Abstract
Growth/differentiation factor-15 (GDF-15) is a distant member of the transforming growth factor-β superfamily and is ubiquitously expressed in the central nervous system. It is prominently upregulated in cerebral cortical and ischemic lesion paradigms. GDF-15 robustly promotes the survival of lesioned nigrostriatal dopaminergic neurons in vivo; GDF-15-deficient mice exhibit progressive postnatal motor and sensory neuron losses implying essential functions of GDF-15 in neuronal survival. We show that GDF-15 mRNA and protein are, respectively, six-fold and three-fold upregulated in the murine retina at 1 day after optic nerve crush, slightly elevated mRNA levels being maintained until day 28. However, the magnitude and time course of retinal ganglion cell (RGC) death are indistinguishable in knockout and control mice. Selected mRNAs implicated in the regulation of the death vs. survival of RGCs, including ATF3, Bad, Bcl-2 and caspase-8, were similarly regulated in both knockout and control retinae. Immunohistochemistry for tyrosine hydroxylase and choline acetyltransferase revealed no differences in staining patterns in the two genotypes. mRNA and protein levels of galanin, a putative neuroprotective factor and positive regulator of neuron survival and axonal regeneration, were prominently upregulated after crush in knockout retinae at day 3, as compared with control retinae, suggesting that GDF-15 acts as a physiological regulator of galanin. GDF-15 is therefore prominently upregulated in the retina after optic nerve crush but does not directly interfere with the magnitude and temporal progression of RGC death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth/differentiation factor-15 (GDF-15) is a distant member of the transforming growth factor-β superfamily and has a wide distribution in many mammalian tissues, including the central nervous system (CNS). GDF-15 was discovered in our laboratory (Böttner et al. 1999a, 1999b) and we have begun to study it with respect to its functions in the CNS. GDF-15 is a potent pro-survival factor for several neuron populations in vitro and in vivo, including dopaminergic nigrostriatal neurons (Strelau et al. 2000), spinal cord and brainstem motor neurons (Strelau et al. 2009), sensory neurons in spinal ganglia (Strelau et al. 2009) and cerebellar granule neurons (Subramaniam et al. 2003). In a paradigm of CNS ischemia, i.e., occlusion of the middle cerebral artery and in a cold lesion paradigm of the cortex, GDF-15 has been found to be prominently upregulated (Schindowski et al. 2011; Schober et al. 2001) in neurons.
For further analysis of neural functions of GDF-15, we have generated a GDF-15 knockout mouse (Strelau et al. 2009). GDF-15 knockout mice have a normal life span, are fertile and lack an immediately obvious phenotype. Initial analyses suggest that the dopaminergic nigrostriatal system does not seem to be affected but spinal motor and dorsal root ganglionic sensory neurons undergo progressive postnatal death, up to a maximum of 20% being reached at the age of 4.5 months (Strelau et al. 2009). Collectively, these data suggest that GDF-15 acts as a potent pro-survival factor for distinct classes of neurons.
Although GDF-15 is ubiquitous throughout the brain, its mRNA and protein levels are low compared with levels in organs such as the liver, lung and kidney. In the unlesioned CNS, the choroid plexus is the only site in which GDF-15 mRNA and protein can be readily localized by in situ hybridization and immunohistochemistry. However, following lesions, GDF-15 mRNA and protein become easily recognizable in neurons both at the lesion site and in neuron populations projecting to the lesioned area (Schober et al. 2001). Whether GDF-15 is released from neurons and what its functions are in detail are still obscure.
Lesions of the optic nerve constitute both an important clinical entity and a well-established model system for exploring molecular and cellular aspects of de- and regeneration in the CNS (Fischer 2012; Lingor et al. 2012; Schmeer et al. 2012; Thanos et al. 2012). Major advantages over other CNS lesion paradigms include easy access and well-described molecular and cellular local changes, together with retro- and anterograde alterations, within the retina and in the lateral geniculate body and superior colliculus. The alterations include the death of particular cell types, in particular retinal ganglion cells (RGCs), demyelination and formation of myelin debris and complex inflammatory scenarios involving macrophages, microglia, astrocytes and Müller glia of the retina (Sandvig et al. 2004). Surviving RGCs mostly fail to regenerate axons into the lesioned optic nerve, a failure that is attributed to the inhibitory extrinsic environment and a diminished regenerative capacity of mature CNS neurons (Schwab and Bartholdi 1996; Fischer 2012; Borrie et al. 2012; Foscarin et al. 2012; Pernet and Schwab 2012; Stuermer 2012). The lack of regeneration is caused by inhibitory molecules within the myelin, by the injury-induced glial scar (Schwab 2004; Silver and Miller 2004; Yiu and He 2006) and by accumulating macrophages (Horn et al. 2008; Busch et al. 2009). Thus, concepts and practical approaches for overcoming the many adverse conditions for optic nerve regeneration have to address at least RGC survival and the elimination of inhibitory cues for axon growth. Although significant progress has been made in recent years with regard to the neuroprotection and activation of RGCs, including the elucidation of the signaling pathways that activate the intrinsic growth program and overcome inhibitory signaling (Fischer 2012), the goal of satisfactory RGC protection and regeneration is still distant. The present study has been performed to elucidate whether GDF-15 wildtype (wt) and knockout mice will reveal the potential of endogenous GDF-15 to protect RGC and to regulate selected retinal genes that have been implicated in RGC survival and regeneration following optic nerve crush.
Materials and methods
Animal handling
All animal treatments were conducted with permission from the responsible authority of the University Clinic Freiburg. C57BL6/J wt mice were provided by the animal facility of the BioMed Zentrum, University Clinic Freiburg. The GDF-15lacZ/lacZ mouse colony was established in Heidelberg (Strelau et al. 2009) on a C57BL6/J background and was re-established in Freiburg by embryo transfer. The animals were held under standard conditions, with a 12/12-h day/night cycle. Food and water were provided ad libitum. All animals were 8–10 weeks old.
Surgery
Animals were anesthetized by inhalation of isoflurane (Forane). The left optic nerve was injured by intra-orbital optic nerve crush (IONC) with watchmaker forceps (Dumont 5), according to a well-established method (Parrilla-Reverter et al. 2009). Briefly, the episcleral fascia was removed on the temporal side of the eye. The nerve was then crushed for 15 s at 1–2 mm behind the bulbus. The animals were killed by cervical translocation.
Immunohistochemistry
Staining of GDF-15 and retinal markers was performed on cryosections. Eyes were placed in 4% paraformaldehyde (PFA) overnight. Following postfixation, they were then placed in 30% sucrose for up to 24 h for cryoprotection and finally frozen in Tissue-Tec (Sakkura) on dry ice. Cryosections (10 μm) were cut on a cryostat (CM 3050S Leica) and mounted on Superfrost microscope slides. A custom-made rabbit anti-GDF-15 antibody (Immunoglobe) was applied at a 1:200 dilution, followed by biotin-conjugated anti-rabbit secondary antibody (1:500, Jackson 711-065-152) and visualized by Cy-3-labeled Streptavidin (1:500, Jackson 016-160-084). Antibodies against Brn3a (marker of RGC; goat, 1:500, Santa Cruz, sc-31984), choline acetyltransferase (ChAT; goat, 1:100, R&D, AB144) and tyrosine hydroxylase (TH; rabbit, 1:1000, Millipore, AB152) were used to identify subtypes of retinal neurons in wt and knockout mice. Staining was visualized with Cy-2/Alexa-488 conjugates (donkey anti rabbit-Cy2, 1:500, Jackson 711-225-152; donkey anti goat-Alexa 488, 1:500, GE lifesciences A11055).
The density of RGCs was manually determined on retina whole-mounts. The staining procedure has been described by Nadal-Nicolás et al. (2009). Briefly, retinae were dissected immediately after the removal of the eyes and placed in 4% PFA overnight. All the following steps were performed by using PBST (phosphate-buffered saline pH 7.4 with 0.1% Triton X-100). Retinae were washed with PBST (3×15 min), followed by a blocking step with 3% H2O2 in PBST containing 3% normal donkey serum (NDS) for 1 h at room temperature (RT). The tissues were then washed in PBST and blocked in PBST containing 10% NDS for 2–3 h at RT. A polyclonal goat anti-Brn3a antibody (Santa Cruz) was applied at a dilution of 1:300 in PBST/10% NDS for 48 h at 4°C. Tissues were then washed three times for 1 h. The secondary antibody, a donkey anti-goat antibody coupled with biotin (Jackson ImmunoResearch 705-65-147, 1:500), was then applied at 4°C overnight. The ABC reagent (Vector Laboratories, PK-6100) was applied at RT for 3–4 h. Diaminobenzidine was used to visualize the staining.
RGC quantification
The density of RGCs was determined by staining retinal wholemounts against Brn3a. Cells were counted in 20 random fields in each retina at 400× magnification (Zeiss Axioskop). The cells were counted in an area of 300×375 μm2. The density of RGCs was expressed as RGCs per square millimeter. To calculate the density, we considered the mean number of RGCs/field that resulted from all counted fields of three retinae, in each group (for statistics, see below).
Quantitative real-time polymerase chain reaction
The isolated tissues were collected in RNALater and kept at 4°C for up to 2 weeks, until all the groups (unlesioned control and 6 h, 1, 3, 7, 14 and 28 days post-surgery) were collected. The tissues were transferred to TriFast (Peqlab) and homogenized in Precellys tubes (Peqlab). For the isolation of RNA, we used a modification of the classical guanidinium thiocyanate-phenol-chloroform extraction method with bromo-chloro-propane instead of chloroform (Chey et al. 2011). The extracted RNA was purified on columns (RNA Clean & Concentrator-25, Zymo Research). The purified RNA was immediately reverse-transcribed with GoScript Reverse Transcriptase (Promega) according to the recommendations of the manufacturer. Quantitative reverse transcription plus the polymerase chain reaction was performed in MyIQ (Bio-Rad) with GoTaq qPCR Master Mix (Promega). The following primers were used: GDF15, Fw: gagctacggggtcgcttc, Rv: gggaccccaatctcacct; Atf3, Fw: gctggagtcagttaccgtcaa Rv: cgcctccttttcctctcat; caspase-8, Fw: gaggctgacttcctgtatgctt, Rv:aaccacgacccgtccttt; Bad, Fw: ggagcaacattcatcagcag, Rv: tacgaactgtggcgactcc; Bcl-2, Fw: gtacctgaaccggcatct, Rv: ggggccatatagttccacaa; galanin, Fw: tttctcagtttcttgcaccttaaa, Rv: cagtggacatggtctcagga. The fold-change of mRNA expression was calculated by the ΔΔCt method (for statistics, see below).
Western blot
Total protein extraction was performed as described by Chey et al. (2011). Equal amounts of 25 μg protein were loaded per lane on a 15% denaturing SDS-polyacrylamide gel, separated by SDS-polacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Peqlab 39-4010, 0.2 μm) by electroblotting. After a blocking step with 5% non-fat milk, rabbit anti-GDF-15 antibody (1:500, custom-made, Immunoglobe) or a goat anti-galanin antibody (1:300, Santa Cruz sc-16411) was applied and detected by horseradish-peroxidase (HRP)-linked secondary antibody (anti-rabbit-HRP, 1:5000, Cell Signaling 7074s; anti-goat-HRP, 1:10,000, DAKO P0449). D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; rabbit-anti-GAPDH, 1:1000, Cell Signaling 2118) was used as a loading control. The signal was visualized by ECL Plus Western Blotting Detection Reagents (GE Lifesciences RPN2132) and film (GE Lifesciences 28-9068-35). The optical density of the bands on the film was measured by Image J software and used for quantification.
Statistical analysis
Data are presented as means ± SD. Significant differences were determined either by a one-way analysis of variance (ANOVA) test with Tukey’s post hoc test or by the Kruskal-Wallis test together with Dunn’s multiple comparison test by Graphpad Software.
Results and discussion
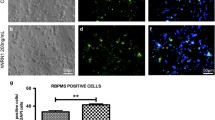
We first explored sites of protein expression of GDF-15 in the retina. As shown in Fig. 1a–f, both RGCs and cells within the inner nuclear layer (INL) were immunopositive for GDF-15. Retinae from knockout mice served as a control. We next investigated whether optic nerve crush had an impact on GDF-15 mRNA and protein expression levels in the retina. As shown in Fig. 2a, GDF-15 mRNA levels were six-fold elevated over control levels at 1 day after lesioning. At 3 days, GDF-15 mRNA was still three-fold increased. A two-fold increase was maintained from 7 days up to 28 days post-lesioning. GDF-15 protein levels were three-fold upregulated at 1 day after lesioning and subsequently normalized (Fig. 2b, c). Figure 1g–l shows that optic nerve lesion increased GDF-15 immunoreactivity in both RGCs and INL. Together, these data suggest that GDF-15 mRNA and protein levels in the retina are increased in response to optic nerve crush, with a peak at 1 day and a moderate chronic elevation of mRNA levels until 4 weeks.
a–f Growth/differentiation factor-15 (GDF-15) immunoreactivity (red) in the retina. Note positive retinal ganglion cells (RGCs) and cells in the inner nuclear layer (INL). Retinae from knockout (ko) mice served as a control (wt wildtype). Unspecific immunoreactivity is located beyond photoreceptor outer segments (DAPI nuclear stain [blue], GCL ganglion cell layer, ONL outer nuclear layer). Bars 20 μm. g–l Increase in GDF-15 immunoreactivity induced in RGCs and INL at 1 day after optic nerve lesion (Brn3a marker of RGC [green]). Bar 20 μm
Expression of GDF-15 in the murine retina at various time points (h hours, d days) following optic nerve crush. a GDF-15 mRNA levels in the retina, normalized to D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), compared with the unlesioned (control) retina. b GDF-15 protein levels in the retina following optic nerve crush. Values were determined by relative optical density of the Western blot signal, normalized to GAPDH and compared with control retinae. Values in a, b represent means ± SD from three independent experiments. Statistical significance of differences was determined by one-way analysis of variance (ANOVA) followed by Tukey’s test. *P<0.05, **P<0.01, *** P<0.001. c Representative Western blot
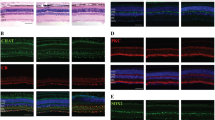
GDF-15 has been shown to act as a neurotrophic factor on several neuron populations in vitro and in vivo, including dopaminergic neurons in the substantia nigra (Strelau et al. 2000), spinal cord and brain stem motoneurons, sensory neurons of spinal ganglia and sympathetic neurons (Strelau et al. 2009). We therefore investigated whether the acute elevation of GDF-15 expression in the retina at 1 day post-lesioning and the consecutive chronic upregulation over 1 month counteracted the apoptotic cell death that RGCs undergo after optic nerve injury (Allcutt et al. 1984; Villegas-Pérez et al. 1993; Berkelaar et al. 1994; Fischer et al. 2004; Fischer 2012). As shown in Fig. 3a–f, whole-mount retinae from wt and GDF-15 knockout mice stained with a Brn3a antibody clearly revealed substantial neuron losses but no overt differences in the densities and patterns of RGC at 1 week and 2 weeks after optic nerve crush. Quantification of RGC numbers (Fig. 3g) showed the established temporal and quantitative pattern of RGC loss over 1 week and 2 weeks, with no significant differences between wt and GDF-15 knockout mice. Thus, GDF-15 expressed and upregulated in the retina after optic nerve crush does not alter the temporal decline of RGC losses after optic nerve lesion.
Loss of RGCs following optic nerve crush over time. a–f RGCs were visualized by staining with an antibody to Brn3a. a–c GDF-15 wildtype (wt) mice. d–f GDF-15 knockout (ko) mice. Bar 100 μm. g Quantification (con control, w week). Values represent means ± SD from three independent experiments. Statistical significance was determined by Kruskal-Wallis test followed by Dunn’s multiple comparison test. *P<0.05, **P<0.01, ***P<0.001
We next investigated whether optic nerve crush induced distinct patterns in the expression of genes involved in the life/death decision and putative regenerative ability of retinal neurons in GDF-15 wt and knockout mice. Atf3 is a member of the ATF/c-AMP-responsive element-binding protein (CREB) family of bZIP transcription factors (Moore and Goldberg 2011). Atf3 expression levels are low in most cell types but increase following injury, such as optic nerve trauma and chronic glaucoma (Takeda et al. 2000; Guo et al. 2011). Figure 4a documents a more than five-fold upregulation of Atf3 mRNA at 1 and 7 days after optic nerve crush and a similar upregulation occurred in GDF-15-deficient animals (Fig. 4b). Upregulation of Atf3 might be indicative of the successful prevention of apoptosis (Zhang et al. 2011) and the promotion of axon regeneration (Seijffers et al. 2007). However, other scenarios are also found, e.g., its forced overexpression in neurons, which fails to overcome their inability to grow axons on inhibitory substrates or regenerate axons into the CNS (Seijffers et al. 2007). In the present context, the identical upregulation of Atf3 mRNA in GDF-15 wt and knockout mice probably reflects indistinguishable RGC losses in the two phenotypes and the inability of upregulated GDF-15 to protect a significant number of RGCs from death.
mRNA levels of selected genes with relevance to life-death decisions of neurons determined by quantitative polymerase chain reaction in GDF-15 wildtype (wt; a) and knockout (ko; b) retinae at days 1 and 7 following optic nerve crush. Fold-changes were normalized by GAPDH relative to the control. With the exception of caspase-8 mRNA (Caspase8), levels of Atf3 (ATF3), Bad (BAD) and Bcl-2 (BCL2) were not significantly different in GDF-15 wt and knockout mice. Values represent means ± SD from three independent experiments. Statistical significance of differences was determined by one-way ANOVA followed by Tukey’s test. *P<0.05, **P<0.01, *** P<0.001
The Bcl-2 family of signaling proteins acts as effectors of death or survival signals. Bad is a proapoptotic member of the Bcl-2 family, although its proapoptotic function is inhibited by phosphorylation at multiple sites (Chipuk et al. 2010; Hojabrpour et al. 2012). Bcl-2 is also critical for the apoptotic process (Reed 1994). Following optic nerve crush, Bad and Bcl-2 mRNAs were not significantly upregulated at 1 and 7 days post-lesioning, either in GDF-15 wt or GDF-15 knockout mice (Fig. 4a, b). Moreover, immunostaining of the retina with an antibody to phosphorylated Bad at 1 and 7 days post-lesioning did not reveal overt differences in staining patterns and intensities in the two genotypes (not shown).
Caspases are a family of cysteine proteases that regulate apoptosis (Inoue et al. 2009; Yi and Yuan 2009). Caspase-8 is a downstream effector of caspase-6 and is activated downstream of caspase-6 in the injured retina (Monnier et al. 2011). Its specific inhibition promotes the survival of RGCs and regeneration to a similar extent as blockade of caspase-6. We found that caspase-8 was not significantly upregulated on day 1 and 7 in wt retinae after optic nerve crush but was slightly induced on day 7 in knockout retinae (Fig. 4a, b).
To investigate whether the presence of GDF-15 had implications for the status of the dopaminergic and cholinergic systems in the retina following optic nerve crush, we compared TH and ChAT immunostaining in GDF-15 wt and knockout retinae. Retinal dopamine, co-released with gamma aminobutyric acid (GABA; Hirasawa et al. 2012), plays many roles in high-resolution light-adapted vision, contrast sensitivity, acuity and circadian rhythms as revealed by a recent analysis of a retina-specific deletion of TH (Jackson et al. 2012; Yang et al. 2013). Cholinergic transmission in the retina is responsibe, inter alia, for generating cholinergic retinal waves; acetylcholine-GABA cotransmission is crucial for motion sensitivity and direction selectivity (Elstrott et al. 2008; Lee et al. 2010; Ford et al. 2012). Our analysis of TH and ChAT immunoreactivities failed to reveal overt differences between both GDF-15 genotypes (not shown).
The neuropeptide galanin is upregulated in several types of neurons upon peripheral nerve injury and in CNS lesions and can act as a neuroprotective and growth-promoting factor (Ubink et al. 2003; Hökfelt and Tatemoto 2010; Holm et al. 2012; Zigmond 2001). Galanin and galanin receptors are also expressed in the retina (Hökfelt et al. 1992; Borowsky et al. 1998). As shown in Fig. 5a, galanin mRNA is robustly upregulated in the retina of GDF-15 knockout mice at 3 days after optic nerve crush and is still significantly elevated after 7 days. In contrast, galanin mRNA levels are not altered in GDF-15 wt mice. Peak mRNA expression at 3 days coincides with a robust increase in protein expression (Fig. 5b). Whereas these data might suggest a role for GDF-15 as a physiological regulator of galanin expression in the retina, they are unlikely to support the notion of galanin being a neurotrophic factor for RGCs.
Expression of galanin mRNA (a) and protein (b) in GDF-15 wildtype (wt) and knockout (ko) mice at days 3 and 7 after optic nerve crush. Note the robust upregulation of galanin mRNA in knockout retinae at day 3 after injury, with no significant regulation occurring in wt retinae. Values represent means ± SD from three independent experiments. Statistical significance of differences was determined by one-way ANOVA followed by Tukey’s test. *P<0.05, **P<0.01, *** P<0.001
References
Allcutt D, Berry M, Sievers J (1984) A qualitative comparison of the reactions of retinal ganglion cell axons to optic nerve crush in neonatal and adult mice. Brain Res 318:231–240
Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ (1994) Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 14:4368–4374
Borowsky B, Walker MW, Huang LY, Jones KA, Smith KE, Bard J, Branchek TA (1998) Cloning and characterization of the human galanin GALR2 receptor. Peptides 19:1771–1781
Böttner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C (1999a) Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene 237:105–111
Böttner M, Suter-Crazzolara C, Schober A, Unsicker K (1999b) Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res 297:103–110
Borrie SC, Baeumer BE, Bandtlow CE (2012) The Nogo-66 receptor family in the intact and diseased CNS. Cell Tissue Res 349:105–117
Busch SA, Horn KP, Silver DJ, Silver J (2009) Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci 29:9967–9976
Chey S, Claus C, Liebert UG (2011) Improved method for simultaneous isolation of proteins and nucleic acids. Anal Biochem 411:164–166
Chipuk JE, Moldoveanu T, Llambi F, Parson MJ, Green DR (2010) The Bcl-2 family reunion. Mol Cell 37:299–310
Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB (2008) Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves.Neuron 58:499–506
Fischer D (2012) Stimulating axonal regeneration of mature retinal ganglion cells and overcoming inhibitory signaling. Cell Tissue Res 349:79–85
Fischer D, Petkova V, Thanos S, Benowitz LI (2004) Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci 24:8726–8740
Ford KJ, Félix AL, Feller MB (2012) Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves.J Neurosci 32:850–863
Foscarin S, Rossi F, Carulli D (2012) Influence of the environment on adult CNS plasticity and repair. Cell Tissue Res 349:161–167
Guo Y, Johnson EC, Cepurna WO, Dyck JA, Doser T, Morrision JC (2011) Early gene expression changes in the retinal ganglion cell layer of a rat glaucoma model. Invest Ophthalmol Vis Sci 52:1460–1473
Hirasawa H, Betensky RA, Raviola E (2012) Corelease of dopamine and GABA by a retinal dopaminergic neuron. J Neurosci 32:13281–13291
Hökfelt T, Tatemoto K (2010) Galanin: a multitalented neuropeptide. EXS 102:1–5
Hökfelt T, Aman K, Arvidsson U, Bedecs K, Ceccatelli S, Hulting AL, Langel U, Meister B, Pieribone V, Bartfai T (1992) Galanin message-associated peptide (GMAP)- and galanin-like immunoreactivities: overlapping and differential distributions in the rat. Neurosci Lett 142:139–142
Hojabrpour P, Waissbluth I, Ghaffari M, Cox M, Duronio V (2012) CaMK22-gama mediates phosphorylation of BAD at Ser170 to regulate cytokine-dependent survival and proliferation. Biochem J 442:139–149
Holm L, Hilke S, Adori C, Theodorsson E, Hökfelt T, Theodorsson A (2012) Changes in galanin and GalR1 gene expression in discrete brain regions after transient occlusion of the middle cerebral artery in female rats. Neuropeptides 46:19–27
Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J (2008) Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci 28:9330–9341
Inoue S, Browne G, Melino G, Cohen GM (2009) Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ 16:1053–1061
Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG (2012) Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci 32:9359–9368
Lee S, Kim K, Zhou ZJ (2010)Role of ACh-GABA cotransmission in detecting image motion and motion direction.Neuron 68:1159–1172
Lingor P, Koch JC, Tönges L, Bähr M (2012) Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res 349:289–311
Monnier PP, D’Onofrio PM, Magharious M, Hollander AC, Tassew N, Szdlowska K, Tymianski M, Koeberle PD (2011) Involvement of caspase-6 and caspase-8 in neuronal apoptosis and the regenerative failure of injured retinal ganglion cells. J Neurosci 31:10494–10505
Moore DL, Goldberg JL (2011) Multiple transcription factor families regulate axon growth and regeneration. Dev Neurobiol 71:1186–1211
Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Nieto-López L, Cánovas-Martínez I, Salinas-Navarro M, Vidal-Sanz M, Agudo M (2009) Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci 50:3860–3868
Reed JC (1994) Bcl-2 and the regulation of programmed cell death. J Cell Biol 124:1–6
Sandvig A, Berry M, Barrett LB, Butt A, Logan A (2004) Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia 46:225–251
Schmeer CW, Wohl SG, Isenmann S (2012) Cell-replacement therapy and neural repair in the retina. Cell Tissue Res 349:363–374
Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Pérez MP, Vidal-Sanz M (2009) Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res 89:32–41
Pernet V, Schwab ME (2012) The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res 349:97–104
Schindowski K, von Bohlen und Halbach O, Strelau J, Ridder DA, Herrmann O, Schober A, Schwaninger M, Unsicker K (2011) Regulation of GDF-15, a distant TGF-β superfamily member, in a mouse model of cerebral ischemia. Cell Tissue Res 343:399–409
Schober A, Böttner M, Strelau J, Kinscherf R, Bonaterra GA, Barth M, Schilling L, Fairlie WD, Breit SN, Unsicker K (2001) Expression of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. J Comp Neurol 439:32–45
Schwab ME (2004) Nogo and axon regeneration. Curr Opin Neurobiol 14:118–124
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76:319–370
Seijffers R, Mills CD, Woolf CJ (2007) ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci 27:7911–7920
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156
Strelau J, Sullivan A, Böttner M, Lingor P, Falkenstein E, Suter-Crazzolara C, Galter D, Jaszai J, Krieglstein K, Unsicker K (2000) Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J Neurosci 20:8597–8603
Strelau J, Strzelczyk A, Rusu P, Bendner G, Wiese S, Diella F, Altick AL, von Bartheld CS, Klein R, Sendtner M, Unsicker K (2009) Progressive postnatal motoneuron loss in mice lacking GDF-15. J Neurosci 29:13640–13648
Stuermer CA (2012) How reggies regulate regeneration and axon growth. Cell Tissue Res 349:71–77
Subramaniam S, Strelau J, Unsicker K (2003) Growth differentiation factor-15 prevents low potassium-induced cell death of cerebellar granule neurons by differential regulation of Akt and ERK pathways. J Biol Chem 278:8904–8912
Takeda M, Kato H, Takamiya A, Yoshida A, Ikyama H (2000) Injury-specific expression of activating transcription factor-3 in retinal ganglion cells and its colocalized expression with phosphorylated c-Jun. Invest Ophthalmol Vis Sci 41:2412–2421
Thanos S, Böhm MR, Schallenberg M, Oellers P (2012) Traumatology of the optic nerve and contribution of crystallins to axonal regeneration. Cell Tissue Res 349:49–69
Ubink R, Calza L, Hökfelt T (2003) “Neuro”-peptides in glia: focus on NPY and galanin. Trends Neurosci 26:604–609
Villegas-Pérez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ (1993) Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol 24:23–36
Yang J, Pahng J, Wang GY (2013) Dopamine modulates the off pathway in light-adapted mouse retina. J Neurosci Res 91:138–150
Yi CH, Yuan J (2009) The Jekyll and Hyde functions of caspases. Dev Cell 16:21–34
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627
Zhang S-J, Buchthal B, Lau D, Hayer S, Dick O, Schwaninger M, Veltkamp R, Zou M, Weiss U, Bading H (2011) A signalling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage. J Neurosci 31:4978–4990
Zigmond RE (2001) Can galanin also be considered as growth-associated protein 3.2? Trends Neurosci 24:494–496
Acknowledgments
We thank Ioannis Patrozos, Attila Magyar and Katrin Huber-Wittmer for support and constructive discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Petar Charalambous and Xiaolong Wang contributed equally to this work.
We thank the German Research Foundation (DFG) for supporting our research on GDF-15.
Rights and permissions
About this article
Cite this article
Charalambous, P., Wang, X., Thanos, S. et al. Regulation and effects of GDF-15 in the retina following optic nerve crush. Cell Tissue Res 353, 1–8 (2013). https://doi.org/10.1007/s00441-013-1634-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1634-6