Abstract
The oriental fruit moth Cydia molesta is an important pest and the behavioural role of olfactory signals such as pheromones and plant volatiles have been studied extensively in both sexes. To understand odour processing further, however, detailed knowledge of the anatomy of the olfactory system is crucial. In the present study, an atlas of the antennal lobe (AL) is presented based on the three-dimensional reconstructions of both ALs of three male and three female brains by means of neuroanatomical and computational approaches. We identified 48–49 "ordinary" glomeruli and one large glomerulus situated at the entrance of the antennal nerve in males, and 49–52 "ordinary" glomeruli and one large glomerulus in the ventro-medial part of the AL in females. Anomalous supernumerary, anomalous missing and sexually dimorphic glomeruli were found in the studied individuals in greater numbers than in other lepidopteran species. Male and female maps were compared with respect to glomerular size and position with 45 glomeruli being matched, indicating a conserved glomerular pattern between the sexes. Three additional glomeruli were sexually dimorphic in size and five male-specific and six female-specific glomeruli were also found. Palp backfills resulted in the staining of a unique glomerulus in both sexes identified as the sexually dimorphic glomerulus 45. This glomerulus was never stained from antennal backfills, which stained the other glomeruli of the AL. The three-dimensional atlas can now be used to elucidate the functional role of individual glomeruli in both sexes of C. molesta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In insects, odour information is detected and coded in olfactory receptor neurons (ORNs) housed in sensilla mainly located on the antenna. ORNs send axonal projections to the first structure that processes olfactory information in the brain, the antennal lobe (AL). The ALs are divided into structural units named glomeruli. These glomeruli have emerged as important functional structures in which synaptic interactions take place. They are readily accessible for experiments because of their small number and large size. The distinctiveness of glomeruli varies greatly among different species and their number is species-specific (e.g. Rospars 1988; Hildebrand and Shepherd 1997; Rospars and Hildebrand 2000). For most insect species, the AL contains between 40 and 100 glomeruli, as opposed to the vertebrate primary olfactory centre, the olfactory bulb, that often contains more than 1000 glomeruli. Nonetheless, important similarities have been found in the neuroanatomy and physiology between the olfactory systems of mammals and insects (Hildebrand and Shepherd 1997) and an increasing number of studies suggests that the quality of an odour stimulus is represented across the glomerular array, both in insects (Joerges et al. 1997; Vickers et al. 1998; Mustaparta 2005) and in vertebrates (Jourdan et al. 1980; Kauer 2002; Bozza et al. 2002).
In Lepidoptera, sex-pheromone-related information is mostly processed within the macroglomerular complex (MGC), a group of glomeruli that are male-specific and typically enlarged in this sex, whereas plant-odour-related information is known to be processed in the so-called “ordinary” glomeruli present in both males and females (Anton and Hansson 1994, 1995; Christensen and Hildebrand 2002). The number of glomeruli forming the MGC is often similar to the number of behaviourally relevant pheromone components, with each ORN type projecting to one MGC glomerulus in several moth species (e.g. Hansson et al. 1992; Ochieng et al. 1995; Todd et al. 1995; Berg et al. 1998). By contrast, the representation of non-pheromone odours is less well understood. Although some attempts have been made to stain plant-odour-responding ORNs from the antennae of various moth species (Hillier et al. 2006; Hillier and Vickers 2007; Todd and Baker 1996) without success with regard to clear functional mapping, the specificity of non-pheromone-receptor projections has been primarily examined indirectly via the expression of olfactory membrane receptors (e.g. Gao et al. 2000; Vosshall et al. 2000; Bhalerao et al. 2003) and optical imaging studies in moths, fruit flies and honeybees (Galizia et al. 2000; Carlsson et al. 2002; Meijerink et al. 2003; Wang et al. 2003). In these studies, the functional representation of general odours has been shown to be similar in both sexes (Carlsson et al. 2002). Anatomical matching of isomorphic glomeruli between the sexes has been carried out in the cockroach Blaberus craniifer (Rospars and Chambille 1981), the noctuid moth Mamestra brassicae (Rospars 1983) and the sphingid moth Manduca sexta (Rospars and Hildebrand 2000) and in heliothine moths (Skiri et al. 2005).
To improve our understanding of the spatial component of odour processing in glomeruli, we need to know their exact arrangement within the AL. For this purpose, three-dimensional (3D) maps have been constructed for several insect species (Rospars 1983; Flanagan and Mercer 1989; Rospars and Chambille 1989; Stocker et al. 1990; Galizia et al. 1999; Laissue et al. 1999; Rospars and Hildebrand 2000; Chiang et al. 2001; Berg et al. 2002; Sadek et al. 2002; Smid et al. 2003; Greiner et al. 2004; Ignell et al. 2005; Huetteroth and Schachtner 2005; Masante-Roca et al. 2005; Skiri et al. 2005; Ghaninia et al. 2007; Zube et al. 2008).
The oriental fruit moth Cydia molesta (Busck) (Lepidoptera: Tortricidae), previously known as Grapholita molesta, is a major pest of stone fruits worldwide and recently also of apples and pears (Rothschild and Vickers 1991; Il’ichev et al. 2004; Reis et al. 1988; Hickel and Ducroquet 1998; Kovanci et al. 2004). Its sex pheromone blend comprises four components: (Z)-8 dodecenyl acetate (Z8–12:Ac), (E)-8 dodecenyl acetate (E8–12:Ac), (Z)-8 dodecen-1-ol (Z8–12:OH) and dodecan-1-ol (12:OH) (Roelofs et al. 1969; Cardé et al. 1979), the first three being required to evoke pheromone-mediated flight in males (Baker et al. 1981; Charlton and Cardé 1981; Linn and Roelofs 1983). Pheromone-based techniques are well established to monitor C. molesta and, since the implementation of mating disruption, the effect of the sex pheromone on the behaviour of males has been extensively studied (Charlton and Cardé 1981; Figueredo and Baker 1991; Rumbo and Vickers 1997; Stelinski et al. 2005). In addition, the effect of male pre-exposure to the sex pheromone has been studied. Stelinski et al. (2005) have found that the mean durations of sustained flights of pre-exposed males are shorter than for naïve males and that the effect of the pheromone pre-exposure decays over time. The effect of sex pheromone on the behaviour of females has only recently begun to be investigated; only the major component (Z8–12:Ac) has been reported to have an effect in females by advancing their calling time by 2 h (Stelinski et al. 2006). Plant-derived stimuli are no less important for phytophagous insects than is the sex pheromone. Mated females have been shown to fly to peaches and apples and to butyl hexanoate, a major apple volatile, in a double-choice arena test (Natale et al. 2004; Piñero and Dorn 2007).
Although the behaviour of C. molesta has been well studied, the structure and function of its peripheral and central olfactory system are largely unknown. Nonetheless, calcium-imaging studies have shown that benzaldehyde (a minor constituent of a host plant-derived synthetic mixture), which elicits a behavioural response in mated females, plays a key role in the behavioural discrimination and in the neural representation of mixtures (Piñero et al. 2007). The behavioural data available make this species well suited for functional studies of plant and pheromone processing and its plasticity.
In the present study, we have established the 3D glomerular map for males and females of C. molesta as a tool for future functional studies. We have aimed at a precise comparison of male and female maps in a species in which the detection of pheromone and plant compounds is well described in both sexes. Backfills from palps and antennae have been performed to differentiate between target glomeruli of functionally discrete receptor neuron populations. Precise maps are a prerequisite for an understanding of the discrimination of olfactory signals. The 3D map should serve to identify target glomeruli of physiologically characterized and stained ORNs and projection neurons in this species. It should also provide a common frame for the interpretation of calcium-dependent responses in optical imaging studies and for the localization of glomeruli that are involved in various kinds of plasticity.
Materials and methods
Insects
Pupae of Cydia molesta were obtained from Piacenza, Italy (courtesy of Dr. Fabio Molinari). Rearing on an artificial diet was carried out at Lleida, Spain and the adults were allowed to emerge in an environmental chamber kept under a 16 h light:8 h dark cycle at 25±1°C. Male and female adults used for anatomical experiments were no more than 3 days old.
Antibody staining procedure
In order to visualize individual glomeruli and to study the anatomy of C. molesta, we first punctured the insect head capsule and then fixed it for 3 h at room temperature in a solution of 4% paraformaldehyde in 0.1 M phosphate buffer solution (PBS) at pH 7.4 to facilitate the dissection. Once dissected, the brains were kept overnight in a fixative of 4% paraformaldehyde with 0.25% Triton X-100 in PBS (PBST) at 4°C. After fixation, the brains were rinsed in PBST and left in 2% normal goat serum (Sigma-Aldrich Quimica, Madrid, Spain) in PBST for 2 h. Afterwards, they were incubated for 48 h on a rotator in a synapsin antibody produced in mouse (DSHB, Iowa, USA; Klagges et al. 1996) diluted 1:50 in PBST with 2% normal goat serum at 4°C, rinsed in PBST and incubated in Alexa-fluor-488 goat anti-mouse conjugate (Invitrogen) applied at a dilution of 1:150 in PBST for 48 h on a rotator at 4°C. The brains were then rinsed with PBST, cleared in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif., USA) for 24 h, mounted as whole-mounts on glass slides with a spacer (0.12 mm) between the slide and the coverslip and visualized under a laser scanning confocal microscope (see below).
Palp and antennal backfills
Male and female moths of 1 or 2 days of age were restrained in a plastic pipette tip with the head protruding. The head was immobilized with dental wax. The pipette tip was then placed horizontally inside a Petri dish containing a moist paper tissue. The tip of the antennae or the tip of the palp was cut and a glass capillary filled with 1% solution of neurobiotin (Neurobiotin Tracer, Vector Laboratories) in 0.25 M KCl was also placed horizontally allowing the cut end of the insect antennae or the palp to enter the glass capillary. The Petri dish was then kept at 4ºC for 24 h for antennal backfills and 6 h for palp backfills.
Neurobiotin-stained brains were subsequently dissected in Millonig’s buffer (pH 7.2) and fixed in 4% paraformaldehyde in Millonig’s buffer at room temperature overnight. The brains were then rinsed in Millonig’s buffer and dehydrated and rehydrated in ethanol and propylene oxide to increase membrane permeability. Neurobiotin was then visualized by incubation in buffered Oregon green-avidin conjugate (Oregon Green, Invitrogen, Barcelona, Spain) with 0.25% Triton X and 1% bovine serum albumin. After incubation, the brains were again rinsed in Millonig’s buffer, cleared and mounted in Vectashield as described above.
Confocal microscopy
Whole-mount brains were viewed with a FluoView 500 Olympus confocal microscope (Hamburg, Germany) equipped with an argon laser that permitted the visualization of structures labelled with Alexa-fluor-488. For the overview scanning of the whole brain, a 20× dry objective was used with optical sections at a thickness of 2 µm, whereas detailed scanning was performed with a 40× dry objective at 1-µm-thick optical sections. All confocal images were scanned and stored at a resolution of 1024×1024 pixels.
Maximum projections were performed from partial stacks of backfill image series obtained with the confocal microscope by means of Image J software (NIH, USA).
Segmentation and measurements on sections
The brains that presented good fluorescent staining in all sections of the entire stack were selected for 3D reconstruction. In both the right and the left ALs of three males (M1–3) and three females (F1–3), the outlines of each individual glomerulus were manually delineated in every optical section of the high magnification scans by using a custom-made program (K. Kiêu, L. Couton and J.P. Rospars, unpublished) developed in the Matlab language (The MathWorks, Natick, Mass., USA). The volume v and the coordinates x, y, z of the centre of mass of each glomerulus were calculated from its outlines. The data relative to a glomerulus were then reduced to four values: the coordinates x, y, z and the radius R of the sphere having the same volume. The x- and y-axes corresponded to the bottom and left edges of each image, respectively. The z-axis corresponded to the succession of the intersections of the x- and y-axes, at bottom-left, and defined the location of every section within the stack. The z-dimension (thickness) had to be corrected by a factor of 1.6 because of the refractive index mismatch caused by the air objectives (Bucher et al. 2000). The voxel size obtained for the overview scanning was 0.550×0.550×3.2 µm (x, y, z). For detailed scanning, the voxel size was 0.275×0.275×1.6 µm (x, y, z).
Identification and final coordinates of glomeruli
To identify glomeruli and to calculate their standardized coordinates, we proceeded as explained previously (Rospars 1983; Couton et al. 2009). Only a summary will be given here. After all visible glomeruli had been delineated in both ALs, a preliminary number was given to each one. However, two large glomeruli, one at the entrance of the antennal nerve (AN) in males and another one located medio-ventrally in females, could be recognized immediately in all individuals of the same sex. These glomeruli were given the same number in all three individuals of the same sex and were used as landmark glomeruli.
A comparison was then made to match homologous right and left glomeruli (intra-individual). This comparison was achieved by comparing (1) the confocal sections directly at different levels of the z-axis throughout the AL, (2) realistic 3D reconstructions and (3) standardized views showing the ALs from various sides (lateral, medial, anterior, posterior, dorsal and ventral) after correction of the orientation of the brains with respect to their plane of symmetry. In these views, the left ALs were represented as their mirror image with respect to the plane of symmetry of the brain in oder to be directly comparable with the right ALs. After matching the right and left glomeruli in ALs of the same individual, an average AL was produced by calculating the mean coordinates and mean radii of the matched glomeruli.
The inter-individual matchings were achieved in three steps. First, we compared the standardized view of the three average ALs of males. To compensate for the different optical sectioning planes in the various preparations, we rotated the average ALs around the medio-lateral X-axis, which is perpendicular to the plane of symmetry. At this stage, the position of each glomerulus was defined in a coordinate system with its origin at the AL centre and with axes X medio-lateral, Y postero-anterior and Z ventro-dorsal. We calculated an average male AL based on the means of coordinates X, Y, Z and radii of all matched glomeruli. Secondly, we performed the same procedure for the females and oriented the ALs identically to the male ALs in order to obtain an average female AL. Finally, we compared the standardized views of the two reconstructed average ALs (male and female) to determine possible differences between them. We additionally checked these inter-sexual matchings on the six average male and female ALs.
Statistical tests
The sizes of matched male (6 radii) and female (6 radii) glomeruli were compared by the non-parametric Wilcoxon test at the 1% significance level. The glomeruli that, in this test, showed a significant difference were considered as sexually dimorphic. The global equality in size of homologous glomeruli in two lobes was tested at the 5% significance level by using the coefficients of correlation of their radii. The conservation of their spatial location was tested in the same way by using the distance of each glomerulus to the centre of the lobe in which it resided. All tests were carried out with the Matlab Statistical Toolbox.
Nomenclature
For naming glomeruli, the following rules were used: glomeruli found in several lobes were given a number (1–45), those found in a single sex (sex-specific) were given a letter (a–e in males, f–n in females) and, finally, the anomalous glomeruli found in a single lobe were also given a letter (o–q). Hence, all numbered glomeruli were found in both sexes, whereas glomeruli designated by a letter were found in only one sex, one individual or one lobe.
Results
Three-dimensional reconstruction
The small size of the C. molesta brains made it possible to visualize easily the entire AL as a single stack of sequential optical sections (Figs. 1, 2). The ALs were spherical and their antero-posterior diameter was 70–85 µm for males and 60–75 µm for females. Left and right ALs were situated touching each other and the main cell body cluster was located ventrally in both sexes (Figs. 1, 2). The ordinary glomeruli surrounded a central fibre core devoid of any glomeruli (Figs. 1, 2). Most glomeruli were uniform in shape, size and relative position on comparison of the different ALs and could therefore be identified and recognized individually.
Series of confocal sections of the antennal lobe (AL) from anterior (a) to posterior (f) at various depths (z distance from anterior pole of the AL) through the left AL of Cydia molesta M1 reconstructed in Fig. 3a–f (an antennal nerve, cb cell body cluster, d dorsal, l lateral, m medial, v ventral, 1–45 and a–e male glomeruli; see Table 1). Bar 50 µm
Series of confocal sections from anterior (a) to posterior (e) at various depths (z distance from anterior side of the AL) through the left AL of C. molesta F1 reconstructed in Fig. 3g–l (an antennal nerve, cb cell body cluster, d dorsal, l lateral, m medial, v ventral, 1–45 and f–n female glomeruli; see Table 1). Bar 50 µm
Male ALs
In the six male ALs studied, we found 49–50 glomeruli; 47 could be systematically identified in all six ALs, one of them being the enlarged glomerulus located at the AN entrance (44=cumulus, Cu; Fig. 1, 3a). The remaining glomeruli were anomalous in the studied samples and belonged to two categories: missing and supernumerary (Table 1, left columns). We considered a glomerulus as missing when it was absent from a single individual or from a single AL. Glomerulus 42 was missing in both lobes of M2 and was the smallest glomerulus in M1 and M3 with a radius (R) of 9 µm. Glomerulus 36 was missing in the left lobe of M3, and glomerulus 40 in its right lobe (Table 1). Conversely, we considered a glomerulus as supernumerary when it was present in a single individual, such as q, which was found only in M2 (Table 1) and which was also a small glomerulus (R=9 µm).
Three-dimensional maps of the left AL of male M1 (a–f) and female F1 (g–l). Views are perpendicular to the medio-lateral X, postero-anterior Y and ventro-dorsal Z-axes. Small sketches within each view (bottom left or right) show the orientation of the ALs (red circles) with respect to the brain. Glomeruli are coloured according to the categories in Table 1: yellow anomalous missing (not present in at least one individual of the two sexes: 36–42, l, n), red male-specific (a–e), blue female-specific (f–k), green sexually dimorphic (43–45), grey sexually isomorphic (1–35). Anomalous supernumerary (present in only one individual) are not shown (al antennal lobe, an antennal nerve, cb cell body cluster, ol optic lobe, sog suboesophageal ganglion; isomorphic glomeruli, same as in Fig. 2. Bars 50 µm
The radii of the male glomeruli are mostly in the range of 9 to 16 µm (Fig. 4, thick line). The Cu was the largest structure within all ALs with a radius of 25, 22 and 27 µm in M1, M2 and M3, respectively. It was bordered by two large glomeruli, one laterally close to Cu (11 with R=16 µm) and another dorsally (12 with R=15 µm; Fig. 3c, e). Two of the smallest male glomeruli lay on opposite sides of the most posterior AL region, one ventral (35 with R=9 µm) and the other dorsal (42 with R=10 µm) (Fig. 3b, d).
Histograms of glomerular sizes in males (thick line) and females (thin line). In both sexes, the radius of each identified glomerulus was calculated as the mean of the available measurements (6 per sex for non-anomalous glomeruli). Almost all glomeruli follow a normal distribution in the range 9 to 16 µm. The two right-most glomeruli are the sexually dimorphic 44 (25 µm in males) and 45 (22 µm in females)
The sizes of homologous glomeruli in the left and right ALs of the same brain and across male individuals were similar, as shown by their coefficients of correlation (Table 2, Fig. 5a, c). The same conclusion held for the distances of homologous glomeruli to the centre of their respective AL (Table 2, Fig. 5b, d), i.e. identified glomeruli from the same and different individuals had similar sizes and were situated at a similar position.
Comparison of sizes and spatial locations of homologous glomeruli in C. molesta. Location is quantified by the distance of each glomerulus to the centre of the corresponding AL. Radii (a) and distances (b) between the right and left ALs of M1 (intra-individual comparison). Radii (c) and distances (d) between averages of right and left lobes for males M1 and M2 (inter-individual comparison). Comparison of mean radii (e) and mean distances (f) for 6 male lobes and 6 female lobes. Each plot shows the two regression lines x/y and y/x. For radii (a, c, e), the sex dimorphic glomeruli (43, 44 and 45) were not included in the determination of the regression lines
Female ALs
In the six female ALs studied, we found 50–53 glomeruli; 47 of them were identified in all female ALs. Additional glomeruli were only found in some ALs within our sample (Table 1, right columns). As in males, these anomalous glomeruli were supernumerary or missing. Two were apparently supernumerary: o (present only in the right lobe of F2), and p (only in the right lobe of F3; Table 1). Other glomeruli were missing unilaterally or bilaterally in one individual: glomerulus m was missing in F1, whereas l and 41 were missing in F2 and n and 37 in F3 (Table 1). Interestingly, l is the smallest female glomerulus (R=7 µm; Fig. 3g–i).
The best identifiable structure was an enlarged glomerulus located medio-ventrally, viz. the large glomerulus 45 (Figs. 2, 3i, j). This exceptional glomerulus had a radius of 21, 20 and 23 µm in females F1, F2 and F3, respectively. The smallest female glomeruli were l (R=7 µm), n (R=9 µm) and 14 (R=10 µm). As in males, the radii of the other female glomeruli were in the range 9 to 16 µm (Fig. 4, thin line) and the intra- and inter-individual correlations of the radii and of the distances to the AL centre of homologous glomeruli were highly significant (Table 2).
Comparison of male and female ALs
Systematic comparisons of male and female ALs revealed three main qualitative classes of glomeruli within our sample: sex isomorphic, sex dimorphic and sex-specific.
The isomorphic glomeruli were present in both sexes in the same position and with the same size. They formed the largest class (35 glomeruli). In both sexes, glomeruli 1, 2 and 4 were situated close to each other in all ALs studied (Fig. 3c, i). Two of the largest and most elongated glomeruli (11 and 12) lay antero-ventrally and dorsally. The most anterior glomeruli seemed more densely packed than glomeruli in the rest of the AL. Seven other glomeruli (36–42) could be placed in this class, although they were classified as anomalous missing in one or two individuals. In particular, this was the case of glomeruli 38 and 39 of female F3, which were not found in F1 and F2. Indeed, the male-female comparisons suggested that they corresponded to male glomeruli 38 and 39. For this reason, they were considered as anomalous missing in two of the females (F1 and F2) and present in the other four individuals (M1–3 and F3). The radius histograms of isomorphic glomeruli were similar in males and females with an approximately Gaussian distribution in the range 9 to 16 µm (Fig. 4).
Sex dimorphic glomeruli were also present in both sexes but with significantly different radii (P-value less than 1%). We found three glomeruli in this class (Table 1) that were at least 25% larger in one sex than in the other. One of them, 44 (= Cu; Fig. 3a, g), was 93% larger in males than in females, with average radii of 25 and 13 µm, respectively. It was located at the entrance of the AN in both sexes. Two glomeruli, 43 and 45, were larger in females than in males. The average radius of glomerulus 43 was 13 µm in males and 16 µm in females, i.e. 25% larger in females. Glomerulus 45, located medio-ventrally (Fig. 3c, i), was 37% larger in females (16 µm in males and 22 µm in females). Two of the sexually dimorphic glomeruli (44 and 45) were far from the Gaussian distribution of the other glomeruli (Fig. 4), whereas the third one, glomerulus 43, was included in the distribution, indicating that sexual dimorphism was not restricted to “macroglomeruli”.
Sex-specific glomeruli were found in only one sex, five in males (a–e) and six in females (f–k). Three “anomalous” glomeruli (l, m and n) could be placed in the female-specific class in our sample; each was missing bilaterally in one female and in all males. These glomeruli were also found in different locations within the AL.
Based on this interpretation, which resulted from the study of 12 ALs, the 10 anomalous missing glomeruli listed in Table 1 could be finally assigned to two of the “regular” classes, the sex isomorphic (36–42 missing in one or two individuals) and the female-specific (l, m, n missing in one female) classes.
Finally, the location and size of the 45 homologous (isomorphic and sexually dimorphic) glomeruli were globally compared in males and females by using regression plots (Fig. 5e, f) and coefficients of correlation (Table 2). Both methods showed that the location of glomeruli (as measured by their distance to the centre of the lobe) were well conserved between sexes, although less well than between individuals of the same sex. Similarly, the inter-sexual coefficient of correlation of the radii of homologous glomeruli in the male and the female average ALs were found to be larger than the intra-sexual coefficients of correlation. However, all correlations were still significant (Table 2, Fig. 5e, f).
Palp and antennal backfills
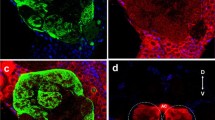
Out of 39 attempted backfills in the palp, 19 (49%) were successful, whereas out of 12 attempted backfills from the antennae, seven (58%) resulted in stained fibres in the brain. Unilateral palp backfills revealed bilateral projections ascending to the AL in both males and females. Within each AL, only one glomerulus in a medio-ventral position received stained fibres (Fig. 6a, b). In males, the glomerulus stained was identified as number 45, which, according to the male-female comparison, also corresponded to glomerulus 45 stained in our palp backfill preparations in females (Fig. 6a, b).
Maximum projections of stacks of confocal sections of the deuto- and tritocerebrum and the suboesophageal ganglion of C. molesta showing male (a z: 7–47 µm) and female (b z: 21–38 µm) palp backfills and male (c z: 6–54 µm) and female (d z: 9–58 µm) antennal backfills (al antennal lobe, an antennal nerve, sog suboesophageal ganglion, 45 ordinary glomerulus receiving input from the palps). Bar 50 µm
Unilateral antennal backfills revealed ipsilateral staining in a large number of, but not in all, glomeruli in both males and females. None of the successful antennal backfills had stained branches in glomerulus 45 in males or in females (Fig. 6c, d).
Discussion
In the present paper, a complete AL atlas for both sexes of the tortricid moth C. molesta is presented based on the systematic anatomical matching of glomeruli within and between the sexes. Although sexual isomorphism of so-called "ordinary" glomeruli has been known for a long time (Rospars and Chambille 1989), we show here that, even though a large percentage of glomeruli seem to be isomorphic between sexes, many differences exist with respect to the presence, absence or size of glomeruli for this lepidopteran species. This has permitted us to distinguish, in our sample, anomalous supernumerary, anomalous missing, sexually dimorphic, and sexually-specific glomeruli.
Number of glomeruli in Cydia molesta
The AL structure of C. molesta resembles the glomerular organization of other insects, i.e. glomeruli that surround a central fibre core. The number of glomeruli is known to vary among insect species, ranging from ∼50 to 61 in mosquitoes (Ignell et al. 2005; Ghaninia et al. 2007), 50 in fruit flies (Laissue et al. 1999; Couto et al. 2005; Fishilevich and Vosshall 2005), 99 in cockroaches (Chiang et al. 2001), 156 in honeybees (Flanagan and Mercer 1989; Galizia et al. 1999) and up to 1000 in locusts or social wasps (for references, see Ignell et al. 2001). In Lepidoptera, the number of glomeruli is constant, independently of the family or the size of the animals, and varies between 60 and 70 (Rospars 1983; Rospars and Chambille 1989; Rospars and Hildebrand 1992, 2000; Berg et al. 2002; Sadek et al. 2002; Ai and Kanzaki 2004; Greiner et al. 2004; Masante-Roca et al. 2005).
In C. molesta, the number of “ordinary” glomeruli found, i.e. 50 in males and 54 in females (not including anomalous supernumerary glomeruli) is almost the same as that found in the sibling species Cydia pomonella, i.e. 50 in males and 52 in females (Ansebo 2004). This is slightly lower than the numbers found in other lepidopteran species: 64–68 in various Noctuid species (Rospars 1983; Berg et al. 2002; Sadek et al. 2002; Greiner et al. 2004), 64 in the Sphingid Manduca sexta (Rospars and Hildebrand 1992, 2000) and 60–71 in the Tortricid moth Lobesia botrana (Masante-Roca et al. 2005). The mentioned lepidopteran species investigated so far belong to distantly related families and, thus, differences in the number of glomeruli within the AL are therefore not surprising. However, the two Tortricid genera Cydia and Lobesia are closely related and the difference in the number of glomeruli is unexpected. An explanation of this difference might involve the different life styles of the investigated species. C. molesta and C. pomonella, which are oligophagous insects, are considered as major pests of peach and apple trees, respectively, and may use a narrower range of olfactory cues to find their hosts than Lobesia botrana, a polyphagous insect that can undergo development on more than 40 species of plants (Gabel et al. 1992; Ben-Yehuda et al. 1993; Katerinopoulos et al. 2005), even though it is considered as a major grapevine pest. In an insect species, the number of glomeruli is closely related to the number of expressed olfactory receptors (Couto et al. 2005) and, consequently, to the number and complexity of odours that they can discriminate. This is confirmed by data from highly social insects such as bees and ants, which have relatively large numbers of glomeruli and use a large variety of chemical cues (Galizia et al. 1999; Kleineidam et al. 2005).
Sexual dimorphism in the AL of Cydia molesta
We have found that a high percentage of glomeruli (88% in males, 45 out of 50; 83% in females, 45 out of 53) can be matched in the same position in both male and female ALs. This population can be subdivided into two classes: the sex-isomorphic glomeruli (42) and the sex-dimorphic glomeruli (3). Moreover, both sexes possess glomeruli that cannot be matched across sexes; these form a class of sex-specific glomeruli (5 in males and 9 in females in our sample).
Macroglomeruli in males
Large glomeruli are commonly found in species that use sex pheromone communication, such as moths. These glomeruli have been shown to receive exclusive input from ORNs sensitive to sex pheromone and the number of macroglomerular substructures in Lepidoptera is thought to be correlated to the number of behaviourally active components forming the pheromone blend of the respective species (Hansson et al. 1992; Ochieng et al. 1995; Todd et al. 1995; Berg et al. 1998). In C. molesta, we have found one enlarged glomerulus at the entrance of the AN in males. At least three pheromone components, Z8–12:Ac, E8–12:Ac and Z8–12:OH, are needed to elicit upwind flight in C. molesta males (Figueredo and Baker 1991; Lacey and Sanders 1992; Rumbo and Vickers 1997; Stelinski et al. 2005). Therefore, we hypothesize that other glomeruli might be part of a more complex macroglomerular structure that cannot be identified by anatomical criteria alone.
Female enlarged glomeruli
Even though plant volatiles generally seem to be more important to females than the sex pheromone, pheromone auto-detection is known from a few species (e.g. Ljunberg et al. 1993). In the case of C. molesta, females advance their calling period by 2 h when they detect sex pheromone released by conspecific females (Stelinski et al. 2006). No functional study, in males or females, has yet been performed to distinguish which glomeruli are involved in the processing of the sex pheromone or plant volatiles in this species. Although antennal electrophysiological responses and behavioural activation by the major pheromone component (Z8–12:Ac) have been demonstrated (Stelinski et al. 2006), no macroglomerulus-like structure is located at the entrance of the AN in females. However, one glomerulus (44, Fig. 3) is located in the same position as the male Cu at the entrance of the AL. In the noctuid moth Spodoptera littoralis, females have receptor neurons that detect their own pheromone and behavioural antagonists (Ljunberg et al. 1993). Such ORNs sensitive to the major sex pheromone component of their own pheromone have been shown to project to a small glomerulus located at the entrance of the AL (Ochieng et al. 1995). Moreover, intracellular recordings have revealed that projection neurons arborizing in the female glomeruli that occupy a position equivalent to the male MGC always respond to the pheromone components (Anton and Hansson 1994). We speculate that the same situation occurs in C. molesta as in S. littoralis. However, the functional role of the glomeruli in both males and females can only be elucidated by electrophysiological studies with subsequent staining or imaging experiments.
Other glomeruli differing between the sexes
Male moths need to find mating partners and they therefore have specific, often enlarged, glomeruli dedicated to sex pheromone processing. Mated females, on the other hand need to find oviposition sites. Therefore, we suggest that plant volatiles are more important for females than the sex pheromone and that this could account for the slightly higher number of “ordinary” glomeruli found in females than in males and also for the two sexually dimorphic glomeruli (43 and 45), which are larger in females than in males.
One of these glomeruli (45) has a larger size than “ordinary” glomeruli and is unlikely to be a member of the pheromone-sensitive glomeruli because of its position. According to our palp and antennal backfill preparations, this glomerulus, both in males and in females, receives input from the palp and not from the antennae. It is found in a position corresponding to the “labial pit organ glomerulus” previously described for M. sexta (Kent et al. 1986; Rospars and Hildebrand 2000) or H. assulta and H. virescens in which it is easily identified because of its large size and ventral position (Berg et al. 2002). Receptor neurons from the maxillary palps and labial palps of holometabolous insects usually target specific ventral glomeruli within the AL and have been shown to process information on CO2 levels in the environment (Guerenstein and Hildebrand 2007). More specifically, receptor neuron projections from the labial pit organ in moths and from maxillary sensilla in flies arborize uni- or bilaterally within one to three medio-ventrally situated glomeruli in each AL (Anton and Homberg 1999; Guerenstein et al. 2004; Ignell et al. 2005; Ghaninia et al. 2007; Guerenstein and Hildebrand 2007).
The coefficients of correlation of glomerular radii in intra-sexual comparisons are larger than the coefficient in the inter-sexual comparison (Table 2). This indicates that the so-called “isomorphic” glomeruli are not identical in both sexes, although the pairwise differences in size between homologous male and female glomeruli are not statistically significant at the 1% level when compared one at a time. The inter-sexual differences only clearly appear when studied collectively with plots (compare Fig. 5e, f) and coefficients of correlation (Table 2). This diffuse kind of sexual dimorphism has also previously been observed in other species (Rospars and Chambille 1989). It may reflect sexual differences in antennal olfactory sensilla, so that ORNs, expressing a given receptor type and projecting in any given glomerulus, might differ more in number between sexes than within the same sex.
Concluding remarks
In the case of C. molesta, data are available on the effect of pheromone and specific host-plant compounds on the behaviour of males and females, providing an excellent basis for functional studies. The map of the AL of C. molesta will be a useful tool (1) for the identification of target glomeruli of physiologically characterized ORNs and/or AL neurons and (2) for future functional studies allowing a better interpretation of the individual steps of the olfactory code.
Abbreviations
- 3D:
-
Three-dimensional
- AL:
-
Antennal lobe
- AN:
-
Antennal nerve
- Cu:
-
Cumulus
- MGC:
-
Macroglomerular complex
- ORN:
-
Olfactory receptor neuron
- PBS:
-
Phosphate buffer solution
- Z8–12:Ac:
-
(Z)-8 dodecenyl acetate
- E8–12:Ac:
-
(E)-8 dodecenyl acetate
- Z8–12:OH:
-
(Z)-8 dodecen-1-ol
- 12:OH:
-
Dodecan-1-ol
References
Ai H, Kanzaki R (2004) Modular organization of the silkmoth antennal lobe macroglomerular complex revealed by voltage-sensitive dye. J Expe Biol 207:633–644
Ansebo L (2004) Odour perception in the codling moth Cydia pomonella L.—from brain to behaviour. PhD thesis, Swedish University of Agricultural Sciences, Alnarp
Anton S, Hansson B (1994) Central processing of sex pheromone, host odour, and oviposition deterrent information by interneurons in the antennal lobe of female Spodoptera littoralis (Lepidoptera: Noctuidae). J Comp Neurol 350:199–214
Anton S, Hansson B (1995) Sex pheromone and plant-associated odour processing in the antennal lobe interneurons of male Spodoptera littoralis (Lepidoptera: Noctuidae). J Comp Physiol 176:773–789
Anton S, Homberg U (1999) Antennal lobe structure. In: Hansson BS (ed) Insect olfaction. Springer, Berlin, pp 98–125
Baker TC, Meyer W, Roelofs WL (1981) Sex-pheromone dosage and blend specificity of response by oriental fruit moth males. Entomol Exp Appl 30:269–279
Ben-Yehuda S, Izhar Y, Wyosoki M, Argaman Q (1993) The grape berry moth, Lobesia botrana Denis & Schiffenmueller (Lepidoptera: Tortricidae), in pear orchards, in Israel. J Pest Manag 39:149–151
Berg BG, Almaas TJ, Bjaalic JG, Mustaparta H (1998) The macroglomerular complex of the antennal lobe in the tobacco budworm Heliothis virescens: specified subdivision in four compartments according to information about biologically significant compounds. J Comp Physiol 183:669–682
Berg BG, Galizia CG, Brandt R, Mustaparta H (2002) Digital atlases of the antennal lobe in two species of tobacco budworm moths, the oriental Helicoverpa assulta (male) and the American Heliothis virescens (male and female). J Comp Neurol 446:123–134
Bhalerao S, Sen A, Reinhard S, Rodrigues V (2003) Olfactory neurons expressing identified receptor genes project to subsets of glomeruli within the antennal lobe of Drosophila melanogaster. J Neurosci 54:577–592
Bozza T, Feinstein P, Zheng C, Mombaerts P (2002) Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci 22:3033–3043
Bucher D, Scholz M, Stetter M, Obermayer K, Pflüger HJ (2000) Correction methods for three-dimensional reconstructions from confocal images. I. Tissue shrinking and axial scaling. J Neurosci Methods 100:135–143
Cardé AM, Baker TC, Cardé RT (1979) Identification of a four-component sex pheromone of the female oriental fruit moth Grapholita molesta (Lepidoptera: Tortricidae). J Chem Ecol 5:423–427
Carlsson MA, Galizia CG, Hansson BS (2002) Spatial representation of odours in the antennal lobe of the moth Spodoptera littoralis (Lepidoptera: Noctuidae). Chem Senses 27:231–244
Charlton RE, Cardé RT (1981) Comparing the effectiveness of sexual communication disruption in the oriental fruit moth (Grapholita molesta) using different combinations and dosages of its pheromone blend. J Chem Ecol 7:501–508
Chiang AS, Liu YC, Chiu SL, Hu SH, Huang CY, Hsieh CH (2001) Three-dimensional mapping of brain neuropils in the cockroach, Diplotera punctata. J Comp Neurol 440:1–11
Christensen TA, Hildebrand JG (2002) Pheromonal and host-odor processing in the insect antennal lobe: how different? Curr Opin Neurobiol 12:393–399
Couto A, Alenius M, Dickson BJ (2005) Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol 15:1535–1547
Couton L, Minoli S, Kiêu K, Anton S, Rospars JP (2009) Constancy and variability of identified glomeruli in antennal lobes: computational approach in Spodoptera littoralis. Cell Tissue Res. doi:10.1007/s00441-009-0831-9
Figueredo AJ, Baker TC (1991) Reduction of the response to sex pheromone in the oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae) following successive pheromonal exposures. J Insect Behav 5:347–363
Fishilevich E, Vosshall LB (2005) Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol 15:1548–1553
Flanagan D, Mercer AR (1989) An atlas and 3-D reconstruction of the antenal lobes in the worker honeybee, Apis mellifera L. (Hymenoptera: Apidae). Int J Insect Morphol Embryol 18:145–159
Gabel B, Thiéry D, Suchy V, Marion-Poll F, Hradsky P, Farkas P (1992) Floral volatiles of Tanacetum vulgare L. attractive to Lobesia botrana Den. and Schiff. females. J Chem Ecol 18:693–701
Galizia CG, McIlwrath SL, Menzel R (1999) A digital three-dimensional atlas of the honeybee antennal lobe based on optical sections acquired by confocal microscopy. Cell Tissue Res 295:383–394
Galizia CG, Sachse S, Mustaparta H (2000) Calcium responses to pheromone and plant odours in the antennal lobe of the male and female moth Heliothis virescens. J Comp Physiol [A] 186:1049–1063
Gao Q, Yuan B, Chess A (2000) Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurobiol 3:780–785
Ghaninia M, Hansson BS, Ignell R (2007) The antennal lobe of the African malaria mosquito, Anopheles gambiae—innervation and three-dimensional reconstruction. Arthropod Struct Dev 36:23–39
Greiner B, Gadenne C, Anton S (2004) Three-dimensional antennal lobe atlas of the male moth, Agrotis ipsilon: a tool to study structure-function correlation. J Comp Neurol 475:202–210
Guerenstein PG, Hildebrand JG (2007) Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol 53:20–40
Guerenstein PG, Christensen TA, Hildebrand JG (2004) Sensory processing of ambient CO2 information in the brain of the moth Manduca sexta. J Comp Physiol [A] 190:707–725
Hansson BS, Ljunberg H, Hallberg E, Löfstedt C (1992) Functional specialization of olfactory glomeruli in a moth. Science 256:1313–1315
Hickel ER, Ducroquet JPHJ (1998) Monitoring and control of Grapholita molesta in Alto Vale do Rio do Peixe. Agropecuaria Catarin 11:8–11
Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20:595–631
Hillier NK, Vickers NJ (2007) Physiology and antennal lobe projections of olfactory receptor neurons from sexually isomorphic sensilla on male Helitohis virescens. J Comp Physiol [A] 193:649–663
Hillier NK, Kleineidam C, Vickers NJ (2006) Physiology and glomerular projections of olfactory receptor neurons on the antenna of female Heliothis virescens (Lepidoptera: Noctuidae) responsive to behaviourally relevant odors. J Comp Physiol [A] 192:199–219
Huetteroth W, Schachtner J (2005) Standard three-dimensional glomeruli of the Manduca sexta antennal lobe: a tool to study both developmental and adult neuronal plasticity. Cell Tissue Res 319:513–524
Ignell R, Anton S, Hansson B (2001) The antennal lobe of orthoptera: anatomy and evolution. Brain Behav Evol 57:1–17
Ignell R, Dekker T, Ghaninia M, Hansson BS (2005) Neuronal architecture of the mosquito deutocerebrum. J Comp Neurol 493:207–240
Il’ichev AL, Williams DG, Milner AD (2004) Mating disruption barriers in pome fruit for improved control of oriental fruit moth Grapholita molesta Busck (Lepidoptera: Tortricidae) in stone fruit under mating disruption. J Appl Entomol 128:126–132
Joerges J, Küttner A, Galizia CG, Menzel R (1997) Representations of odours and odour mixtures visualized in the honeybee brain. Nature 387:285–288
Jourdan F, Duveau A, Astic L, Holley A (1980) Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res 188:139–154
Katerinopoulos HE, Pagona G, Afratis A, Stratigakis N, Roditakis N (2005) Composition and insect attracting activity of the essential oil of Rosmarinus officinalis. J Chem Ecol 31:111–122
Kauer JS (2002) On the scents of smell in the salamander. Nature 417:336–342
Kent KS, Harrow ID, Quartararo P, Hildebrand JG (1986) An accessory olfactory pathway in Lepidoptera: the labial pit organ and its central projection in Manduca sexta and certain other sphinx moths and silk moths. Cell Tissue Res 245:237–245
Klagges BRE, Heimbeck G, Godenschwege TA, Hofbauer A, Plufgfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E (1996) Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J Neurobiol 16:3154–3165
Kleineidam CJ, Obermayer M, Halbich W, Rossler W (2005) A macroglomerulus in the antennal lobe of the leaf-cutting ant workers and its possible functional significance. Chem Senses 30:383–392
Kovanci OB, Walgenbach JF, Kennedy GG (2004) Evaluation of extended-season mating disruption of the oriental fruit moth Grapholita molesta (Busck) (Lep., Tortricidae) in apples. J Appl Entomol 128:664–669
Lacey MJ, Sanders CJ (1992) Chemical composition of sex pheromone of oriental fruit moth and rates of release by individual female moths. J Chem Ecol 18:1421–1435
Laissue PP, Reiter C, Hiesinger PR, Halter S, Fischbach KF, Stocker RF (1999) Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol 405:543–552
Linn CE, Roelofs WL (1983) Effect of varying proportions of the alcohol component on sex pheromone blend discrimination in male oriental fruit moths. Physiol Entomol 8:291–306
Ljunberg H, Anderson P, Hansson BS (1993) Physiology and morphology of pheromone-specific sensilla on the antennae of male and female Spodoptera littoralis (Lepidoptera: Noctuidae). J Insect Physiol 39:253–260
Masante-Roca I, Gadenne C, Anton S (2005) Three-dimensional antennal lobe atlas of male and female moths, Lobesia botrana (Lepidoptera: Tortricidae) and glomerular representation of plant volatiles in females. J Exp Biol 208:1147–1159
Meijerink J, Carlsson MA, Hansson BS (2003) Spatial representation of odorant structure in the moth antennal lobe: a study of structure-response relationships at low doses. J Comp Neurol 467:11–21
Mustaparta H (2005) Innate and changed responses to plant odours in moths and weevils. Chem Senses 30:1297–1298
Natale D, Mattiacci L, Pasqualini E, Dorn S (2004) Apple and peach fruit volatiles and the apple constituint butyl hexanoate attract female oriental fruit moth, Cydia molesta, in the laboratory. J Appl Entomol 128:22–27
Ochieng SA, Anderson P, Hansson BS (1995) Antennal lobe projection patterns of olfactory receptor neurons involved in sex pheromone detection in Spodoptera littoralis (Lepidoptera: Noctuidae). Tissue Cell 27:221–232
Piñero JC, Dorn S (2007) Synergism between aromatic compounds and green leaf volatiles derived from the host plant underlies female attraction in the oriental fruit moth. Entomol Exp Appl 125:185–194
Piñero JC, Galizia CG, Dorn S (2007) Synergistic behavioral responses of female oriental fruit moths (Lepidoptera: Tortricidae) to synthetic host plant-derived mixtures are mirrored by odor-evoked calcium activity in their antennal lobes. J Insect Physiol 54:333–343
Reis FW, Nora I, Melzer R (1988) Population dynamics of Grapholita molesta, Busck, 1916, and its adaptation on apple in south Brazil. Acta Hort 232:204–208
Roelofs WL, Comeau A, Selle R (1969) Sex pheromone of the oriental fruit moth. Nature 224:723
Rospars JP (1983) Invariance and sex-specific variations of the glomerular organization in the antennal lobes of a moth, Mamestra brassicae, and a butterfly, Pieris brassicae. J Comp Neurol 220:80–96
Rospars JP (1988) Structure and development of the insect antennodeutocerebral system. Int J Insect Morphol Embryol 17:243–294
Rospars JP, Chambille I (1981) The deutocerebrum of the cockroach Blaberus craniifer Burm. Quantitative study and automated identification of the glomeruli. J Neurobiol 12:221–247
Rospars JP, Chambille I (1989) Identified glomeruli in the antennal lobes of insects: invariance, sexual variation and postembryonic development. In: Singh RN, Strausfeld NJ (eds) Neurobiology of sensory systems. Plenum, New York, pp 355–375
Rospars JP, Hildebrand JG (1992) Anatomical identification of glomeruli in the antennal lobes of the male sphinx moth Manduca sexta. Cell Tissue Res 270:205–227
Rospars JP, Hildebrand JG (2000) Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem Senses 25:119–129
Rothschild GHL, Vickers RA (1991) Biology, ecology and control of the oriental fruit moth. In: Van der Geest LPS, Evenhus HH (eds) World crop pest, tortricid pests: their biology, natural enemies and control, vol 5. Elsevier, Amsterdam, pp 389–412
Rumbo ER, Vickers RA (1997) Prolonged adaptation as possible mating disruption mechanism in oriental fruit moth, Cydia (= Grapholita) molesta. J Chem Ecol 23:445–457
Sadek MM, Hansson BS, Rospars JP, Anton S (2002) Glomerular representation of plant volatiles and sex pheromone components in the antennal lobe of the female Spodoptera littoralis. J Exp Biol 205:1363–1376
Skiri HT, Ro H, Berg BG, Mustaparta H (2005) Consistent organization of glomeruli in the antennal lobes of related species of heliothine moths. J Comp Neurol 491:367–380
Smid HM, Bleeker MAK, Loon JJA van, Vet LEM (2003) Three-dimensional organization of the glomeruli in the antennal lobe of the parasitoid wasps Cotesia glomerata and C. rubecula. Cell Tissue Res 312:237–248
Stelinski LL, Vogel KJ, Gut LJ, Miller JR (2005) Seconds-long preexposures to pheromone from rubber septum or polyethelene tube dispensers alters subsequent behavioral responses of male Grapholita molesta (Lepidoptera: Tortricidae) in a sustained-flight tunnel. Environ Entomol 34:696–704
Stelinski LL, Il’ichev AL, Gut LJ (2006) Antennal and behavioural responses of virgin and mated oriental fruit moth (Lepidoptera: Tortricidae) females to their sex pheromone. Ann Entomol Soc Am 99:898–904
Stocker RF, Lienhard MC, Borst A, Fischbach KF (1990) Neuronal architecture of the antennal lobe in D. melanogaster. Cell Tissue Res 262:9–34
Todd JL, Baker TC (1996) Antennal lobe partitioning of behaviourally active odors in female cabbage looper moths. Naturwissenschaften 83:324–326
Todd JL, Anton S, Hansson BS, Baker TC (1995) Functional organization of the macroglomerular complex related to behaviourally expressed olfactory redundancy in male cabbage looper moths. Physiol Entomol 20:349–361
Vickers NJ, Christensen TA, Hildebrand JG (1998) Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J Comp Neurol 400:35–56
Vosshall LB, Wong AM, Axel R (2000) An olfactory sensory map in the fly brain. Cell 102:147–159
Wang JW, Wong AM, Flores J, Vosshall LB, Axel R (2003) Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112:271–282
Zube C, Kleineidam CJ, Kirschner S, Neef J, Rossler W (2008) Organization of the olfactory pathway and odor processing in the antennal lobe of the ant Camponotus floridanus. J Comp Neurol 506:425–441
Acknowledgements
We thank Romina Barrozo for helpful comments on the manuscript and with the preparation of figures. The monoclonal antibody developed by Erich Buchner was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nélia Varela and Louise Couton contributed equally to this work.
This study was supported by research grants from INRA (Projet Jeune Equipe and Projet S.P.E.) to S.A. and J.P.R. and by the Spanish Ministry of Science and Technology (research grant AGL2004-05812/AGR to J.A. and C.G., Spanish Science and Education Department, MEC). N.V. was financed by fellowship no. BES-2005-7605 (MEC, Spain).
Rights and permissions
About this article
Cite this article
Varela, N., Couton, L., Gemeno, C. et al. Three-dimensional antennal lobe atlas of the oriental fruit moth, Cydia molesta (Busck) (Lepidoptera: Tortricidae): comparison of male and female glomerular organization. Cell Tissue Res 337, 513–526 (2009). https://doi.org/10.1007/s00441-009-0839-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0839-1