Abstract
Cerebellar Purkinje cells (PCs), the sole output neurons in the cerebellar cortex, play an important role in the cerebellar circuit. PCs appear to be rather sensitive to aging, exhibiting significant changes in both morphology and function during senescence. This article reviews such changes during the normal aging process, including a decrease in the quantity of cells, atrophy in the soma, retraction in the dendritic arborizations, degeneration in the subcellular organelles, a decline in synapse density, disorder in the neurotransmitter system, and alterations in electrophysiological properties. Although these deteriorative changes occur during aging, compensatory mechanisms exist to counteract the impairments in the aging PCs. The possible neural mechanisms underlying these changes and potential preventive treatments are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebellar Purkinje cells (PCs), the sole output neurons in the cerebellar cortex, undergo significant age-related morphological and functional alterations. These alterations may relate to age-related cerebellar disabilities, such as dysfunctions in postural control (Hogan 2004), deficits in motor activities (Hilber and Caston 2001; Mattay et al. 2002; Taniwaki et al. 2007), a decline in the intellect (Lee et al. 2005), and impairments in cognitive functions (Hogan 2004; Paul et al. 2009) in the elderly. Moreover, a multitude of age-related cerebellar diseases are accompanied by obvious alterations in the morphology and function of PCs, such as essential tremor (ET) (Louis et al. 2009), Alzheimer's disease (AD) (Sjöbeck and Englund 2001), and Lewy Body disease (LBD) (Tu et al. 1997). This review focuses on PC modifications that occur during the normal aging process, which have not been reviewed previously, and will increase our understanding of the mechanisms underlying aging in degenerative diseases.
Age-related PC loss
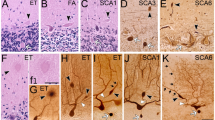
PCs are among the most vulnerable neurons in the brain (Patrick and Anderson 2000; Woodruff-Pak 2006; Servais et al. 2007; Dhar et al. 2007; Woodruff-Pak et al. 2010) and are prone to aging insult (Quackenbush et al. 1990; Hadj-Sahraoui et al. 2001; Andersen et al. 2003; Zhang et al. 2006). A complicated and disputed issue is whether the quantity of PCs decreases or remains constant during the normal aging process. Some evidence has shown that PCs exhibit significant age-related neuronal loss (Ogata et al. 1984; Rogers et al. 1984; Woodruff-Pak 2006; Pires et al. 2010; Woodruff-Pak et al. 2010). Hall and Millerakh Corsellis (1975) reported a ∼2.5% PC loss per decade during aging, whereas other studies found no obvious age-related decline in PC number (Drüge et al. 1986; Bakalian et al. 1991). Another report demonstrated that PCs remained constant in most cerebellar parts, except in the anterior lobe, where a highly significant decline in the number of cells of 40.9% occurred during aging (Andersen et al. 2003). While it becomes more and more prevalent that many brain regions, such as striate cortex (Hua et al. 2008) and hippocampus (von Bohlen und Halbach and Unsicker 2002), undergo no significant neuron loss during the aging process, the cerebellar PCs might not comply with the same rule. Woodruff-Pak et al. (2010) used unbiased stereology to estimate the total number of PCs in cerebellum and pyramidal neurons in the hippocampus, the results revealed significant loss of PCs but stable pyramidal neurons during brain aging. We speculate that the discrepancies among these literatures might be due to variations in species, cerebellar regions and counting methods (for example, using the unbiased stereological technique or by visual examination), or depend on whether an undistinguishable aging disease (such as an early AD) or shrinking factors caused by tissue processing were considered or not (Andersen et al. 2003).
It has been shown that a number of factors might account for age-related PC loss, such as excessive amino acids (Felici et al. 1989), a decrease in neuroglobin (a kind of neuroprotective protein) (Sun et al. 2005) and loss of reciprocal interaction with target neurons (Huang et al. 1999). Neuronal loss is usually viewed as one of the important causes of age-related deteriorations of neurological functions. Because PCs are responsible for cerebellar cortical-dependent forms of behavior (Woodruff-Pak 2006), age-related PC loss will lead to significant cerebellar dysfunctions, such as a delay in eyeblink conditioning (Woodruff-Pak 2006) and deficits in spontaneous motor activity (Larsen et al. 2000). Loss of PCs in the cerebellum also is considered to be a factor in cerebellar atrophy during normal aging (Woodruff-Pak et al. 2001).
Atrophy in the PC soma
While there is a dispute on age-related PC loss, it is evident that the aging process will result in a decisive shrinkage in the PC soma. Ogata et al. (1984) has reported that the PC volume from the rats aged 18, 24 and 30 months significantly decrease as compared with that of 3-month-old counterparts, and Andersen et al. (2003) estimated a significant decrease of 33% in the PC somatic volume in aged human cerebellum. A decrease in PC volume may be related to the decline of nuclear elements (Ogata et al. 1984) and the degeneration of other organelles or loss of the cytoplasmic matrix in the perikaryon (Monteiro 1991). These changes suggest a significant decline in substance synthesis as well as trophic deficiencies and possibly neuronal dysfunction in the aged PCs.
Retraction in PC dendritic arborizations
PC dendritic arborizations are profuse and approach the pial surface in young cerebellum, whereas old dendrites appear significantly atrophied (Quackenbush et al. 1990; Hadj-Sahraoui et al. 2001; Zhang et al. 2006). The height of PC dendrites (measured from the maximal point in the arbors perpendicularly to the PC layer) accounts for a large percentage of the molecular layer thickness in younger cerebellum, whereas that of the old PC dendrites occupies significantly less of the molecular layer thickness, despite a significant atrophy in the molecular layer during cerebellar aging (Hadj-Sahraoui et al. 2001). The width of PC dendrites (measured as the extent of the arbors along an axis parallel to the PC layer in the molecular layer) is also significantly smaller in the elderly compared to the young (Hadj-Sahraoui et al. 2001). In addition, the branches from young PCs are nearly homogeneous in each segment, whereas the aging dendrites exhibit abundant vacuolar profiles and membrane swirls (Chen and Hillman 1999). This phenomenon indicates cytoarchitecture aberrations in aging PC dendrites, which greatly affect the small distal dendrites and decrease substance transport to distal terminals, thereby accounting for the preferential loss of distal branchlets during PC dendritic aging (Rogers et al. 1984; Quackenbush et al. 1990).
Reduction in PC dendrites may attenuate the exchanges of neural information, because PCs receive synaptic inputs mainly through their dendritic networks. Age-related loss of PC arborizations could directly reduce the amount of information input, reduce afferent efficacy, and affect information integration and signal transmission in the aged cerebellum, consequently leading to a reduction in cerebellar function. In addition, loss of PC dendritic arborizations might also contribute to the decline in the thickness of the molecular layer in the aged cerebellum (Zhang et al. 2006).
The causes of age-related loss of PC dendrites remain unclear. An important aspect is the effect of age-related decrease of inputs from parallel fibers to PCs (Dlugos and Pentney 1994; Huang et al. 1999, 2006b). The parallel fibers arise from granule cells in the cerebellar cortex and form synapses onto the dendrites of Purkinje cells. Thus, loss of parallel fibers (Huang et al. 1999) or granule cells (Pentney et al. 2002; Zhang et al. 2006) may trigger the dendritic loss in aging PCs. In addition, since specific trophic factors, such as insulin-like growth factor-1 (IGF-1) and brain-derived neurotrophic factor (BDNF), can promote dendritic growth (Niblock et al. 2000; Binder 2007), but exhibit age-related reduction in aging brain (Markowska et al. 1998; Erickson et al. 2010), it is reasonable to propose that age-related declines of PC dendrites might also be associated with such reduced trophic support during cerebellar aging.
Degeneration of PC organelles
The ultrastructures of PCs reportedly undergo significant age-related alterations (Ogata et al. 1984; Monteiro 1991; Monteiro et al. 1994; Fattoretti et al. 1996). Mitochondria in the aging PCs showed significant morphological degeneration, such as a decrease in mitochondrial volume, numerical density and volume density (Fattoretti et al. 1996, 1998). This degeneration might affect mitochondrial respiratory chain activity (Ojaimi et al. 1999) and lead to a reduced energy metabolism in the aging PCs (Atamna 2004). The smooth endoplasmic reticulum, an important calcium storage organelle (Monteiro et al. 2000), exhibits dilation in aged PCs (Dlugos 2005), which is a threat to PC calcium homeostasis. The lipofuscin in the cytoplasm markedly increased in aging PCs (Ogata et al. 1984; Monteiro et al. 1994), which might interfere with the intraneuronal configuration and might speed up the death of PCs (Monteiro et al. 1994). In addition, it has been shown that the aging nuclei become pyknotic and undergo regression (Ogata et al. 1984; Monteiro et al. 1994). The nucleolar volume decreases with age (Ogata et al. 1984) and the “nucleus-ribosome system” becomes impaired (Nosal 1979), including changes in the nucleolar texture, repartition in the the nucleolus, variations in interchromatin/perichromatin granules and redistribution of free ribosomes with age, which could possibly reflect the molecular dysfunction related to the production of various types of RNA and neuronal proteins in the old (Nosal 1979). Other PC organelles, such as the degeneration in Golgi apparatus and ground substance, emergence of dense bodies and vacuolations, have also been reported to occur during aging (Monteiro 1991; Nosal 1979). In summary, these age-related degenerations in PC organelles might affect neuronal functions of energy metabolism, substance synthesis and intraneuronal homeostasis. These vital alterations might lead to PC apoptosis and subsequently cause cerebellar dysfunctions in senile individuals.
Changes in PC synapses
Synapses are not immutable and static structures in the nervous system but are prone to be remodeled on both the morphology and function as a consequence of environmental stimulations during aging (Bertoni-Freddari et al. 1996; Poe et al. 2001) and behavior experience (Gruart et al. 2006; Lee et al. 2007). In the cerebellum, PC synapses are mostly formed with the parallel fibers (Dlugos and Pentney 1994; Huang et al. 1999, 2006a). A high correlation has been reported between synaptic density and PC density (Rogers et al. 1984); therefore, if the number of PCs declines and/or their dendritic arborizations is lost during aging, the synapses might also be lost. These synaptic losses may trigger other age-related cell losses in the aged cerebellum (Huang et al. 1999). Other studies reported significant loss of approximately 33% of synapses in PC dendritic spines (counted per tissue square area of electron micrograph) during aging, which might result from the impairment with advanced age of specific afferent neurons and/or a selective age-related vulnerability of dendritic spines (Glick and Bondareff 1979). In addition, the fluidity of the lipid bilayers of synaptosomal membranes degenerates in the aged (Ohyama et al. 1995), which suggests that the synapse is prone to collapse and could lead to synapse loss in senile PC. In summary, in addition to a reduced number of synapses and the degenerating junctional zones, any alterations at synaptic terminal regions may affect synaptic dynamic morphology and impair energy or information receiving in aged PCs (Bertoni-Freddari et al. 1986, 1996).
Altered response ability to neurotransmitters of aged PCs
It has been shown that both the concentration of the most neurotransmitters and the neuronal response ability to the neurotransmitters change with brain aging (Bickford 1993; Bickford et al. 1985; Bickford-Wimer et al. 1988), which greatly affect neuromodulatory actions. A widely studied neurotransmitter that affects the response of aging PCs is norepinephrine (NE), which preferentially inhibits the spontaneous activity in the young PCs rather than in the senescent (Bickford-Wimer et al. 1988). Electrophysiological and electrochemical techniques showed an age-related loss of noradrenergic function in postsynaptic compartments (Bickford-Wimer et al. 1988), which may relate to behavioral deficits in senescence (Bickford et al. 1985, 1999; Bickford-Wimer et al. 1988). NE is also an important neuromodulator in the cerebellar cortex that amplifies the action of gamma-aminobutyric acid (GABA) (Bickford 1993). Therefore, an age-related decrease in NE content will attenuate the GABAergic inhibitions to aging PCs and subsequently affect cerebellar functions. Alterations of neurotransmitters in aging PCs might be related to the changes of neurotransmitter enzymatic abilities, for example, age-related decline of glutamate dehydrogenase activity might result in overproduction of amino acids and contribute to cell loss, whereas age-related increase in monoaminooxidase function can reduce the neuron energetic production (Felici et al. 1989; Tranquilli Leali et al. 2003).
Alteration of the electrophysiological features in aged PCs
Age-related changes in the electrophysiological features of PCs have been widely reported (Rogers et al. 1981; Chung et al. 2003), for example, aging leads to deficits in the PC activation threshold and increases the inhibitory threshold (Rogers et al. 1981). Many factors might cause these changes, such as a declined receptor response (Parfitt 1988), decreased postsynaptic sensitivity to neurotransmitters (Marwaha et al. 1980), and functional alterations in many ion channels (Chung et al. 2001a, b, 2003).
Ion channels have been extensively studied for investigation of electrophysiological alterations in the aged PCs. The sodium channels are involved in the integration of synaptic input in cerebellar PCs (Schaller and Caldwell 2003). Modification of sodium channel availability is a candidate for age-related alteration of the action potential (Chung et al. 2003). In aged PCs, Na 1.1 immunoreactivity is greatly increased, which likely contributes to the mechanisms underlying the age-related alteration in action potential (Chung et al. 2003). In addition, immunoreactivities for Kv1.1 and Kv1.2 are increased specifically in aged PCs, which might also affect PC functions and cause age-related disorders (Chung et al. 2001a). Many neuronal processes are regulated by calcium influx through voltage-gated calcium channels (VGCCs), including firing patterns of action potentials (Chung et al. 2000). In aged PCs, immunoreactivity of the VGCC alpha (1C) subunits is increased whereas that of the alpha (1D) subunits is decreased, which might reflect a gradual loss of regulation of Ca2+ channels in the senescent period (Chung et al. 2001b). Takahashi et al. (2009) reported that age-dependent alterations in Cav2.1 channel function might result in aberrant synaptic signaling, leading to motor dysfunction, because Cav2.1 channels control neurotransmitter release and impair synaptic transmission (Kodama et al. 2006). These alterations on ion channels indicate a complex feature on the electrophysiological response during PC aging.
Behavior deficits as PC aging
Impairments of PCs will directly generate cerebellar dysfunctions under various situations, which were widely reported in Lurcher or Staggerer mutant mice (Hilber and Caston 2001; Caston et al. 2003; Porras-García et al. 2005, 2010; Chintala et al. 2009). These mutant animals are characterized by premature and aberrant apoptosis in the cerebellum, such as great loss of Purkinje cells and granule cells, which appears to be a good model of progressive neurodegeneration in aging cerebellum (Hilber and Caston 2001). As Lurcher mutant mice showed a decrease in both motor skills and motor learning (Hilber and Caston 2001), which was mainly caused by loss of PCs, we speculate that age-related loss in PCs could also result in motor skill and motor learning dysfunctions in the elderly. Lurcher mice also deficit in spatial orientation (water maze) and associative learning (eyelid classical conditioning) tasks (Porras-García et al. 2005), suggesting a similar age-related degeneration of orientation ability and associative learning activities in the old. However, not all the cerebellar dysfunctions in these mutants are related to PC loss. Stagger mice showed weaker motor behavior and working memory than those of the wild mice at the age when PC number was quite normal and similar to that of wild mice, indicating a fine structural and/or biochemical changes might precede PC death (Caston et al. 2003, 2004).
Compensatory mechanisms for age-related PC dysfunctions
Although PCs undergo significant age-related morphological and functional degeneration, a compensatory mechanism exists to counteract the degeneration. Age-related hyperplasia and hypertrophy of astrocytes (Sabbatini et al. 1999; Zhang et al. 2006) have been reported to provide neuroprotective effects on aging neurons, and might also exert protection for the aging PCs. Some compensatory mechanisms might be invoked by the aging brain. For example, despite the terminal dendritic segments of aging PCs were preferentially lost, the proximal regions of the network exhibited regrowth and reorganization, which might serve as a kind of compensation for age-related dendritic loss (Quackenbush et al. 1990). Although the number and total surface area of synapses decrease significantly with age, the average synaptic size exhibits a closely negative correlation to the synapse number during aging (Bertoni-Freddari et al. 1986, 1996; Chen and Hillman 1999), which helps maintain synaptic transmission between aging neurons. Such a correlation indicates a compensatory mechanism of dendritic deafferentation (Monteiro et al. 1992). In addition, the upregulation of the IGF-I receptor in the aged cerebellum suggests promotion of the survival of a degenerated population of PCs during aging (Chung et al. 2002).
Current strategies for preventing degeneration of PC aging
Aging is a progressive physiological syndrome that is unavoidable. However, proper treatments can slow down the aging process. Suppression of oxidative stress is considered to be an important mechanism for suspending neuronal vulnerability to aging effects (Joseph et al. 1998). 5-lipoxygenase (5-LOX), the concentration of which is increased in elderly brains, may participate in neurodegeneration during aging. Thus, decreasing the level of 5-LOX (such as by regulating hormonal level of melatonin or hyperglucocorticoidemia), might be a way to delay age-related neurodegeneration (Manev et al. 2000). In addition, administration of certain hormones, (e.g., steroid hormones; Schumacher et al. 2003; George et al. 2006), antioxidant-rich foods (Joseph et al. 1998; Bickford et al. 1999; Ho et al. 2009; Seo et al. 2009), neurotrophic factors (Lärkfors et al. 1994; Tolbert and Clark 2003), active exercise (Larsen et al. 2000; Martinez Gagliardo et al. 2008; Chae and Kim 2009), transplantation of endogenous stem/progenitor cells (Rao and Mattson 2001; Jin and Galvan 2007), and medications (Binstock et al. 2006) can also benefit aging neurons in different brain regions. Similarly, these manipulations might have similar effects in preventing degeneration of cerebellar PCs during senescence. Probably, with the development of the therapeutic techniques, transplantation of neural stem/progenitor cells to the aging cerebellum (Alcock et al. 2007) might be an optional treatment for age-related cerebellar dysfunctions in the future. The postnatal cerebellar cortex has been reported to have at least two distinct types of progenitor cells (Jankovski et al. 1996). These stem/progenitor cells are able to acquire the position and mature electrophysiological properties after transplantation (Klein et al. 2005). Therefore, it is reasonable to speculate that prevention of aging PC degeneration could be achieved by increasing the generation of endogenous stem/progenitor cells and/or transplantation of stem/progenitor cells. Recent evidence has shown that aging leads to deterioration of DNA repair mechanisms, such as lowering the efficiency of mismatch repair, base excision repair, nucleotide excision repair and double-strand break (Gorbunova et al. 2007), which will increase cell apoptosis, mutation or death in many tissues. These age-related changes could similarly occur in cerebellar PCs and lead to their degenerative changes during aging. As treating DNA oligonucleotide might correct age-associated decline of DNA repair capacity (Goukassian et al. 2002), such treatment can potentially improve DNA repair and delay PC aging.
In summary, normal aging does lead to a significant functional deficit of cerebellar PCs. However, the underlying neural mechanisms remain poorly understood. Some changes of morphology at cellular–subcellular level as well as modifications of neural transmitters and its corresponding receptors may contribute to functional decline of PCs that accompany senescence, whereas compensatory mechanisms expected to counteract PCs function degeneration are also proven to exist. Subsequent studies with integrative techniques of behavior assessment, electrophysiology, morphology and molecular tools are needed to clarify these issues so as to uncover potential strategies to prevent PC aging and related motor ability reduction in aged individuals.
References
Alcock J, Scotting P, Sottile V (2007) Bergmann glia as putative stem cells of the mature cerebellum. Med Hypotheses 69:341–345
Andersen BB, Gundersen HJ, Pakkenberg B (2003) Aging of the human cerebellum: a stereological study. J Comp Neurol 466:356–365
Atamna H (2004) Heme, iron, and the mitochondrial decay of ageing. Ageing Res Rev 3:303–318
Bakalian A, Corman B, Delhaye-Bouchaud N, Mariani J (1991) Quantitative analysis of the Purkinje cell population during extreme ageing in the cerebellum of the Wistar/Louvain rat. Neurobiol Aging 12:425–430
Bertoni-Freddari C, Giuli C, Pieri C, Paci D (1986) Age-related morphological rearrangements of synaptic junctions in the rat cerebellum and hippocampus. Arch Gerontol Geriatr 5:297–304
Bertoni-Freddari C, Fattoretti P, Paoloni R, Caselli U, Galeazzi L, Meier-Ruge W (1996) Synaptic structural dynamics and aging. Gerontology 42:170–180
Bickford P (1993) Motor learning deficits in aged rats are correlated with loss of cerebellar noradrenergic function. Brain Res 620:133–138
Bickford PC, Hoffer BJ, Freedman R (1985) Interaction of norepinephrine with Purkinje cell responses to cerebellar afferent inputs in aged rats. Neurobiol Aging 6:89–94
Bickford PC, Shukitt-Hale B, Joseph J (1999) Effects of aging on cerebellar noradrenergic function and motor learning: nutritional interventions. Mech Ageing Dev 111:141–154
Bickford-Wimer PC, Granholm AC, Gerhardt GA (1988) Cerebellar noradrenergic systems in aging: studies in situ and in in oculo grafts. Neurobiol Aging 9:591–599
Binder DK (2007) Neurotrophins in the dentate gyrus. Prog Brain Res 163:371–397
Binstock RH, Fishman JR, Juengst ET (2006) Boundaries and labels: anti-aging medicine and science. Rejuvenation Res 9:433–435
Caston J, Hilber P, Chianale C, Mariani J (2003) Effect of training on motor abilities of heterozygous staggerer mutant (Rora(+)/Rora(sg)) mice during aging. Behav Brain Res 141:35–42
Caston J, Chianale C, Mariani J (2004) Spatial memory of heterozygous staggerer (Rora(+)/Rora(sg)) versus normal (Rora(+)/Rora(+)) mice during aging. Behav Genet 34:319–324
Chae CH, Kim HT (2009) Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochem Int 55:208–213
Chen S, Hillman DE (1999) Dying-back of Purkinje cell dendrites with synapse loss in aging rats. J Neurocytol 28:187–196
Chintala S, Novak EK, Spernyak JA, Mazurchuk R, Torres G, Patel S, Busch K, Meeder BA, Horowitz JM, Vaughan MM, Swank RT (2009) The Vps33a gene regulates behavior and cerebellar Purkinje cell number. Brain Res 1266:18–28
Chung YH, Shin C, Park KH, Cha CI (2000) Immunohistochemical study on the distribution of neuronal voltage-gated calcium channels in the rat cerebellum. Brain Res 865:278–282
Chung YH, Shin CM, Kim MJ, Lee BK, Cha CI (2001a) Age-related changes in the distribution of Kv1.1, Kv1.2 channel subunits in the rat cerebellum. Brain Res 897:193–198
Chung YH, Shin CM, Kim MJ, Shin DH, Yoo YB, Cha CI (2001b) Differential alterations in the distribution of voltage-gated calcium channels in aged rat cerebellum. Brain Res 903:247–252
Chung YH, Shin CM, Joo KM, Kim MJ, Cha CI (2002) Age-related upregulation of insulin-like growth factor receptor type I in rat cerebellum. Neurosci Lett 330:65–68
Chung YH, Joo KM, Kim MJ, Cha CI (2003) Age-related changes in the distribution of Na(v)1.1 and Na(v)1.2 in rat cerebellum. NeuroReport 14:841–845
Dhar P, Mohari N, Mehra RD (2007) Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology 234:10–20
Dlugos C (2005) Analyses of smooth endoplasmic reticulum of cerebellar parallel fibers in aging, ethanol-fed rats. Alcohol 35:67–73
Dlugos CA, Pentney RJ (1994) Morphometric analyses of Purkinje and granule cells in aging F344 rats. Neurobiol Aging 15:435–440
Drüge H, Heinsen H, Heinsen YL (1986) Quantitative studies in ageing Chbb: THOM (Wistar) rats. II. Neuron numbers in lobules I, VIb+c and X. Bibl Anat 28:121–137
Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF (2010) Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci 30:5368–5375
Fattoretti P, Bertoni-Freddari C, Caselli U, Paoloni R, Meier-Ruge W (1996) Morphologic changes in cerebellar mitochondria during aging. Anal Quant Cytol Histol 18:205–208
Fattoretti P, Bertoni-Freddari C, Caselli U, Paoloni R, Meier-Ruge W (1998) Impaired succinic dehydrogenase activity of rat Purkinje cell mitochondria during aging. Mech Ageing Dev 101:175–182
Felici L, Bronzetti E, Amenta F (1989) Enzyme histochemistry of glutamate dehydrogenase in ageing rat cerebellar cortex. Mech Ageing Dev 47:199–205
George O, Vallée M, Le Moal M, Mayo W (2006) Neurosteroids and cholinergic systems: implications for sleep and cognitive processes and potential role of age-related changes. Psychopharmacology 186:402–413
Glick R, Bondareff W (1979) Loss of synapses in the cerebellar cortex of the senescent rat. J Gerontol 34:818–822
Gorbunova V, Seluanov A, Mao Z, Hine C (2007) Changes in DNA repair during aging. Nucleic Acids Res 35:7466–7474
Goukassian DA, Bagheri S, Keeb L, Eller MS, Gilchrest BA (2002) DNA oligonucleotide treatment corrects the age-associated decline in DNA repair capacity. FASEB J 16:754–756
Gruart A, Muñoz MD, Delgado-García JM (2006) Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci 26:1077–1087
Hadj-Sahraoui N, Frederic F, Zanjani H, Delhaye-Bouchaud N, Herrup K, Mariani J (2001) Progressive atrophy of cerebellar PC dendrites during aging of the heterozygous staggerer mouse (Rora+/sg). Dev Brain Res 126:201–209
Hall TC, Millerakh Corsellis JAN (1975) Variations in the human Purkinje cell population according to age and sex. Neuropathol Appl Neurobiol 1:267–292
Hilber P, Caston J (2001) Motor skills and motor learning in Lurcher mutant mice during aging. Neuroscience 102:615–623
Ho YS, Yu MS, Yik SY, So KF, Yuen WH, Chang RC (2009) Polysaccharides from Wolfberry Antagonizes Glutamate Excitotoxicity in Rat Cortical Neurons. Cell Mol Neurobiol 29:1233–1244
Hogan MJ (2004) The cerebellum in thought and action: a fronto-cerebellar aging hypothesis. New Ideas in Psychol 22:97–125
Hua T, Kao C, Sun Q, Li X, Zhou Y (2008) Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain Res Bull 75:119–125
Huang CM, Brown N, Huang RH (1999) Age-related changes in the cerebellum: parallel fibers. Brain Res 840:148–152
Huang CM, Miyamoto H, Huang RH (2006a) The mouse cerebellum from 1 to 34 months: parallel fibers. Neurobiol Aging 27:1715–1718
Huang CM, Wang L, Huang RH (2006b) Cerebellar granule cell: ascending axon and parallel fiber. Eur J Neurosci 23:1731–1737
Jankovski A, Rossi F, Sotelo C (1996) Neuronal precursors in the postnatal mouse cerebellum are fully committed cells: evidence from heterochronic transplantations. Eur J Neurosci 8:2308–2319
Jin K, Galvan V (2007) Endogenous neural stem cells in the adult brain. J Neuroimmune Pharmacol 2:236–242
Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC (1998) Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci 18:8047–8055
Klein C, Butt SJ, Machold RP, Johnson JE, Fishell G (2005) Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development 132:4497–4508
Kodama T, Itsukaichi-Nishida Y, Fukazawa Y, Wakamori M, Miyata M, Molnar E, Mori Y, Shigemoto R, Imoto K (2006) A CaV2.1 calcium channel mutation rocker reduces the number of postsynaptic AMPA receptors in parallel fiber–Purkinje cell synapses. Eur J Neurosci 24:2993–3007
Lärkfors L, Lindsay RM, Alderson RF (1994) Ciliary neurotrophic factor enhances the survival of Purkinje cells in vitro. Eur J Neurosci 6:1015–1025
Larsen JO, Skalicky M, Viidik A (2000) Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. Comp Neurol 428:213–222
Lee JY, Lyoo IK, Kim SU, Jang HS, Lee DW, Jeon HJ, Park SC, Cho MJ (2005) Intellect declines in healthy elderly subjects and cerebellum. Psychiatry Clin Neurosci 59:45–51
Lee KJ, Jung JG, Arii T, Imoto K, Rhyu IJ (2007) Morphological changes in dendritic spines of Purkinje cells associated with motor learning. Neurobiol Learn Mem 88:445–450
Louis ED, Faust PL, Vonsattel JP, Honig LS, Henchcliffe C, Pahwa R, Lyons KE, Rios E, Erickson-Davis C, Moskowitz CB, Lawton A (2009) Older onset essential tremor: more rapid progression and more degenerative pathology. Mov Disord 24:1606–1612
Manev H, Uz T, Sugaya K, Qu T (2000) Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J 14:1464–1469
Markowska AL, Mooney M, Sonntag WE (1998) Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 87:559–569
Martinez Gagliardo K, Clebis NK, Stabille SR, De Britto MR, De Sousa JM, De Souza RR (2008) Exercise reduces inhibitory neuroactivity and protects myenteric neurons from age-related neurodegeneration. Auton Neurosci 141:31–37
Marwaha J, Hoffer B, Pittman R, Freedman R (1980) Age-related electrophysiological changes in rat cerebellum. Brain Res 201:85–97
Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR (2002) Neurophysiological correlates of age-related changes in human motor function. Neurology 58:630–635
Monteiro RA (1991) Age-related quantitative changes in the organelles of rat neocerebellar Purkinje cells. Histol Histopathol 6:9–20
Monteiro RA, Rocha E, Marini-Abreu MM (1992) Age-related quantitative changes in inhibitory axo-somatic synapses on Purkinje cells of rat neocerebellum (Crus I and Crus II). J Submicrosc Cytol Pathol 24:351–357
Monteiro RA, Rocha E, Marini-Abreu MM (1994) Heterogeneity and death of Purkinje cells of rat neocerebellum (Crus I and Crus II): hypothetic mechanisms based on qualitative and quantitative microscopical data. J Hirnforsch 35:205–222
Monteiro RA, Henrique RM, Rocha E, Silva MW, Oliveira MH (2000) Quantitative age-changes in endoplasmic reticulum and nucleus of cerebellar granule cells. Neurobiol Aging 21:97–105
Niblock MM, Brunso-Bechtold JK, Riddle DR (2000) Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J Neurosci 20:4165–4176
Nosal G (1979) Neuronal involution during ageing. Ultrastructural study in the rat cerebellum. Mech Ageing Dev 10:295–314
Ogata R, Ikari K, Hayashi M, Tamai K, Tagawa K (1984) Age-related changes in the Purkinje's cells in the rat cerebellar cortex: a quantitative electron microscopic study. Folia Psychiatr Neurol Jpn 38:159–167
Ohyama H, Hiramatsu M, Ogawa N, Mori A (1995) Age-related differences in synaptosomal membrane fluidity. Biochem Mol Biol Int 37:133–140
Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E (1999) Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev 111:39–47
Parfitt KD (1988) Age-related electrophysiological changes in cerebellar noradrenergic receptors. Age 11:120–127
Patrick GW, Anderson WJ (2000) Dendritic alterations of cerebellar Purkinje neurons in postnatally lead-exposed kittens. Dev Neurosci 22:320–328
Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, Clark CR, Kukla M, Mulligan R, Gordon E (2009) Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiol Aging 30:457–465
Pentney RJ, Mullan BA, Felong AM, Dlugos CA (2002) The total numbers of cerebellar granule neurons in young and aged Fischer 344 and Wistar–Kyoto rats do not change as a result of lengthy ethanol treatment. Cerebellum 1:79–89
Pires RS, Real CC, Folador TS, Tellini NR, Torrão AS, Britto LR (2010) Differential response of AMPA and NMDA glutamate receptors of Purkinje cells to aging of the chicken cerebellum. Neurosci Lett 478:146–149
Poe BH, Linville C, Brunso-Bechtold J (2001) Age-related decline of presumptive inhibitory synapses in the sensorimotor cortex as revealed by the physical disector. J Comp Neurol 439:65–72
Porras-García E, Cendelin J, Domínguez-del-Toro E, Vozeh F, Delgado-García JM (2005) Purkinje cell loss affects differentially the execution, acquisition and prepulse inhibition of skeletal and facial motor responses in Lurcher mice. Eur J Neurosci 21:979–988
Porras-García E, Sánchez-Campusano R, Matínez-Vargas D, Domínguez-Del-Toro E, Cendelín J, Vozeh F, Delgado-Garcia JM (2010) Behavioral characteristics, associative learning capabilities, and dynamic association mapping in an animal model of cerebellar degeneration. J Neurophysiol 104:346–365
Quackenbush LJ, Ngo H, Pentney RJ (1990) Evidence for nonrandom regression of dendrites of Purkinje neurons during aging. Neurobiol Aging 11:111–115
Rao SM, Mattson PM (2001) Stem cells and aging: expanding the possibilities. Mech Ageing Dev 122:713–734
Rogers J, Zornetzer SF, Bloom FE (1981) Senescent pathology of cerebellum: Purkinje neurons and their parallel fiber afferents. Neurobiol Aging 2:15–25
Rogers J, Zornetzer SF, Bloom FE, Mervis RE (1984) Senescent microstructural changes in rat cerebellum. Brain Res 292:23–32
Sabbatini M, Barili P, Bronzetti E, Zaccheo D, Amenta F (1999) Age-related changes of glial fibrillary acidic protein immunoreactive astrocytes in the rat cerebellar cortex. Mech Ageing Dev 108:165–172
Schaller KL, Caldwell JH (2003) Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum 2:2–9
Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y (2003) Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol 71:3–29
Seo MY, Chung SY, Choi WK, Seo YK, Jung SH, Park JM, Seo MJ, Park JK, Kim JW, Park CS (2009) Anti-aging effect of rice wine in cultured human fibroblasts and keratinocytes. J Biosci Bioeng 107:266–271
Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G (2007) Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci USA 104:9858–9863
Sjöbeck M, Englund E (2001) Alzheimer's disease and the cerebellum: a morphologic study on neuronal and glial changes. Dement Geriatr Cogn Disord 12:211–218
Sun Y, Jin K, Mao XO, Xie L, Peel A, Childs JT, Logvinova A, Wang X, Greenberg DA (2005) Effect of aging on neuroglobin expression in rodent brain. Neurobiol Aging 26:275–278
Takahashi E, Niimi K, Itakura C (2009) Motor coordination impairment in aged heterozygous rolling Nagoya, Cav2.1 mutant mice. Brain Res 1279:50–57
Taniwaki T, Okayama A, Yoshiura T, Togao O, Nakamura Y, Yamasaki T, Ogata K, Shigeto H, Ohyagi Y, Kira J, Tobimatsu S (2007) Age-related alterations of the functional interactions within the basal ganglia and cerebellar motor loops in vivo. Neuroimage 36:1263–1276
Tolbert DL, Clark BR (2003) GDNF and IGF-I trophic factors delay hereditary Purkinje cell degeneration and the progression of gait ataxia. Exp Neurol 183:205–219
Tranquilli Leali FM, Artico M, Potenza S, Cavallotti C (2003) Age-related changes of monoaminooxidases in rat cerebellar cortex. Eur J Histochem 47:81–86
Tu PH, Robinson KA, de Snoo F, Eyer J, Peterson A, Lee VM, Trojanowski JQ (1997) Selective degeneration of Purkinje cells with Lewy body-like inclusions in aged NFHLACZ transgenic mice. J Neurosci 17:1064–1074
von Bohlen und Halbach O, Unsicker K (2002) Morphological alterations in the amygdala and hippocampus of mice during ageing. Eur J Neurosci 16:2434–2440
Woodruff-Pak DS (2006) Stereological estimation of Purkinje neuron number in C57BL/6 mice and its relation to associative learning. Neuroscience 141:233–243
Woodruff-Pak DS, Vogel RW 3rd, Ewers M, Coffey J, Boyko OB, Lemieux SK (2001) MRI-assessed volume of cerebellum correlates with associative learning. Neurobiol Learn Mem 76:342–357
Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KP, Comalli DM, Kennard JA, Agelan A, Thompson RF (2010) Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci USA 107:1624–1629
Zhang CZ, Hua TM, Zhu ZM, Luo X (2006) Age-related changes of structures in cerebellar cortex of cat. J Biosci 31:55–60
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Anhui Provincial Education Bureau (No. KJ2007B330ZC) and the Key Natural Science Foundation of Anhui Provincial Education Bureau (No. KJ2009A167).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, C., Zhu, Q. & Hua, T. Aging of cerebellar Purkinje cells. Cell Tissue Res 341, 341–347 (2010). https://doi.org/10.1007/s00441-010-1016-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1016-2