Abstract
Orthodontic force application is well known to induce sterile inflammation, which is initially caused by the compression of blood vessels in tooth-supporting apparatus. The reaction of periodontal ligament cells to mechanical loading has been thoroughly investigated, whereas knowledge on tissue reactions of the dental pulp is rather limited. The aim of the present trial is to analyze the effect of orthodontic treatment on the induction and cellular regulation of intra-pulpal hypoxia. To investigate the effect of orthodontic force on dental pulp cells, which results in circulatory disturbances within the dental pulp, we used a rat model for the immunohistochemical analysis of the accumulation of hypoxia-inducible factor-1α in the initial phase of orthodontic tooth movement. To further examine the regulatory role of circulatory disturbances and hypoxic conditions, we analyze isolated dental pulp cells from human teeth with regard to their specific reaction under hypoxic conditions by means of flow cytometry, immunoblot, ELISA and real-time PCR on markers (Hif-1α, VEGF, Cox-2, IL-6, IL-8, ROS, p65). In vivo experiments showed the induction of hypoxia in dental pulp after orthodontic tooth movement. The induction of oxidative stress in human dental pulp cells showed up-regulation of the pro-inflammatory and angiogenic genes Cox-2, VEGF, IL-6 and IL-8. The present data suggest that orthodontic tooth movement affects dental pulp circulation by hypoxia, which leads to an inflammatory response inside treated teeth. Therefore, pulp tissue may be expected to undergo a remodeling process after tooth movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During orthodontic treatment, dental crowns are subjected to a continuous force, which results in bone resorption on the compression side and appropriate bone apposition on the tension side to achieve tooth movement. The process of tissue remodeling to resolve necrotic tissue and to induce the repair of these tissues has been described in detail for periodontal apparatus (Meikle 2006). The special role of periodontal ligament cells (PDL cells) in the homeostasis and induction of PDL as well as in alveolar bone remodeling processes during orthodontic tooth movement has also been well established (Henneman et al. 2008). Besides the already well-described process of periodontal remodeling, a number of reports have addressed the effects of dental pulp on the process of orthodontic tooth movement (von Böhl et al. 2012; Yamaguchi and Kasai 2007). Since, in orthodontic therapy, vital teeth are moved over long distances, dental pulp tissue needs to adapt. The initial response of dental pulp tissue after the application of orthodontic force still remains unclear.
Orthodontic force is assumed to evoke transient circulatory disturbances in the periodontal tissue as well as in the dental pulp, since the initial force application is believed to compress blood vessels (Hamersky et al. 1980; Goz et al. 1992). In response to orthodontic force application, dental pulp tissue has been shown to decrease its rate of tissue respiration, while reduced alkaline phosphatase activity, vacuolisation of odontoblasts and apoptosis have been observed (Stenvik and Mjor 1970; Hamersky et al. 1980; von Böhl et al. 2012). Kvinnsland et al. (1989) reported mild inflammatory responses to orthodontic force in the dental pulp. Loss of tooth vitality during orthodontic treatment has been described occasionally, which was presumably preceded by a previous dental trauma (Brin et al. 1991; Bauss et al. 2010). Moreover, some authors have described increased enzymatic activity of aspartate amino transferase in dental pulp tissue, indicating metabolic changes and possible cell damage due to orthodontic force application (Perinetti et al. 2004; Veberiene et al. 2009). The production of neuropeptides, such as substance P, neurokinin A and calcitonin gene-related peptide, is thought to be induced by a mechanical force, which promotes neurogenic inflammation in dental pulp (Yamaguchi et al. 2004; Caviedes-Bucheli et al. 2011). These neuropeptides were abundant in both the pulp tissue and the periodontal ligament. The increased release of these mediators induces vasodilation but also promotes the production of pro-inflammatory cytokines, such as IL-6 (Caviedes-Bucheli et al. 2008). Furthermore, Kojima et al. (2006) observed in vitro the promoting effect of substance P on the synthesis of prostaglandin-E2 (PGE2) and the receptor activator of the nuclear factor κB Ligand (RANKL) by human dental pulp fibroblasts.
The aim of the present trial was to evaluate in vivo the effect of orthodontic tooth movement on inducing circulatory disturbances and hypoxic conditions in dental pulp. These findings were transferred to an in vitro model to further analyze the cellular response of dental pulp fibroblasts after orthodontically-induced short-time hypoxia. We hypothesized that dental pulp circulation would change after the application of orthodontic force and that these alterations would modify the cellular response of dental pulp cells over the course of tooth movement. To identify the specific cellular response and to evaluate the immunomodulatory function of dental pulp fibroblasts during hypoxic cell stress, we analyzed the activation and expression of pro-inflammatory factors after induction of hypoxic cell stress by different in vitro models. We hypothesized that hypoxic cell stress leads to enhanced expression of the hypoxia marker Hif-1α, of pro-inflammatory cytokines and of the vascular growth factor VEGF that are induced by the activation of the NF-κB pathway, thus activating an immune-related tissue response.
Materials and methods
In vivo experiments of orthodontic tooth movement
Ten 12-week-old male Wistar rats weighing 300–350 g each (Harlan Winkelmann, Borchen, Germany) were used in the experiment. They were provided with food and water ad libitum. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the local district government and the Animal Care Commissioner of the University of Bonn, Germany. The rats were anaesthetized with 0.01 ml Rompun (Bayer, Leverkusen, Germany) and 0.24 ml Ketavet (Pharmacia and Upjohn, Erlangen, Germany). By inserting a NiTi-coil spring (GAC International®, Germany) between the blocked incisivi and the first maxillary molar (Fig. 1), the molar was moved mesially by a constant force of 0.5 N for 4 h. The molars of 5 untreated rats served as controls. Upon completion of the experiments, the anesthetized animals were killed and then perfused with phosphate-buffered saline supplemented with 4 % paraformaldehyde for fixation purposes. Afterwards, the maxilla of each animal was dissected, divided into two halves and prepared for light microscopical examination as recently described (Jäger et al. 2005).
Histology and immunohistochemistry
Before being processed for paraffin histology, specimens were decalcified in neutral 10 % ethylene diamine tetra-acetic acid. For orientation purposes, 5- to 7-μm serial sagittal sections were prepared and selected sections were stained with hematoxylin and eosin. Tissue sections were processed for immunohistochemical detection of Hif-1α protein expression using a monoclonal primary antibody of mouse origin against rat Hif-1α (anti-HiF-1α sc-53546; Santa Cruz Biotechnology, USA) in a 1:50 working solution and a secondary HRP conjugate antibody (Dako Invision) or fluorescence antibody (texas red) according to previously established protocols (Gotz et al. 2008; Abuduwali et al. 2013).
Semiquantitative assessment of the Hif-1α protein expression
Randomly chosen light microscopical images (microscope: Axioscope 2 Microscope; camera: Axio-Cam MRC; Carl Zeiss, Germany) were captured per specimen at the dental pulp of the first root of maxillary first molars (magnification ×100). Hif-1α immunoreactivity was analyzed semi-quantitatively in relation to total pulp space by the help of the analyzing software axio vison (Carl Zeiss).
Semiquantitative assessment of the intra-pulpal Hif-1α expression was performed according to a modification of the previously published protocol (Wolf et al. 2012). In brief, the chosen sections of specimen were analyzed for the immune reactivity for Hif-1α. Immunoreactivity was determined semi-quantitatively by assigning one of the following grades to the specimen: 0, no Hif-1α immunoreactivity; 1, weak immunoreactivity with only single cells presenting faint immunoreactions; 2, moderate immunoreactivity with about 50 % close to the tooth dentin area showing a visible Hif-1α protein expression; 3, strong immunoreactivity with about 75 % of the cells close to the tooth dentin; and 4, very strong immunoreactivity: 75 % of cells close to the tooth dentin and in the other pulpal tissue showing a visible Hif-1α protein expression. Reproducibility of the readouts was ensured by analyzing selected specimens in duplicate. An intraobserver error was demonstrated to happen in less than 4 % of the cases and the deviation did not exceed one grade. All measurements were performed by the same investigator. To avoid bias, the investigator was blinded with respect to the origin of the specimens under analysis.
Isolation and cultivation of human dental pulp fibroblasts
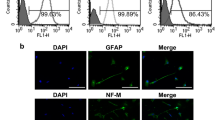
Teeth extracted for orthodontic reasons from healthy individuals (19–30 years old) were collected after informed consent. The collection of teeth was approved by the Ethics Committee of the University of Regensburg. The teeth were washed with sterile phosphate-buffered saline (PBS) immediately after extraction and cracked by a sharp blow with a hammer. The dental pulp tissue was removed from the cracked teeth and washed again with sterile PBS. Then, the pulp tissue was minced, placed onto a 35-mm well culture dish and covered with a sterilized glass cover slip. The tissue was cultured in Dulbecco modified Eagle medium with 10 % FCS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37 °C in a humidified atmosphere of 5 % CO2. After the confluent outgrowth of cells, the dental pulp fibroblasts were trypsinized and propagated onto cell culture flasks. Only cells of the fourth and fifth passage were used for the trial. The successful isolation of dental pulp fibroblasts was confirmed by their fibroblast-like morphology (Fig. 2a) and the expression of characteristic marker genes (Fig. 2b), such as osteonectin (Tsukamoto et al. 1992; Martinez and Araujo 2004), type I and III collagen (Suguro et al. 2008), vimentin (Suguro et al. 2008) and alkaline phosphatase (Tsukamoto et al. 1992).
Induction of in vitro hypoxia in human dental pulp cells
For hypoxic treatment, dental pulp cells were transferred to GasPak® pouches for anaerobic cultures (Becton-Dickinson, Heidelberg, Germany) for 6 h as previously described by Steinbach et al. (2003). Hypoxic cells and supernatants were collected and further processed for transcriptomic and proteomic analyses. All experiments were repeated in triplicates.
Treatment of human dental pulp cells with ROS
To investigate the contribution of oxidative stress to the production of pro-inflammatory cytokines, we stimulated human dental pulp cells with 100 μM hydrogen peroxide (H2O2). The activation of NF-κB protein p65 and Hif-1α were analyzed with the immunoblot technique. The stimulation of cytokine and growth factor expression (IL-6, IL-8, Cox-2 and VEGF) was proved by reverse transcription real-time PCR.
Measurement of oxidative stress by DCF staining in flow cytrometric analysis
Intracellular reactive oxidative species (ROS) was measured using 2′,7′-dichlorodihydrofluorescein-diacetat (DCFH-DA). This organic compound diffuses across cell membranes into cells, in which it is converted to fluorescent dichlorofluorescein by a ROS-mediated chemical reaction. Cells were incubated under hypoxic conditions for 6 h. Then, cells were stained in the medium with 50 μM DCFH-DA (Sigma Aldrich, Taufkirchen, Germany) at 37 °C for 30 min in a humidified atmosphere of 5 % CO2. Cells were detached by trypsin, resuspended in culture medium and collected by centrifugation (3,000g for 5 min). The cell pellet was then washed three times with PBS. After resuspension, fluorescence was determined by flow cytometry (BD FACSCanto; BD Biosciences, Heidelberg, Germany) at an excitation wavelength of 495 nm and an emission wavelength of 530 nm (FL-1).
Protein isolation and immunoblot
Protein from the cytoplasma and nucleus was extracted with the NE-PER Nuclear and Cytoplamic Extraction Kit (Thermo Scientific, USA) and blotted to a PVDF-membrane. The primary antibody used in this trial was rabbit polyclonal anti-p65, anti-actin, anti-lamin a/c and anti-Hif-1α antibody (Abcam, Cambridge, UK). The secondary antibody was anti-rabbit IgG-horseradish-peroxidase (antibodies-online.com; Aachen, Germany). After the membrane had been washed thoroughly, immuno-positive bands were detected by means of chemiluminescence using the Luminata™ Crescendo Western HRP Substrate (EMD Millipore).
RT - Real time PCR analysis
The cell monolayers were rinsed with 1 ml Tri-Reagent (Sigma-Aldrich, St. Louis, USA) and further processed according to the manufacturer’s recommendations to obtain the total RNA of each group. The cDNA synthesis (QuantiTect®Reverse Transcriptase, Qiagen, Germany) comprised 1 μg of the total RNA of each group. Real time PCR was conducted with the SYBR® Green JumpStart™ Taq ReadyMix™ (Sigma-Aldrich) with triplets for each cDNA in Mastercycler® ep realplex (Eppendorf, Hamburg, Germany). Real-time amplifications included an initial step of 10 min at 95 °C (polymerase heat activation) followed by 45 cycles of 10 s at 95 °C (denaturation), 8 s at 60 °C (annealing) and 9 s at 72 °C (elongation and data collection). Gene expression was calculated according to the ΔΔCT-method by Livak and Schmittgen (2001). We chose appropriate intron spanning primers for PCR amplification of RNA-Polymerase polypeptide A (forward primer: 5′-gcaccacgtccaatgaca-3′, reverse primer: 5′-agccatcaaaggagatgacg-3′), Cox-2 (forward primer: 5′-cttcacgcatcagtttttcaag-3′, reverse primer: 5′-tcaccgtaaatatgatttaagtccac-3′), IL-6 (forward primer: 5′-caggagcccagctatgaact-3′, reverse primer: 5′-agcaggcaacaccaggag-3′), IL-8 (forward primer: 5′-agacagcagagcacacaagc-3′, reverse primer: 5′-atggttccttccggtggt-3′) and VEGF (forward primer: 5′-ccttgctgctctacctccac-3′, reverse primer: 5′-ccacttcgtgatgattctgc-3′) using the online Universal ProbeLibrary Assay Design Center from Roche (http://www.roche-applied-science.com/webapp/wcs/stores/servlet/CategoryDisplay?catalogId=10001&tab=Assay+Design+Center&identifier=Universal+Probe+Library&langId=-1#tab-3) to avoid the co-amplification of genomic DNA.
Cytokine quantification in the cell supernatant
The cell supernatant of normoxia-treated and hypoxia-treated cells were collected in Eppendorf tubes and further processed for ELISA detection of IL6 and IL-8 according to the manufacturer’s instructions. The ELISA quantification of IL-6 and IL-8 was conducted by the Institute of Clinical Chemistry at the University Medical Center Regensburg.
Statistical analysis
Data are presented as mean and standard deviation (SD). Student’s t test was employed to compare the results of the reference and test groups.
Results
Detection of hypoxic conditions in dental pulp tissue during the initial phase of orthodontic tooth movement in vivo
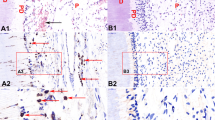
Immunohistochemical analyses of the dental pulp revealed a slight basal expression of Hif-1α immune reactivity within the dental pulp tissue, which was mainly located close to the dentin surface of the tooth root (Fig. 3a, c, e). Following the application of orthodontic forces, an increase in the hypoxia-regulated Hif1-α inside the dental pulp of teeth could be observed (Fig. 3b, d, e). In comparison to the pulp tissue of untreated animals, the amount of Hif1-α-positive cells was observed to be higher within the pulp tissue of orthodontically moved teeth. In treated animals, Hif-1α immunoreactivity was mostly visible in pulp cells at the surface of the root and—to some extent—also in the entire tissue of the root canal (Fig. 3).
Immunohistochemical and florescence staining (anti-Hif-1α sc-53546; Santa Cruz, USA) shows the root canal and the pulp side of the first maxillary molar of the rat before (a, c) and after (b, d) a 4-h orthodontic treatment. In untreated specimens, a weak basal Hif-1α immune reactivity (arrows) could be observed, which was up-regulated after the application of orthodontic forces, DAB staining with magnification ×200, fluorescence staining with magnification ×100; dentin (d), dental pulp (p). e Semiquantitative assessment of orthodontic tooth movement-induced changes in Hif-1α protein expression. Each value represents the mean ± SD for five animals per group. *P < 0.05, experimental groups versus untreated control
Cellular effects of short-term hypoxia (6 h) on human dental pulp cells
The induction of hypoxic conditions in dental pulp cells resulted in enhanced up-regulation of the typical hypoxic marker Hif-1α in immunoblot analyses (Fig. 4). Hif-1α was not stably expressed under normoxic conditions in control experiments in human dental pulp fibroblasts. Hypoxic conditions in dental pulp cells further induced pro-inflammatory and angiogenic responses as shown by the significant increase in IL-6, IL-8, Cox-2 and VEGF mRNA expression in response to hypoxia (Fig. 5a). In addition, the inflammatory response was also observed by means of the enhanced secretion of IL-6 and IL-8 levels in the cell supernatants of the treated cells (Fig. 5b). After hypoxic treatment, a significant change in DCF fluorescence could be detected due to increased oxidative stress (Fig. 6).
Immunoblot analyses of the hypoxic marker Hif-1α (anti- Hif-1α; Abcam, Cambridge, UK) in dental pulp fibroblasts. Hypoxia was induced by cultivating cells in the absence of oxygen in an anaerobic chamber for 6 h. Hypoxia enhances the expression of the intracellular hypoxic marker Hif1α in treated cells. Untreated controls did not show any Hif1α expression
a Changes in the expression of the pro-inflammatory factors Cox-2, IL-6 and IL-8 and the angiogenic growth factor VEGF at a transcriptional level. Gene expression: VEGF was 4.2 ± 0.1-fold up-regulated, IL-6 was 4.9 ± 0.7-fold up-regulated, IL-8 was 4.5 ± 0.5-fold up-regulated and Cox-2 was 7.1 ± 0.2-fold up-regulated; n = 6. b Quantification of released pro-inflammatory cytokines to cell supernatant after hypoxic treatment in an anaerobic chamber. Hypoxic conditions induced the release of a significant amount of IL-6 (62.6 ± 2.3 pg/ml) and IL-8 (2,418.5 ± 148.9 pg/ml) proteins into the cell supernatant compared to untreated controls (IL-6: 48.5 ± 3.1 pg/ml; IL-8: 1,942.6 ± 129.9 pg/ml). The bar graph shows the mean ± SD of 6 independent cultures that were assayed in duplicate. *P < 0.05, hypoxia treated group versus vehicle control
Evidence of enhanced oxidative stress in hypoxic dental pulp fibroblasts shown by a a curve shift of DCF fluorescence and b a comparison of the geometric mean of DCF fluorescence in flow cytometric analyses. After 6 h of hypoxic cell culture conditions, DCF fluorescence was significantly changed compared to untreated control cells. *P < 0.05, hypoxia treated group versus vehicle control
Effect of oxidative stress on human dental pulp cells
After stimulation with oxidative stress, we observed increased expression of the cytokine marker IL-6, IL-8 and Cox-2 and of the angiogenic growth factor VEGF (Fig. 7). Furthermore, our data clearly showed that oxidative stress leads to enhanced accumulation of the NF-κB marker p65 and Hif-1α in the nuclear fraction under normoxic conditions (Fig. 8a, b).
Stimulatory effect of H2O2 treatment on pro-inflammatory cytokine and angiogenic growth factor expression. After a 6-h treatment, all investigated markers showed a significant increase compared to untreated controls. These changes were comparable to those of cell culture experiments in the anaerobic chamber. Data are representative of two independent experiments, both yielding comparable results. Each value is the mean ± SD for 6 independent cultures. *P < 0.05, experimental group versus vehicle-treated control
a Effect of oxidative stress on the accumulation of NF-κB by means of changes of p65 in nuclear fraction after induction of oxidative cell stress (100 μM H2O2) for 3 and 6 h. b Effect of H2O2 treatment on transient Hif-1α stability. The induction of oxidative stress by the administration of 100 μM H2O2 resulted in transient stability of Hif-1α after a 3-h treatment compared to untreated cell cultures under normoxic conditions. The figure exemplarily represents the result of 6 independent cultures assayed in duplicate
Discussion
To evidence the transient depletion of oxygen during the initial phase of orthodontic treatment in dental pulp tissue, we investigated the expression of the transcription factor Hif-1α. The expression of this transcription factor enables the cell to survive under hypoxic conditions by up-regulating numerous genes, such as the vascular endothelial growth factor (VEGF), erythropoietin (EPO), or lactate dehydrogenase (LDH), which allow adaptive reactions by stimulating neoangiogenesis and by regulating glycolysis (Weidemann and Johnson 2008). The stable expression of Hif-1α is regulated by oxygen-sensing Hif-hydroxylases. These hydroxylases are active under normoxic conditions and hydroxylate the Hif-1α on prolyl residues, which promote the Von Hippel-Lindau tumor suppressor protein to recognize the Hif-1α for ubiquitination and rapid degradation by the proteasom (Weidemann and Johnson 2008). In the case of hypoxia, Hif-1α escapes from the oxygen-requiring hydroxylation process and translocates into the nucleus to trigger the hypoxic response. The up-regulation of Hif-1α expression in the dental pulp tissue of orthodontically-treated teeth in vivo consequently shows an adaptive response of the dental pulp and also ensures the vitality of the tissue under orthodontic force application. The fact that there is also a faint basal expression of Hif-1α under untreated conditions, which is up-regulated under hypoxia, agrees with the findings of Stroka et al. (2001). These authors observed stable Hif-1α protein expression in different nomoxic tissues, such as the heart muscle, liver, brain and kidney. These authors suggested that certain human tissues under normoxic conditions stably express Hif-1α in order to regulate tissue homeostasis. A further possibility for the weak basal Hif-1α expression is the assumption that the oxygen concentration in the dental pulp might be below a threshold that enables weak stability of Hif-1α, since Hif-1α was not expressed in dental pulp fibroblast under normoxic conditions in vitro. In accordance with our findings, data published by Derringer and Linden (2004) also showed the enhanced expression of angiogenic factors, such as the fibroblast growth factor-2 (FGF-2), the platelet-derived growth factor (PDGF), the transforming growth factor-β (TGF-β) and VEGF, in pulp tissues subjected to orthodontic force, which are under transcriptional control of the transcription factor Hif-1α (Weidemann and Johnson 2008).
Besides the increase of the hypoxic factor Hif-1α, a further important finding was the increased expression of pro-inflammatory factors, such as Cox-2, IL-6 and IL-8, by dental pulp fibroblasts in response to hypoxia. The production of pro-inflammatory cytokines in the dental pulp tissue in response to orthodontic loading has been discussed as being exclusively caused by neurogenic inflammation (Norevall et al. 1995; Yamaguchi et al. 2004). A number of different neuropeptides, including substance P and calcitonin gene-related peptide, were reported by Norevall et al. (1995) to be involved in the inflammation of the dental pulp during orthodontic tooth movement. These neuropeptides are derived from nerve fibers that supply the periodontium and the dental pulp. Moreover, substance P and calcitonin gene-related peptide were found to be potent to stimulate dental pulp cells to produce IL-1β, IL-6, prostaglandin-E2 and RANKL (Yamaguchi et al. 2004; Kojima et al. 2006). In our cell biological trial, we observed that short-term hypoxia due to circulatory disturbances may be an important factor for stimulating inflammation in the dental pulp in the initial phase of orthodontic tooth movement. Numerous trials have shown that hypoxia affects the activity of the nuclear factor-kB (NF-κB) transcription factor family (Eltzschig and Carmeliet 2011). Members of the NF-κB proteins (p65, RelB, RelC, p50, p52) regulate cellular homeostasis, anti-apoptosis and apoptosis and inflammation. For instance, NF-κB controls the expression of pro-inflammatory cytokines (IL-1, IL-6, IL-8 and TNF-α), adhesion molecules (E-selectin) and pro-inflammatory enzymes (e.g., Cyclooxygenase-2 or nitric oxide synthase). Moreover, the hypoxic activation of NF-κB is considered to enable cells to survive a period of hypoxic conditions by activation of anti-apoptotic genes. Hypoxic activation of NF-κB is, at least, due to the activation of IkB kinase (IKK) activation in the canonical pathway. But other alternative activation pathways, such as the oxygen-sensing hydroxylases responsible for directing Hif-1α stability and activity, are considered to regulate components of the NF-κB pathway (Cockman et al. 2006; Cummins et al. 2006). Based on this information and the present data, a regulatory effect of hypoxic stress in dental pulp cells can be expected over the course of orthodontic tooth movement.
A characteristic feature of hypoxic cells is the generation of reactive oxygen species (ROS). Cellular ROS, similar to superoxide or hydrogen peroxide, is generated in response to hypoxia by the mitochondrial electron transport chain. By means of dental pulp cells, we showed the supporting effect of oxidative stress on the expression of pro-inflammatory factors. Similarly, Chae et al. (2011) proved that anti-oxidants reduce the production of pro-inflammatory cytokines in PDL cells and slow the mobility of orthodontically treated molars. A number of trials have indicated that ROS influence the activity of redox-responsive transcription factors. We demonstrated in our trial that dental pulp cells accumulate NF-κB protein p65 upon challenge with moderate concentrations of H2O2. The stimulatory effect of H2O2 on NF-κB activation highly depends on the cell type and oxidative stress does not generally stimulate NF-κB activation (Li and Karin 1999). It is conceivable that the hypoxia-induced production of ROS may be a further factor for enhancing the activation of NF-κB and for promoting both anti-apoptotic and inflammatory processes.
Conclusion
We have shown up-regulation of the transcription factor Hif-1α upon oxygen depletion in dental pulp tissue as a result of circulatory disturbances in the initial phase of orthodontic tooth movement in vivo. We provided further information about the regulatory effects of short-term hypoxia on dental pulp fibroblasts in vitro. These findings showed that orthodontic tooth movement induces periodontal, as well as intra-pulpal tissue reactions in treated teeth. Furthermore, our findings suggest that orthodontic force application during orthodontic therapy may result in oxygen depletion in dental pulp tissue, which activates adaption responses, as well as inflammatory processes. The regulation of hypoxia-induced protein expression shows the immunomodulatory role of dental pulp fibroblasts during the course of orthodontic tooth movement. This shows the involvement of the dental pulp in the remodeling process during orthodontic tooth movement, thus extending the well-accepted knowledge of hypoxia-related remodeling within the periodontal ligament.
References
Abuduwali N, Lossdorfer S, Winter J, Wolf M, Gotz W, Jager A (2013) Autofluorescent characteristics of human periodontal ligament cells in vitro. Ann Anat. doi:10.1016/j.aanat.2013.03.007
Bauss O, Schafer W, Sadat-Khonsari R, Knosel M (2010) Influence of orthodontic extrusion on pulpal vitality of traumatized maxillary incisors. J Endod 36(2):203–207
Brin I, Ben-Bassat Y, Heling I, Engelberg A (1991) The influence of orthodontic treatment on previously traumatized permanent incisors. Eur J Orthod 13(5):372–377
Caviedes-Bucheli J, Moreno JO, Ardila-Pinto J, Del Toro-Carreno HR, Saltarin-Quintero H, Sierra-Tapias CL, Macias-Gomez F, Ulate E, Lombana-Sanchez N, Munoz HR (2011) The effect of orthodontic forces on calcitonin gene-related peptide expression in human dental pulp. J Endod 37(7):934–937
Caviedes-Bucheli J, Munoz HR, Azuero-Holguin MM, Ulate E (2008) Neuropeptides in dental pulp: the silent protagonists. J Endod 34(7):773–788
Chae HS, Park H, Hwang HR, Kwon A, Lim W, Yi WJ, Han D, Kim YH, Baek J (2011) The effect of antioxidants on the production of pro-inflammatory cytokines and orthodontic tooth movement. Mol Cell 32(2):189–196
Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, Pugh CW, Oldham NJ, Masson N, Schofield CJ, Ratcliffe PJ (2006) Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH). Proc Natl Acad Sci USA 103(40):14767–14772
Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT (2006) Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 103(48):18154–18159
Derringer KA, Linden RWA (2004) Vascular endothelial growth factor, fibroblast growth factor 2, platelet derived growth factor and transforming growth factor beta released in human dental pulp following orthodontic force. Arch Oral Biol 49(8):631–641
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364(7):656–665
Gotz W, Gerber T, Michel B, Lossdorfer S, Henkel K, Heinemann F (2008) Immunohistochemical characterization of nanocrystalline hydroxyapatite silica gel (NanoBone(r)) osteogenesis: a study on biopsies from human jaws. Clin Oral Implants Res 19(10):1016–1026
Goz GR, Rahn BA, Schulte-Monting J (1992) The effects of horizontal tooth loading on the circulation and width of the periodontal ligament–an experimental study on beagle dogs. Eur J Orthod 14(1):21–25
Hamersky PA, Weimer AD, Taintor JF (1980) The effect of orthodontic force application on the pulpal tissue respiration rate in the human premolar. Am J Orthod 77(4):368–378
Henneman S, von den Hoff JW, Maltha JC (2008) Mechanobiology of tooth movement. Eur J Orthod 30(3):299–306
Jäger A, Zhang D, Kawarizadeh A, Tolba R, Braumann B, Lossdörfer S, Götz W (2005) Soluble cytokine receptor treatment in experimental orthodontic tooth movement in the rat. Eur J Orthod 27(1):1–11. doi:10.1093/ejo/cjh089
Kojima T, Yamaguchi M, Kasai K (2006) Substance P stimulates release of RANKL via COX-2 expression in human dental pulp cells. Inflamm Res 55(2):78–84
Kvinnsland S, Heyeraas K, Ofjord ES (1989) Effect of experimental tooth movement on periodontal and pulpal blood flow. Eur J Orthod 11(3):200–205
Li N, Karin M (1999) Is NF-kappaB the sensor of oxidative stress? FASEB J 13(10):1137–1143
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25(4):402–408
Martinez EF, Araujo VC (2004) In vitro immunoexpression of extracellular matrix proteins in dental pulpal and gingival human fibroblasts. Int Endod J 37(11):749–755
Meikle MC (2006) The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 28(3):221–240
Norevall LI, Forsgren S, Matsson L (1995) Expression of neuropeptides (CGRP, substance P) during and after orthodontic tooth movement in the rat. Eur J Orthod 17(4):311–325
Perinetti G, Varvara G, Festa F, Esposito P (2004) Aspartate aminotransferase activity in pulp of orthodontically treated teeth. Am J Orthod Dentofac Orthop 125(1):88–92
Steinbach JP, Wolburg H, Klumpp A, Probst H, Weller M (2003) Hypoxia-induced cell death in human malignant glioma cells: energy deprivation promotes decoupling of mitochondrial cytochrome c release from caspase processing and necrotic cell death. Cell Death Differ 10(7):823–832
Stenvik A, Mjor IA (1970) Pulp and dentine reactions to experimental tooth intrusion. A histologic study of the initial changes. Am J Orthod 57(4):370–385
Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D (2001) HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15(13):2445–2453
Suguro H, Asano M, Kaneko Y, Omagari D, Ogiso B, Moro I, Komiyama K (2008) Characterization of human dental pulp-derived cell lines. Int Endod J 41(7):609–616
Tsukamoto Y, Fukutani S, Shin-Ike T, Kubota T, Sato S, Suzuki Y, Mori M (1992) Mineralized nodule formation by cultures of human dental pulp-derived fibroblasts. Arch Oral Biol 37(12):1045–1055
Veberiene R, Smailiene D, Danielyte J, Toleikis A, Dagys A, Machiulskiene V (2009) Effects of intrusive force on selected determinants of pulp vitality. Angle Orthod 79(6):1114–1118
von Böhl M, Ren Y, Fudalej PS, Kuijpers-Jagtman AM (2012) Pulpal reactions to orthodontic force application in humans: a systematic review. J Endod 38(11):1463–1469
Weidemann A, Johnson RS (2008) Biology of HIF-1alpha. Cell Death Differ 15(4):621–627
Wolf M, Lossdorfer S, Abuduwali N, Meyer R, Kebir S, Gotz W, Jager A (2012) Effect of intermittent PTH(1–34) on human periodontal ligament cells transplanted into immunocompromised mice. Tissue Eng A 18(17–18):1849–1856
Yamaguchi M, Kasai K (2007) The effects of orthodontic mechanics on the dental pulp. Semin Orthod 13(4):272–280
Yamaguchi M, Kojima T, Kanekawa M, Aihara N, Nogimura A, Kasai K (2004) Neuropeptides stimulate production of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in human dental pulp cells. Inflamm Res 53(5):199–204
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Römer, P., Wolf, M., Fanghänel, J. et al. Cellular response to orthodontically-induced short-term hypoxia in dental pulp cells. Cell Tissue Res 355, 173–180 (2014). https://doi.org/10.1007/s00441-013-1739-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1739-y