Abstract
Within a few decades, the repair of long neuronal pathways such as spinal cord tracts, the optic nerve or intracerebral tracts has gone from being strongly contested to being recognized as a potential clinical challenge. Cut axonal stumps within the optic nerve were originally thought to retract and become irreversibly necrotic within the injury zone. Optic nerve astrocytes were assumed to form a gliotic scar and remodelling of the extracellular matrix to result in a forbidden environment for re-growth of axons. Retrograde signals to the ganglion cell bodies were considered to prevent anabolism, thus also initiating apoptotic death and gliotic repair within the retina. However, increasing evidence suggests the reversibility of these regressive processes, as shown by the analysis of molecular events at the site of injury and within ganglion cells. We review optic nerve repair from the perspective of the proximal axon stump being a major player in determining the successful formation of a growth cone. The axonal stump and consequently the prospective growth cone, communicates with astrocytes, microglial cells and the extracellular matrix via a panoply of molecular tools. We initally highlight these aspects on the basis of recent data from numerous laboratories. Then, we examine the mechanisms by which an injury-induced growth cone can sense its surroundings within the area distal to the injury. Based on requirements for successful axonal elongation within the optic nerve, we explore the models employed to instigate successful growth cone formation by ganglion cell stimulation and optic nerve remodelling, which in turn accelerate growth. Ultimately, with regard to the proteomics of regenerating retinal tissue, we discuss the discovery of isoforms of crystallins, with crystallin beta-b2 (crybb2) being clearly upregulated in the regenerating retina. Crystallins are produced and used to promote the elongation of growth cones. In vivo and in vitro, crystallins beta and gamma additionally promote the growth of axons by enhancing the production of ciliary neurotrophic factor (CNTF), indicating that they also act on astrocytes to promote axonal regrowth synergistically. These are the first data showing that axonal regeneration is related to crybb2 movement within neurons and to additional stimulation of CNTF. We demonstrate that neuronal crystallins constitute a novel class of neurite-promoting factors that probably operate through an autocrine and paracrine mechanism and that they can be used in neurodegenerative diseases. Thus, the post-injury fate of neurons cannot be seen merely as inevitable but, instead, must be regarded as a challenge to shape conditions for initiating growth cone formation to repair the damaged optic nerve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The optic nerve
One of the marked features of the optic nerve, equally advantageous for experimentalists and clinicians, is its extracranial location and its experimental accessibility from the earliest stages of development and throughout life. The optic nerve thus still serves as an outstanding model for tackling fundamental questions in brain development, axonal guidance, nerve injuries and nerve repair. The first compartmentalization of the eye primordial and optic stalk is visible at early developmental stages, when the prosencephalic neural tube protrudes bilaterally to form the optic vesicles. The protrusion of the neural tube occurs at different, yet corresponding, developmental stages in representative species, e.g. at about 12.5 days in mouse and rat, at 22 days in the human embryo and at about 30 h in the chick embryo.
Retinal ganglion cell (RGC) axons grow out during the early formation of the secondary eye cup and are immediately directed towards the optic stalk to form the optic nerve, thereby preserving their intraretinal positional information, which is then used to terminate axonal growth at topographically correct target areas. Much research and experimental variations are devoted to unravelling the cellular and molecular mechanisms of retinal axon growth and guidance and axonal interaction with cells and non-cellular cues along the intraretinal course and within the optic stalk. Initial retinofugal axons are first anatomically rearranged into a tube-like order within the optic nerve. When approaching the presumptive optic chiasm in the ventral diencephalons, the axons interact with a scaffold of local glioblasts and glial cells and, naturally, with retinal axons arriving from the contralateral eye cup. After passing through the chiasm midline, retinal axons continue to grow and form the optic tract, which terminates within certain retinoreceptive nuclei within the ventral, lateral and dorsal thalamus and more caudally within the midbrain. Structural development of the visual system is mainly completed at the time of birth and during a species-specific postnatal critical period of weeks to months.
The primary projection of retinal axons within thalamic and midbrain centres maintains a topographic order (e.g. retinotopy) both at structural (e.g. axons from neighbouring ganglion cells terminate on neighbouring brain cells) and functional levels (e.g. neighbouring visual field spots stimulate neighbouring ganglion and brain cells). Because of the differing degree of decussation observed among mammalian species, some reorganization of the topography takes place to assure binocular vision. This feature of the visual system, however, involves higher cortical areas of vision. In the human optic nerve, ganglion cell axons segregate retinotopically at the level of the optic nerve head. This retinotopic segregation of axons arising from particular retinal regions changes near the chiasm (Fitzgibbon and Taylor 1996). Nasal axons (∼52%) cross to the contralateral side and temporal axons (∼48%) remain ipsilateral (Schmid et al. 2000).
Quantitatively, the typical primate optic nerve consists in about one million RGC axons, all of which are myelinated by oligodendrocytes, surrounded by astrocytes, nutrified by capillaries, in communication with microglial cells and in contact with the extracellular matrix (ECM; Honjin et al. 1977; Drenhaus et al. 1997; Sadun and Wang 2011). In rodents, fewer axons occur, with rats having numbers ranging from 105 to 2×105 and with mice having even a fewer number (Galindo-Romero et al. 2011); this is determined by the transcription factor PAX2 (Alur et al. 2008). Approximately 20% of the volume of the adult central nervous system (CNS) and hence comparably of the optic nerve, is occupied by an extracellular space filled with a combination of proteins and polysaccharides that comprises the ECM (Nicholson and Sykova 1998). The cellular and non-cellular organization of the optic nerve resembles that in other regions of the cerebral and spinal white matter. The oligodendrocyte-containing optic nerve differs from peripheral nerves, in that the latter contain myelinating Schwann cells and is therefore considered a central tract located outside the confines of the cranial grooves.
Anatomically, the optic nerve is formed at the ophthalmologically visible optic nerve head, travels within the muscle conus formed by the extraocular muscles and, after about 30 mm, passes into the optic canal (which is a 5- to 12-mm-long boney canal lying superonasal to the superior orbital fissure) and enters the cranium. Some sympathetic axons destined for the orbit and the dura-covered ophthalmic artery located at the inferolateral aspect of the optic nerve lie within the optic canal close to the nerve. Within the canal and posterior to it, meningeal tissue is tethered to the optic nerve with little free space. This tight packing of tissue within the optic canal might be the reason that some traumatic optic and compressive neuropathies occur without radiographically detectable changes to the associated bone.
Developmental aspects of the optic nerve as related to injury in adulthood
Ganglion cell birth and axonal outgrowth
In all vertebrates, the eye primordia arise as lateral protrusions from the prosencephalon, at about embryonic day 12.5 (E12.5) in the rat and mouse and at about E22 in the human embryo. As the eye vesicles approach the ectoderm, they induce the invagination of the lens placode and initiate the formation of the secondary eye vesicle (e.g. eye cup). These processes are controlled by the Pax family of genes, which encode transcription factors and in particular by Pax-6, the homologue to the eye less gene in Drosophila (McDonald and Wilson 1996). At around the time of primary optic vesicle formation, retinal neuroblasts leave the cell cycle in a specific order with respect to their prospective functional cell type: the first cells to become post-mitotic are RGCs followed by photoreceptors, horizontal cells, amacrines and bipolar cells. Of all retinal neurons, the RGCs have received the greatest interest from developmental biologists and clinicians, because these neurons provide a model system for the investigation of topographic projections (for a review, see Thanos and Mey 2000), for nerve regeneration and for their role in the sensory physiology of vision. After their final mitosis, RGC neuroblasts detach from the outer ventricular surface and migrate vertically towards the inner limiting membrane where they form the ganglion cell layer. One of the crucial steps towards the directed growth of axons is the transition of non-polarized to polarized cells by retraction of the vitreal process and extension of a process, which is defined as the axon, towards the inner plexiform layer. The polarization process is probably influenced by chondroitin sulphate proteoglycans (CSPGs), whereas growth-associated protein-43 (GAP-43) is expressed in these earliest RGCs. Several factors are involved in the early cell polarization and outgrowth of axons including laminin-1, various collagens and growth factors (for a review, see Thanos and Mey 2000).
Factors supporting axonal growth
Instructive molecules account for growth cone guidance within the retina and within the optic stalk. Either such local cues are present on neuroepithelial cells, glial endfeet and neighbouring axons or they can be secreted into the ECM. Anatomically, growth cones are located adjacent to Müller glia endfeet, whereas in the optic nerve, tract and beyond, they seem to grow along preformed adhesive pathways on neuroepithelial endfeet (Silver and Rutishauser 1984). In addition to interacting with their neighbouring axons and growth cones, an intimate interaction occurs with the neuroepithelial endfeet, with the ECM and with soluble ligands, which might cause directed growth or repulsion. In this respect, the highly complex composite of various factors and of different species (e.g. mouse, rat, chick, human) represents only a fragment of the knowledge in this field and further factors have been described in former reviews (e.g. Thanos and Mey 2000). Evans and Gage (2005) have proposed that sonic hedgehog diffuses from the midline to activate Pax2 into the optic stalk and forms a boundary between the optic stalk and the optic cup to repress Pax6. Later in the development of the optic stalk, Pitx2 is expressed in the neural-crest-derived mesenchyme cells around the optic stalk to activate signaling that induces the elongation of the optic stalk (Evans and Gage 2005). Receptor-type-protein phosphatases (RPTPs) are also required for the growth of axons in Drosophila. The vertebrate homologues, protein tyrosine phosphatases (PTPs), have also been implied to regulate axonal growth in the limb (Stepanek et al. 2005) but whether they operate in a similar fashion within the optic nerve remains to be shown.
Although new cells are born within the retina and although axons enter the optic stalk, the elongation of axons within the presumptive optic nerve is accompanied by the addition of more axons rendering the nerve a fasciculated structure. Parallel to the fibre invasion, the development of glioblasts proceeds in the retina-diencephalic direction of the optic nerve. Axons elongate through the extracellular space, which is organized by a framework of radial glial processes (Silver 1984; Navascuès et al. 1987). The directionality of axonal growth is probably determined by CSPG-containing ECM, specific proteolytic processes involving glioblast- and growth-cone-derived enzymes and cell death within the ventral optic stalk wall. Growth-permissive and growth-supporting molecules such as laminin-1 and the laminin alpha-2 chain are expressed on the endfeet of neuroepithelial cells along the developing optic nerve and the chiasm (Morissette and Carbonetto 1995). In addition, a number of cell adhesion molecules and cadherins are expressed in the chiasm and are putative candidates to influence chiasm development. The homeobox gene Pitx2 is also required for the normal development of the optic stalk, ocular anterior segment and neural crest (Evans and Gage 2005). We can therefore plausibly assume that Pitx2 co-regulates the microenvironment to influence the growth of axons, because mutations in the gene result in changed optic nerve phenotypes. Moreover, a number of integrins are expressed on the axons and seem to bind to ECM molecules during development. With maturation of the optic nerve, RGCs lose the response to laminin (Cohen et al. 1986). Fibroblast growth factor (FGF) signaling sustains the expression of guidance cues such as semaphorin 3A and xslit1 throughout Xenopus optic tract development suggesting a sustained role of morphogens (Atkinson-Leadbeater et al. 2010).
Molecules inhibiting axonal growth
In addition to the molecules supporting growth cone movement, repellent molecules are involved in axonal navigation. Such repulsive ligands, which might also be of cellular or ECM origin, bind to membrane-bound receptors and activate, within the growth cones, the RhoA-GTpases, which control Rho-kinases and result in the depolymerization of actin and the collapse of growth cone filopodia. Repulsive ligands might be cell-specific (Kapfhammer and Raper 1987) and can alter the growth direction of axons by selectively inhibiting filopodia across the growth cone area (Walter et al. 1990). Several molecules have been identified to mediate collapse such as collapsing 1, neuropilin and semaphorins (for a review, see Thanos and Mey 2000). The data summarized over the last four decades converge to give the “multifactorial” axon navigation theory through the optic nerve.
More recently, additional inhibitors to axonal growth have been identified. The lipid sulphatide, a major constituent of CNS myelin, is inhibitory to mice optic nerve axons and contributes to axon-regenerative failure in vivo (Winzeler et al. 2011). In addition, transactivation of the epidermal growth factor receptor (EGFR) with pharmacological inhibitors has shown that the phosphorylated-EGFR is not present on regenerating axons, suggesting a role in signaling inhibition mediated by glial cells (Berry et al. 2011). In addition to known growth inhibitors, such as neurite outgrowth inhibitor (Nogo), oligodendrocyte glycoprotein and myelin-associated glycoprotein (MAG), the paired immunoglobulin-like receptor B responds to myelin-associated inhibitors and mediates the inhibition of axon growth by requiring the P75 receptor for signal-transduction as shown in mice carrying a mutation in the P75 gene (Fujita et al. 2011; Akbik et al. 2011). Several additional inhibitors probably remain to be discovered.
Target-dependent axonal guidance: retinotopy
One of the basic features of visual field representation is the precise cellular topographic projection of neighbouring ganglion cell axons on the thalamic and mesencephalic centres. RGCs residing in close proximity within the retina will send axons in neighbouring thalamic neurons thereby keeping the retinal coordinates both in relationship to each other and in relationship to the visual field represented by the total number of RGCs. However, neither the retina nor the thalamus provides a simple two-dimensional area. Both areas have different sizes, different metric coordinates and different curvatures and hence do not allow us to devise a simple Cartesian model. The endeavour to construct such models requires a synthesis of several methodological approaches. These include an understanding of the intraretinal growth mechanisms, the guidance of axons through the optic nerve, the segregation of axons at the chiasm, the navigation and reorganization within the optic tract and the target-dependent guidance of axons within the three-dimensional thalamic nuclei. To this end, ephrins and ephrin-receptors have been identified to be relevant within the targets (for a review, see O’Leary and Wilkinson 1999; see also Marler et al. 2008; Suetterlin et al. 2012).
Disturbances of the optic nerve and some clinical aspects of traumatic optic neuropathy
Traumatic optic neuropathy (TON) is the injury-induced damage of the optic nerve function that results in sudden loss of vision and can occur after direct or indirect injury along the ascending optic pathway, with an incidence of 2%–5% after facial injury. Direct injuries result from either section or compression (boney pieces, oedemas, haemorrhages) and are caused by penetrating stab wounds and orbit-penetrating foreign bodies such as bullets, knives and sharp bone fragments derived from periorbital bone fractures (Acheson 2004). Indirect injuries result from shearing forces that are transmitted through the bones and from abnormal eye shakings in relation to the nerve or blood vessels (Levin et al. 1999) and from secondary vasospasm and swelling within the rigid optic canal. The confines of the optic canal can result in a compartment syndrome that accounts for most of the indirect optic neuropathies, because the nerve is tethered to the dural sheath and, hence, has a higher sensitivity to shearing. At the cellular level, the damage resulting from either direct or indirect injury consists in bidirectional (anterograde/ascending and retrograde/descending) Walerian degeneration of axons and RGCs, followed by glial scarring. The optic nerve head is considered the most vulnerable part of the optic nerve, most probably because of the anatomical transition from the eye cup to the narrow optic nerve, passing through the netlike lamina cribrosa, or the transition from the non-myelinated intraretinal state to the myelinated state within the optic nerve.
In spite of the relatively uniform structure along the prechiasmatic optic nerve path, traumatic optic neuropathies exhibit substantial variations in their clinical outcome. Clear cuts of optic nerve axons are relatively rare in the human optic nerve, even during accidental penetration by bullets or knives or iatrogenic cuts during the removal of nerve infiltrating tumors in the surroundings; compressive TONs are more common and result from haematomas and oedemas. Although TONs resulting from acute cuts and compression share common clinical implications, the former typically result in immediate complete or incomplete loss of vision, whereas the latter may also result in delayed and slower visual impairment. Experimental cuts have been performed over the last century in order to examine the mechanisms of optic nerve degeneration (Ramón y Cajal 1928) or to study neurotrophic and neuroprotective agents (for a review, see Isenmann et al. 2003) and neural regeneration (Heiduschka et al. 2005).
Ophthalmologically, TONs result in changes of virtually all optic nerve functions including visual acuity, visual fields, pupillary reflexes and funduscopically detectable morphological changes (especially after injuries close to the optic nerve head and the peripapillary vessels). Usually, the injuries are not restricted to the optic nerve and involve ocular muscles thereby influencing ocular motility. Depending on the patient’s medical condition and the circumstances of examination, the evaluation may be restricted to some basic procedures but should always include an assessment of pupillary reflexes to disclose an afferent pupillary defect. Incomplete TONs are characterized by a moderate-to-severe reduction of visual acuity. No visual acuity is expected when the temporal half of the optic nerve is affected, for it comprises axons from the fovea and macula.
The body of bibliography on the case series relating to TON includes hundreds of eyes treated in neuroophthalmological service centres and departments of plastic and reconstructive surgery, orbital surgery, neurological surgery, otorhinolaryngology, neurosurgery and general ophthalmology. Most of the prospective studies are nonrandomized and lack control groups. Difficulties are therefore encountered when comparing the studies, even qualitatively, because of the relatively isolated information provided by each study on the spontaneous visual recovery that appears with a relatively high frequency after TON. The effects and safety of surgical interventions such as decompression in the management of TON between 1966 and August 2005 have not been beneficial according to the Cochrane Central Register of Controlled Trials (Yu Wai Man and Griffiths (2005). Moreover, surgery is associated with a risk of defined complications such as leakage of cerebrospinal fluid and meningitis.
The International Optic Nerve Trauma Study (IONTS) was designed as a comparative and nonrandomized interventional study with concurrent treatment groups involving a total of 133 TON cases (Levin et al. 1999) whose visual function was assessed within 3 days of injury. On the basis of treatment received within 7 days of trauma, the authors concluded that neither the dosage nor the timing of corticosteroid treatment nor even the timing of surgery was associated with an increased probability of visual improvement. The study found that visual acuity recovered in 57% of the untreated group, 32% of the surgery group and 52% of the steroid group and found no clear benefit for either steroid therapy or decompression surgery (Levin et al. 1999). The authors recommended that it is clinically reasonable to decide upon treatment on an individual/case-by-case basis.
Cellular changes within the optic nerve and histopathology of TON
The onset of cellular changes following various types of injury to the optic nerve might be influenced by both the severity of the lesion and its distance from the ganglion cell bodies. The responses to injury are suggested to be more rapid for transection of the optic nerve at the globe than for those at a more posterior location involving the intracranial optic tract or the chiasm. Radius and Anderson (1978) found that disc pallor developed as early as 2 weeks after a proximal photocoagulation-induced injury in the monkey. However, the ganglion cells and the intraretinal axon segments survived longer. By 3 weeks, changes were perceptible in the ganglion cells and the nerve fibre layer, leading to a decrease in ganglion cell population, with this becoming significant after 4 weeks. The time of onset and the progression of the ganglion cell atrophy were similar after optic nerve transection in the posterior orbit in owl monkeys (Quigley et al. 1977). Both of these studies show that the timing of the atrophy of the ganglion cell bodies is independent of the location of injury along the optic nerve.
The optic atrophy of the later stages of TON is characterized by the loss of axons in both directions from the site of injury and by the gradual loss of oligodendrocytes including their myelin sheaths. The typical organization of glial columns between the parallel nerve fascicles is disrupted and the astrocytes begin to proliferate (gliosis) together with a profound thickening of the interseptal pial membranes. In spite of gliotic proliferation and meningeal thickening, the optic nerve diameter decreases and the subarachnoid space widens. The ramified resident microglial cells phagocytose degenerating neuronal debris and myelin, thereby transforming themselves into lipid-loaded ameba-shaped macrophages.
Retrograde apoptosis of ganglion cells and atrophy occur as early as 2 weeks after injury, whereas adjacent cells such as astroglial and microglial cells respond earlier. Very early changes have been demonstrated in an eye enucleated 30 h following optic nerve transection, 24 mm behind the globe. Immunohistological changes culminate in responses of astrocytes, macrophages and microglial cells. However, no apoptotic profiles or disruption of the retinal layers occur. As expected, a necrotic zone is present in the vicinity of the optic nerve transection, with an accumulation of macrophages and axonal debris and the disruption of myelin. In the rare case of a foreign body within the optic nerve, Prokosch et al. (2011) have reported that, even 30 years after lesion, complex cellular reactions can be identified.
Retrograde degeneration of the RGCs is the final common outcome underlying TON, wherever the initial site or whatever the mechanism of the injury. The axon injury initiates ganglion cell disease and death. The molecular responses at the site of axon injury involve an interruption of axonal transport, local excitotoxicity from physiological or pathological levels of glutamate, the formation of free radicals, a decrease in the flow of neurotrophic factors from targets to the ganglion cells, a leakage of potentially toxic constituents at the axonal stump, the activation of microglial cells, the proliferation of astrocytes, an accumulation of retrogradely transportable molecules and the local breakdown of the blood–brain barrier. Multiple mechanisms certainly account for the axonal response and the influence of diverse factors can also be assumed.
Further, the presence and post-traumatic expression of receptors to neurite inhibitors such as Nogos (NogoR; Wang et al. 2002), which are myelin-associated glycoproteins, can be seen as an additional mechanism leading to the death of ganglion cells. Evidence is accumulating that additional inhibitors are located within the ECM of the optic nerve and belong to the CSPGs that prevent repair of the damaged CNS (for reviews, see Busch and Silver 2007; Sherman and Back 2007). These inhibitors prevent the successful formation of axonal growth cones at the tips of cut axons and thereby convert a primarily anabolic response (e.g. chromatolysis; Cragg 1970) into a suicide response. This might occur in conjunction with the deprivation of neurotrophic factors, which are blocked by the injury and by additional factors that are normally retrogradely transported to the ganglion cell body.
Apoptosis of the ganglion cell body seems to be the final fate. Whereas proteases are activated in typical cascades to allow for clearance of cytoplasmic proteins, DNases are activated within the nucleus to prevent further translation and transcription. At the same time, calcium homeostasis is disturbed both intracellularly and extracellularly. Neighbouring microglial cells are activated to become phagocytic, ingesting disintegrating ganglion cells (Thanos et al. 1993). There is actually no possible replacement of dying ganglion cells, despite the recent hopes that intraretinal or other intraocular stem cells can be used to substitute the ganglion cell layer.
Diagnosis of TON
Advanced TON is visible ophthalmoscopically at the optic nerve head, which shows a differential pattern of pallor depending on the location and severity of the injury. Although loss of vision can occur immediately after injury, pallor of the optic nerve head occurs with a delay of weeks (Fig. 1). Ophthalmoscopy is therefore not the diagnostic tool of first choice, although it is recommendable for examining the status immediate after injury. Assessments of the visual acuity, visual field (in conscious and cooperative patients) and pupillary reflexes are essential to determining further management. Examination of both eyes with comparative evaluations of visual acuity, visual field and fundoscopic status are necessary to exclude the bilateral TON that results from injury to both optic nerves at the optic canal level.
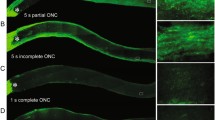
Examples of traumatic optic neuropathy (TON). a Freshly injured optic nerve with intrapapillar bleeding (arrow) and subretinal bleeding in the fundus. b Partial atrophy (arrow) of the temporal optic nerve with visible lamina cribrosa. c Larger partial retrograde atrophy of the optic nerve head (arrow) attributable to injury at the optic canal. d Complete palor of the atrophied optic nerve head (arrow) caused by cutting of the prechiasmatic optic nerve
Intraorbital injuries close to the optic nerve head result in a descending atrophy of the ganglion cells within 2 to 4 weeks and ascending atrophy within 4 to 6 weeks. Ophthalmoscopically visible atrophy of the nerve head becomes apparent at a few weeks after injury proximal to the optic canal and is clearly visible 3 months later, even in the case of a partial lesion (Fig. 1). Examination of experimentally induced TON in monkeys has shown a similar chronological sequence of cellular responses, with the ganglion cells degenerating by 4–5 weeks after optic nerve section and the intraretinal glial cells proliferating over the same period. Optic nerve myelin degenerates more slowly, some remnants of myelin being still detectable at 6 months after injury (Kupfer 1963). Although these changes might also occur in the human TON, the diagnosis of TON should be based on various investigative methods, including trauma history, assessment of visual function, ultrasonography, magnetic resonance imaging (MRI) and computed tomography (CT).
High-resolution MRI is the preferred imaging technique for evaluating soft tissue lesions, in particular those within the orbital apex and intracranially. CT is necessary to search for bone fractures around the orbit (Tsai et al 2005; Leung et al. 2006), in the optic canal, at the orbital apex (Yeh and Foroozan 2004) and intracranially in order to plan surgical intervention or when MRI is contra-indicated. Ultrasonography can assess anterior orbital fractures including rim and zygoma injuries, in particular when combined with ocular trauma.
A complete ophthalmic examination is essential, including slit-lamp microscopy, fundoscopy and the pupillary reflexes to light, the latter being especially useful in assessing an unconscious patient. However, most partial (Fig. 1a-c) or total (Fig. 1d) atrophies of the optic nerve can be easily diagnosed by fundoscopy. Measurement of visually evoked potentials is recommendable for functionality assessments in conscious and motile patients, in particular if remnant potentials can be detected. Recent introduced methods of scanning laser polarimetry might be helpful in assessing the optic nerve fibre layer after TON. Whenever possible, the measurement of the nerve fibre layer is a reliable and specific parameter for detecting intraretinal changes after TON.
Summary of current clinical therapeutic concepts of TON
Surgical and pharmacological tools are available for the fast treatment of TON. Surgical decompression at the optic canal has been recommended as having beneficial effects on visual acuity and is considered by some authors to be the therapy of choice after an initial unsuccessful use of steroids, either alone or in combination with decompression. However, a critical evaluation of retrospective case series has revealed that most of the studies are nonrandomized and lack reliable control groups, such as an untreated group. Such inclusions are mandatory, since there is a high incidence of spontaneous recovery of visual acuity in untreated TON. New approaches might be developed as new surgical techniques evolve and research into the pathophysiology of TON progresses. Endoscopic optic nerve decompression has been considered a minimally invasive procedure with no adverse cosmetic effects but this remains to be verified given that nonrandomized studies have been used to demonstrate its efficacy. Specifically, any new therapy has to be assessed in a prospective and randomized clinical trial in order to avoid ill-founded recommendations with a potential risk of deleterious effects.
Steroids have often been cited to be effective in treating CNS injuries including spinal cord, head trauma and TON by inhibiting oxygen free-radical-induced lipid peroxidation. Papers published in the 1990s and thereafter often cite the National Acute Spinal Cord Injury Study (NASCIS) 2 and 3 trials as evidence that high-dose methylprednisolone is an effective therapy in acute spinal cord injury. However, these trials are questionable from various points of view and the evidence therefrom is now insufficient to support the use of prednisolone in the standard treatment of acute spinal cord injury. Steroids continue to be given to individuals suffering acute spinal cord injury and some adverse effects have been reported (Hurlbert 2006). Steroids are also given to TON patients, even though a critical review of their effects by the IONTS (Levin et al. 1999) has found no significant visual acuity improvement compared with either spontaneous recovery or surgical treatment. Further, Steinsapir et al. (2000) have demonstrated that high-dose methylprednisolone exacerbates axonal loss following optic nerve damage in animals. The concept of using high-dose steroids must thus be reconsidered until prospective, controlled and randomized trials deliver decisive lines of evidence.
Neuroprotecting substances form a heterogeneous group of treatments derived from a wealth of studies involving experimental models on ganglion cell death under various circumstances, including glaucoma, ischaemia and the crush and cut of the optic nerve. Neurotrophic factors have been introduced to enhance the survival of axotomized RGCs in rats (Mey and Thanos 1993). However, none of these agents has successfully entered the clinical phase of testing, although some are still considered to have positive effects in diverse models. In our opinion, the potential of neurotrophic therapy is limited to its use as a complementary therapy.
Both conservative and surgical treatments of TON remain controversial despite numerous reports in favour of either approach and, hence, a prospective, randomized and multicentric study appears mandatory for drawing definite conclusions. Prompt and accurate ophthalmic diagnosis is essential. Results of initial ophthalmic examination (visual acuity, ophthalmoloscopy and visual field defects) might influence the choice of therapy and are necessary for the inclusion/exclusion of the patient in a prospective and randomized study. The necessity for further research into the pathophysiology of both ganglion cell death and TON and the development of low-risk surgical techniques and neuroprotection is obvious in the current state of controversy.
Experimental injuries to the optic nerve
Injury, survival and regeneration at postnatal stages
Maturation of the visual system is different at the time of birth when compared between species. Newborn rodents have an immature optic nerve with retinal maturation extending up to the second week of life. Rat RGCs are generated between E13 and E19 and the first axons reach the midbrain at E16/16.5. The latest peripheral axons born at E19 reach the midbrain at P4/P5 (Dallimore et al. 2002). Development is similar in the mouse. At the day of eye opening (P14), the retinofugal system is considered mature. In addition to the retinal differentiation and formation of synapses, both within the retina and within visual centres, the intraretinal microglial network is formed during the first postnatal days in mice. Optic nerve cut in mice at postnatal day 5 is not associated with regenerative axon growth. Even the overexpression of antiapoptotic bcl-2 in transgenic mice is not sufficient to increase the regenerative potential of RGCs (Lodovichi et al. 2001), indicating a programmed irreversible decay of ganglion cells.
However, concurrent induction of bcl-2 at P4 and attenuation of reactive gliosis in mice result in rapid axonal regeneration over long distances and thus bcl-2 is only important in association with the maturation of astrocytes and post-traumatic reactive gliosis (Cho et al. 2005). One further reason for postnatal optic nerve response to injury is the reaction of oligondrocytes, which upregulate specific white matter markers such as N-acetylaspartate (Bjartmar et al. 2002). The postnatal glial reaction to injury is also mediated by calcium signalling involving purinoreceptors as shown at P1 in the rat optic nerve (James and Butt 2001). The typical gliotic scar formation observed in mature optic nerves resulted in marked controversial discussions in the past. However, astrocytes in the postnatal optic nerve have the ability to form a scar as in the adult (Trimmer and Wunderlich 1990). Neuroprotective measures at postnatal nerves might involve oxidative modification of sulphhydrogel groups as shown at 8 weeks of life in rats (Swanson et al. 2005). Regeneration-supporting measures in rat optic nerves at P8/P9 involve the neutralization of myelin-associated neurite growth inhibitors, which allow axons to grow distances of between 2.5 and 3.2 mm (Weibel et al. 1994). By the day of eye opening, e.g. at P14, retinal axons show a “regenerative window” that lasts until the end of the third postnatal week. This growth window is characterized by continuous down-regulation of GAP-43, which seems to be controlled by the transcription factor translin-associated protein-X (Trax; Schröer et al. 2007). After the third week of life, the rodent retinofugal projection gains mature features and any kind of axotomy results in bidirectional degeneration of RGCs and their axons. However, the postnatal rat retina is populated with nestin-positive Müller glial cells whose number increases after the cutting of the optic nerve (Xue et al. 2006). Likewise in humans and monkeys, no spontaneous regeneration of axons has been reported to occur. In analogy to rodents, a decline in the regenerative ability has been observed in the monkey retina (Rose et al. 2008). In contrast to rodents, monkey ganglion cells continue to express GAP-43 throughout life and possess a certain degree of axonal regrowth without preconditioning lesions.
The human retina and optic nerve express neurotrophin receptors (Trk A, Trk B and Trk C) in fetal stages, at 1 month after birth and in adult stages (Nag and Wadhwa 1999) indicating that, in humans, development of the visual system is responsive to growth-stimulating neutrophins. Although the human visual pathway might be different from that in rodents, as shown by the example of the formation of the chiasm at the midline (Neveu and Jeffery 2007), genetic models of animals with changed routes of axons should greatly improve our understanding of the mechanisms of chiasmal decussation (for a review, see Herrera an Garcia-Frigola 2008).
Injury, survival and regeneration in adulthood
Injury to the adult optic nerve caused mechanically or by diseases can be studied in a common model in order to understand mechanisms of axonal response to injury, retrograde death of RGCs and potential measures to support regeneration of axons. As for clinical TON, we shall distinguish between direct and indirect traumatic injury. Direct TON includes complete or incomplete optic nerve transection (Fig. 2a, b), compression (e.g. crush) through the meninges or after sheath opening, ischaemia and compression at the optic nerve head (e.g. glaucoma). Indirect TON has been rarely used experimentally in monkeys (Quiqley et al. 1977). Secondary TON occurs after lesions are placed within the thalamus, the midbrain or the cortex. Of relevance to whether axon regeneration is expected to occur are, however, direct injuries associated with axotomy along the prechiasmal optic nerve course, e.g. cuts or crushes (Fig. 2a, b).
Experimental TON. a Section through the rat optic nerve at the level of the optic nerve head with growth-associated protein-43 (GAP-43)-stained axonal stumps (green), which form “clubs” at the site of injury. b Higher magnification of the boxed area in a showing the axonal stumps with terminal clubs (blue nuclear counterstaining). c Representation of the molecular panoply required for growth cone formation and movement. Two hypothetical retinal axonal growth cones express a number of molecules to interact with each other, with the extracellular matrix of neuroepithelical cell end feet and with soluble ligands within the extracellular space; the last-mentioned might be attractive or repulsive. Modified from Thanos and Mey (2001)
The early signs of traumatic degeneration, as accurately described by Ramón y Cajal (1928) and later by Liebermann (1971), are observed within the cut optic nerve. One can distinguish a necrotic zone within 24 h after lesion and a metamorphic axonic region with the stumps is thickened and intensely labelled with antibodies against GAP-43. Within the metamorphic region, the axon stumps are varicose and elongated into Ramón y Cajal’s “clubs” or retraction buds. The phenomenon of club formation is similar within the proximal and distal portion of the optic nerve, with the varicosed segments becoming shorter with time and retracting towards the eye. According to the vast bibliography on the optic nerve, clubs, rings, bead-like formations, disruption of myelin, glial scarring, activation of microglial cells and phagocytosis of axomic remnants and lipoid bodies can be seen in both portions of the optic nerve (Fig. 2b).
A review of the literature makes it clear that little uniformity exists between the types of optic nerve lesion employed, ranging from a simple mechanical crush during which the ensheathed nerve is exposed (Barron et al. 1986; Stevenson 1987) to complete axotomy during which the nerve sheath is opened (Lavie et al. 1990; Sivers et al. 1987); sometimes, the type of crush is not even specified. The apparent lack of conformity between various crush studies makes a comparison of the merits of these studies difficult. All future reports should therefore include an exact description of the mode of the crush, e.g. temporal versus dorsal exposure of the optic nerve, mechanical or other form of crush, crush through the optic nerve sheath versus opened sheath, duration of crush and exact distance of the crush behind the optic lobe. Concerning the RGCs size with regard to the various methods of optic nerve lesion, a crush (open or blind) increases the sizes of RGCs, whereas axotomy reduces the size of RGCs (Moore and Thanos 1996). This indicates the crucial role of the meningeal sheath in the response of RGCs to injury.
One of the most accurate descriptions with regard to the response of the optic nerve to injuries was given by Ramón y Cajal (1928), who examined various regions of the peripheral and central nervous system including the injured optic nerve: “It is to be assumed that the retina and optic nerve will react to violence not like peripheral nerves but like the brain or spinal cord … that is with small frustrated acts of growth … because of the absence of cells of Schwann which emit powerful neurotrophic agents…” RGCs indeed exhibit only a limited and transient sprouting reaction after transection and they fail to regrow axons through the interior of the optic nerve. This failure of regeneration has been attributed to inhibitory factors associated with optic nerve myelin (e.g. Nogo) and/or glial scarring, which also produces growth-inhospitable ECM proteins (for reviews, see Chen et al. 2000; Busch and Silver 2007). Most likely, multiple factors cooperate to inhibit the regrowth of axons. Therefore, an accurate examination of both stumps of the optic nerve is mandatory, in order to understand the cause(s) of non-regeneration.
The question as to why the axons do not regrow after being cut can therefore be separated into four aspects. The first involves the RGCs, which receive retrograde signals and respond accordingly. This aspect has been addressed in a comprehensive review (Isenann et al. 2006). The second concerns the proximal stump of the axon, which responds immediately by forming a necrotic and a metamorphic zone with irregular varicosities and clubs (see above). Understanding the molecular events within this proximal portion will enhance our chances of “forcing” such clubs to become growth cones. The third aspect, which is considered to represent an obstacle to growth cone formation, is the site of the cut, which is associated with multiple glial, neuronal, myelin, macrophages, microglial and vascular reactions that direct the ECM towards scar formation. The fourth aspect to be considered is the distal optic nerve, which is separated from the retina. Within this distal nerve, the first signs of axomic irregularities appear 3 to 4 days after injury and neurofibrillar granulations become visible by 1 week after transection. Signs of degradation are evident by 2 weeks after cutting and myelin is phagocytosed by macrophages and microglial cells.
Axonal regeneration after optic nerve injury
The ephemeral sprouting of ganglion cell axons within the cut optic nerve, together with the subsequent cascades of degeneration and scarring has prompted numerous studies to gain an understanding of the mechanisms of failure to achieve permanent optic nerve repair. Various hypotheses have been postulated from the very onset of experimental neurology (for a review, see Ramón y Cajal 1928). The attempts of axons to form growth cones and arborizations within the scar suggest that the inherent ability of RGCs to repair their axons is not lost during maturation but is down-regulated for reasons as yet to be defined. The requirements for the successful formation of growth cones might replicate the formation of growth cones during development and are schematically shown in Fig. 2c. At the descriptive level, the frequent presence of voluminous “balls” and “buds” in the cut nerve and at the edges of the scar shows that the sprouts experience difficulties in progressing and that they additionally suffer the onset of atrophy before they are able to move past the scar. The numerous free “rings” and axomic balls within the scar and the nerve itself represent exploratory sprouting, as if the nerve edge itself presents an obstacle that the cones are unable to cross. Re-activation of axonal growth by providing hospitable environments would show that this assumption is plausible.
In the following, the strategies applied to examine regenerative ability are reviewed starting with the RGCs in vitro and then moving on to deal with complex models such as the grafting of peripheral nerves and the stimulation of optic nerve repair in situ.
Axonal growth in dissociated RGC cultures
Deciphering whether the mature RGCs retain or regain the molecular machinery for axonal regeneration is a daunting task. Therefore, the dissociation of RGCs to analyse these neurons in vitro has narrowed this focus and promises to provide insights into the mechanisms of, hopefully, successful regeneration. However, sooner or later, the lessons learned from such reductive approaches have to be integrated into an understanding of the way that the RGCs behave in the intact retina.
Expanding on experience gained from embryonic neuron cultures on laminin (Manthorpe et al. 1983), Wigley and Berry (1988) have dissociated RGCs from the adult rat retina and shown that the regeneration of axons depends on the presence of either cortical or indigenous neonatal retinal glial monolayers. The major outcome of this approach is the demonstration that adult RGCs can regenerate axons in culture. In spite of some limitations of cultured RGCs, they provide some advantages. Numerous later studies have revealed that the receptors to various neurotrophins, such as high-affinity TrkA, TrkB and TrKc and P75, are expressed in cultured RGCs (García et al. 2002, 2003) indicating the responsiveness of the cells to neurotrophins (Fig. 3a). Factors secreted by retinal Müller cells also appear to have beneficial effects on the survival of RGCs; factors such as ciliary neurotrophic factor (CNTF) or brain-derived neurotrophic factor (BDNF) have specific effects on axon outgrowth and these effects are dissociable from effects on cell survival, thus indicating that they have axonogenesis effects on RGCs (Jo et al. 1999). Further molecules controlling axonogenesis are cAMP, calcium and extracellular-signal-regulated kinases (Chierzi et al. 2005). RGCs in vitro seem to remain invulnerable to glutamate or N-methyl-D-aspartate (Ullian et al. 2004), thus providing an excellent cellular model for axonogenesis. However, the responsiveness to excitotoxic influences or growth inhibitors is age-dependent (Fig. 3b). In addition to the optic-nerve-derived myelin and ECM inhibitors (for a review, see Goldberg and Barres 1998), loss of the amacrine-signaled axon growth ability is responsible for regeneration failure (Barres et al. 1988; Goldberg et al. 2002a, 2002b). Moreover, the loss of responsiveness to trophic agents after axotomy (Shen et al. 1999) might be restored by cAMP elevation, which rapidly recruits TrkB to the plasma membrane in CNS neurons including RGCs (Meyer-Franke et al. 1988, 1995). These studies indicate that the strategy of dissociating and culturing RGCs in vitro is an unavoidable approach that enriches our molecular knowledge and allows us to tackle a number of mechanisms concerning neurotrophins and their cellular control.
Molecules decorating regenerating growth cones and responding to repulsive and supporting ligands. a Modified representation showing the relationship when retinal axons are confronted with growth-promoting Schwann cells and growth-promoting laminin. As examples, α6-integrin, GAP-43 and crystallin beta b2 (crybb-2) are encircled to illustrate their expression. In particular, crybb-2 has been shown to traffic in and out of regenerating growth cones (Liedkte et al. 2007a, 2007b). b Crybb-2 expression in growth cone protrusions (arrows) in cultured RGC-5 cells. c GAP-43 expression (green) in a retinal section of an aged human. The expression of GAP-43 is associated with the ability of the aged retina to regenerate axons in culture
Regeneration in organotypic retinal cultures and crystallins
In vitro models of retinal regeneration have also been developed (Bähr et al. 1988; Wizenmann and Bähr 1998; Johnson et al. 1988, 1989; Stupp et al. 2005). They allow the exploration of the mechanisms of axonal growth on various substrates, the testing of neurotrophins and the examination of the effects of lens injury, as revealed by co-culture experiments (Fischer et al. 2000, 2008; Stupp et al. 2005). Moreover, primate tissue can be examined in vitro (Fig 3a, c); recent experiments have indeed revealed that monkey RGCs also have a reasonable potential to regrow their axons from retinal explants. Although the rate of axonal regeneration declines physiologically with increasing age of the monkey, axonal regeneration is still possible to a certain degree, even in adulthood (Rose et al. 2008). The advantage of retinal organotypic explants is the opportunity to examine the contribution of retinal glial cells. As an example, Fischer et al. (2008) have shown that glial-derived CNTF is produced in explants in response to substitution with crystallins.
The results of injuring the lens (Fischer et al. 2000, 2001; Leon et al. 2000; Yin et al. 2003) were simultaneously published by two different groups. Whereas in the hands of one of the groups, axonal growth seemed to be stimulated with lentogenic crystallins (Fischer et al. 2000, 2001, 2008), the second group emphasized the importance of inflammation and macrophage-derived factors (Leon et al. 2000; Yin et al. 2003, 2006).
Based on the mutual inflammatory mechanism of lens injury, stimulation of ocular macrophages with intravitreal injections of zymosan (Yin et al. 2003) was also found to support axonal growth. Explorations of alternative noninflammatory mechanisms have revealed that the co-culturing of dissociated RGCs with injured lens tissue devoid of macrophages also improves the growth of axons (Lorber et al. 2002). In an attempt to localize the growth factors within the lens, a co-culture of retinal stripes with intact lens epithelium cells has also been shown to support axonal growth in vitro (Stupp et al. 2005), suggesting that independent mechanisms can operate. Consequently, axon regeneration in RGCs might require multiple approaches, such as (1) the inactivation of growth-inhibiting signals, since this naturally occurs in the aforementioned models; (2) the activation of the intrinsic growth state of neurons, as this occurs by injuring the lens; and (3) the adjustment of the microenvironment to permit the formation of growth cones, since this occurs in vitro. The gene cohort that is activated when mature RGCs are transformed from a degenerative to a regenerative state in the lens injury model in vivo has recently been investigated in fluorescently prelabelled, dissociated and enriched RGCs (Fischer et al. 2004).
Numerous lens-derived factors can be considered to be operative in this context. In addition to potential trophic factors, the transparent lens of vertebrates contains large amounts of crystallins, which are grouped into various classes according to their size, structure and function. Alpha-crystallins include two subunits with molecular masses of 20 and 22 kDa. The αA-subunit is expressed mainly in lens fibres, whereas the αB-subunit is found predominantly in the epithelium of the lens. The function of αA-crystallin is the maintenance of lens transparency, whereas αB-crystallin acts as an antiphotooxidant (Sax and Piatigorsky 1994; Wistow 1990). The β- and γ-crystallins belong to the second major class of lens proteins. Because of their high homology, they form a common superfamily. The exceptional stability of these proteins is attributable to their relationship to stress proteins. Some members of this superfamily are also expressed outside the lens and, in particular, within retinal and hippocampal neurons (Liedtke et al. 2007a, 2007b). Their functional role in these neurons is still unknown. Several β-crystallin isoforms are secreted by cultured retinas during axonal regeneration and seem to be involved in the elongation process of RGC axons (Liedtke et al. 2007a, 2007b). The addition of crystallin βb2 (crybb2) to the culture medium enhances the growth of axons in retinal explants, in the RGC-5 cell line and in primary hippocampal neurons. These results suggest that some crystallins constitute a new group of factors that operate either directly or indirectly on neurons and promote axon outgrowth (Liedtke et al. 2007a, 2007b).
Growth factors required in vitro
An intermediate strategy between dissociated cells in culture and in vivo is provided by the examination of RGCs in their natural environment, the retina. To this purpose, the multilayered mammalian retinal tissue can be cultured organotypically. The method was previously established for goldfish retina (Landreth and Agraroff 1979), which is a tissue that possesses an indigenous ability for axonal growth. The potential for axonal growth from adult rat retinal explants was shown in chemically defined media following a protocol of in vivo preconditioning by optic nerve crush and the prelabelling of RGCs with fluorescent dyes (Bähr et al. 1988). According to a similar approach by Johnson et al. (1989), the outgrowth process was not laminin-dependent but was related to the “priming” caused by the crush in vivo (Bähr et al. 1988; Johnson et al. 1988). When different substrates were used, however, laminin-1 turned out to be the optimal substrate to facilitate axonal regeneration in culture (Bähr et al. 1988, 1991). Among the cells supporting this regenerative growth, Schwann cells appeared to produce neurotrophic factors and laminin and to myelinate regenerating axons in culture (Bähr et al. 1991; Leaver et al. 2006a, 2006b). One of the advantages of this culture system is its ability to be replicated in other species including the human and the monkey retina (Thanos and Thiel 1990; Rose et al. 2008). A second advantage is that purified neurotrophic factors can be tested (Mey and Thanos 1993; Thanos et al. 1993; Fischer et al. 2000, 2008; Böcker-Meffert et al. 2002; Stupp et al. 2005; Wong et al. 2006). A third advantage is that retinal tissue from subjects with defined diseases such as diabetes (Takano et al. 1999) and glaucoma (Lasseck et al. 2007) can be used for examining their effects on axonal regeneration. Last but not least, indirect effects mediated by chemotaxis (Cen et al. 2007) or neutralization of inhibitory MAGs (Fischer et al. 2004; Wong et al. 2003) can be monitored in retinal explants. Our understanding of the mechanisms of axonal regeneration has benefited from retinal explants at both the molecular and cellular levels (Fig. 3) but many central questions of regenerative failure in vivo remain unanswered, mainly because additional factors operate within the intact and injured optic nerve in vivo.
The regrowth of axons in vitro has been demonstrated to be associated with the release of intraretinal factors that in turn trigger growth. This was accomplished by using either optic nerve crush or combined optic nerve crush with lens injury in vivo to transform RGCs into a regenerative state (Fischer et al. 2004). The retinas were then explanted and cultured in vitro to allow them to regrow their axons, thus transforming them into a state of axonogenesis. After the quantification of successful axonal regeneration, the culture supernatants were collected, concentrated and subjected to proteomic analysis to obtain the profiles of proteins that were released from the retina during axonal regeneration. Several proteins were reproducibly identified and analysed, among which retinal crystallins showed a remarkable abundance. Examination of the most-upregulated crystallin, crybb2, revealed that it promoted axonal growth. This was the first study that indicated that the process of axonal regrowth from RGCs is associated with releasable neuronal crystallins that operate through an autocrine mechanism facilitating axonal growth from explants and dissociated neurons from the retina and the hippocampus.
The strong neuroprotective and axon-growth-promoting effects of lens injury on axotomized RGCs are well documented (Fischer et al. 2000, 2001, 2004; Müller et al. 2007; Yin et al. 2003, 2006). The complex cellular responses to lens injury in the inner eye comprise the activation of several cell types including an infiltration of activated macrophages into the inner eye and the activation of microglia, retinal astrocytes and Müller cells (Hauk et al. 2008; Leon et al. 2000; Lorber et al. 2002; Müller et al. 2007; Pernet and Di Polo 2006; Pernet et al. 2005). Activated macrophages have recently been suggested to be the source of factors mediating these effects (Yin et al. 2003). However, next to macrophage-derived factors, lens-derived factors have also been previously shown to facilitate axon regrowth of RGCs in culture, suggesting that lens-derived factors either directly or indirectly exert additional effects on retinal neurons, effects that are triggered by a macrophage-independent mechanism (Liedtke et al. 2007a, 2007b; Lorber et al. 2008; Stupp et al. 2005). Lens injury effects have also been shown to occur in the absence or presence of low numbers of macrophages (Hauk et al. 2008) and that astrocyte-derived CNTF, which is upregulated in retinal glia and released after lens injury or zymosan injection, is a key-contributing factor mediating the beneficial effects of intraocular inflammation (Müller et al. 2007). However, the lens-derived factors that either induce intraocular inflammation or CNTF expression in vivo or that facilitate axon outgrowth in macrophage-free cultures have not yet been identified. To address this issue, lens proteins were separated into their characteristic crystallin fractions by gel filtration chromatography and their identity and purity were confirmed by using SDS-polyacrylamide gel electrophoresis and Western blot analysis. The purification results and the identification of lens proteins were consistent with those of earlier reports (Srivastava et al. 1994). As for lens injury, intravitreal injections of crystallins of the beta/gamma (b/g)-superfamily induced inflammatory reactions associated with an infiltration of macrophages and activation of retinal glia, whereas comparable amounts of lenticular a-crystallins or bovine serum albumin (BSA) failed to induce inflammation. Similarly, only crystallins of the b/g-superfamily induced CNTF upregulation in retinal astrocytes. Thus, intravitreal application of the purified fractions of b- and g-crystallins was correlated with the characteristic hallmarks that have been associated with the beneficial effects of lens injury or zymosan treatment. Consistently, the intravitreal application of b- or g-crystallins switched RGCs to a robust regenerative state, whereas treatment with BSA or a-crystallins remained mostly ineffective. When the regenerative state of RGCs was assessed 5 days after surgery by measuring axon outgrowth from retinal explants, a single intravitreal injection of b- or g-crystallins at the time of nerve injury was as or even more effective than lens injury. Intravitreal injections were also sufficient to induce a vigorous growth of axons within a peripheral nerve graft. However, a single application resulted in the regeneration of fewer axons than that observed after lens injury. This was probably the result of differences in the bioavailability of crystallins, since a single injection of 1 mg water-soluble crystallins represents only about 12% of the total amount of lens crystallins available and, in contrast to a single injection, native lens crystallins are continuously released from the injured lens over several days into the vitreous body, maintaining high intravitreal concentrations and making the proteins available to retinal cells. In accordance with this idea, we found that intravitreal injections of b-crystallins were almost as effective as lens injury regarding the number of axons regenerating into the crushed optic nerve when the first application was delayed by 3 days and the injection repeated 7 days after oncomodulin treatment. Next to in vitro explants, replacement of the inhospitable optic nerve environment has been performed.
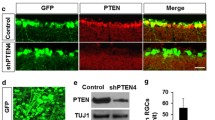
Based on the effects of lens injury, oncomodulin produced by attracted macrophages was identified as one of the factors stimulating axonal regrowth (Yin et al. 2003, 2006, 2009). The induction of a controlled intraocular inflammatory response, combined with the elevation of intracellular cAMP and deletion of the gene Pten (phosphatase and tensin homologue; Park et al. 2008) enabled RGCs to regenerate axons to the full length of the optic nerve in mature mice (Kurimoto et al. 2010; Filbin 2006). The asumed mechanism is that an elevation of intracellular cAMP increased the ability of oncomodulin to bind to receptors and therefore to augment inflammation (Kurimoto et al. 2010). In addition to the PTEN/target of rapamycin (TOR) pathway (Park et al. 2008), Krüppel-like-factor-4 (KLF4) seems to be crucially involved in axon regeneration. RGCs lacking KLF4 show increased axonal growth both in vivo and in vitro (Moore et al. 2009). Further regulators of axonal growth seem to be enzymatic responses within the cell, as shown in the example of Rho/ROCK (Rho-associated kinase; Linger et al. 2008) and with antagonists or Rho (Bertrand et al. 2005, 2007).
Lengthy axonal elongation within autologous peripheral nerve grafts
This model has now been applied to the rat, hamster, mouse and cat optic nerve and shows the intrinsic ability of ganglion cells to regenerate axons and rebuild synaptic contacts with functional significance (Vidal-Sanz et al. 1987; Carter et al. 1989; Heiduschka et al. 2005). As predicted by Ramón y Cajal (1928), the possibility of using peripheral nerve grafts to “bridge” the distance between the proximal optic nerve stump and brain is a realistic approach. Tello (1907) was the first who undertook such experiments and grafted peripheral nerves in the wounds of the optic nerve of the rabbit to examine whether cut axons can sprout through the scar and enter the bands of Büngner in the grafted peripheral nerve. Because of technical problems, such as the dislocation of the grafts, Leoz Ortín and Arcaute (1914) who repeated this experiment saw “in one case, by chance, the graft remained very close to the edge of the optic nerve stump, by means of an embryonic bridge of small extent, one could clearly see some bundles of sprouts which had grown considerably and which, after a few turns, crossed the scar and insinuated themselves into the graft, within which they travelled for long distances” (cited in Ramón y Cajal 1928).
The challenge of studying axonal growth through peripheral nerves was revived in the 1980s when ganglion cell axons were seen to regrow approximately their normal length (2 cm) when autologous peripheral nerve segments were inserted into the retina of adult rats (So and Aguayo 1985). When such segments were opposed and sutured at the ocular stump of the cut optic nerve of rats, axons extended throughout the peripheral nerve segment and penetrated the superior colliculus forming synaptic contacts (Vidal-Sanz et al. 1987). The presence of living Schwann cells and their trophic influences within a peripheral nerve segment (Fig. 4a) accounts for the lengthy regrowth of optic nerve axons (Vidal-Sanz et al. 1987; Berry et al. 1988). In accordance with their natural importance, the Schwann cells myelinated optic nerve axons in rats (Vidal-Sanz et al. 1987) and in cats, as shown later. Myelination is specifically attributed to Schwann cells and no other engrafted cells such as olfactory ensheathing cells can myelinate regenerating optic nerve fibres (Li et al. 2007) or astrocytes, which appear to be non-supportive for growth (Campbell et al. 2003). The terminal arborizations formed within the SC are responsive to light in hamster (Sauvé et al. 1995) and rat (Thanos 1992). Reconnection of the retina with the brain results in type-specific stabilization of RGCs and target-dependent survival of these cells in rats (Thanos and Mey 1995). Further cells influencing the regrowth of optic nerve axons are intact sensory fibres (King et al. 2006), macrophages (Chen et al. 2008; Yin et al. 2003, 2006) and miroglial cells (Thanos et al. 1993; Raibon et al. 2002). Undoubtedly, trophic factors such as BDNF, CNTF and macrophage-derived factors and the regulation of numerous factors within the ganglion cell body and the surrounding cells co-determine the fate of the cut axons (for a review, see Isenmann et al. 2003). The stimulation of RGCs is perhaps one of the most promising approaches combining enhanced post-injury survival and the chance to form axons within a peripheral nerve graft, as shown in the paradigm of multifactorial stimulation by factors released into the vitreous body and retina after lens injury (Fischer et al. 2000, 2001). Contemporary approaches to analyse the gene expression in adult RGCs (Ivanov et al. 2006) and to address the role of certain pathways during the period of axonal regrowth will certainly help to solve the problem.
Molecular decoration of retinal growth cones within a peripheral nerve graft (a) and within a distal part of the optic nerve (b). a Growth is supported by Schwann cells and a growth-permissive extracellular matrix (ECM). Attractive ligands are associated with growth cone movement. b In spite of the repulsive factors derived from oligodendrocytes and the inhospitable environment formed by the central ECM, astrocytes and microglia, growth is facilitated when RGC bodies are stimulated, e.g. after lens injury (Fischer et al. 2000; Leon et al. 2000) or by modifying the genes controlling growth (Park et al. 2008)
Notably, the use of peripheral nerve grafts within the visual system has revolutionized the vast lines of evidence of successful regeneration and greatly contributed to our understanding that optic nerve repair is, in principle, possible. The technical challenge is to find a means of translating this knowledge into the regeneration of axons within the distal optic nerve and tract.
Although the aspect of optic nerve regeneration has been addressed only in experimental models, valuable lines of evidence have been collected to encourage further research into the mechanisms initiating and maintaining axonal growth after TON. To this end, the challenge is to transfer such studies into preclinical or clinical applications, for instance by using autologous peripheral nerve grafts in severe cases of optic nerve transections. Apposition of such a peripheral nerve graft at the site of injury might result in the ingrowth of the sectioned optic nerve axons and the retrograde stabilization of the ganglion cell bodies, which otherwise are inevitably lost. Stabilized and regenerating ganglion cells might then be surgically reconnected with the lateral geniculate body to rebuild synaptic contacts.
Regeneration within the distal optic nerve and contribution of crystallins
Cutting of the optic nerve in rats and simultaneous injury to the intraocular lens stimulate ganglion cell axons to grow within their own distal optic nerve and to reach central targets, thus eliciting positive visually-evoked potential responses (Fischer et al. 2000, 2001). Both inflammatory responses involving macrophages (Yin et al. 2003, 2006) and direct lentogenic factors have been shown to facilitate axonal regeneration (Lorber et al. 2002, 2008; Stupp et al. 2005). These experiments have revealed that the intrinsic ganglion cell ability to regrow an axon can be supported by the external substitution of neurotrophic agents (Fig. 4b). However, combinational treatments seem inevitable, in order to overcome inhibitors (Fig. 4b) and to activate the RGCs-intrinsic growth state (Benowitz and Yin 2007).
Crystallins were originally defined as a group of structural proteins of the vertebrate lens (Wistow 1990; Graw 2009) and of some extralenticular tissues, including the nervous system and the retina (Sax and Piatigorsky 1994). Within the lens, they are synergistically responsible for refractive functions such as maintaining the transparency of the lens throughout life via hydration and preventing opacification and the formation of cataracts. The ubiquitous occurrence of crystallins in several tissues and cell types (including RGCs) and their homology and relationship to heat-shock proteins have resulted in some of them being classified as stress proteins, although they are also vital to normal tissue differentiation (Wistow 1990; Graw 2009; Sax and Piatigorski 1994). Various crystallins are also temporarily expressed within the rat retina after injury (Vázquez-Chona et al. 2004), indicating their involvement in post-injury repair. The neuronal survival and neurite growth-promoting effects of eye lens puncture on adult and early postnatal rat RGCs (Leon et al. 2000; Fischer et al. 2000, 2001; Yin et al. 2003; Lorber et al. 2002) have led to suggestions of a role for crystallins. However, the exact mechanism for these effects might involve either inflammatory events and the activation of macrophages, as shown by the examination of macrophage factors (Leon et al. 2000; Yin et al. 2003) or noninflammatory effects postulated from the results of co-culturing lens and retinal tissue (Lorber et al. 2002; Stupp et al. 2005).
Crybb2 protein has been detected within the retina, brain and testis and crybb2 mRNA has been reported in various tissues including the retina. Ganguly et al. (2008) have identified a novel allele of crybb2 in the mouse and examined its expression in the brain demonstrating the participation of the protein in Ca2+−dependent pathological processes. Crybb2 is involved in both cAMP-dependent and cAMP-independent phosphorylation within the lens. This coupling to phosphorylation pathways indicates its important functional roles in retinal tissue. The results of the study of Ganguly et al. (2008) are consistent with the above investigations and show that various isoforms of crybb2 are present in adult retinal tissue. In addition, their study has revealed the localization of crybb2 within RGCs and their regenerating axonal processes, especially in filopodia and growth cones. This is the first study to show a nonrefractive role of crybb2 in regenerative axonogenesis and to suggest that crybb2 moves from the RGCs into the extracellular space and backwards into the cells, although the underlying mechanisms remain to be elucidated. The colocalization of crybb2 with synaptotagmin 1, which is a leading calcium sensor within the retina and possibly triggers exocytosis, indicates a similar mechanism of secretion into the culture medium. Colocalization of crybb2 with calmodulin, which is the major calcium-binding protein in the retinal ganglion cell layer and in the RGC-5 cell line, provides further evidence that crybb2 also operates through calcium binding. Double-staining for crybb2 and tau-1 protein, which is a specific marker for axons, has shown that crybb2 is translocated into the processes, whereas tau-1 remains confined to the cell body. Finally, the similarity between RGCs and hippocampal neurons indicates that similar functions can be attributed to β-crystallins within the CNS. Interactions between these and other proteins involved in the growth of axons might be possible but they remain to be demonstrated experimentally.
The known expression and function of crystallins are changing in retinodegenerative disorders demonstrating the potentially crucial role of these proteins. As demonstrated in the model of optic nerve injury in axonal regeneration, an emerging concept highlights β- and γ-crystallins as key factors supporting axonal repair. Crybb-2 is expressed in RGCs and is upregulated in regenerating cells to participate actively in the elongation of axons. An understanding of this new role of the involvement of crystallins in nerve repair could lead to novel therapeutic opportunities for a wide range of nerve disorders.
References
Acheson JF (2004) Optic nerve disorders: role of canal and nerve sheath decompression surgery. Eye 18:1169–1174
Akbik F, Cafferty WB, Strittmatter SM (2011) Myelin associated inhibitors: a link between injury-induced and experience-dependent plasticity. Exp Neurol 235:43–52
Alur RP, Cox TA, Crawford MA, Gong X, Brooks BP (2008) Optic nerve axon number in mouse is regulated by PAX2. JAAPOS 12:117–121
Atkinson-Leadbeater K, Bertolesi GE, Hehr CL, Webber CA, Cechmanek PB, McFarlane S (2010) Dynamic expression of axon guidance cues required for optic tract development is controlled by fibroblast growth factor signaling. J Neurosci 30:685–693
Bähr M, Vanselow J, Thaos S (1988) In vitro regeneration of adult rat ganglion cell axons from retinal explants. Exp Brain Res 73:393–401
Bähr M, Hopkins JM, Bunge RP (1991) In vitro myelination of regenerating adult rat retinal ganglion cell axons by Schwann cells. Glia 4:529–533
Barres BA, Silverstein BE, Corey DP, Chun LL (1988) Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1:791–803
Barron KD, Dentinger MP, Krohel G, Easton SK, Mankes R (1986) Qualitative and quantitative ultrastructural observations on retinal ganglion cell layer of rat after intraorbital optic nerve crush. J Neuroscytol 15:345–362
Benowitz LI, Yin Y (2007) Combinatorial treatments for promoting axon regeneration in the CNS: Strategies for overcoming inhibitory signals and activating neurons’intrinsic growth state. Dev Neurobiol 67:1148–65
Berry M, Rees L, Hall S, Yiu P, Sievers J (1988) Optic axons regenerate into sciatic nerve isografts only in the presence of Schwann cells. Brain Res Bull 20:223–231
Berry M, Ahmed Z, Douglas MR, Logan A (2011) Epidermal growth factor receptor antagonists and CNS axon regeneration: mechanisms and controversies. Brain Res Bull 84:289–299
Bertrand J, Winton MJ, Rodriguez-Hernandez N, Campenot RB, McKerracher L (2005) Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J Neurosci 25:1113–1121
Bertrand J, Di Polo A, McKerracher L (2007) Enhanced survival and regeneration of axotomized retinal neurons by repeated delivery of cell-permeable C3-like Rho antagonists. Neurobiol Dis 25:65–72
Bjartmar C, Battistuta J, Terada N, Dupree E, Trapp BD (2002) N-acetylaspartate is an axon-specific marker of mature white matter in vivo: a biochemical and immunohistochemical study on the rat optic nerve. Ann Neurol 51:51–58
Böcker-Meffert S, Rosenstiel P, Röhl C, Warneke N, Held-Feindt J, Sievers J, Lucius R (2002) Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci 43:2021–2026
Busch SA, Silver J (2007) The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol 17:120–127
Campbell G, Kitching J, Anderson PN, Lieberman AR (2003) Different effect of astrocytes and Schwann cells on regenerating retinal axons. Neuroreport 14:2085–2088
Carter DA, Bray GM, Aguayo AJ (1989) Regenerated retinal ganglion cell axons can form well-differentiated synapses in the superior colliculus of adult hamsters. J Neurosci 9:4042–4050
Cen LP, Luo JM, Zhang CW, Fan YM, Song Y, So KF et al (2007) Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Invest Ophthalmol Vis Sci 48:4257–66
Chen L, Holland GN, Yu F, Levinson RD, Lampi KJ, Horwitz J, Gordon LK (2008) Associations of seroreactivity against crystalline proteins with disease activity and cataract in patients with uveitis. Invest Ophthalmol Vis Sci 49:4476–4481
Chen MS, Huber AB, Haar ME van der, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403:434–439
Chierzi S, Ratto GM, Verma P, Fawcett JW (2005) The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur J Neurosci 21:2051–2062
Cho KS, Yang L, Lu B, Feng Ma H, Huang X, Pekny M, Chen DF (2005) Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J Cell Sci 118:863–872
Cohen J, Burne JF, Winter J, Bartlett P (1986) Retinal ganglion cells lose response to laminin with maturation. Nature 322:465–467
Cragg BG (1970) What is the signal for chromatolysis? Brain Res 23:1–21
Dallimore EJ, Cui Q, Beazley LD, Harvey AR (2002) Postnatal innervation of the rat superior colliculus by axons of late-born retinal ganglion cells. Eur J Neuosci 16:1295–1304
Drenhaus U, Von Gunten A, Rager G (1997) Classes of axons and their distribution in the optic nerve of the tree shrew (Tupaia belangeri). Anat Rec 249:103–116
Evans AL, Gage PJ (2005) Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet 14:3347–3359
Filbin MT (2006) How inflammation promotes regeneration. Nat Neurosci 9:715–717
Fischer D, Pavlidis M, Thanos S (2000) Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci 41:3943–3954
Fischer D, Heiduschka P, Thanos S (2001) Lens-injury stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol 172:257–272
Fischer D, Petkova V, Thanos S, Benowitz LI (2004) Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci 24:8726–40
Fischer D, Hauk T, Müller A, Thanos S (2008) Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci 37:471–479
Fitzgibbon T, Taylor SF (1996) Retinotopy of the human retinal nerve fibre layer and optic nerve head. J Comp Neurol 375:238–251
Fujita Y, Endo S, Takai T, Yamashita T (2011) Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of TrK activity. EMBO J 30:1389–1401
Galindo-Romero C, Avilés-Trigueros M, Jiménez-López M, Valiente-Soriano FJ, Salinas-Navarro M, Nadal-Nicolás F, Villegas-Pérez MP, Vidal-Sanz M, Agudo-Barriuso M (2011) Axotomy-induced retinal ganglion cell death in adult mice: quantitative and topographic time course analyses. Exp Eye Res 92:377–387
Ganguly K, Favor J, Neuhäuser-Klaus A, Sandulache R, Puk O, Beckers J, Horsch M, Schädler S, Vogt WD, Wurst W, Graw J (2008) Novel allele of crybb2 in the mouse and its expression in the brain. Invest Ophthalmol Vis Sci 49:1533–1541
García M, Forster V, Hicks D, Vecino E (2002) Effects of müller glia on cell survival and neuritogenesis in adult porcine retina in vitro. Invest Ophthalmol Vis Sci 43:3735–3743
García M, Forster V, Hicks D, Vecino E (2003) In vivo expression of neurotrophins and neurotrophin receptors is conserved in adult porcine retina in vitro. Invest Ophthalmol Vis Sci 44:4532–4541
Goldberg JL, Barres BA (1998) Neuronal regeneration: extending axons from bench to brain. Curr Biol 8:310–312
Goldberg JL, Espinosa JS, Xu Y, Davidson N, Novacs GT, Barres BA (2002a) Retinal gangion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron 33:689–702
Goldberg JL, Klassen MP, Hua Y, Barres BA (2002b) Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296:1819–1820
Graw J (2009) Genetics of crystallins: cataract and beyond. Exp Eye Res 88:173–189
Hauk TG, Muller A, Lee J, Schwendener R, Fischer D (2008) Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp Neurol 209:469–482
Heiduschka P, Fischer D, Thanos S (2005) Recovery of visual evoked potentials after regeneration of cut retinal ganglion cell axons within the ascending visual pathway in adult rats. Restor Neurol Neurosci 23:303–312
Herrera E, Garcia-Frigola (2008) Genetics and development of the optic chiasm. Front Biosci 13:1646–1653
Honjin R, Sakato S, Yamashita T (1977) Electron microscopy of the mouse optic nerve: a quantitative study of the total optic nerve fibers. Arch Histol Jpn 40:321–332
Hurlbert RJ (2006) Strategies of medical intervention in the management of acute spinal cord injury. Spine 31:S16–S21
Isenmann S, Kretz A, Cellerino A (2003) Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Ret Eye Res 22:483–543
Ivanov D, Dvoriantchikova G, Nathanson L, McKinnon SJ, Vi S (2006) Microarray analysis of gene expression in adult retinal ganglion cells. FEBS Lett 580:331–335
James G, Butt AM (2001) Changes in P2Y and P2X purinoceptors in reactive glia following axonal degeneration in the rat optic nerve. Neurosci Lett 312:33–36
Jo SA, Wang E, Benowitz LI (1999) Ciliary neurotrophic factor is an axogenesis factor for retinal ganglion cells. Neuroscience 89:579–591
Johnson AR, Wigley CB, Gregson NA, Cohen J, Berry M (1988) Neither laminin nor prior optic nerve section are essential for the regeneration of adult mammalian retinal ganglion cell axons in vitro. J Neurocytol 17:95–104
Johnson AR, Gregson NA, Wigley CB, Berry M (1989) The conditioning effect of optic nerve injury upon axonal regrowth from adult rat retinal ganglion cells explanted in vitro. Neurosci Lett 97:63–68
Kapfhammer J, Raper JA (1987) Interactions between growth cones and neurites growing from different neural tissues in culture. J Neurosci 7:1595–1600
King C, Barlett C, Sauve Y, Lund R, Dunlop S, Beazley L (2006) Retinal ganglion cell axons regenerate in the presence of intact sensory fibres. Neuroreport 17:195–199
Kupfer C (1963) Retinal ganglion cell degeneration following chiasmal lesions in man. Arch Ophthalmol 70:256–260
Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, Moko L, Kügler S, Benowitz LI (2010) Long distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci 30:15654–15663
Landreth GE, Agranoff BW (1979) Explant culture of adult goldfish retina: a model for the study of CNS regenerations. Brain Res 161:39–55
Lasseck J, Schröer U, Koenig S, Thanos S (2007) Regeneration of retinal ganglion cell axons in organ culture is increased in rats with hereditary buphthalmos. Exp Eye Res 85:90–104
Lavie V, Murray M, Solomon A, Ben-Bassat S, Belkin M, Rumlet S, Schwartz M (1990) Growth of injured rabbit optic axons within their degenerating optic nerve. J Comp Neurol 298:293–314
Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR (2006a) AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther 13:1328–1341
Leaver SG, Harvey AR, Plant GW (2006b) Adult olfactory ensheathing glia promote the long-distance growth of adult retinal ganglion cell neuritis in vitro. Glia 53:467–476
Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI (2000) Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 20:4615–26
Leoz Ortín GL, Arcaute LR (1914) Processos regenerativos del nervio óptico y retina con ocasíon de injertos nerviosos. Trab Lab Invest Biol Madrid 11:239–254
Leung DY, Kwong YY, Lam DS (2006) The outcome of 48 eyes with indirect traumatic optic neuropathy and periorbital facial bone fracture. J Trauma 60:685
Levin LA, Beck RW, Joseph MP, Seiff S, Kraker R (1999) The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma Study. Ophthalmology 106:1268–1277
Li Y, Li D, Raisman G (2007) Transplanted Schwann cells, not olfactory ensheathing cells, myelinate optic nerve fibres. Glia 55:312–316
Lieberman AR (1971) The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol 14:49–124
Liedtke T, Naskar R, Eisenacher M, Thanos S (2007a) Transformation of adult retina from the regenerative to the axonogenesis state activates specific genes in various subsets of neurons and glial cells. Glia 55:189–201
Liedtke T, Schwamborn JC, Schröer U, Thanos S (2007b) Elongation of axons during regeneration involves retinal crystallin beta b2 (crybb2). Mol Cell Proteomics 6:895–907
Lingor P, Tönges I, Pieper N, Bermel C, Barski E, Planchamp V, Bähr M (2008) ROCK inhibition and CNTF interact on intrinsic signaling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 131:250–263
Lodovichi C, Di Cristo G, Cenni MC, Maffei L (2001) Bcl-2 overexpression per se does not promote regeneration of neonatal crushed optic fibers. Eur J Neurosci 13:833–838
Lorber B, Berry M, Logan A, Tonge D (2002) Effect of lens lesion on neurite outgrowth of retinal ganglion cells in vitro. Mol Cell Neurosci 21:301–11
Lorber B, Berry M, Logan A (2008) Different factors promote axonal regeneration of adult rat retinal ganglion cells after lens injury and intravitreal peripheral nerve grafting. J Neurosci Res 86:894–903
Manthorpe M, Engvall E, Ruoslahti E, Longo FM, Davis GE, Varon S (1983) Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol 97:1882–1890
Marler KJ, Becker-Barroso E, Martínez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U (2008) A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci 28:12700–12712
McDonald R, Wilson SW (1996) Pax proteins and eye development. Curr Opin Neurobiol 6:49–56
Mey J, Thanos S (1993) Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res 602:304–317
Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG Jr, Reichardt LF, Barres BA (1988) Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 21:681–693
Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA (1995) Characterization of the signalling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 15:805–819
Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL (2009) KLF family members regulate intrinsic axon regeneration ability. Science 326:298–301
Moore S, Thanos S (1996) Differential increases in rat retinal ganglion cell size with various methods of optic nerve lesion. Neurosci Lett 207:117–120
Morissette N, Carbonetto S (1995) Laminin alpha 2 chain (M-chain) is found within the pathway of avian and murine retinal projections. J Neurosci 15:8067–8082
Müller A, Hauk TG, Fischer D (2007) Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain 130:3308–3320
Nag TC, Wadhwa S (1999) Neurotrophin receptors (Trk A, Trk B, and Trk C) in the developing and adult human retina. Brain Res Dev Brain Res 117:179–189
Navascuès J, Martin-Partido G, Alvàrez LS, Rodriguez-Gallardo G-M (1987) Gliobast migration in the optic stalk of the chick embryo. Anat Embryol 176:79–85
Neveu MM, Jeffery G (2007) Chiasm formation in man is fundamentally different from that in the mouse. Eye 21:1264–1270
Nicholson C, Sykova E (1998) Extracellular space structure revealed by diffusion analysis. Trends Neurosci 21:207–215
O’Leary DDM, Wilkinson DG (1999) Ephrin receptors and ephrins in neural development. Curr Opin Neurobiol 9:65–73
Park K, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOr pathway. Science 322:963–966
Pernet V, Di Polo A (2006) Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain 129:1014–1026
Pernet V, Hauswirth WW, Di Polo A (2005) Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo. J Neurochem 93:72–83
Prokosch V, Spieker T, Merte RL, Thanos S (2011) Concomitant cellular reactions in optic nerve siderosis existing for 30 years. Opthalmologe 108:372–377
Quiqley HA, Davis EB, Anderson DR (1977) Descending optic atrophy in primates. Invest Ophthalmol Vis Sci 16:841–849
Radius RL, Anderson DR (1978) Retinal ganglion cell degeneration in experimental optic atrophy. Am J Ophthalmol 86:673–679
Raibon E, Sauve Y, Carter DA, Gaillard F (2002) Microglial changes accompanying the promotion of retinal ganglion cell axonal regeneration into peripheral nerve grafts. J Neurocytol 31:57–71
Ramón y Cajal S (1928) Degeneration and regeneration of the nervous system (translation by May RM). Oxford University Press, London
Rose K, Schröer U, Volk GF, Schlatt S, König S, Feigenspan A, Thanos S (2008) Axonal regeneration-specific protein expression in the maturating and adult monkey retina in organotypic culture. Restor Neurol Neurosci 26:249–266
Sadun AA, Wang MY (2011) Abnormalities of the optic disc. Handb Clin Neurol 102:117–157
Sauvé Y, Sawai H, Rasminsky M (1995) Functional synaptic connections made by regenerated retinal ganglion cell axons in the superior colliculus of adult hamsters. J Neurosci 15:665–675
Sax CM, Piatigorsky J (1994) Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Relat Areas Mol Biol 69:155–201
Schmid R, Wilhelm B, Wilhelm H (2000) Naso-temporal asymmetry and contraction on anisocoria in the pupillomotor system. Graefes Arch Clin Exp Ophthalmol 238:123–128
Schröer U, Volk GF, Liedtke T, Thanos S (2007) Translin-associated factor-X (Trax) is a molecular switch of growth-associated protein (GAP)-43 that controls axonal regeneration. Eur J Neurosci 26:2169–2178
Shen S, Wiemelt AP, McMorris FA, Barres BA (1999) Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron 23:285–295
Sherman LS, Back SA (2007) A “GAG” reflex prevents repair of the damaged CNS. Trends Neurosci 31:44–51
Silver J (1984) Studies on the factors that govern directionality of axonsl growth in the embryonic optic nerve and at the chiasm of mice. J Comp Neurol 223:238–251
Silver J, Rutishauser U (1984) Guidance of optic axons in vivo by a performed adhesive pathway on neuroepithelial endfeed. Dev Biol 106:485–499
Sivers J, Hausmann B, Unsicker K, Berry M (1987) Fibroblast growth factors promote the survival of adult rat retinal ganglion cells after transection of the optic nerve. Neurosci Lett 76:157–162
So KF, Aguayo AJ (1985) Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res 328:349–354
Steinsapir KD, Goldberg RA, Sinha S, Hovda DA (2000) Methylprednisolone exacerbates axonal loss following optic nerve trauma in rats. Restor Neurol Neurosci 17:157–163
Stepanek L, Stoker AW, Stoeckli E, Bixby JL (2005) Receptor tyrosine phosphatases guide vertebrate motor axons during development. J Neurosci 25:3813–3823
Stevenson JA (1987) Growth of retinal ganglion cell axons following optic nerve crush in adult hamsters. Exp Neurol 97:77–89
Stupp T, Pavlidis M, Busse H, Thanos S (2005) Lens epithelium supports axonal regeneration of retinal ganglion cells in a coculture model in vitro. Exp Eye Res 81:530–538
Suetterlin P, Marler KM, Drescher U (2012) Axonal ephrinA/EphA interactions, and the emergence of order in topographic projections. Semin Cell Dev Biol 23:1–6
Swanson KI, Schlieve CR, Lieven CJ, Levin LA (2005) Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest Ophthalmol Vis Sci 46:3737–3741
Takano M, Sango K, Horie H, Sato M, Iijima Y, Ohno S, Inoue S, Ishikawa Y (1999) Diabetes alters neuritis regeneration from mouse retinal explants in culture. Neurosci Lett 275:175–178
Tello F (1907) La regeneration dans les voies optiques. Trab Lab Invest Biol Univ Madr 5:237–248
Thanos S, Mey J (1995) Type-specific stabilization and target-dependent survival of regenerating ganglion cells in the retina of adult rats. J Neurosci 15:1057–1079
Thanos S, Mey J (2001) Development of the visual system of the chick. II. Mechanisms of axonal guidance. Brain Res Rev 35:205–245
Thanos S, Pavlidis C, Mey J, Thiel HJ (1992) Specific transcellular staining of microglia in the adult rat after traumatic degeneration of carbocyanine-filled retinal ganglion cells. Exp Eye Res 55:101–117
Thanos S, Mey J, Wild M (1993) Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci 13:455–466
Trimmer PA, Wunderlich RE (1990) Changes in astroglial scar formation in rat optic nerve as a function of development. J Comp Neurol 296:359–378
Tsai HH, Jeng SF, Lin TS, Kueh NS, Hsieh CH (2005) Predictive value of computed tomography in visual outcome in indirect traumatic optic neuropathy complicated with periorbital facial bone fracture. Clin Neurol Neurosurg 107:200–206
Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA (2004) Invulnerability of retinal gangion cells to NMDA excitotoxicity. Mol Cell Neurosci 26:544–557
Vázquez-Chona F, Song BK, Geisert EE Jr (2004) Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis SCi 45:2737–2746
Vidal-Sanz M, Bray M, Villegas-Perez MP, Thanos S, Aguayo AJ (1987) Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci 7:2894–2909
Walter J, Allsopp TE, Bohnhoffer F (1990) A common denominatior of growth cone guidance and collapse? Trends Neurosci 13:447–452
Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z (2002) Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417:941–944
Weibel D, Cadelli D, Me S (1994) Regeneratin of lesioned rat optic nerve fibers is improved after neutralization of myelin-associated neurite growth inhibitors. Brain Res 642:259–266
Wigley CB, Berry M (1988) Regeneration of adult rat retinal ganglion cell processes in monolayer culture: comparison between cultures of adult and neonatal neurons. Brain Res 470:85–98
Winzeler AM, Mandemakers WJ, Sun MZ, Stafford M, Phillips CT, Barres BA (2011) The lipid sufatide is a novel myelin-associated inhibitor of CNS axon outgrowth. J Neurosci 31:6481–6492
Wistow G (1990) Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol 30:140–145
Wizenmann A, Bähr M (1998) Growth preferences of adult rat retinal ganglion cell axons in retinotectal cocultures. J Neurobiol 35:379–387
Wong EV, David S, Jacob MH, Jay DG (2003) Inactivation of myelin-associated glycoprotein enhances optic nerve regeneration. J Neurosci 23:5391
Wong WK, Cheung AW, Cho EY (2006) Lens epithelial cells promote regrowth of retinal ganglion cells in culture and in vivo. Neuroreport 17:699–704
Xue LP, Lu J, Cao Q, Kaur C, Ling EA (2006) Nestin expression in Müller glial cells in potnatal rat retina and its upregulation following optic nerve transection. Neuroscience 143:117–127
Yeh S, Foroozan R (2004) Orbital apex syndrome. Curr Opin Ophthalmol 15:490–498
Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI (2003) Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci 23:2284–2293
Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI (2006) Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci 9:843–852
Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, LI Z, Zaverucha-do-Valle C, He H, Petkova V, Zack DJ, Benowitz LI (2009) Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci USA 106:19587–19592
Yu Wai Man P, Griffiths PG (2005) Surgery for traumatic optic neuropathy. Cochrane Database Syst Rev 4:CD005024
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thanos, S., Böhm, M.R.R., Schallenberg, M. et al. Traumatology of the optic nerve and contribution of crystallins to axonal regeneration. Cell Tissue Res 349, 49–69 (2012). https://doi.org/10.1007/s00441-012-1442-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1442-4