphyxia, cerebral palsy and persistent pulmonary hypertension of the newborn (Galan 2011; Galan et al. 2005; Jacobsson and Hagberg 2004; Rosenberg 2008). In addition, several studies have reported a long-term sequelae of IUGR complications, including adult hypertension, heart disease, stroke and diabetes (Barker 1993; Barker et al. 1993; Holemans et al. 1993; Phipps et al. 1993; Reusens-Billen et al. 1989). Placentae from growth-restricted pregnancies are characterized by a number of pathologic findings such as reduced syncytiotrophoblast surface area, decreased trophoblast invasion possibly due to elevated apoptosis and increased mTOR protein (Hung et al. 2002; Ishihara et al. 2002; Levy and Nelson 2000; Mayhew et al. 2003; Smith et al. 1997).
Cigarette smoking during pregnancy is associated with a number of serious obstetric complications including increased rates of spontaneous abortion, premature delivery and IUGR (Arffin et al. 2012). In fact, research suggests that prenatal nicotine exposure could affect the fetal central nervous system, brain development and increase infant mortality rates (Guan et al. 2009; Wahabi et al. 2013). Exogenous antenatal exposure is also associated with the adult onset of diabetes and hypertension (Wahabi et al. 2013). In contrast to studies of direct smoking during pregnancy, studies detailing effects of secondhand smoke (SHS) during pregnancy are limited. Currently, there is conflicting information about the induction of IUGR by SHS. A correlation was demonstrated between SHS and the induction of IUGR in a recent study conducted by Whabi et al. (2013). Linkages between IUGR and tobacco exposure likely exist despite earlier studies that did not detect a correlation between IUGR and pregnant women exposed to smoke (Subramoney et al. 2010; Wahabi et al. 2013).
While the exact correlation between SHS and IUGR remains to be determined, research has revealed that many harmful constituents found in SHS readily exchange between the mother and the developing fetus. Nicotine’s ability to cross the placenta is a well-known phenomenon, as is the induction of localized hypoxia in fetuses exposed to tobacco smoke (Dempsey and Benowitz 2001). In the sheep model, studies showed that infusion of low-dosage nicotine leads to fetal hypoxia and elevated fetal blood pressure without affecting maternal blood gases or cardiovascular systems (Guan et al. 2009). Studies further suggested that SHS significantly increases the risk for spontaneous abortion, preterm delivery, sudden infant death syndrome (SIDS) and still birth (Dempsey and Benowitz 2001; Khader et al. 2011; Subramoney et al. 2010). Commonality exists such that these studies and others suggest a vulnerability of the fetus to the effects of tobacco smoke.
The mammalian target of rapamycin (mTOR) protein is a phosphatidylinositol kinase-regulated protein kinase that regulates cell growth in response to nutritive insults and growth factors (Blume-Jensen and Hunter 2001; Jansson et al. 2006; Volarevic and Thomas 2001; Wullschleger et al. 2006). Downstream effectors in the mTOR pathway include the 70-kDa ribosomal protein S6kinase 1 (p70S6K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1; Roos et al. 2007; Volarevic and Thomas 2001). Activation through the phosphorylation of these intermediate proteins by mTOR is known to regulate translation initiation and protein synthesis of numerous targets (Blume-Jensen and Hunter 2001; Patti et al. 1998). Regulation of mTOR and p70S6K activity is mediated by phosphatidylinositol-3 kinase/AKT signaling (Chung et al. 1997; Ming et al. 1994; Wen et al. 2005). Under physiologic conditions, the activation of p70S6K is also mediated by the induction of the extracellular regulated kinase (ERK) pathway (Eguchi et al. 1999; Iijima et al. 2002). In the human placenta, mTOR has been localized to syncytiotrophoblast cells, suggesting a role for this protein in nutrient sensing during pregnancy (Aiko et al. 2002).
Studies have shown that total mTOR protein in the placenta is increased in human IUGR, whereas placental phospho (p)-p70S6K protein is down-regulated during the progression of IUGR. Despite a clear necessity for further research, these data suggest diverse mechanistic effects potentially required for the regulation of target proteins during IUGR.
An increase of placental apoptosis is observed during IUGR (Ishihara et al. 2002). The X-linked inhibitor of apoptosis protein (XIAP) is a factor that knocks down proteins that regulate cell death (Holcik and Korneluk 2001; Li et al. 2000; Suzuki et al. 2001). This protein is present in trophoblast throughout placental development but its expression is significantly decreased near term when apoptosis is maximal highlighting a role for this protein in the temporal regulation of trophoblast apoptosis (Gruslin et al. 2001). Abrogation of apoptosis via XIAP signaling relies on the inhibition of caspases 3, 7 and 9 (Suzuki et al. 2001). Thus, controlling the activity of pro-apoptotic caspases appears to be essential for precisely balancing cell death and survival. Studies have recently shown that XIAP expression is decreased near term during hyperthermia-induced IUGR suggesting elevated apoptosis is likely to occur through the mediation of XIAP (Arroyo et al. 2008). However, the expression profile of XIAP in both the placenta and mesometrial compartment during IUGR has yet to be elucidated.
This study sought to better understand the effects of SHS during hemochorial placentation. Utilization of the murine model provided an essential tool to better evaluate trophoblast biology in the uterine mesometrial compartment, the potential impact of mTOR signaling and apoptotic profiles in the context of SHS exposure.
Materials and methods
Animals and tissue preparation
Animal use was approved by the Institutional Animal Care and Use Committee at Brigham Young University. C57 Black 6 (C57BL/6) mice were purchased from Charles River Laboratories, Wilmington, MA, USA. To obtain timed pregnancies, females were caged with males overnight. Placentae and mesometrial compartments were dissected from pregnant mice at 18.5 days of gestation (dGA). Placentae and fetuses were weighed and tissues were snap frozen in liquid nitrogen for protein analysis. Whole conceptuses were frozen in dry ice-cooled heptane for immunohistochemistry analysis. All tissue samples were stored at −80 °C until used.
Secondhand smoke treatment (SHS)
The generation of the IUGR pregnancy occurred following exposure of pregnant mice (n = 7) to SHS conditions as previously shown by Winden et al. (W2014). Pregnant mice were placed in the nose-only Scireq InExpose cigarette-smoking robot starting at day 14.5 dGA and exposed for 4 days with necropsy at 18.5 dGA. To induce IUGR, pregnant mice were placed in soft restraints and connected to an exposure tower, wherein a computer-controlled puff of smoke generated every minute resulted in 10 s of SHS exposure (from six cigarettes; 2R1; University of Kentucky, Lexington, KY, USA) followed by 50 s of room air (fresh air) daily to induce IUGR. This procedure was done daily for 10 min during the time of treatment. Control animals (n = 7) were placed in soft restraints and exposed only to room air daily for 10 min.
Immunofluorescence (IF)
Immunofluorescence (IF) was performed on frozen whole conceptus sections. In summary, slides were washed in a 1× Tris buffer solution (TBS) and blocked with Background Sniper (Biocare Medical, Concord, CA, USA) for 1 h. This was followed by incubation overnight with a primary antibody for Cytokeratin 7 (Dako, Carpinteria, CA, USA). Slides were then incubated for 1 h with donkey anti-mouse TR (Biocare Medical). 4’,6-diamidino-2-phenylindole, dihydrochloride (DAPI) was used for counterstaining prior to mounting with glass coverslips. Slides were viewed using a Texas Red excitation and emission filter.
Western blot analysis
Western blot analysis was used to determine expression levels of active caspase 3, active XIAP and the mTOR family of proteins in the placenta of control and SHS animals as previously described (Arroyo et al. 2009). Cell lysates (50 μg) were separated on 4–12 % Bis-Tris gels and transferred to nitrocellulose membranes. Membranes were incubated with antibodies against cleaved caspase 3, phospho-XIAP, phospho-mTOR (Ser2448), total mTOR, phospho-p70 S6 kinase (SK6) (Thr389), total p70SK6, phospho-4EBP1 (Thr37/46), and total 4EBP1 (all from Cell Signaling Technology, Danvers, MA, USA; excluding total p70 from Epitomics, Burlingame, CA, USA and phospho-XIAP from Abcam, Cambridge, MA, USA). Membranes were then incubated with a secondary horseradish peroxidase (HRP)-conjugated antibody for 1 h at room temperature. The membranes were incubated with ECL substrate and the emission of light was detected using x-ray film. To determine loading consistencies, each membrane was stripped and reprobed with an antibody against mouse β actin (Sigma Aldrich, St. Louis, MO, USA). Expression levels of the proteins were quantified by densitometry normalized to β actin expression and changes in expression were reported by comparing to the untreated controls.
Invasive trophoblast cell culture and cigarette smoke extract (CSE)
A first-trimester cytotrophoblast cell line Sawn71 (SW71; n = 9) was used for invasive cytotrophoblast studies. SW71cells were maintained and cultured in RPMI medium (Mediatech, Manassas, VA, USA). Cell medium was supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin and streptomycin. CSE was generated as previously described by Reynolds et al. (2006). The solution was generated as follows: two 2RF4 research cigarettes (University of Kentucky) were continuously smoked with a vacuum pump into 20 ml of RPMI medium (Mediatech). The smoke-bubbled medium was filtered through a 0.22-μm filter to remove large particles. The resulting medium was defined as 100 % CSE. The total particulate matter content of 2RF4 cigarettes is 11.7 mg/cigarette, tar 9.7 mg/cigarette and nicotine 0.85 mg/cigarette. Dilutions were made using DMEM medium to a concentration of 10 % CSE.
Cell treatments
SW71 cells were detached and 20,000 cells/ml were incubated in 2 % FBS medium alone or medium supplemented with 0.5 %, or 1.0 % CSE for 24 h (Reynolds et al. 2006, 2008). Real time cell invasion was determined following these treatments.
Real time cell invasion determination
An xCELLigence was utilized to determine real time invasion of trophoblast cells (Keogh 2010; Rahim and Uren 2011). Invasion was assessed in 16-well CIM-Plates (n = 9) composed of an upper and lower chamber, each containing 16 wells. The top wells were coated with a 1:40 matrigel concentration and incubated for 4 h. Trophoblast cells were plated in the top chamber at a concentration of 20,000 cells/well in 2 % FBS RPMI in a total volume of 100 μL in the presence or absence of CSE (0.5 or 1.0 %). The bottom chamber wells were filled with 160 μL of 10 % FBS RPMI. The cells were then placed in the RTCA DP instrument and invasion readings were done every 15 min for 5 h.
Statistical analysis
Results were checked for normality and data were shown as means ± SE. Mann–Whitney tests were used to compare weights, protein expression and invasion indexes. Significant differences between groups were noted at p < 0.05.
Results
Fetal and placental weights
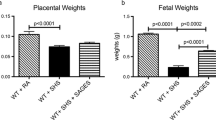
Decreased fetal and placental weights are characteristics of IUGR. We initially investigated the effects of SHS on placental and fetal weights after SHS treatment of near-term mice (18 days of gestation; dGA) for 4 days. There was a significant 1.4-fold reduction in placental weight (p < 0.0003) and a 2.3-fold reduction in fetal weight (p < 0.0002) in SHS exposed mice compared to room air controls (Fig. 1a, b). These data implicate SHS in fetal and placental weight deviations coincident with IUGR.
Trophoblast invasion and apoptosis
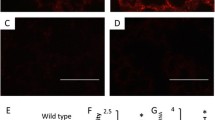
We next investigated trophoblast invasion and placental apoptosis following antenatal SHS treatment. IF for cytokeratin 7 (CK7) was utilized to localize trophoblast cells within the mesometrial compartment of pregnant mice. There was decreased invasion of trophoblast cells into the mesometrial compartment in the SHS treated animals (Fig. 2b) when compared to controls (Fig. 2a). The no-primary negative staining control is also presented in Fig. 2c. Immunoblotting for active caspase 3 was next performed in order to determine apoptosis in the placenta. We found a 1.3-fold (p < 0.02) decrease in placental active caspase 3 in SHS animals when compared to controls (Fig. 2d). In contrast, we observed a significant increase (3.6-fold; p < 0.05) in phospho (active) XIAP, an inhibitor of caspase 3 activation (Fig. 2e). Our results suggest that SHS is likely involved in decreased trophoblast invasion and elevated XIAP-mediated apoptosis observed in this model of IUGR.
Trophoblast invasion and apoptosis during SHS induces IUGR. a–c CK7 IHC showed decreased trophoblast invasion into the uterine mesometrial compartment of treated animals (a) as compared to controls (b). Samples were also compared to the negative control (c). d Active caspase 3 was increased (1.3-fold; p < 0.02) with SHS in the placenta of treated animals as compared to controls. e Phospho-XIAP was significantly increased (3.6-fold; p < 0.05) in the placenta of SHS-treated animal as compared to controls
MTOR family of proteins in the placenta
The mTOR family of proteins has been shown to regulate cell growth during altered availability of nutrients (Roos et al. 2007). Previous results revealed increased placental mTOR expression during IUGR (Knuth et al. 2014). Accordingly, we next investigated the expression of the active mTOR family of proteins in the placenta in the context of SHS exposure. Placental expression of active mTOR (2.1-fold; p < 0.05), active p70 (1.9-fold; pp0.05) and active 4EBP1 (1.3-fold; p < 0.05) were significantly decreased in SHS exposed animals compared to controls (Fig. 3a–c). These data suggested a correlation exists between SHS-induced IUGR and decreased activation of placental mTOR family members.
Trophoblast invasion and CSE
Nicotine is known to be a risk factor for IUGR (Detmar et al. 2008). A hallmark of IUGR is decreased trophoblast invasion (Arroyo and Winn 2008). Published reports have already demonstrated enhanced cell invasion in both cancer and trophoblast cells following CSE exposure (Dasgupta et al. 2009; Kraus et al. 2014). We therefore wanted to quantitatively determine the direct effects of CSE on trophoblast invasion in culture. As expected, trophoblast invasion was significantly decreased when cells were treated with 0.5 % (5.9-fold; p < 0.02), or 1.0 %(6.0-fold; p < 0.02) CSE when compared to cells incubated in fresh media alone (Fig. 4).
Discussion
IGUR remains one of the leading causes of fetal mortality. Research in the recent past has focused on diverse factors including maternal exposure to primary tobacco smoke; however, very little is known in relation to the effects of secondhand smoke during pregnancy. Pointing to the robustness of involuntary smoke exposure, we discovered that a secondhand smoke model of IUGR in the mouse resulted in significant reductions in both fetal and placental weights that coincided with decreased trophoblast invasion. These data were supported by recent work by Vivarigou et al. that highlighted birth size deviations while the number of conceptuses were not affected (Mund et al. 2013; Varvarigou et al. 2009). In fact, our work involving secondhand smoke exposure suggests that, like primary smoking, exposure to SHS is also capable of inducing IUGR (Esposito et al. 2008).
An interesting discovery related to the decreased activation of the pro-apoptotic molecule caspase 3, a finding that potentially sheds light on a plausible mechanistic driver of the IUGR phenotypes we observed. Increased activity of caspase 3 was expected as activation of placental caspase 3 has already been shown to be involved in the development of IUGR (Arroyo et al. 2008, 2010; Kimball et al. 2015). Further, XIAP protein was also augmented in our exposed animals, which was also anticipated due to its effects in the regulation of caspase 3. Reports have shown that XIAP protein is differentially regulated in diverse models of placental disease including hypoxia and exposure to polycyclic aromatic hydrocarbons (Detmar et al. 2008; Jeon et al. 2013). Even more interesting is the recent discovery that nicotine treatment of cultured cells confers protection from excessive apoptosis, suggesting a complex mechanism of cellular apoptosis downstream of the addictive nicotine and the wide array of other noxious agents that destroy tissue integrity (Dasgupta et al. 2006). In the current model of IUGR, we observed increased XIAP protein in the placenta of SHS-treated animals when compared to controls. This suggested that perhaps increased XIAP is involved in the negative modulation of active caspase 3, potentially as a trophoblast survival mechanism during treatment with SHS.
Decreased nutrient availability and diminished expression of mTOR family members in the placenta are observed during the development of IUGR (Arroyo et al. 2009; Jansson et al. 2006, 2012; Knuth et al. 2014; Roos et al. 2007, 2009; Ross et al. 1996). In the current report, SHS decreased the activation of mTOR protein and the secondary messengers p70 and 4EBP1. This suggests that mTOR signaling may be a common pathway, among others, that drives the nutritional aspect of the IUGR phenotype. Recently published work by our laboratory demonstrated a role for mTOR in trophoblast invasion (Knuth et al. 2014). Since SHS caused decreased trophoblast invasion and decreased mTOR activity in vivo, confirmatory studies in vitro were undertaken in order to dissect the invasive properties of trophoblast cells in culture. We observed that trophoblasts treated with CSE had decreased invasion in culture. These data implicated tobacco smoke in the control of trophoblast invasion and confirmed the work of others that demonstrated CSE pathways in the orchestration of pre-eclampsia and fetal growth restriction (Ahmed 2014). Additional work in the near future should aim to test the hypothesis that mechanistic control of decreased invasion is due in part to decreased mTOR activation during SHS treatment.
SHS exposure is a very common occurrence but the consequences of SHS during pregnancy are not well established. In fact, this is the first report that establishes an in vivo correlation between SHS and the mTOR pathway as a mechanism for the development of IUGR disease. Accordingly, these data clarify the need to discern mTOR-mediated placental abnormalities that likely confer complications that may reemerge in adulthood (Arroyo and Winn 2008). For instance, alterations in the expression and activity of nutrient transporters in affected placentae may not only restrict fetal growth but compromise other mTOR regulated processes including post natal growth, maturation and aging (Arroyo et al. 2009; Roos et al. 2007, 2009; Ross et al. 1996). As cigarette smoke exposure correlates with the development of IUGR, a more robust evaluation of mTOR and its temporal effects during gestation and after birth is necessary (Milnerowicz-Nabzdyk and Bizon 2014; Triche and Hossain 2007). For instance, Milnerowicz-Nabzdyk and Bizon (2014) further speculated that different chemical fractions of smoke may be more centrally involved in the development of general IUGR as compared to idiopathic IUGR. This phenomenon may explain why differences in mTOR activation are observed in the various models of IUGR present in the literature today (Arroyo et al. 2009; Kimball et al. 2015; Roos et al. 2007, 2009; Ross et al. 1996). In the end, future studies that investigate downstream signaling molecules associated with decreased mTOR and those that seek to elucidate mechanisms of decreased invasion during SHS-induced IUGR are critically necessary. These undertakings are of vital importance, as they should provide new therapeutic avenues that could help in the alleviation of IUGR symptoms and thus improve fetal health.
References
Ahmed A (2014) Molecular mechanisms and therapeutic implications of the carbon monoxide/hmox1 and the hydrogen sulfide/CSE pathways in the prevention of pre-eclampsia and fetal growth restriction. Pregnancy Hypertension 4:243–244
Aiko K, Tsujisawa T, Koseki T, Hashimoto S, Morimoto Y, Amagasa T, Nishihara T (2002) Involvement of cytochrome c and caspases in apoptotic cell death of human submandibular gland ductal cells induced by concanamycin A. Cell Signal 14:717–722
Arffin F, Al-Bayaty FH, Hassan J (2012) Environmental tobacco smoke and stress as risk factors for miscarriage and preterm births. Arch Gynecol Obstet 286:1187–1191
Arroyo JA, Winn VD (2008) Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol 32:172–177
Arroyo JA, Anthony RV, Galan HL (2008) Decreased placental X-linked inhibitor of apoptosis protein in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 199:80–88
Arroyo JA, Brown LD, Galan HL (2009) Placental mammalian target of rapamycin and related signaling pathways in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 201(616):e611–e617
Arroyo JA, Li C, Schlabritz-Loutsevitch N, McDonald T, Nathanielsz P, Galan HL (2010) Increased placental XIAP and caspase 3 is associated with increased placental apoptosis in a baboon model of maternal nutrient reduction. Am J Obstet Gynecol 203(364):e313–e368
Barker DJ (1993) Fetal origins of coronary heart disease. Br Heart J 69:195–196
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet 341:938–941
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411:355–365
Brar HS, Rutherford SE (1988) Classification of intrauterine growth retardation. Semin Perinatol 12:2–10
Chung T, Crilly KS, Anderson WH, Mukherjee JJ, Kiss Z (1997) ATP-dependent choline phosphate-induced mitogenesis in fibroblasts involves activation of pp 70 S6 kinase and phosphatidylinositol 3'-kinase through an extracellular site. Synergistic mitogenic effects of choline phosphate and sphingosine 1-phosphate. J Biol Chem 272:3064–3072
Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S (2006) Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A 103:6332–6337
Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, Haura E, Chellappan S (2009) Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer 124:36–45
Dempsey DA, Benowitz NL (2001) Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf 24:277–322
Detmar J, Rennie MY, Whiteley KJ, Qu D, Taniuchi Y, Shang X, Casper RF, Adamson SL, Sled JG, Jurisicova A (2008) Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. Am J Physiol Endocrinol Metab 295:E519–E530
Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T (1999) Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem 274:36843–36851
Esposito ER, Horn KH, Greene RM, Pisano MM (2008) An animal model of cigarette smoke-induced in utero growth retardation. Toxicology 246:193–202
Galan HL (2011) Timing delivery of the growth-restricted fetus. Semin Perinatol 35:262–269
Galan HL, Anthony RV, Rigano S, Parker TA, de Vrijer B, Ferrazzi E, Wilkening RB, Regnault TR (2005) Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 192:272–279
Gray PH, O’Callaghan MJ, Harvey JM, Burke CJ, Payton DJ (1999) Placental pathology and neurodevelopment of the infant with intrauterine growth restriction. Dev Med Child Neurol 41:16–20
Gruslin A, Qiu Q, Tsang BK (2001) X-linked inhibitor of apoptosis protein expression and the regulation of apoptosis during human placental development. Biol Reprod 64:1264–1272
Guan J, Mao C, Xu F, Zhu L, Liu Y, Geng C, Zhang L, Xu Z (2009) Low doses of nicotine-induced fetal cardiovascular responses, hypoxia, and brain cellular activation in ovine fetuses. Neurotoxicology 30:290–297
Holcik M, Korneluk RG (2001) XIAP, the guardian angel. Nat Rev Mol Cell Biol 2:550–556
Holemans K, Van BR, Verhaeghe J, Aerts L, Van Assche FA (1993) In vivo glucose utilization by individual tissues in virgin and pregnant offspring of severely diabetic rats. Diabetes 42:530–536
Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ (2002) Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 90:1274–1281
Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, McDermott PJ, Kuppuswamy D (2002) c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem 277:23065–23075
Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T (2002) Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 186:158–166
Jacobsson B, Hagberg G (2004) Antenatal risk factors for cerebral palsy. Best Pract Res Clin Obstet Gynaecol 18:425–436
Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T (2006) Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 576:935–946
Jansson T, Aye IL, Goberdhan DC (2012) The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 33(Suppl 2):e23–e29
Jeon SY, Lee HJ, Na KH, Cha DH, Kim JK, Park JW, Yoon TK, Kim GJ (2013) Hypoxia-induced downregulation of XIAP in trophoblasts mediates apoptosis via interaction with IMUP-2: implications for placental development during pre-eclampsia. J Cell Biochem 114:89–98
Keogh RJ (2010) New technology for investigating trophoblast function. Placenta 31:347–350
Khader YS, Al-Akour N, Alzubi IM, Lataifeh I (2011) The association between second hand smoke and low birth weight and preterm delivery. Matern Child Health J 15:453–459
Kimball R, Wayment M, Merrill D, Wahlquist T, Reynolds PR, Arroyo JA (2015) Hypoxia reduces placental mTOR activation in a hypoxia-induced model of intrauterine growth restriction (IUGR). Physiol Rep 3(12)
Knuth A, Liu L, Nielsen H, Merril D, Torry DS, Arroyo JA (2014) Placenta Growth Factor Induces Invasion and Activates p70 during Rapamycin Treatment in Trophoblast Cells. Am J Reprod Immunol 73(4):330–340
Kraus DM, Feng L, Heine RP, Brown HL, Caron KM, Murtha AP, Grotegut CA (2014) Cigarette smoke-induced placental adrenomedullin expression and trophoblast cell invasion. Reprod Sci 21:63–71
Levy R, Nelson DM (2000) To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta 21:1–13
Li J, Sasaki H, Sheng YL, Schneiderman D, Xiao CW, Kotsuji F, Tsang BK (2000) Apoptosis and chemoresistance in human ovarian cancer: is Xiap a determinant? Biol Signals Recept 9:122–130
Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS (2003) Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta 24:219–226
Milnerowicz-Nabzdyk E, Bizon A (2014) Effect of cigarette smoking on vascular flows in pregnancies complicated by intrauterine growth restriction. Reprod Toxicol 50:27–35
Ming XF, Burgering BM, Wennstrom S, Claesson-Welsh L, Heldin CH, Bos JL, Kozma SC, Thomas G (1994) Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature 371:426–429
Mund M, Louwen F, Klingelhoefer D, Gerber A (2013) Smoking and pregnancy--a review on the first major environmental risk factor of the unborn. Int J Environ Res Public Health 10:6485–6499
Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR (1998) Bidirectional modulation of insulin action by amino acids. J Clin Investig 101:1519–1529
Phipps K, Barker DJ, Hales CN, Fall CH, Osmond C, Clark PM (1993) Fetal growth and impaired glucose tolerance in men and women. Diabetologia 36:225–228
Pollack RN, Divon MY (1992) Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol 35:99–107
Rahim S, Uren A (2011) A real-time electrical impedance based technique to measure invasion of endothelial cell monolayer by cancer cells. J Vis Exp Apr 1;(50)
Reusens-Billen B, Remacle C, Hoet JJ (1989) The development of the fetal rat intestine and its reaction to maternal diabetes. II. Effect of mild and severe maternal diabetes. Diabetes Res Clin Pract 6:213–219
Reynolds PR, Cosio MG, Hoidal JR (2006) Cigarette smoke-induced Egr-1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am J Respir Cell Mol Biol 35:314–319
Reynolds PR, Kasteler SD, Cosio MG, Sturrock A, Huecksteadt T, Hoidal JR (2008) RAGE: developmental expression and positive feedback regulation by Egr-1 during cigarette smoke exposure in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 294:L1094–L1101
Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T (2007) Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582:449–459
Roos S, Powell TL, Jansson T (2009) Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans 37:295–298
Rosenberg A (2008) The IUGR newborn. Semin Perinatol 32:219–224
Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G (1996) Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol 270:E491–E503
Smith SC, Baker PN, Symonds EM (1997) Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol 177:1395–1401
Subramoney S, d’Espaignet ET, Gupta PC (2010) Higher risk of stillbirth among lower and middle income women who do not use tobacco, but live with smokers. Acta Obstet Gynecol Scand 89:572–577
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R (2001) X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and −7 in distinct modes. J Biol Chem 276:27058–27063
Triche EW, Hossain N (2007) Environmental factors implicated in the causation of adverse pregnancy outcome. Semin Perinatol 31:240–242
Varvarigou AA, Asimakopoulou A, Beratis NG (2009) Impact of maternal smoking on birth size: effect of parity and sex dimorphism. Neonatology 95:61–67
Volarevic S, Thomas G (2001) Role of S6 phosphorylation and S6 kinase in cell growth. Prog Nucleic Acid Res Mol Biol 65:101–127
Wahabi HA, Alzeidan RA, Fayed AA, Mandil A, Al-Shaikh G, Esmaeil SA (2013) Effects of secondhand smoke on the birth weight of term infants and the demographic profile of Saudi exposed women. BMC Public Health 13:341
Wen HY, Abbasi S, Kellems RE, Xia Y (2005) mTOR: a placental growth signaling sensor. Placenta 26 Suppl A:S63-S69
Winden DR, Barton DB, Betteridge BC, Bodine JS, Jones CM, Rogers GD, Chavarria M, Wright AJ, Jergensen ZR, Jimenez FR, Reynolds PR (2014) Antenatal exposure of maternal secondhand smoke (SHS) increases fetal lung expression of RAGE and induces RAGE-mediated pulmonary inflammation. Respir Res 15:129
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124:471–484
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported in part by grant 7R00HD055054-06
Conflict of interest
The authors report no conflict of interest.
Rights and permissions
About this article
Cite this article
Mejia, C., Lewis, J., Jordan, C. et al. Decreased activation of placental mTOR family members is associated with the induction of intrauterine growth restriction by secondhand smoke in the mouse. Cell Tissue Res 367, 387–395 (2017). https://doi.org/10.1007/s00441-016-2496-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2496-5