Abstract
In the context of cell-based oral hard tissue regeneration, especially assumed plasticity of oral host tissue cells in response to human mesenchymal stem cells (hMSCs), is poorly understood. To investigate this area, we assess osteogenic features in various oral cell types during hMSC coculture, including human alveolar osteoblasts (hOAs), periodontal ligament cells (hPDLs) and gingival fibroblasts (hGFs). Interactive hMSC coculture globally enhanced the transcription of osteogenic genes, in all oral cell types under study, as revealed by qRT-PCR and did not affect oral cell proliferation compared with controls in a transwell coculture system as evaluated by 5-bromo-2′-deoxyuridine proliferation assay. 3D gel-derived hMSC cocultures exhibited an abundance of bone-related key molecules in oral cells, which followed the ranking hOAs > hGFs > hPDLs. Compared to matched controls, this hierarchy also applied for the presence of higher amounts of extracellular matrix deposits and mineralization nodules in interactive hMSC coculture. Our results show for the first time that in the context of prospective periodontal tissue regeneration strategies, hMSCs influence oral cells by gradually shaping their plasticity, particularly features associated with an osteogenic phenotype. These novel findings contribute another piece to the conceptual hMSC action puzzle and valuably support the notion that hMSCs trigger osteogenesis in the oral cell context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the context of the currently intense and interdisciplinary search for regeneration strategies that aim at restoring damaged or diseased tissues, human bone marrow-derived mesenchymal stem cells (hMSCs) appear to be promising aspirants for oral periodontal tissue regeneration (Kawaguchi et al. 2004; Li et al. 2009; Sanchez-Lara et al. 2012). Periodontal regeneration has enormous clinical significance, because periodontitis, which manifests as inflammation of the tooth holding apparatus, is widely regarded as the second most common disease worldwide and the most common cause for tooth loss in adults. Due to their inherent mesenchymal nature, hMSCs are attractive candidates for regenerating a truly functional periodontium comprising hard and soft tissue elements, i.e., bone, periodontal ligament and gingival connective tissue. Bone preservation is of particular importance because the presence of an ample bone socket is crucial for tooth anchorage. In this respect, the hMSCs’ provenance from the bone marrow site makes them a perfect match for bone regeneration purposes (Jones and Yang 2011). Nevertheless, although encouraging findings are accumulating, MSCs are reported to rapidly undergo apoptosis (Kelm et al. 2012) and to show low engraftment rates in vivo (Prockop 2009). However, new findings suggest that MSCs contribute more significantly to tissue repair by actions different from mere replacement of the damaged cells of the recipient (Phinney and Prockop 2007; Uccelli et al. 2011). In this respect, the concept that multiple hMSC-induced processes including trophic effects, angiogenesis and immunosuppressive actions (Barry and Murphy 2004) are responsible for tissue regeneration becomes increasingly important (Si et al. 2011). This variety of hMSC effects on immune and blood vessel cells strongly suggests that hMSCs may similarly affect further host tissue cells. However, minimal attention has so far been paid to the effects that MSCs have on the residing site-specific parenchyma cells of potential recipient host tissues, particularly in the context of oral tissues which play an exceptional role due to their unique developmental origin as ectomesenchymal derivatives (Chai et al. 2000; Zhang et al. 2012). During periodontal tissue regeneration, these cells come in contact and will interact with therapeutically applied MSCs. As a general assumption, cells that are arranged in spatial vicinity may interact via multiple mechanisms, which can be assigned to either paracrine growth factor-mediated communication pathways, cell–cell adhesion, or biomechanical stimulation (Engler et al. 2009). Thus, in order to evaluate the effects of human MSC (hMSC) interaction with various oral cells by allowing numerous kinds of potential interaction mechanisms, we generated interactive coculture systems, comprising hMSCs and osteoblasts from the alveolar bone (hOAs), periodontal ligament cells (hPDLs) and gingival fibroblasts (hGFs), respectively. Basically, these oral cell types provide the fundamental tissue components of the human periodontium and are gradually competent of hard tissue formation (Nanci and Bosshardt 2006), led by hOAs that are natural bone generators, followed by hPDLs that are also capable of expressing bone-associated biomarkers if required, e.g., if orthodontic forces are applied (Choi et al. 2011; Krishnan and Davidovitch 2006). In addition, even the soft tissue components, hGFs, have been reported to be osteogenicly inducible (Mostafa et al. 2011; Zhou et al. 2008). Hence, oral cells appear to harbor a certain inherent plasticity and, in this light, the question of how they are influenced by hMSC interaction appears even more urgent. For this purpose, interactive coculture systems are useful tools for an in-depth assessment of cellular crosstalk effects by mimicking tissue-specific cell-to-cell interactions (Roesch-Ely et al. 2006).
To our best knowledge, this is the first report using interactive coculture systems for a systematic comparison of hMSC-induced proliferation behavior in conjunction with osteogenic features in various oral cells including gene expression of crucial bone biomarkers, i.e., RUNX2, collagen type I, osteonectin and osteopontin (Zhou et al. 2008; Giannopoulou and Cimasoni 1996; Ivanovski et al. 2001). Furthermore, the comparison enabled the final tissue-level outcome of an oral cell interaction with hMSCs to be evaluated in terms of osteogenic matrix components and calcium deposition-indicating mineralization.
Materials and methods
All chemicals were purchased from Sigma-Aldrich, Munich, Germany, unless otherwise indicated. For a detailed description of the experiments performed, see the Electronic supplementary material.
Cell isolation and culture
All experiments were carried out in accordance to the guidelines of the World Medical Association Declaration of Helsinki and were approved by the Committee of Ethics of the Medical Faculty of the Albert Ludwigs-University Freiburg, Germany (vote number 411/2008) and, for tissue harvest at the Medical Faculty of the Heidelberg University Hospital, from the local institutional Committee of Ethics (vote number 148/2003; renewal 30.09.2005). hMSCs were purchased (PromoCell, Heidelberg, Germany) and cultivated in hMSC proliferation medium supplemented with 10 % fetal calf serum, 50 ng/ml amphotericin and 50 μg/ml gentamicin (all Provitro, Berlin, Germany). The hMSC-inherent clonogenic and multilineage potential was assessed as reported previously (Proksch et al. 2012). Human oral cells were derived from operative biopsies of donors (n = 12) with informed consent, i.e., n = 4 tissue fragments each for osteoblasts from the alveolar bone (hOAs), periodontal ligament fibroblasts (hPDLs) and gingival fibroblasts (hGFs), respectively. In brief, tissue fragments were plated as explants in DMEM low glucose (containing 25 mM HEPES and L-glutamine; PAA, Pasching, Austria) supplemented with 10 % fetal bovine serum (FBS; Biochrom, Berlin, Germany), 1 % kanamycin and 1 % glutamax (Invitrogen, Darmstadt, Germany).
Interactive coculture systems
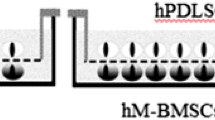
For the generation of interactive coculture systems, oral cells were established in 96-well plates (Corning Costar, Amsterdam, The Netherlands) at a density of 1 × 103 cells/well for proliferation assessment, while 1 × 105 cells/well were plated in 12-well plates (BD Biosciences, Heidelberg, Germany) for gene expression analysis. 5 × 103 hMSCs were inoculated per 96-well insert or 4 × 105 cells in compatible 12 well–culture inserts with a porous uncoated PET membrane (3.0 μm). This set up facilitates hMSC interactive crosstalk with hOAs, hPDLs, or hGFs, respectively, for the given culture periods and the subsequent examination of BrdU incorporation or mRNA expression in oral cells, devoid of hMSC BrdU or mRNA contamination.
For the generation of cell-containing matrices, hMSCs calibrated to 1 × 105 cells were suspended in FBS and added to a collagen I solution (4 mg/ml; Life Technologies, Darmstad, Germany) supplemented with 10 % Hank’s buffered saline solution. The resulting gel was titrated with sodium hydroxide to pH 7.4, polymerized for 2 h at 37 °C and covered with culture medium for 24 h. Then, the gels were supplied with each 5 × 105 hOAs, hPDLs, or hGFs, respectively and lifted to 1.0-μm polyethylene terephthalate membrane cell culture inserts (Millipore, Schwalbach, Germany) after 48 h in order to guarantee an optimal all-side culture medium nutriment supply during a culture period of 21 days. All cell cultures of each specimen and combination were duplicated for all experiments.
BrdU proliferation assay
The BrdU incorporation into newly synthesized DNA in interactive cocultures was measured using a cell proliferation ELISA kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. In brief, cells were fixed after T = 1, 3, 7, 10 and 14 days in hMSC coculture or monoculture control, respectively and DNA was denatured with a one-step ready-to-use solution supplied by the kit for 30 min at RT. After thoroughly removing the solution, a peroxidase (POD)-labeled anti-BrdU antibody was added (1:100, 90 min, RT). The immune complexes were detected by adding tetramethyl-benzidine (30 min, RT), the substrate reaction was stopped by adding 1 M H2SO4 (1:5) and the reaction product was quantified by immediately measuring the absorbance at 450 nm (reference wavelength 690 nm).
qRT-PCR
After accurately washing the cells with PBS, total cellular RNA was purified using a guanidium–thiocyanate method (RNeasy Mini kit; Qiagen, Hilden, Germany) and stored at −80 °C. Genomic DNA contamination was eliminated by DNase digestion according to the manufacturer’s instructions (RNase free DNase kit; Qiagen). The RNA integrity and quantity were verified using the Experion RNA StdSens chip microfluidic technology according to the manufacturer’s instructions and cDNA was synthesized from 500 ng of total RNA each by using the iScript reaction mix in a C1000 Thermal Cycler (all Bio-Rad, München, Germany). For real-time PCR, cDNA samples were amplified in duplicate in a 25-μl reaction mixture using pre-validated RT2 qPCR primer assays (SABiosciences, Frederick, MD, USA) with a iQ-SYBR Green Super Mix in a CFX96 cycler (both Bio-Rad). Data were collected and analyzed using CFX96 Manager Software version 1.0 (Bio-Rad).
Indirect immunofluorescence stains (IIF)
The expression of osteogenic proteins in monocultured controls and hMSC-cocultured oral cells was checked by IIF on gel cryosections. After blocking unspecific binding sites, mouse anti-human RUNX2 (Abnova, Taipei, Taiwan), rabbit anti-human collagen 1a1 and osteopontin (Abcam, Cambridge, UK), or osteonectin (Immunodiagnostik, Bensheim, Germany) primary antibodies were applied (all Abcam), which were detected by goat anti-mouse or anti-rabbit IgG1 Alexa Fluor 488 and 594 antibodies (Invitrogen) alternated by intense wash steps. Cell nuclei were counterstained using DAPI and the cells were mounted (Fluoromount G; Southern Biotech, Birmingham, USA) and photographed with a Biozero BZ-9000 fluorescence microscope equipped with a CCD camera (Keyence, Neu-Isenburg, Germany).
Histological stains
All experiments were performed at room temperature. For anilinblue/nuclear fast red staining, cryosections were fixed with 75 % ethanol (20 min), rinsed, stained with nuclear fast red (0.1 % in 5 % aluminium sulphate, 10 min), washed with distilled water, counterstained with aniline blue (30 s), washed, differentiated in 96 % ethanol (60 s) and air-dried. For Masson-Goldner trichrome staining, the specimens were fixed with Bouin’s solution (1 h), washed with running tap water, stained with Weigert’s iron hematoxylin (2 min; Carl Roth, Karlsruhe, Germany) and again washed with running tap water. Subsequently, the specimens were stained using a Masson-Goldner trichrome staining kit (Carl Roth) according to the manufacturer’s instructions. For von Kossa staining, cryosections were fixed with 4 % paraformaldehyde, rinsed, stained with AgNO3 (5 %), washed with distilled water, differentiated with pyrogallol (1 %, 2 min), washed, fixed with sodium thiosulfate (5 %, 5 min), washed and counterstained with hematoxylin. For microscopic evaluation, immersion oil droplets were placed on the air-dried specimens and evaluated with a SZH10 microscope (Olympus, Münster, Germany) equipped with a CCD Colour view III camera. The resulting images were analyzed using the cell* software (both Olympus).
Statistics
To evaluate the effect of groups (interactive cocultures and controls) and time (culture periods), a Repeated Measures Analysis was performed for each outcome of interest. For this purpose, linear models were fitted (one model per outcome) within which the structure of the data, i.e., repeated measurements per group (repeatedly measured at several time points), was accounted for. The group effects and differences of least-square means (LSM) are calculated with their 95 % confidence intervals. Several multiple comparisons of LSM in group and time combinations were conducted and p values were adjusted by the methods of Benjamini-Hochberg, Tukey, and Bonferroni, respectively, where appropriate. All calculations were performed with the statistical software SAS 9.1.2. using PROC MIXED.
Results
Various periodontal cell types differ in mineralization potential and surface marker expression
The oral cells used in this study were derived from explant cultures of 12 donors and expanded in number. Subsequently, they underwent precise characterization in terms of mineralization potential and surface marker expression. A systematic screening of surface markers, typically associated with mesenchymal cell types showed that hOAs, hPDLs and hGFs were homogeneously positive for CD13, CD73, CD90 and CD105 but virtually lacked CD45, CD146, alkaline phosphatase (ALP), c-kit and Stro-1. However, differences (% of positive expression) were found with respect to CD10, CD34, CD44 and CD166 (supplemental Fig. S1a), all of which were expressed at lower levels in hOAs compared to hPDLs and hGFs. This finding may be attributed to hOAs being essentially hard tissue cells, whereas hPDLs and hGFs are the main constituents of soft connective periodontal tissues. In addition, the oral cells revealed a mineralization potential with different degrees of intensity: hOAs greater than other cell types, followed by hPDLs and hGFs, which had similar mineralizing nodule deposition after 21 days (supplemental Fig. S1b).
Human mesenchymal stem cells fulfil the standard characterization criteria
Considering hMSCs, the definition is hampered by the current confusing terminology (Bianco et al. 2008). Here, we employed the standard hMSC characterization criteria of the International Society for Cellular Therapy (ISCT) and assured that hMSCs, which were subsequently used for interactive cocultures, were plastic-adherent, emanated from colonies, were able to be directed into osteoblasts, adipocytes and chondrocytes and expressed the typical surface markers including CD73, CD90 and CD105 but lacked CD14, CD19, HLA-DR, CD34 and CD45, as reported elsewhere (Proksch et al. 2012).
Interactive hMSC coculture does not modify the proliferation pattern of oral cells
Among the multiple ways that general cell features appear to respond to environmental stimuli, proliferation quantification is one of the most obvious. Hence, the proliferation performance of solitary grown and hMSC-cocultured hOAs, hPDLs and hGFs, respectively, was determined by means of BrdU incorporation at 1, 3, 7, 10 and 14 days of culture. We found that solitary grown control hOAs, hPDLs and hGFs potently proliferated and that the proliferation pattern in all cell populations appeared in a peak-to-valley manner. However, inter-group comparison revealed that hMSC coculture did not significantly modify the proliferation pattern either of hOAs, hPDLs, or hGFs.
With regard to control hOAs, the BrdU incorporation significantly increased at day 3 (p = 0.0481), day 7 (p < 0.0001) and day 10 (p = 0.0081) compared to baseline (Fig. 1a, black squares). The same applied to hMSC-cocultured hOAs, revealing significance in BrdU augmentation at day 3 (p = 0.0081), day 7 (p < 0.0001) and day 10 (p = 0.0481; Fig. 1a, white squares).
Proliferation dynamics of oral cell types in response interactive hMSC coculture. Line graphs showing the least square (LS) mean BrdU-incorporation with 95 % confidence intervals measured as optical density units (y-axis, 450 nm: measuring wavelength, 690 nm: reference wavelength) of solitary grown (controls) and hMSC-cocultured (+hMSCs) oral cells at different time points (x-axis), compared by Repeated Measures Analysis with post-test p value adjustment by the method of Benjamini-Hochberg. Differences in BrdU incorporation were considered significant at *p < 0.05, **p < 0.01 and ***p < 0.0001. a The BrdU incorporation was significantly elevated at day 3, day 7 and day 10 in control hOAs (black squares, connected by a dashed line) and hOAs cocultured with hMSCs (white squares, connected by a solid line) compared to baseline. b In control hPDLs (black circles, connected by a dashed line), the BrdU incorporation was significantly increased at day 3 and day 7 and in hMSC-cocultured hPDLs at day 7 compared to baseline (white circles, connected by a solid line). c hGFs proliferated well (controls: black triangles, connected by a dashed line) independently of hMSC coculture (white triangles, connected by a solid line), such that the BrdU incorporation was significantly augmented at day 3 and day 7 compared to baseline in both groups
For control hPDLs, significance of BrdU incorporation was observed at day 3 (p = 0.0299) and day 7 (p = 0.0039; Fig. 1b, black circles) and interaction with hMSCs left this profile unchanged (BrdU incorporation at day 7, p = 0.0498; Fig. 1b, white circles).
With hGFs, significant BrdU uptake was detected for both control and hMSC-cocultured cells at day 3 (p = 0.0108 control; Fig. 1c, black triangles; and p = 0.0166 coculture; Fig. 1c, white triangles) and day 7 (p = 0.0013 control and p = 0.0072 coculture; Fig. 1c).
The observation that hMSC coculture obviously did not modify oral cell proliferation during the period of investigation suggests that hMSCs neither exert striking restriction nor regulatory control on the respective oral cell type. Nevertheless, the slight coculture-related proliferation reduction trend led us to assess whether oral cell differentiation, a parameter of outstanding importance in the context of oral periodontal tissue regeneration, may be affected by interactive hMSC coculture.
Interactive hMSC coculture emphasizes the transcription of osteogenic genes preferentially in hPDLs
To test whether the osteogenic differentiation capacity of oral cells is influenced by interactive hMSC coculture, we first quantified mRNA levels after culture periods of 7 and 14 days, found to be the most interesting time points regarding the previously described proliferation changes. As bone retrieval is considered the main goal of hMSC-based periodontal regeneration, we selected a panel of genes associated with osteogenic differentiation, including runt-related transcription factor 2 (RUNX2), collagen 1 alpha 1 (Col1a1), osteonectin and osteopontin. The transcription profile tended to vary individually for each cell type, such that differences in temporal and inter-group gene expression comparisons were generally close to having significance. As a general result, we observed the trend that hMSCs enhanced the gene expression of osteogenic biomarkers in the cocultured oral cell type (Fig. 2a–l). This trend became most obvious in hPDLs, in which hMSC coculture yielded an up-regulation of all four bone-related genes under study, followed by hGFs and hOAs, which showed a slightly higher expression of two genes after 14 days if compared to controls. This is an important aspect, since hGFS are soft tissue cells of mesenchymal origin (Pitaru et al. 1995).
Expression of bone-associated mRNA in hMSC-cocultured oral cells. Least square mean (LSM) ΔΔCt values with 95 % confidence intervals (y-axis) of RUNX2 (a–c), collagen 1a1 (Col1a1, d–f), osteonectin (g–i) and osteopontin (j–l) expression in control (black, connected by a dashed line) and hMSC-cocultured (white, connected by a solid line) hOAs (squares), hPDLs (circles) and hGFs (triangles), respectively, each at day 0, day 7 and day 14 (x-axis; d day). The relative gene of interest quantities were normalized to the corresponding relative beta-actin quantities (ΔΔCt). The respective LSM ΔΔCt values were compared by Repeated Measures Analysis with post-test p value adjustment by the method of Bonferroni for inter-group comparisons and differences in gene expression were considered significant at *p < 0.05, **p < 0.01 and ***p < 0.0001. a, d, g, j In hOAs, the expression of Col1a1 and osteonectin was sustained by hMSC coculture, while the transcription of RUNX2 and osteopontin was enhanced at day 14 only in the hMSC coculture group. b, e, h, k Regarding hPDLs, interactive hMSC coculture tended to increase the transcription of all genes under study, especially of RUNX2 and osteonectin. c, f, i, l In hGFs, the expression of RUNX2, Col1a1 and osteonectin increased by time in a peak-to-valley manner irrespective of hMSC coculture, while the down-regulation of osteopontin transcription in controls was counteracted by hMSCs
Regarding hOAs, the transcription of all genes exhibited a slowly increasing trend with prolonged culture duration irrespective of hMSC coculture (Fig. 2a, d, g, j). Interestingly, the transcription of RUNX2 (p = 0.0373; Fig. 2a) and osteopontin (Fig. 2j) was increased in hMSC-cocultured hOAs at day 14 compared to baseline, while the expression remained stable in control hOAs (Fig. 2d). This finding suggests that shaping of osteogenic features in hOAs following hMSC coculture may reflect an expression reinforcement of the analyzed genes.
In hPDLs, manifest coculture-dependent effects became obvious. In the control group, the transcription of RUNX2 (Fig. 2b), Col1a1 (Fig. 2e), osteonectin (Fig. 2h) and osteopontin (Fig. 2k) remained quite constant in time but considerably increased in the hMSC coculture group from baseline to day 14 (RUNX2 p = 0.0319; Fig. 2b, osteopontin p = 0.0477; Fig. 2h). This increase suggests an hPDL-immanent plasticity regarding the realization of osteogenic genes, which is apparently shaped by the interaction with cocultured hMSCs.
To our surprise, hGFs also expressed osteogenic mRNAs and the expression followed a peak-to-valley pattern. With respect to RUNX2, a slight transcription increase observed in control hGFs was emphasized by interactive hMSC coculture (Fig. 2c). The increase in Col1a1 expression was significant in control and in hMSC-cocultured hGFs at day 7 (p = 0.0110 control and p = 0.0304 coculture, respectively; Fig. 2f). Most prominently, osteonectin expression was increased significantly both at day 7 (p = 0.0003 control and p = 0.0043 hMSC coculture; Fig. 2i) and day 14 (p = 0.0023 control and p = 0.0048 hMSC coculture; Fig. 2i). Importantly, hMSC coculture exhibited a counter-rotating gene expression profile concerning osteopontin, which slightly and constantly increased upon interactive hMSC coculture, while gene expression increased at day 7 but decreased considerably at day 14 in hGF controls (Fig. 2l). This gene expression pattern in conjunction with the others mentioned above suggests an intrinsic capacity of soft connective tissue-descending hGFs for expressing genes, which may be assigned to the osteogenic phenotype and being shaped by hMSC coculture interactions.
The fact that osteogenic genes are increasingly transcribed in hOAs is not surprising; however, hPDLs and hGFs also showed an osteogenic potential irrespective of hMSC induction. Consequently, as a general assumption, our data suggest that oral cells possess a certain degree of plasticity. The observation of osteogenic gene transcription trending to be emphasized by hMSC coculture provides the first evidence that hMSC interaction shapes oral cells to adopt an osteogenic outcome.
Interaction with hMSCs enhances expression of matrix proteins in 3D systems
Motivated by the observed hMSC-induced enhancement of osteogenic gene expression in oral cells, we assessed the tissue-level outcome of hMSC coculture in terms of protein translation of bone-related mRNA expression. To this end, 3D coculture gel-systems containing embedded hMSCs were surface-populated with hOAs, hPDLs, or hGFs, respectively, while gels that lacked hMSCs served as a control. We observed that during the culture period of 21 days, both control and interactive coculture gels contracted in size depending on the respective oral cell type, such that gels supplied with hOAs were only 0.3–0.4 mm, hPDLs-seeded gels were 0.8–1.0 mm and hGFs-furnished gels approximately 0.4–0.5 mm in diameter, respectively.
By indirect immunofluorescence staining (IIF) for RUNX2, collagen 1, osteonectin and osteopontin, we found that the oral cell types under study showed a hierarchical fashion of bone-related protein expression both in hMSC cocultures and controls, i.e., hOAs > hGFs > hPDLs, thus substantiating our conclusion drawn from qPCR data that oral cells display a certain degree of plasticity. Of note, IIF stains revealed that all bone-related markers under investigation were localized close to oral cell seeding sites of the 3D gels.
Protein translates of RUNX2, a key transcription factor of osteogenesis, were detected in all oral cell types under study (Fig. 3a–f). As indicated by localization in the nuclei (supplemental Fig. S2a–f) of cells clustered at the edges and in the centre of the 3D gels, RUNX2 expression was clearly increased by hMSC coculture (Fig. 3a–c; supplemental Fig. S2a–c) compared to controls (Fig. 3d–f; supplemental Fig. S2d–f). This effect was most evident in hOAs, followed by hGFs and finally hPDLs.
Expression of matrix molecules in reaction to interactive hMSC coculture. Representative immunofluorescence stains of bone-related proteins (white asterisks, a–f green (Alexa Fluor 488): RUNX2, g–l green (Alexa Fluor 488): collagen 1, m–r green (Alexa Fluor 488): osteonectin, s–x red (Alexa Fluor 594): osteopontin) in hMSC-cocultured (top row each) and control (bottom row each) oral cells [hMSC-cocultured (a, g, m, s) and control hOAs (d, j, p, v), hMSC-cocultured (b, h, n, t) and control hPDLs (e, k, q, w) and hMSC-cocultured (c, i, o, u) and control hGFs (f, l, r, x)], blue cell nuclei (DAPI), scale bar 200 μm. After 21 days, the expression each of RUNX2, collagen 1, osteonectin and osteopontin was remarkably increased in interactive hMSC cocultures (a, g, m, s) compared to hMSC-lacking control hOAs (d, j, p, v). In hPDLs, interactive hMSC coculture (b, h, n, t) yielded expression levels of all matrix proteins under study similar to matched controls (e, k, q, w). In hGFs, an increase in protein expression was observable upon hMSC interaction (c, i, o, u), compared to hMSC-lacking controls (f, l, r, x), which also expressed bone-related proteins. Of note, all investigated osteogenic proteins were detected preferably at the central clusters and borders of the 3D gels, representing the oral cell seeding sites. Together, all oral cell types disposed of an inherent capacity to express osteogenic proteins and their responsiveness to hMSC coculture in terms of protein translates was ranked in the following hierarchy hOAs > hGFs > hPDLs
Interestingly, hOAs expressed more green-colored collagen 1 molecules upon hMSC interaction (Fig. 3g; supplemental Fig. S2g) compared to hMSC-lacking controls (Fig. 3j; supplemental Fig. S2j). This hMSC coculture-dependent expression was marginal in hPDLs (Fig. 3h, k; supplemental Fig. S2h, k), but became evident in hGFs (Fig. 3i, l; supplemental Fig. S2i, l). In line with RUNX2 expression sites, collagen 1 was detected mainly at the margins and in the central area of cell clusters in the 3D gels.
The expression of green-labelled osteonectin was remarkably augmented in hMSC-cocultured (Fig. 3m–o; supplemental Fig. S2m–o) hOAs, hPDLs and hGFs compared to matched controls (Fig. 3p–r; supplemental Fig. S2p–r) while inter-group comparison revealed that osteonectin expression followed the same ranking. In correspondence to RUNX2 and collagen 1 expression, osteonectin molecules were located mainly at the edges and as clusters at the centre of 3D gels.
Similar to RUNX2, collagen 1 and osteonectin, red-labelled osteopontin was detectable both in interactive hMSC cocultures (Fig. 3s–u; supplemental Fig. S2s–u) and control gels (Fig. 3v–x; supplemental Fig. S2v–x) of each oral cell type. In hMSC-cocultured hOA (Fig. 3s; supplemental Fig. S2s) and hGF gels (Fig. 3u; supplemental Fig. S2u), considerably more osteopontin fluorescence was detected than in matched controls (Fig. 3v, x; supplemental Fig. S2v, x). This trend was also obvious in hPDLs (Fig. 3t, w; supplemental Fig. S2t, w), such that the expression levels of osteopontin followed the same ranking as detected for the other bone-related markers, namely hOAs > hGFs > hPDLs.
Hence, IIF detection of bone-related protein expression strongly supports our gene expression data. The observed size reduction of oral cell 3D cultures followed the same oral cell type-dependent hierarchy as monitored for gene and protein expression, of which the latter was clearly increased by hMSC coculture and importantly, the IIF-detected bone-related proteins were focused at the oral cell seeding sites. These observations suggest that the increase in bone protein expression may be attributed to hMSC-induced oral cell-innate triggering of osteogenic differentiation, providing strong evidence for the existence of individual oral cell plasticity in response to hMSC crosstalk.
Matrix mineralization nodules deposition is pronounced by hMSCs interaction
On the basis of our gene transcription and protein expression results, we further assessed the hallmark of osteogenic differentiation in oral cells triggered by hMSCs, as exemplified by the status of osteoid deposition and extracellular matrix mineralization. For this purpose, histological stains enabled visualizing of the interactive coculture cell distribution, gel morphology and the discrimination of connective tissue, osteoid-like and mineralized bone matrix (Fig. 4, supplemental Fig. S3). Cell distribution and gel morphology of hMSC cocultures were found to conform to matched controls. However, extracellular matrix deposition and mineralization events were clearly induced by hMSC coculture. In addition, we found that oral cell-based tissue-analogues differed gradually with hOA gels ranking first followed by hGF and hPDL, hence substantiating our protein expression results.
Cell distribution and deposition of osteoid and calcium nodules in oral cells cocultured with hMSCs. Representative aniline blue/nuclear fast red (a–f), Masson-Goldner trichrome (g–l) and von Kossa stains (m–r) of bone matrix components in hMSC-cocultured (top row each) and control (bottom row each) oral cells [hMSC-cocultured (a, g, m) and control hOAs (d, j, p), hMSC-cocultured (b, h, n) and control hPDLs (e, k, q) and hMSC-cocultured (c,i,o) and control hGFs (f, l, r)], scale bar: 1,000 μm. Aniline blue/nuclear fast red results in blue staining of collagen and red staining of the cell nuclei. Masson Goldner trichrome method yields the following stains: cell nuclei brown-black, connective tissue green, osteoid red, mineralized matrix: green. Von Kossa technique results in brown-shaded staining of connective tissue and black staining of mineralized matrix. Compared to matched controls, hMSC-cocultured hOAs display an uniform cell distribution with a right hand-sided cell cluster, representing the hOA seeding site (a, d) and showing a flexed morphology tightened by the hOA cell cluster. The most intriguing finding was that interactive hMSC coculture tremendously increased the deposition of osteoid (white asterisks, g) and calcium nodules (white arrows, m) in hOAs compared to matched controls (j, p), with emphasis on hOA seeding sites. Cultures seeded with hPDLs showed an even cell distribution without notable cell clustering both in hMSC cocultures (b) and controls (e). Furthermore, virtually neither osteoid (h, k) nor calcium deposition (n, q) was detectable in hPDLs, irrespective of hMSC coculture. Independent from hMSC coculture, cells are similarly distributed and exhibited cell clustering at the hGF seeding site, bending the gels (c, f). The expression of osteoid (white asterisks, i) and mineralization (white arrows, o) is obviously enhanced by hMSC coculture compared to non-hMSC controls (l, r). Similar to hOAs, both osteoid deposition and mineralization sites are focused on hGF cluster sites
In detail, aniline blue/nuclear fast red stains revealed that cell distribution and gel morphology were notably different with regard to the oral cell type but rather uniform with respect to hMSCs coculture (Fig. 4a-f). Cell clusters were detected at the oral cell seeding sites and their size followed a hierarchical order hOAs > hGFs > hPDLs (Fig. 4a-f). These clusters served as central hubs to which the gels appeared to be tightly pulled (supplemental Fig. S3a-c), while the intensity of this phenomenon followed the same ranking. These findings corroborate our hypothesis that gel size reduction is a consequence of bone matrix protein expression, which is particularly emphasized at oral cell seeding sites. Hence, these results further substantiate individual osteogenic oral cell plasticity.
Masson Goldner Trichrome staining (MGT) yielded even green-colored staining of the gel bodies (Fig. 4g-l). Most notably in hOA gels, MGT stains also exhibited a dense red matrix surrounding the cell conglomerates (Fig. 4g) that are interpreted as osteoid-like accumulations. Of note, these presumable osteoid accumulations were clearly intensified with hMSCs as an interaction partner (Fig. 4g, supplemental Fig. S3e) compared to matched controls (Fig. 4j, supplemental Fig. S3f). In hPDLs, no osteoid accumulation was observable independent of interactive hMSC coculture (Fig. 4h,k). In contrast, osteoid formation observable in hGFs was obviously augmented by hMSC coculture (Fig. 4i, compared to hMSC-lacking controls Fig. 4l), thereby paralleling the situation of hOAs (Fig. 4g, j).
Von Kossa staining permitted detection of mineralized matrix deposits and appeared most explicitly in interactive hMSC-hOA cocultures (Fig. 4m, supplemental Fig. S3h) compared to hOA-only gels (Fig. 4p, supplemental Fig. S3i). Most of the black-coloured mineralization nodes were localized inside the superficial cell clusters. On the other hand, interactive hMSC coculture as well as hMSC-lacking control gels that were colonized with hPDLs showed few mineralization nodes (Fig. 4n, q). In addition, with hGFs (Fig. 4o, r) we also detected black-colored mineralized deposits more pronounced with hMSC as an interactive partner (Fig. 4o).
Next, the observed differences in osteoid levels (Fig. 5a) and calcium nodule detection (Fig. 5b) were quantified and both normalized to total specimen area-covering global collagen. Indeed, hMSC coculture significantly increased osteoid formation and matrix mineralization with oral cell type-dependent ranking, whereas in non-hMSC controls both osteoid and calcium deposition were consistently low. In detail, hMSC interaction significantly enhanced osteoid deposition in hOAs compared with matched controls (p < 0.0001), such that the detected osteoid levels in hMSC-cocultured hOAs were more prominent than in hMSC-cocultured hGFs (p = 0.0015) or hPDLs (p < 0.0001), respectively (Fig. 5a). Accordingly, mineralization events were significantly more numerous in hMSC-cocultured hOAs and even in hGFs compared with controls (p < 0.0001 for hOAs and p = 0.0074 for hGFs), respectively (Fig. 5b). Thus, the quantity of calcium nodules in hMSC cocultures followed the same oral cell type-dependent hierarchy as observed for osteoid formation (p = 0.0055 hMSC-cocultured hOAs vs. hGFs and p < 0.0001 hMSC-cocultured hOAs vs. hPDLs; Fig. 5b).
Osteoid and calcification levels in hMSC cocultured oral cells. Least square mean (LSM) a osteoid/collagen and b calcium/collagen ratios with 95 % confidence intervals (y-axis) of control (black, left-handed) and hMSC-cocultured (white, right-handed each) hOAs (squares), hPDLs (circles) and hGFs (triangles), respectively (x-axis). Mean osteoid/collagen or calcium/collagen ratios were compared by Repeated Measures Analysis with post-test p value adjustment by the method of Tukey and differences were considered significant at *p < 0.05, **p < 0.01 and ***p < 0.0001. a In hOAs, hMSC coculture significantly increased osteoid deposition in relation to the global collagen quantity compared to matched controls. By trend, hMSC coculture enhanced osteoid formation also in hGFs but not hPDLs. In controls, the osteoid levels were not different. However, hMSC coculture yielded a significant oral cell type-dependent increase in osteoid formation, following the ranking hOAs > hGFs > hPDLs. b Coculture with hMSCs entailed a significantly increased extracellular matrix mineralization in hOAs and hGFs compared to controls. In analogy to osteoid detection, hMSC coculture resulted in a significant oral cell type-dependent hierarchical increase in calcium nodule formation
Altogether, our data allow hypothesizing that the osteogenic differentiation progress appeared sustainably reinforced by hMSC coculture in hOAs. The most striking observation is that hGFs outrivaled hPDLs in terms of extracellular matrix (ECM) protein expression, ECM deposits and mineralization nodules. Hence, oral cells display an osteogenic differentiation capacity that is inducible by hMSCs; however, the hMSC impact on hPDLs remained limited to the gene expression level.
Discussion
The therapeutic administration of stem cells employed for tissue regeneration initiates multiple events affecting the recipient tissue site (Prockop 2009; Tolar et al. 2010). In oral regenerative medicine, to our best knowledge this is the first report to describe a systematic comparison of the responses of different human periodontal cell types, namely osteoblasts from the alveolar bone (hOAs), periodontal ligament (hPDLs) and gingival fibroblasts (hGFs), to the interaction with human bone marrow-derived mesenchymal stem cells (hMSCs). An innovative in vitro approach, based on interactive cocultures facilitating multiple physiologically relevant cell-to-cell interactions that may occur in vivo (Stark et al. 2004), indicated that hMSCs sustained a proliferation pattern in oral cells similar to matched controls but clearly shaped their differentiation status in a graduated manner towards an osteogenic direction. We hypothesize that the enhanced osteogenesis may be attributed to oral cells rather than to hMSCs, since the latter act as an inductor of osteogenic gene expression in oral cells, while, vice versa, oral cell coculture results in decreasing osteogenic gene expression in hMSCs (Proksch et al. 2012). These results together with the observation that oral cell-only controls display a certain degree of osteogenesis, which is completely missing in hMSC-only controls, lead us to the assumption that the greatly enhanced osteogenic protein expression, matrix deposition and mineralization in hMSC-oral cell cocultures may represent hMSC-mediated osteogenic oral cell shaping.
Irrespective of spatial restriction as a possible reason for the late stage proliferation decrease, the hMSC coculture did not modify oral cell proliferation behavior. Sufficient cell numbers are crucial for clinical regeneration purposes, hence the finding that hMSCs do not restrict the number of putative hard tissue-forming cells while simultaneously emphasizing their osteogenic differentiation seems important. In this context, the observed contraction of the 3D coculture gels seems to be attributed more to an ongoing differentiation processes than to cell proliferation because it may result from enhanced ECM deposition, probably concomitant with integrin-mediated cell–matrix interaction and contractility (Engler et al. 2009). Since hMSCs effectively prevent apoptosis upon interaction with oral cells (Proksch et al. 2012), the enhanced gel contraction may additionally reflect hMSC-triggered enhancement of cell survival.
The common developmental origin of hOAs, hPDLs and hGFs as descendants of neural crest cells (Palmer and Lubbock 1995; Kuratani et al. 1997) provides a plausible explanation for their vast phenotype similarity in terms of surface molecule expression. Nevertheless, considering that these cells originate from sites that are either hard or soft tissues, it is not surprising that their differentiation potentials differ in reaction to hMSC coculture, as found in the hierarchy of all parameters investigated. This observation reveals two interesting outcomes, namely that oral cells dispose of a certain degree of intrinsic plasticity and that the hMSC-triggered differences in osteogenic differentiation may be attributed to a distinct responsiveness of the diverse oral cell types. In other words, the plasticity of oral cells can be shaped towards an osteogenic phenotype (Zhou et al. 2008) via hMSC interaction. With regard to hOAs, the observed hMSC-related osteogenic induction reinforces their inherent osteogenic potential. Nevertheless, the most significant finding was that hGFs, representatives of pure soft connective tissue cells, displayed robust potential for osteogenic differentiation (Mostafa et al. 2011; Carnes et al. 1997). This is an interesting aspect because hGFs, being neural crest derivatives (Chai et al. 2000; Palmer and Lubbock 1995), display greater similarity to hPDLs and hOAs than to dermal fibroblasts, particularly in terms of gene expression (Lallier et al. 2005). Although Lim and co-workers found osteogenic medium additives to motivate hPDLs rather than hGFs to mineralize their extracellular matrix (Zhou et al. 2008), hGFs were reported to possess a robust osteogenic potential (Mostafa et al. 2011) and to be superior to hPDLs in the prevention of osteoclast formation (de Vries et al. 2006). In this context, our data suggest that 3D culture and hMSC interaction encourage hGFs to top hPDLs with respect to their inherent mineralizing potential.
In the framework of hMSC coculture, hPDLs display an enhanced responsiveness with regard to osteogenic gene transcription but reduced protein expression and matrix maturation. This reversal in gene transcription and downstream differentiation processes imply a time delay between these steps. However, but importantly, osteogenic differentiation events in hPDLs rely on further stimuli including biomechanical cues, e.g., mechanical forces applied to the periodontal cells and particularly hPDLs during orthodontic tooth movement (Krishnan and Davidovitch 2006).
In summary, our results show that oral cells possess a discriminative inherent plasticity that, with the exception of proliferation, is individually shaped by interactive hMSC coculture in terms of gene transcription, matrix protein deposition, osteoid formation and mineralization trends. Although we admittedly did not identify specific pathways, we speculate that, at a mechanistic level, the observed hMSC influence on oral cell realization of osteogenic features may be orchestrated by non-direct contact-innate cell-to-cell-interactions (Osugi et al. 2012; Ruschke et al. 2012), provided by the coculture approach in this study. Thus, our results contribute substantially to the understanding of the cellular processes, occurring during hMSC-based oral tissue regeneration and explicitly portray the urgent need for fine tuning the complex interactions of the different cell types. Such knowledge will be precious for prediction of the outcome of hMSC-based in vivo periodontal regeneration therapies. In this light, the insights revealed from our study are particularly important in terms of bone preservation as the main clinical objective in periodontal regeneration.
References
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584
Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2:313–319
Carnes DL, Maeder CL, Graves DT (1997) Cells with osteoblastic phenotypes can be explanted from human gingiva and periodontal ligament. J Periodontol 68:701–707
Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127:1671–1679
Choi MH, Noh WC, Park JW, Lee JM, Suh JY (2011) Gene expression pattern during osteogenic differentiation of human periodontal ligament cells in vitro. J Periodont Impl Sci 41:167–175
de Vries TJ, Schoenmaker T, Wattanaroonwong N, van den Hoonaard M, Nieuwenhuijse A, Beertsen W, Everts V (2006) Gingival fibroblasts are better at inhibiting osteoclast formation than periodontal ligament fibroblasts. J Cell Biochem 98:370–382
Engler AJ, Humbert PO, Werle-HAller B, Weaver VM (2009) Multiscale modeling of form and function. Science 324:208–212
Giannopoulou C, Cimasoni G (1996) Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res 75:895–902
Ivanovski S, Li H, Haase HR, Bartold PM (2001) Expression of bone associated macromolecules by gingival and periodontal ligament fibroblasts. J Periodont Res 36:131–141
Jones E, Yang X (2011) Mesenchymal stem cells and bone regeneration: current status. Injury 42:562–568
Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H (2004) Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol 75:1281–1287
Kelm JM, Breitbach M, Fischer G, Odermatt B, Agarkova I, Fleischmann BK, Hoerstrup SP (2012) 3D microtissue formation of undifferentiated bone marrow mesenchymal stem cells leads to elevated apoptosis. Tissue Eng Part A 18:692–702
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 129:e1–32
Kuratani S, Matsuo I, Aizawa S (1997) Developmental patterning and evolution of the mammalian viscerocranium: genetic insights into comparative morphology. Dev Dyn 209:139–155
Lallier TE, Spencer A, Fowler MM (2005) Transcript profiling of periodontal fibroblasts and osteoblasts. J Periodontol 76:1044–1055
Li H, Yan F, Lei L, Li Y, Xiao Y (2009) Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs 190:94–101
Mostafa NZ, Uludag H, Varkey M, Dederich DN, Doschak E-BTH (2011) In vitro osteogenic induction of human gingival fibroblasts for bone regeneration. Open Dent J 5:139–145
Nanci A, Bosshardt DD (2006) Structure of periodontal tissues in health and disease. Periodontol 40:11–28
Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M (2012) Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. Tissue Eng Part A 18:1479–1489
Palmer RM, Lubbock MJ (1995) The soft connective tissues of the gingiva and periodontal ligament: are they unique? Oral Dis 1:230–237
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells 25:2896–2902
Pitaru S, McCulloch CA, Narayanan SA (1995) Cellular origins and differentiation control mechanisms during periodontal development and wound healing. J Periodont Res 29:81–94
Prockop DJ (2009) Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17:939–946
Proksch S, Steinberg T, Stampf S, Schwarz U, Hellwig E, Tomakidi P (2012) Crosstalk on cell behavior in interactive cocultures of hMSCs with various oral cell types. Tissue Eng Part A 18:2601–2610
Roesch-Ely M, Steinberg T, Bosch FX, Müssig E, Whitaker N, Wiest T, Kohl A, Komposch G, Tomakidi P (2006) Organotypic co-cultures allow for immortalized human gingival keratinocytes to reconstitute a gingival epithelial phenotype in vitro. Differentiation 74:622–637
Ruschke K, Hiepen C, Becker J, Knaus P (2012) BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res 347:512–544
Sanchez-Lara PA, Zhao H, Bajpai R, Abdelhamid AI, Warburton D (2012) Impact of stem cells in craniofacial regenerative medicine. Front Physiol 3:188
Si YL, Zhao YL, Hao HJ, Fu XB, Han WD (2011) MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev 10:93–103
Stark HJ, Szabowski A, Fusenig NE, Szabowski NM (2004) Organotypic cocultures as skin equivalents: a complex sophisticated in vitro system. Biol Proc Online 6:55–60
Tolar J, Le Blanc K, Keating A, Blazar BR (2010) Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 28:1446–1455
Uccelli A, Morando S, Bonanno S, Bonanni I, Leonardi A, Mancardi G (2011) Mesenchymal stem cells for multiple sclerosis: does neural differentiation really matter? Curr Stem Cell Res Ther 6:69–72
Zhang P, Men J, Fu Y, Shan T, Ye J, Wu Y, Tao Z, Liu L, Jiang H (2012) Contribution of SATB2 to the stronger osteogenic potential of bone marrow stromal cells from craniofacial bones. Cell Tissue Res 350:425–437
Zhou Y, Hutmacher DW, Sae-Lim V, Zhou Z, Woodruff M, Lim TM (2008) Osteogenic and adipogenic induction potential of human periodontal cells. J Periodontol 79:525–534
Acknowledgments
We are grateful to Dr. Ulrich Schwarz, Center for Dental Medicine, University Medical Center Freiburg, Dpt. of Oral and Maxillofacial Surgery, for generously providing bone tissue specimens. We acknowledge the excellent technical assistance of Yrgalem Abreha who supported the cell culturing and the courtesy of Prof. Dr. Mike Swain, Professor Emeritus at the University of Sydney, Faculty of Dentistry and Research Associate at Center for Dental Medicine, University Medical Center Freiburg, for his valuable proofreading of the manuscript. The authors declare that they do not have any conflict of interest. The study was funded by the Deutsche Gesellschaft für Zahn-, Mund- und Kieferheilkunde (DGZMK, 11/2010).
Author disclosure statement
The authors declare that they have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 118 KB)
Supplemental Fig. S1
Human oral cell characterization. Mineralization potential and surface marker expression of hOAs, hPDLs and hGFs. Above: Alizarin red and von Kossa stains following osteogenic induction showed that each oral cell type disposed of an inherent osteogenic potential, headed by hOA showing the strongest matrix mineralization capacity. Below: Flow cytometric analyses of proliferated oral cells (left columns: hOAs; centre columns: hPDLs; right columns: hGFs). Representative histogram plots show that hOAs, hPDLs and hGFs expressed similar levels of mesenchymal surface markers with the exception of CD10, CD34, CD44 and CD166. Green curves: Negative controls, purple curve areas: positive samples. (JPEG 296 kb)
Supplemental Fig. S2
High-magnification images of matrix molecule expression in reaction to interactive hMSC coculture. Representative indirect immunofluorescence stains of bone-related proteins (a-f green (Alexa Fluor 488): RUNX2, g-l green (Alexa Fluor 488): collagen 1, m-r green (Alexa Fluor 488): osteonectin, s-x red (Alexa Fluor 594): osteopontin) in hMSC-cocultured (top row each) and control (bottom row each) oral cells [hMSC-cocultured (a,g,m,s) and control hOAs (d,j,p,v), hMSC-cocultured (b,h,n,t) and control hPDLs (e,k,q,w) and hMSC-cocultured (c,i,o,u) and control hGFs (f,l,r,x)], blue: cell nuclei (DAPI), scale bar: 200 μm. Interactive hMSC coculture enhanced the expression of all osteogenic proteins in oral cells in a cell type-related manner, i.e., mainly in hOAs, followed by hGFs and hPDLs. (a-f) RUNX2 was expressed in cell nuclear localization with emphasis on cells localized at the cell clusters and borders of the 3D gels, i.e., the oral cell seeding sites. (g-l) Collagen 1 was mainly expressed at gel surfaces in co-localization to the oral cell seeding sites. (m-r) Osteonectin and (s-x) osteopontin expression was considerably enhanced by hMSC coculture in all oral cell types compared to controls (JPEG 144 kb)
Supplemental Fig. S3
Comparison of control hMSCs with interactive oral cell-hMSC cocultures and control oral cells, represented by hOAs. (a-c) Representative aniline blue/nuclear fast red (NFR), (d-f) Masson-Goldner trichrome and (g-i) von Kossa stains of 3D control hMSCs (a,d,g), interactive hOA-hMSC cocultures (b,e,h) and control hOAs (c,f,i), scale bar: 1,000 μm. Bone matrix deposition and mineralization were not observed in control hMSCs but were detected in control hOAs and were clearly intensified in interactive cocultures. The size of control hMSC gels considerably exceeded interactive cocultures and control hOAs, which presumably results from the lack of extracellular bone matrix protein-related tightening. Control hMSCs (grey-pink) were surrounded by a weakly stained, lose collagen structure (blue, a), which appears much denser and flexed in direction of a cell cluster located at the hOA seeding site in interactive cocultures (b) and control hOAs (c). While osteoid deposition (red) is completely missing in control hMSCs (d), hMSC-cocultured hOAs showed immensely enhanced osteoid levels in relation to global collagen quantity (green, e), compared to hOA controls (f). While control hMSCs completely lacked mineralization nodule deposition (black-grey, g), hMSC interaction yielded a tremendous increase in matrix calcification in hOA cultures with regard to the global collagen content (brown, h), compared to (i) control hOAs (JPEG 56 kb)
Rights and permissions
About this article
Cite this article
Proksch, S., Steinberg, T., Vach, K. et al. Shaping oral cell plasticity to osteogenic differentiation by human mesenchymal stem cell coculture. Cell Tissue Res 356, 159–170 (2014). https://doi.org/10.1007/s00441-013-1777-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1777-5