Abstract
In the tissue integration of melanocytes and melanoma cells, an important role is attributed to cell adhesion molecules, notably the cadherins. In cultured melanoma cells, we have previously described a more heterogeneous repertoire of cadherins than normal, including some melanoma subtypes synthesizing the desmosomal cadherin, desmoglein 2, out of the desmosomal context. Using biochemical and immunological characterization of junctional molecules, confocal laser scanning, and electron and immunoelectron microscopy, we now demonstrate homo- and heterotypic cell-cell adhesions of normal epidermal melanocytes. In human epidermis, both in situ and in cell culture, melanocytes and keratinocytes are connected by closely aligned membranes that are interspersed by small puncta adhaerentia containing heterotypic complexes of E- and P-cadherin. Moreover, melanocytes growing in culture often begin to synthesize desmoglein 2, which is dispersed over extended areas of intimate adhesive cell-cell associations. As desmoglein 2 is not found in melanocytes in situ, we hypothesize that its synthesis is correlated with cell proliferation. Indeed, in tissue microarrays, desmoglein 2 has been demonstrated in a sizable subset of nevi and primary melanomas. The biological meanings of these cell-cell adhesion molecule arrangements, the possible diagnostic and prognostic significance of these findings, and the implications of the heterogeneity types of melanomas are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the epidermis, melanocytes reside in the basal layer, forming the “epidermal melanin unit” (Montagna and Parakkal 1974; Jimbow et al. 1986). This cell-type homeostasis and pattern is maintained by cell-cell adhesion structures between the melanocytes and the keratinocytes. Disturbances of this pattern may contribute to uncontrolled proliferation of the melanocytes and the development of aberrant structures such as nevi and ultimately malignant melanomas (for recent reviews, see, e.g., Haass et al. 2004, 2005). Cell adhesion molecules of special importance in this respect are the cadherins, calcium-dependent transmembrane glycoproteins, which can mediate intercellular adhesion by both homophilic and heterophilic cis- and trans-interactions, and which specifically can establish homotypic contacts of punctate adhering junctions (puncta adhaerentia) between cells of the same type or heterotypic adhering junctions between different kinds of cells (Niessen and Gumbiner 2002; Duguay et al. 2003; Foty and Steinberg 2005; Troyanovsky 2005).
So far, more than 80 members of the larger cadherin superfamily have been identified, comprising the “classical” type I and type II cadherins as components of adherens junctions (zonulae adhaerentes), the desmosomal cadherins as transmembrane proteins of desmosomes, and the atypical cadherins (e.g., T- and Li-cadherin), protocadherins and cadherin-related proteins (Niessen and Gumbiner 2002; Patel et al. 2003; Goodwin and Yap 2004; Troyanovsky 2005). The type I cadherins include E-cadherin, which is regarded as typical of epithelial cells, N-cadherin, characteristically occurring on mesenchymal and neuronal cells, and P-cadherin, first identified in the placenta, whereas the human type II cadherins are a group of 24 members, including the mesenchymal cadherin 11 and cadherin 5, also termed VE-cadherin. The subfamily of desmosomal cadherins can be subdivided into the cell-type-specific desmoglein isoforms Dsg 1–4 and the desmocollins occurring in three isoforms (Dsc 1–3), each with two splice variants (Koch et al. 1990, 1991, 1992; Buxton et al. 1993; Yin and Green 2004; Troyanovsky 2005).
In normal epidermis, melanocytes and keratinocytes are mostly connected via E-cadherin or P-cadherin (Tang et al. 1994; Nishimura et al. 1999), and the ratio between the two cadherins has been reported to be essential for the location of the melanocytes: whereas melanocytes in the basal layer of the epidermis seem to contain predominantly E-cadherin, those residing in hair follicles are rich in P-cadherin (Nishimura et al. 1999). However, the ultrastructure of the various kinds of heterotypic adherens junctions between melanocytes and keratinocytes has not yet been clarified, either in situ or in co-culture.
Dependent on the developmental stage and the specific microenvironment, the cadherin repertoire of melanocytes can be remarkably variable. During embryonic development, melanocyte precursors provide striking examples of cells migrating over long distances from the neural crest, “homing” to specific epidermal tissues, and en route may also change their character (Le Douarin 1984). In the neural crest, they have been shown to contain cadherin 6B and N-cadherin (Hatta and Takeichi 1986; Nakagawa and Takeichi 1995), which later, i.e., after the onset of migration, can be replaced, at least in part, by cadherin 7. The patterns and the amounts of these three cadherins appear to be important for correct segregation from the neural crest, as alterations have been reported to inhibit the emigration of melanocyte precursors and their migration pathway (Nakagawa and Takeichi 1998; Moore et al. 2004). The general importance of the cadherin-catenin septum in the embryonal migration and homing processes is also indicated by the cell-type-targeted gene ablation study of Hari et al. (2002).
When melanocytes have reached their final position in the epidermis and are in contact with keratinocytes, synthesis of E- and P-cadherin is induced (Nishimura et al. 1999; Jouneau et al. 2000). However, during malignant transformation this process may be reversed; E-cadherin, and often apparently also P-cadherin, have been reported to be downregulated and substituted by N-cadherin (Hsu et al. 1996, 2000a; Sanders et al. 1999; Perlis and Herlyn 2004). This change, also known as the “cadherin switch”, is widely discussed as an important prerequisite not only for the pathogenesis of malignant melanomas, but also for some tumors derived from epithelial cells and has several implications (Hazan et al. 2000; Christofori 2003; Cavallaro and Christofori 2004; Haass et al. 2004, 2005; Perlis and Herlyn 2004). First, it results in a drastic reduction of the E-cadherin-mediated coupling of melanocytic and melanoma precursor cells to keratinocytes, a reduction that then will further result in a reduction of gap junctions between these cell types (Hsu et al. 2000b). Second, it provides the melanoma cells with a novel adhesive repertoire for interaction with new, mostly mesenchymally derived neighbours such as fibroblasts and endothelial cells of blood and lymphatic vessels (Sandig et al. 1997; Li et al. 2001a; Qi et al. 2005; Haemmerling et al. 2006). Third, N-cadherin is widely assumed to promote the survival and migration of melanoma cells and also to provide proliferative and migratory signals (Li et al. 2001a; Kuphal et al. 2004; Qi et al. 2005, 2006; Kuphal and Bosserhoff 2006). Conversely, re-expression of E-cadherin has been reported to restore keratinocyte-mediated growth control and to reverse malignancy (Hsu et al. 2000a; Li et al. 2004). Certain UV-light components have also been reported to reduce E-cadherin not only in melanoma cells, but also in normal human melanocytes (see, e.g., Jamal and Schneider 2002; Perlis and Herlyn 2004).
The functional consequences of the cadherin switch have, for the most part, been studied in cell culture systems and in animal models (Hsu et al. 1996, 2000a; Li et al. 2001a, 2004; Liu et al. 2006). However, immunohistochemical examination of primary melanomas and their metastases has revealed that a proportion of melanoma cells are still E-cadherin-positive and present little, if any, N-cadherin (Danen et al. 1996; Hsu et al. 1996; Silye et al. 1998; Sanders et al. 1999). Therefore, the cadherin switch as an obligatory prerequisite of malignant behaviour and a prognostic marker is still controversial and might also depend on the subtype of melanoma examined (Andersen et al. 2004; Onken et al. 2006).
Similar to E-cadherin, P-cadherin has also been reported to promote adhesion and to counteract the migration of metastatic melanoma cells (Van Marck et al. 2005). This concept is also supported by observations that P-cadherin is frequently lost in advanced melanomas and melanoma metastases (Bachmann et al. 2005; Bauer et al. 2006).
We have recently examined the repertoire of adhesive molecules in cultured melanoma cell lines and found that their cadherin patterns might be more variable than hitherto thought (Schmitt et al. 2007). In particular, we have found that a number of melanoma cell lines synthesize, in the absence of desmosomes, the desmosomal cadherin desmoglein 2 (Dsg2) as a frequent plasma membrane glycoprotein that is not assembled into any junction but is dispersed over large parts of the cell surface. Therefore, we have analyzed the molecular composition and the ultrastructure of the junctions between normal melanocytes and between melanocytes and keratinocytes in situ in human epidermis and in co-cultures. We here demonstrate new forms of close adhesive membrane alignments over extended cell surface areas and small plaque-bearing puncta adhaerentia that connect melanocytes and keratinocytes and that contain E- and P-cadherin and the plaque proteins typical of adherens junctions. Moreover, we show that, when taken into culture, melanocytes might begin to synthesize Dsg2 as a frequent solitary glycoprotein out of the desmosomal context; Dsg2 is dispersed over large surface regions, similar to the distribution patterns recently described in certain types of melanomas (Schmitt et al. 2007).
Materials and methods
Antibodies
Murine monoclonal antibodies (mabs) specific for N-, E-, or P-cadherin, α- and β-catenin, and protein p120ctn were purchased from BD Biosciences Pharmingen (Heidelberg, Germany). Mabs directed against vinculin (clone 11-5) and α-actinin (clone BM-75.2) and rabbit antibodies to α- or β-catenin were obtained from Sigma (Deisenhofen, Germany). Mabs recognizing cadherin 11 and Dsg2 (clone 6D8) and polyclonal rabbit antibodies against proteins ZO-1 and ZO-2 were from Invitrogen (Karlsruhe, Germany). A rabbit antiserum to N-cadherin was obtained from Abcam (Cambridge, UK), and a pan-Dsc rabbit antiserum (clone AHP 322) from Serotec (Duesseldorf, Germany). The following antibodies against junction- and cytoskeleton-associated proteins were purchased from Progen Biotechnik (Heidelberg; see also Schmitt et al. 2007): mabs against desmoplakin 1 and 2 (DP; clones DP-2.15, DP-2.17, and DP-2.20), plakoglobin (clone PG 5.1), plakophilin 1, 2, or 3 (PKP; clones PP1-2D6, PP2/86, and PKP3-270.6.2, respectively), Dsc1 or 3 (clones Dsc1-U100 and Dcs3-U114), Dsg1 (clone P23) or Dsg1 and 2 (clone DG 3.10) , Dsg3 (clone Dsg-G194), vimentin (clone VIM 3B4), and drebrin (clone MX823). As a marker for melanocytes, a mab against Melan A from Progen Biotechnik was used.
A mab against cadherin 5 (VE-cadherin; clone BV9) was a kind gift from Elisabetta Dejana (Department of Biomolecular and Biotechnological Sciences, School of Sciences, University of Milan, Italy; Lampugnani et al. 1992). For the demonstration of plakoglobin, a mab (clone 11E4) generously provided by Margaret J. Wheelock (University of Nebraska Medical Center, Omaha, Neb., USA) was used. Rabbit antibodies against Dsg2 (clone rb5) were kindly provided by Lutz Langbein, and guinea pig antibodies against vimentin by Ilse Hofmann (German Cancer Research Center). Secondary antibodies were as described by Schmitt et al. (2007).
Cell culture
Normal human epidermal melanocytes from foreskin (NHEM-f) and from adult skin (NHEM-a) were obtained from PromoCell (Heidelberg) and cultured in melanocyte growth medium M2 (MGM-M2; PromoCell), which is free of serum and of mitogens such as phorbol-myristate-acetate. For passaging, cells were treated with 0.025% trypsin and 0.01% EDTA, followed by incubation in trypsin neutralization solution (PromoCell). Human melanoma cells of the line MeWo were provided by the American Type Culture Collection (ATTC; Manassas, Va., USA). Simian virus (SV40)-transformed human (“SV80”) fibroblasts and human U333 glioma cells were as previously described (Franke et al. 1979; Achtstaetter et al. 1986). HaCaT keratinocytes were a gift from Petra Boukamp (Genetics of Skin Carcinogenesis, German Cancer Research Center; Boukamp et al. 1988). All of these lines were maintained in Dulbecco’s minimal essential medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum (FCS; Biochrom, Berlin, Germany) and 2 mM glutamine. The isolation and cell culture of human umbilical vein endothelial cells (HUVEC) was as reported by Peitsch et al. (1999). For melanocyte-keratinocyte co-cultures, NHEM-f cells and HaCaT keratinocytes were trypsinized, counted in a Neubauer chamber, and seeded onto glass coverslips contained in plastic dishes. Co-cultures were maintained in MGM-M2 plus 5% FCS for 7 days and were then processed for immunofluorescence or immunoelectron microscopic analysis.
Tissues
Normal human epidermis was obtained during routine pathological diagnoses from the Departments of Dermatology and Pathology of the University Hospital Mannheim, Germany. Samples were either fixed with 4% formaldehyde and embedded in paraffin or snap-frozen in isopentane precooled in liquid nitrogen and stored at −80°C. All procedures were approved by the Medical Ethical Committee of the University Hospital Mannheim, University of Heidelberg, and were performed with the patients’ informed consent. Tissue microarrys were purchased from US Biomax (via BioCat, Heidelberg). The specimens were subjected to heat-induced antigen retrieval (see below) and double-labeled with antibodies to cadherins and to vimentin for cell-type identification, and the proportion of cadherin-positive tumor cells was determined.
Gel electrophoresis, immunoblotting, immunoprecipitation, sucrose gradient centrifugation, and MALDI-TOF analyses
Electrophoresis of total cell proteins in the presence of sodium dodecylsulfate (SDS) was performed in polyacrylamide gels (SDS-PAGE). Immunoblotting of gel-electrophoretically separated polypeptides and immunoprecipitation were conducted as reported (Peitsch et al. 1999, 2005; Schmitt et al. 2007). For immunoprecipitation, a “Triton X-100 immunoprecipitation buffer”, containing 1% Triton X-100, 150 mM NaCl, and 20 mM HEPES (pH 7.4), supplemented with a protease inhibitor cocktail (Complete Mini Inhibitor Tabs, EDTA-free; Roche Diagnostics, Mannheim), was used. MALDI-TOF analyses were performed by Martina Schnoelzer and Tore Kempf (Protein Analysis Facility, German Cancer Research Center; cf. Peitsch et al. 1999).
For fractionation on sucrose gradients, NHEM-f cells were extracted with 1% Triton X-100 buffer. Supernatants obtained after centrifugation at 14,000 rpm for 10 min were loaded on linear 5%–30% sucrose gradients (Peitsch et al. 2001). Bovine serum albumin (BSA), catalase, and thyroglobulin (all from Sigma) were used as size markers and were fractionated on parallel gradients. The gradients were centrifuged in a SW40 rotor (Beckman Instruments, Munich, Germany) at 35,000 rpm for 18 h (4°C). Fifteen fractions of 800 μl were collected from top to bottom and were either supplemented with three-fold-concentrated sample buffer and analyzed by SDS-PAGE or were subjected to immunoprecipitation. For the latter, fractions (F) 5–7, comprising a peak of E- and P-cadherin-containing material, were pooled and diluted to a sucrose concentration of 5%, as determined by using a Zeiss refractometer (Carl Zeiss, Jena, Germany).
RNA isolation, cDNA synthesis, and polymerase chain reaction
Isolation of total RNA from cultured cells and human split skin and the synthesis of cDNA and PCR were conducted as described by Schmitt et al. (2007). RNA was extracted with TriPure Isolation Reagent from Roche Diagnostics according to the manufacturer’s instructions. For polymerase chain reaction (PCR), primers specific for human Dsg1 (forward primer: 5′ AAT ACC AAG GAA CGA TTC 3′; reverse primer: 5′ CTC CTG ATG TGT CAA TGC 3′), for human Dsg2 (forward primer: 5′ GCC AAG AAA GTA CCA GTG TGC TGC 3′; reverse primer: 5′ CTT TCA TCG TGG CTT CCT TGG CCA 3′), and as a control, for the actin-binding protein drebrin (forward primer: 5′ TTT AGA TCT GCC GGC GTC AGC TTC AGC GGC 3′; reverse primer: 5′ CGC ACT TGC GGG CAT CAG GCA CAT 3′) were used. PCR fragments were analyzed on 2% agarose gels. The fragments obtained with Dsg2-specific primers were cloned into the EcoR1 restriction sites of a pCR2.1-TOPO vector by using a TOPO TA Cloning Kit (Invitrogen) and were verified by sequencing.
Transient transfection of NHEM-f cells
The full length human Dsg2 cDNA, generated in this laboratory, was cloned into the NotI sites of a eukaryotic p163/7 expression vector (Schaefer et al. 1994, 1996). This vector contains a major histocompatibility class I H2-2 promotor, which is identical to p164/7 (Niehrs et al. 1992). Transient transfection of NHEM-f cells was performed with Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s recommendations. Cells were analyzed 24 h and 48 h after transfection by using immunostaining with Dsg2 antibodies.
Immunofluorescence and electron and immunoelectron microscopy
Cultured cells grown on glass coverslips were fixed either in 2% formaldehyde in phosphate-buffered saline (PBS) for 5–7 min, followed by permeabilization with 0.1% Triton-X for 2–3 min or with methanol for 5 min at −20°C, followed by 20 s in −20°C acetone. Procedures for immunostaining were as reported by Schmitt et al. (2007). For immunolocalization on paraffin-embedded tissue samples, sections of human epidermis or tissue microarrays were deparaffinized according to standard techniques. To achieve heat-induced antigen retrieval, sections were pretreated by microwaving in 100 mM TRIS-HCl buffer containing 5% urea (pH 9.5, 10 min, 120°C or pH 11, 30 min, 120°C) or in citrate buffer (82 mM sodium citrate and 18 mM citric acid, pH 6, 10 min, 120°C). This was followed by a wash with PBS (5 min, room temperature), incubation with PBS containing 2% milk powder (10 min), and blocking with 10% goat serum and 2% milk powder in PBS (15 min). Primary antibodies were applied for 2 h, and secondary antibodies for 30 min. Microscopic images were recorded with an Axiophot II photomicroscope (Carl Zeiss) equipped with an AxioCam HR (Carl Zeiss), and confocal images with a Zeiss LSM 510 UV microscope.
Electron and immunoelectron microscopy was performed as described (Langbein et al. 2002; Schmitt et al. 2007). For immunoelectron microscopy, NHEM-f cells, NHEM-f-HaCaT co-cultures, and cryostat sections of human epidermis were fixed in 2% formaldehyde (5–7 min), freshly prepared from paraformaldehyde, and permeabilized with 0.1% saponin (1–2.5 min), followed by incubation with primary antibodies for 2 h. After washing steps, the samples were incubated with secondary antibodies conjugated with 1.4-nm gold particles (Nanogold, Biotrend, Cologne, Germany) for 2–4 h, followed by silver enhancement (Langbein et al. 2002). Electron micrographs were taken at 80 kV by using an EM 910 (Carl Zeiss).
Results
Composition and ultrastructure of heterotypic melanocyte-keratinocyte connections in human epidermis
To determine the composition of the heterotypic cell adhesions connecting melanocytes and keratinocytes in situ, we immunostained cryostat and paraffin sections through normal human epidermis with antibodies to the constituents of adherens junctions and desmosomes in combination with Melan A or vimentin as melanocyte markers. Analyses of such sections by confocal microscopy showed an enrichment of E- and P-cadherin and of the plaque proteins of adherens junctions, i.e., α- and β-catenin and protein p120ctn, both at homotypic keratinocyte contacts and at heterotypic adhesions between melanocytes and keratinocytes in the basal epidermis (for examples of E-cadherin and β-catenin, see Fig. 1a,a′,b,b′). By contrast, desmosomal cadherins and the desmosomal plaque protein desmoplakin exhibited the typical punctate staining pattern of desmosomes but appeared to be absent from melanocytes and from melanocyte-keratinocyte contacts. These findings were also obtained with antibodies to Dsg2 (Fig. 1c,c′), the desmosomal cadherin previously identified in a subset of melanoma cells (Schmitt et al. 2007). For comparison, sections of human scalp, showing hair follicles, and of human basal cell carcinomas were double-labeled and examined by confocal microsopy. Here, corresponding observations were made, i.e., an enrichment of α- and β-catenin and E-cadherin at contact sites between melanocytes and follicular epithelia or basal carcinoma cells. Desmosomal proteins, however, including Dsg2, were never detected at these sites (data not shown; for localization of desmosomal proteins in hair follicles, see Franke and Heid 1989; Kurzen et al. 1998).
Double-label confocal microscopy of sections through paraffin-embedded human epidermis, showing cell adhesion proteins at heterotypic melanocyte-keratinocyte contacts. Sections were double-immunostained with antibodies to E-cadherin (red in a, a′), β-catenin (red in b, b′), or Dsg2 (red in c, c′) in combination with antibodies to vimentin (green in a) or Melan A (green in b, c) as markers for melanocytes. E-cadherin and β-catenin are enriched at intercellular junctions between keratinocytes throughout the epidermis and also at the borders between melanocytes and keratinocytes in the basal layer (a′, b′, stars). By contrast, Dsg2 is found at the desmosomes of the basal keratinocytes but is absent from melanocytes and from melanocyte-keratinocyte contacts (c′, stars). a′′–c′′ Phase-contrast images. Bars 20 μm
For a detailed characterization of the heterotypic melanocyte-keratinocyte adhesions, electron and immunoelectron microscopy was performed on cryostat sections of healthy human epidermis. In such electron micrographs, small junctions with an electron-dense plaque, resembling puncta adhaerentia, were observed at sites of contacts between melanocytes and keratinocytes (Fig. 2a,d, arrows). Moreover, the plasma membranes of the melanocytes and keratinocytes were closely aligned to each other over remarkably long distances, with a consistently narrow intercellular space and occasionally a thin cytoplasmic coat (Fig. 2b,c, arrowheads). When the sections were labeled with antibodies to adherens junction proteins such as α- or β-catenin, significant label was seen at the plaques of puncta adhaerentia (e.g., Fig. 2e). Moreover, some catenin-positive reactions were also noted along the plasma membrane alignments of melanocytes and keratinocytes (data not shown). Using mabs reactive with Dsg2 for immunoelectron microscopy, marked enrichment was observed at the intercellular spaces of the desmosomes between keratinocytes. Corresponding to our confocal microscopical observations, the melanocytes and the melanocyte-keratinocyte contacts appeared free of Dsg2 (not shown).
Electron and immunoelectron microscopy showing adhesions between keratinocytes and melanocytes in the basal human epidermis. a Survey of a melanocyte surrounded by keratinocytes in the basal epidermal layer. b–d Conventional electron micrographs of cell-cell contacts between melanocytes and keratinocytes. Both cell types are connected by small, plaque-bearing junctions reminiscent of puncta adhaerentia (arrows in d). d Higher magnification of the boxed region in a). In regions devoid of such puncta, the plasma membranes of melanocytes and keratinocytes are often also closely aligned over long distances, with a narrow intercellular space (b, c, arrowheads). e Immunoelectron microscopy of melanocyte-keratinocyte contacts, demonstrating that β-catenin accumulates at the plaques of puncta adhaerentia. Bars 2 μm (a), 0.25 μm (b–d), 0.125 μm (e)
Cadherins and cadherin complexes in cultured melanocytes
The synthesis and assembly patterns of junctional components were also analyzed in cultured normal human melanocytes, derived from foreskin (NHEM-f). Cell cultures were grown in medium free of serum and of mitogens to avoid transformation, and total protein lysates were prepared for immunoblot analyses after the cultures had reached 50%–70% confluence. Human HaCaT keratinocytes, SV80 fibroblasts, and cultured human melanoma cells of the line MeWo, which previously had been found to contain Dsg2 as a solitary plasma membrane protein (Schmitt et al. 2007), were used for comparison. Immunoblotting indeed demonstrated E- and P-cadherin in NHEM-f melanocytes (Fig. 3, Table 1). By contrast, N-cadherin, endothelial VE-cadherin, which had been reported to occur in a subset of highly aggressive melanoma lines (Hendrix et al. 2001; Hess et al. 2006), and cadherin 11, synthesized by osteoblasts, myofibroblasts, and various other mesenchymally derived cells, including stem cells (Simonneau et al. 1995; Hinz et al. 2004; Wuchter et al. 2007) and also found in one of our melanoma lines (Schmitt et al. 2007), were totally absent. However, the melanocytes contained the set of plaque proteins typical of adherens junctions, i.e., α- and β-catenin, protein p120ctn, vinculin, α-actinin, plus proteins ZO-1 and ZO-2 (Itoh et al. 1993, 1999; Smalley et al. 2005; for the specific problem regarding the complexity of protein p120ctn isoforms, see Aho et al. 2002).
Immunoblot detection of adherens-junction-associated and desmosomal proteins in cultured human melanocytes. Equal amounts of total proteins from human HaCaT keratinocytes (HaCaT), SV80 fibroblasts (SV80), melanoma cells of the line MeWo (MeWo), and normal human epidermal melanocytes from foreskin (NHEM-f) were applied to SDS-polyacrylamide gels, and the separated polypeptides were probed with antibodies against classical cadherins (E-, P-, N- and VE-cadherin, and cadherin 11 [cad cadherin]), plaque proteins of adherens junctions (α- and β-catenin [cat catenin], α-actinin, and vinculin), desmosomal cadherins (Dsg1-3 and Dsc1-3 [Dsg desmoglein, Dsc desmocollin]) and desmosomal plaque proteins (DP1 and 2 [DP desmoplakin], plakoglobin, and PKP1-3 [PKP plakophilin]). NHEM-f cells contain E- and P-cadherin and the plaque proteins typical of adherens junctions. Interestingly, these cells also synthesize the desmosomal cadherin Dsg2, whereas all other desmosomal proteins are absent. This finding has been confirmed by immunoblotting with two different Dsg2 antibodies, one recognizing both Dsg1 and Dsg2 (clone DG3.10) and one specific for Dsg2 (clone 6D8). *For the immunoblot identification of VE-cadherin, whole cell lysates of human umbilical vein endothelial cells (HUVEC) were loaded as a positive control, instead of HaCaT cells. For the detection of cadherin 11, total proteins from astrocytic glioma cells were applied, and for the detection of Dsg1 and Dsc1-3, total proteins of human epidermis were loaded. **After prolonged exposure, trace amounts of plakoglobin are detectable in SV80 and MeWo and in NHEM-f cells
On immunoblotting with antibodies to desmosomal proteins, Dsg2 was seen in both the MeWo and the NHEM-f cells (Fig. 3). By contrast, none of the other desmosomal cadherins or plaque proteins was detected in these experiments, with the exception of small amounts of plakoglobin, a protein known to occur in both adherens junctions and desmosomes (Table 1; Cowin et al. 1986). Thus, we concluded that, as in certain melanoma cell lines (Schmitt et al. 2007), Dsg2 also often occurs in considerable amounts in proliferative human melanocytes, obviously as a solitary plasma membrane glycoprotein, without any of the other known desmosome-specific components.
To clarify whether other types of melanocytes also contained Dsg2, immunoblotting was performed with total cell lysates of cultured melanocytes derived from adult skin (NHEM-a). Indeed, these cells were also Dsg2-positive, as confirmed by immunoblot experiments with two different Dsg2 antibodies (Fig. 4a). Correspondingly, Dsg2 mRNA was demonstrated in NHEM-f and NHEM-a cells (Fig. 4b), and sequencing of the PCR products showed full identity with the human Dsg2 sequence (Schaefer et al. 1994). As another group had reported the occurrence of Dsg1 in melanocytes and melanoma cells (Li et al. 2001b; see, however, also Sanders et al. 1999), we also conducted PCR experiments with primers specific for Dsg1 (Fig. 4b). However, amplification of Dsg1 mRNA was observed neither in NHEM-f nor in NHEM-a cells, in agreement with our immunoblot results (for a discussion, see Schmitt et al. 2007).
Immunoblot and polymerase chain reaction (PCR) identification of Dsg2 in cultured human melanocytes. a Immunoblot analysis of total protein lysates from HaCaT keratinocytes (HaCaT), SV80 fibroblasts (SV80), MeWo melanoma cells (MeWo), and normal human epidermal melanocytes from newborn foreskin (NHEM-f) and from adult skin (NHEM-a). An intense Dsg2-specific band is detected in MeWo cells and in NHEM-f and NHEM-a cells, both with mab DG3.10 and mab 6D8. b PCR analysis with primers specific for Dsg1 and 2 and for the near-ubiquitous actin-binding protein drebrin (Peitsch et al. 1999, 2001, 2005). Dsg2 mRNA is seen both in NHEM-f and NHEM-a cells, whereas Dsg1 mRNA is not. *Human split skin was employed as a positive control for the Dsg1 PCR, HaCaT keratinocytes served as positive control for the Dsg2 PCR, and SV80 fibroblasts were used as a negative control. Size marker bars (left): 517, 396, and 356 bp (Dsg1 and Dsg2 PCR) or 356 and 247 bp (drebrin PCR)
To identify possible interaction partners of Dsg2, extracts of NHEM-f cells were subjected to immunoprecipitation, followed by SDS-PAGE, Coomassie Blue staining, and MALDI-TOF analyses of the specifically enriched proteins. Following immunoprecipitation with Dsg2 antibodies, three bands were observed at about 212, 160, and 42 kDa, which corresponded to non-muscle myosin heavy chain, Dsg2, and actin (Fig. 5a). For comparison, immunoprecipitation was performed with β-catenin antibodies, revealing bands at ~130, ~100, ~90 and 42 kDa, which represented E- and P-cadherin, α- and β-catenin, and actin (Fig. 5b). When polypeptides of β-catenin immunoprecipitates were reacted with antibodies to Dsg2, no significant amounts of co-precipitation products were seen (Fig. 5c). Vice versa, neither α- or β-catenin (Fig. 5c′) nor E- and P-cadherin (not shown) were enriched in Dsg2 immunoprecipitates. By contrast, when the material precipitated with Dsg2 antibodies was probed for plakoglobin, specific enrichment was noted; however, the plakoglobin-specific band seen after the immunoblot reaction was usually weak (data not shown). As Dsg2 is normally linked to intermediate filaments, Dsg2 immunoprecipitates were also analyzed for vimentin, and indeed, this protein showed co-precipitation with Dsg2 (Fig. 5c′′).
Immunoprecipitation and MALDI-TOF analyses, showing cadherin complexes in NHEM-f melanocytes. Proteins immunoprecipitated from NHEM-f lysates with antibodies specific for Dsg2 (a) or for β-catenin (b) were separated on 8% acrylamide gels and stained with Coomassie Blue. In the Dsg2 immunoprecipitate (a), a band of ~160 kDa was seen (band 2), which was identified as Dsg2 by MALDI-TOF analysis. Further bands at ~212 kDa (band 1) and at ~42 kDa (band 3) represent non-muscle myosin and actin. In the β-catenin immunoprecipitate (b), band 1 at ~130 kDa includes both E-cadherin and P-cadherin, with further bands containing α-catenin (band 2), β-catenin (band 3), bovine serum albumin (BSA; band 4), and actin (band 5). Arrows heavy chains of immunoglobulins and BSA (~66 kDa), P material of the preclearing step, IP immunoprecipitate, Ab beads Dynabeads loaded with primary antibodies, M molecular weight markers denoting (top to bottom): 212, 158, 116, 97, 66, 55, and 42 kDa. c–c′′ Immunoprecipitates (IP) from NHEM-f cells, obtained with antibodies to Dsg2 and to β-catenin and immunoblotted for Dsg2 (c), β-catenin (c′) and vimentin (c′′). Dsg2 and β-catenin do not co-precipitate (c, c′), whereas vimentin is specifically enriched in the Dsg2 immunoprecipitate (c′′)
Further to characterize the cadherin complexes present, NHEM-f cell lysates were fractionated by sucrose gradient centrifugation. Immunoblot analyses then showed a peak of E-cadherin-, P-cadherin-, and β-catenin-containing material in F5–F8, with a maximum in F6, corresponding to complexes with a mean S value of ~8 (Fig. 6a). When F5–F7 were pooled and subjected to immunoprecipitation with E-cadherin antibodies, E-cadherin, P-cadherin, and β-catenin were specifically enriched, indicative of heterotypic complexes of E- and P-cadherin (Fig. 6b). By contrast, Dsg2 was observed in gradient F3–F6, corresponding to a monomeric form, but also in ~13S-complexes in F10 and F11 (Fig. 6a). Actin was revealed mostly in monomers and in small complexes, but a portion did co-distribute with Dsg2 in F10 and F11 (Fig. 6a). Taken together with the immunoprecipitation results, these observations indicate that NHEM-f cells contain hetero-complexes of E- and P-cadherin, associated with the plaque proteins of adherens junctions and actin. In addition, they seem to contain two sets of Dsg2 complexes: one in which Dsg2 is linked to vimentin filaments, and another presenting Dsg2 together with actin and non-muscle myosin.
Sucrose gradient fractionation of molecular complexes present in lysates from cultured melanocytes. a Extracts of proteins from cultured human melanocytes (NHEM-f) were centrifuged on linear 5%–30% sucrose gradients, and the fractions were analyzed by SDS-PAGE and immunoblotting with antibodies to E-cadherin, P-cadherin, β-catenin, Dsg1+2 (clone DG3.10), and β-actin (S proteins of the supernatant before fractionation, P pellet). E- and P-cadherin are found, together with β-catenin, in fractions (F) 5–8, with a maximum in F6, i.e., in particles of a mean value of ~8S. Dsg2 immunoreactivity is seen in F3-F6, indicative of Dsg2 monomers but, interestingly, also with a peak in F10 and F11, corresponding to ~13S. Actin appears with a broad peak from F2 to F6, suggestive of a monomer, but is also co-distributed with Dsg2 in F10 and F11, i.e., ~13S. References are: BSA (B: 4.3S), catalase (C: 11.5S), and thyroglobulin (T: 16.5S). b Sucrose gradient F5–F7 were pooled and immunoprecipitated with antibodies to E-cadherin and to the tight junction protein occludin as a control. Note that both P-cadherin and β-catenin are specifically enriched in E-cadherin immunoprecipitates (S supernatant of the pooled F5–F7, P material of the preclearing step, IP immunoprecipitate)
Localization of cadherins in NHEM-f monocultures and NHEM-f-HaCaT co-cultures
The subcellular distribution of cadherins in cultures of NHEM-f cells and in NHEM-f-keratinocyte co-cultures was studied by immunostaining, confocal laser scanning, and immunoelectron microscopy. In NHEM-f monocultures, E- and P-cadherin and the typical plaque proteins of puncta adhaerentia appeared (as expected) predominantly at intercellular junctions, but with relatively lower intensity; such immunoreactions were also seen on free plasma membrane regions (not shown).
To specify the localization of Dsg2, NHEM-f cells were transfected with a eukaryotic expression vector containing the full-length human Dsg2 cDNA, followed by Dsg2 immunostaining and confocal microscopy. Indeed, in the transfected cells, the protein was enriched at the cell periphery, both at cell-cell contacts and along free cell margins (Fig. 7a), reminiscent of the localization seen in the Dsg2-positive melanoma cell lines (Schmitt et al. 2007).
Localization of cell junction proteins in NHEM-f monocultures and in keratinocyte-melanocyte co-cultures. a, a′ NHEM-f cells were transfected with a eukaryotic expression vector containing human Dsg2 cDNA and immunoreacted with Dsg2 antibodies (clone DG3.10). Note Dsg2 accumulations at the contact sites between two melanocytes and at the free cellular margins. b–c′ Co-cultures of NHEM-f and HaCaT keratinocytes labeled for E-cadherin (red in b, b′) or β-catenin (red in c, c′) in combination with vimentin as a melanocyte marker (green in b, c): E-cadherin and β-catenin are seen at homotypic plasma membrane adhesion sites between melanocytes, on the one hand, and keratinocytes, on the other, but also at heterotypic contacts between the two cell types (stars in b, c). Bars 20 μm
When co-cultures of NHEM-f melanocytes and HaCaT keratinocytes were analyzed, an enrichment of E-cadherin (Fig. 7b,b′) and β-catenin (Fig. 7c,c′) not only at homotypic keratinocyte and homotypic melanocyte junctions, but also at heterotypic contacts between NHEM-f and HaCaT cells was seen. To study the intercellular adhesion sites of cultured melanocytes in greater detail, we also examined these homo- and heterotypic cell cultures by electron and immunoelectron microscopy. In monocultures of NHEM-f melanocytes, electron micrographs revealed numerous plaque-bearing junctions of the punctum adhaerens type (Fig. 8a–d, arrows). By immunoelectron microscopy, E-cadherin (e.g., Fig. 8e) and β-catenin (Fig. 8f,g) were seen at both kinds of cell-cell contacts, i.e., at adherens junctions with plaques and at the extended adhesive associations without noticeable plaques.
Electron and immunoelectron microscopy of cultured NHEM-f melanocytes. a–d As seen by conventional electron microscopy, closely parallel cell-cell adhesive alignments between two melanocytes often consist of several distinct small plaque-bearing adhering junctions (arrows in b; b presents a higher magnification of the boxed area in a). Note, at higher magnification (c, d), the equidistance and parallel character of the extended plasma membrane intercepts, in places frequently revealing puncta adhaerentia (arrows) coated by a mostly thin, densely stained plaque. e–g Immunoelectron microscopy of NHEM-f cells, labeled with antibodies to E-cadherin (e) or β-catenin (f, g). Here, enrichment of β-catenin is observed at the plaques of the puncta adhaerentia (f, g), but both E-cadherin (e) and β-catenin (not shown) are also detectable along plaque-free plasma membranes connecting the two cells over long distances, i.e., in a non-junction-bound form. Bars 1 μm (a), 0.25 μm (b, d, e), 0.5 μm (c), 0.125 μm (f, g)
When co-cultures of NHEM-f and HaCaT cells were studied by electron microscopy, small heterotypic cell adhesions were observed that revealed thin dense plaques on either side (Fig. 9a–e), reminiscent of the puncta adhaerentia connecting melanocytes and keratinocytes in human epidermis in situ. Again, immunoelectron microscopy demonstrated enrichment of the junctional markers at these sites (data not shown).
Electron microscopy, presenting heterotypic cell adhesions in melanocytes (M) co-cultured with keratinocytes (K). a, d Survey micrographs of NHEM-f melanocytes co-cultured with HaCaT keratinocytes. Note the numerous cell protrusions in adjacent keratinocytes and melanocytes and the local contacts between the two cell types. b, c, e Details presenting regions of heterotypic cell-cell adhesions: NHEM-f and HaCaT cells are connected by small puncta adhaerentia-type junctions (arrows). b Higher magnification of the boxed region in a. e Detail of the boxed area in d). Bars 1.5 μm (a, d), 0.5 μm (b, e), 0.25 μm (c)
Cadherin patterns in tissue microarrays of primary melanomas and nevi
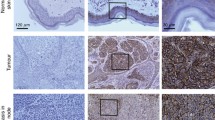
As Dsg2 was detected in melanocytes growing in cell culture but not in those residing in situ in the basal epidermis, we hypothesized that its advent and continual synthesis might be correlated with proliferation. To examine this, tissue microarrays comprising 56 primary melanomas and 24 nevi were immunolabeled with antibodies to Dsg2 in combination with vimentin antibodies for the unequivocal identification of the tumor cells (Fig. 10a,a′, Table 2; a survey presenting all immunostaining results in detail is given in Table S1 as Supplementary Material). Indeed, Dsg2-positive reactions were observed in seven primary melanomas (13.5%) and seven nevi (30.4%). In the Dsg2-positive tumor cells, the protein was again seen at cell-cell contacts. Remarkably, however, the staining patterns within the tumors were heterogeneous, with Dsg2-positive tumor cell clusters next to Dsg2-negative cell groups, and the percentage of Dsg2-containing tumor cells ranged from ~10% to 100% (Table 2).
Cadherin patterns of a primary melanoma, as determined by double-label immunofluorescence confocal microscopy. Tissue microarray samples of a primary melanoma from the left leg (no. 13, cf. Table S1) stained with antibodies to Dsg2 (a, a′, red), N-cadherin (b, b′, red), E-cadherin (c, c′, red), and P-cadherin (d, d′, red), in combination with antibodies to vimentin to identify the tumor cells (green in a–d). Note that most of the melanoma cells contain Dsg2, N-cadherin, and E-cadherin, all accumulated at the cell periphery. By contrast, P-cadherin-positive reactions were observed only in ~20% of the tumor (d, d′). A blood vessel (V) was P-cadherin-negative (d′). a′′–d′′ Phase-contrast images. Bars 20 μm
In parallel, the tissue microarrays were immunolabeled for N-, E-, and P-cadherin in combination with vimentin (Fig. 10b–d, Tables 2, S1). Most of the primary melanomas (68.5%) and nevi (90.9%) contained N-cadherin accumulated at cell-cell boundaries (Fig. 10b,b′, Table 2). In another subtype of such tumors, N-cadherin-positive reactions seemed to occur exclusively in the cytoplasm, an observation difficult to explain on a cell biological basis. Here, however, the specificity and significance of the reaction sites remain to be determined.
A remarkably large group of primary melanomas (65.5%) and nevi (62.5%) was also positive for E-cadherin (see, e.g., Fig. 10c,c′, Table 2), whereas a smaller subset synthesized P-cadherin (27.8% of the primary melanomas and 37.5% of the nevi; Fig. 10d,d′). As noted for Dsg2 (Table 2), E- and P-cadherin often showed strikingly heterogeneous reaction patterns, in that E-cadherin- and P-cadherin-positive and -negative groups of tumor cells occurred next to each other (Table 2; for an example of P-cadherin, see Fig. 10d,d′). Within nevi, the E-cadherin-positive immunoreactions often decreased from epidermal to deeper dermal melanocyte nests. Interestingly, both E- and P-cadherin could occur in the same tumors as N-cadherin, and in a certain subset of primary melanomas, all three classical cadherins were even seen to occur simultaneously (6 of 56, 16.1%), without or with Dsg2 (3 of 56, 5.4%). By contrast, another relatively rare subtype was negative for all of the four cadherins tested (7 of 56, 12.5%; Table S1). In the 24 nevi examined, all three classical cadherins together were found in five tumors (20.8%); two of them were also positive for Dsg2 (8.3%; Table S1). Taken together, these results obtained in tissue microarrays show that the cadherin profile can be highly variable not only among different primary melanomas and nevi, but also within the same tumor.
Discussion
Researchers studying the cell and molecular biology of proliferative or even malignantly transformed melanocytes are often confronted with “unusual” gene expression patterns combining, for example, certain epithelial and mesenchymal cell-type marker molecules or subtype patterns of proteins and structures that make it difficult to assign a distinct cell-type character to the specific cell colony or tumor. For instance, certain melanoma cells and proliferatively active melanocytes, although usually subsumed under neuroectoderm-derived mesenchymal-type cells, are able to synthesize a set of molecules that are characteristic of epithelial-type adhering junctions, such as E-cadherin (Tang et al. 1994; Hsu et al. 2000a; Li et al. 2001a) and Dsg2 (Schmitt et al. 2007). Furthermore, melanocytes and melanoma cells often form heterotypic adhering junctions with keratinocytes or with other epithelial and non-epithelial cells, and some are even able to adhere intimately to each other or to other kinds of cells over extended plasma membrane associations without distinct junctional structures.
Desmoglein-containing adhesive cell-cell alignments of proliferative melanocytes and melanoma cells
We have recently reported that certain melanoma cell culture lines regularly synthesize the desmosomal cadherin Dsg2, which hitherto had been assumed to be absent from melanocytes and melanomas (Schmitt et al. 2007). Remarkably, however, we have now detected Dsg2 as a major and frequent cadherin also in normal, i.e., not malignantly transformed, cultured melanocytes. Like the Dsg2-positive melanoma cell lines (Schmitt et al. 2007), the cultured melanocytes contain no other typical desmosomal proteins, except for occasional small amounts of plakoglobin, in general a widespread junctional plaque protein of both desmosomes and adherens junctions (Cowin et al. 1986).
Desmosomal cadherins, i.e., members of the desmoglein and desmocollin subfamilies of cadherin glycoproteins, have so far been identified only in adhering junction structures such as in desmosomes (Koch et al. 1990, 1991, 1992; Buxton et al. 1993; Godsel et al. 2004; Yin and Green 2004) and in the composite junctions (areae compositae) of mammalian heart muscle cells (Franke et al. 2007; Pieperhoff et al. 2008) or as integral molecules of “half-desmosomes”. The latter have been described in processes of desmosome formation such as exocytosis, or in Ca2+-deficiency-induced junctional splitting and in endocytic vesicle uptake (Cowin et al. 1984; Duden and Franke 1988; Demlehner et al. 1995; Schaefer et al. 1996).
The Dsg2 molecules that we have observed as frequent cell surface components in proliferative melanocytes and in a certain category of melanoma cells are evenly dispersed and do not seem to assemble into distinct adhering junctional structures. Nevertheless, they often appear to be intimately aligned with cell proteins on the surface of a neighboring cell, thus forming a novel homogeneous kind of cell-cell-adhesive association, which often extends over large areas. The importance of these solitary surface-exposed Dsg2 molecules, which are not co-assembled with any other detectable desmosome-specific component or integrated into any distinct junction structures, for cell-cell associations in normal and in pathologically altered tissues, notably in metastatic processes, remains to be determined.
The Dsg2-presenting melanocytes and melanoma cell subtypes described in this and in our previous report (Schmitt et al. 2007) are not the only cells found to synthesize this desmosomal glycoprotein as a solitary molecule and to expose it over large areas of the cell surface. A subline of human fibrosarcoma cells has also been reported to synthesize Dsg2 continuously and to export this protein to the cell surface where it is seen in relatively large regions (Chitaev and Troyanovsky 1997). Only upon the addition of further desmosomal components by injection or cDNA transfection does an organized co-assembly of this pre-existing Dsg2 with the other partners into desmosome-like junctions take place (Koeser et al. 2003).
Obviously, the cell-cell trans-interactions between proliferative melanocytes or melanoma cells with adjacent host tissue cells, e.g., keratinocytes in the case of the epidermis, are stable enough to maintain their direct cross-talk in the architectonic context of the specific tissue or tumor. Indeed, in view of the surprisingly strong adhesive trans-interaction forces of individual desmosomal cadherins (Troyanovsky 2005), these numerous and rather widely spread Dsg2 molecules may be essentially involved in cell-cell recognition and attachment processes, thus also representing a significant factor in the metastatic process. Clearly, the cell-cell interaction strengths of such non-junction-integrated cadherin molecules will have to be experimentally determined in the future. We also propose to consider this and other types of cell-cell attachment forms in diagnoses of melanomas and other melanocyte-related disorders.
E- and P-cadherin heterodimers in melanocyte cultures
Our biochemical analyses of detergent-solubilized cadherins from cultured melanocytes cells have revealed heterotypic complexes of E- and P-cadherin, reminiscent of our previous studies on cultured melanoma cell lines (Schmitt et al. 2007), which have indicated the existence of such E-P-cadherin hetero-complexes. Whereas the binding specificities of cadherins have been traditionally considered as homotypic, it has recently become evident that their interactions can be more promiscuous (Volk et al. 1987; Shan et al. 2000; Shimoyama et al. 2000; Omelchenko et al. 2001; Duguay et al. 2003; Patel et al. 2003; Foty and Steinberg 2005). Specifically, heterodimers of E- and P-cadherin have been identified in cultures of human carcinoma cells of line A431 (Klingelhoefer et al. 2000).
When interacting in cell cultures, cadherins can form both cis-dimers, i.e., lateral dimers in adhering junctions of the same cell (Shan et al. 2000), or trans-dimers between two adjacent cells (Duguay et al. 2003). Obviously, heterotypic trans-cellular cadherin interactions are more frequent than previously thought (Shimoyama et al. 2000; Omelchenko et al. 2001; Duguay et al. 2003; Patel et al. 2003). The heterodimers of E- and P-cadherin described to predominate in cultures of A431 cells are of the cis-type (Klingelhoefer et al. 2000). On the other hand, many of the E- and P-cadherin molecules introduced into fibroblasts (L-cells) by cDNA transfections seem to form “trans E-P-hetero-cadherin” complexes between adjacent cells (Duguay et al. 2003; Foty and Steinberg 2005). Such trans-cellular hetero-cadherin complexes appear to be of a similar strength as the corresponding trans-cellular homo-cadherin complexes. Whether the immunoprecipitable complexes of E- and P-cadherin found in the melanocytes and the melanoma cell cultures in the present and the preceding study (Schmitt et al. 2007) are of the cis- or of the trans-type remains to be examined. Moreover, these complexes will have to be further characterized with respect to their adhesive strength.
Heterotypic adhering junctions between melanocytic cells and keratinocytes
Melanocytes are known for their frequent (often obligatory) heterotypic cell-cell junctions. It is thus all the more surprising to note that the ultrastructure of such heterotypic adhering junctions has not yet been clarified, even for the abundant melanocyte-keratinocyte junctions, other than the repeated statements that they do not include desmosomes (Breathnach 1974; Montagna and Parakkal 1974; for the related problem of the association, often via invaginations, with keratinocytes and melanin transfer between these cells see, e.g., Jimbow et al. 1986).
In this study, we have presented, for the first time, the ultrastructure of the heterotypic adhering junctions connecting normal melanocytes and keratinocytes in the epidermis. Both in the human epidermis in situ and in co-cultures, melanocytes and keratinocytes are connected by small plaque-bearing structures that contain the protein and glycoprotein ensemble typical of adherens junctions; thus, in molecular terms, they represent typical puncta adhaerentia as known from a wide range of other cells (see, e.g., Wuchter et al. 2007 and references cited therein). In puncta adhaerentia, one or two of the classical cadherins can usually be identified that, on the cytoplasmic side, insert into a thin and indistinct coat formed by plaque proteins including α- and β-catenin, protein p120ctn, plakoglobin, and (depending on the specific cell type) a few other, mostly actin-binding proteins. Morphologically, these “mini-junctions” are relatively inconspicuous and only sometimes can be demonstrated to anchor filament bundles on one or both cytoplasmic plaques. In the puncta-type adherens junctions of diverse subtypes of melanoma cells, the junctional plaque can exhibit various cadherin patterns, i.e., N-, E-, or P-cadherin or cadherin 11 or combinations of two or three of these cadherins (Schmitt et al. 2007 and references therein). Some melanoma subtypes, notably of uveal origin, have also been reported to contain VE-cadherin (Hendrix et al. 2001, 2003; Seftor et al. 2002; Hess et al. 2006). In addition, we have now made clear that not only melanoma cells (whether grown in situ or in cell culture), but also proliferative melanocytes in culture can synthesize the desmosomal cadherin, Dsg2. However, this protein is, for the most part, not integrated into any particular junction but is dispersed over the cell surface, without any obvious cytoplasmic coat of anchoring proteins.
Our electron-and immunoelectron-microscopic results indicate that the heterotypic puncta junctions between keratinocytes and melanocytes are primarily based on complexes between E- and P-cadherin, both synthesized in basal keratinocytes and in melanocytes (Tang et al. 1994; Nishimura et al. 1999). Moreover, we have noticed that, in the cells studied, some cadherins, including melanocytic E-cadherin, are not restricted to puncta adhaerentia structures but may also occur at plaque-free plasma membranes, both in tissue-bound and in cultured melanocytes. This indicates that two forms of cadherins should generally be distinguished here: a junction-bound form and a non-junction-bound form. A similar distribution has been reported by some authors for N-cadherin in endothelial cells; here, this cadherin can be enriched at intercellular junctions, apparently often together with VE-cadherin, but may also occur outside of the junctions on the free endothelial surface (for controversial discussions, see, e.g., Salomon et al. 1992; Alexander et al. 1993; Schulze and Firth 1993; Navarro et al. 1998; Jaggi et al. 2002; Luo and Radice 2005; for a review, see Dejana 2004).
Different cadherin profiles in primary melanomas and nevi as determined in tissue microarrys
As we had detected Dsg2 in certain melanomas and in cultured melanocytes but not in melanocytes in situ, we reasoned that its synthesis out of the context with the other desmosomal components might be correlated with cell proliferation or might be induced by some kind of activation characteristic of proliferating melanocytes. The results obtained in tissue microarrays of melanocytic tumors are to a certain degree compatible with this hypothesis. Indeed, Dsg2 has been found in a subset of such tumors, i.e., in 30% of the nevi and in 13% of the primary melanomas, in which it appears enriched at cell-cell contacts, essentially in agreement with a small number of melanoma metastases immunostained for Dsg2 (Schmitt et al. 2007). This subtype of Dsg2-positive melanocytic tumors will have to be specified in the future. In this context, a minor but especially aggressive subtype of melanomas is characterized by the addition of VE-cadherin to normal cadherin complexes and by the appearance of certain kinases (Hendrix et al. 2001, 2003; Seftor et al. 2002; Hess et al. 2006). Whether the presence of Dsg2 in nevi and melanomas allows any prognostic conclusions will have to be examined in future clinically based studies on larger numbers of samples, in which Dsg2 expression will also have to be correlated with the Breslow and the Clark level of the melanomas.
On the other hand, however, marked and systematic heterogeneity has also been noted for P-cadherin. When the microarrays were labeled with antibodies to this cadherin, about 38% of the nevi and 28% of the primary melanomas exhibited P-cadherin-positive reactions along the cell boundaries. This is in correspondence with observations of membrane P-cadherin staining in benign nevi and in initial melanomas, both correlated with a favourable prognosis (Bachmann et al. 2005; Bauer et al. 2006). In contrast to other authors, however, we have not observed “cytoplasmic” immunoreactions for P-cadherin, probably because of differences of the antibodies employed or the protocols used for antigen retrieval and immunostaining.
Both E- and N-cadherin have been detected in a high percentage of the nevi and melanomas (cf. Tables 2, S1). Often these two cadherins occur together in the same tumor, a finding in accordance with our previous observations of melanoma metastases (Schmitt et al. 2007). Plasma membrane E-cadherin immunostaining has also been noted in a high percentage of advanced primary melanomas and of melanoma metastases by other groups (Silye et al. 1998; Sanders et al. 1999). This appears, at first glance, at variance with the prevailing hypothesis that a switch from E- to N-cadherin is essential for the progression of highly malignant melanomas (see Introduction). On the other hand, a possible explanation for this result might be that E-cadherin may only temporarily be down-regulated during certain steps of tumor cell segregation and invasion and then re-expressed in the advanced metastatic tumor.
To our surprise, we have also frequently noted, for E- and P-cadherin and for Dsg2, heterogeneous staining patterns within the same tumor, i.e., groups of tumor cells strongly positive for the specific cadherin next to tumor regions negative for this glycoprotein (cf. Tables 2, S1, Fig. 10d). In nevi labeled for E-cadherin, the immunoreaction often shows a gradual decrease from the epidermal layers to deeper dermal nests, reminiscent of other reports (Krengel et al. 2004). However, in the melanomas of our microarray studies, such gradients have not been observed, possibly because the samples were taken from the very centers of the melanomas. Here, the heterogeneous cadherin patterns suggest that one and the same melanoma can contain multiple small cell colonies with strikingly different adhesion protein profiles. Mosaic patterns of junctional proteins have also been observed for desmosomal cadherins (Kurzen et al. 2003), the desmosomal plaque protein plakophilin 1 (Moll et al. 1997), the adherens junction-associated drebrin (Peitsch et al. 2005), and several tight junction molecules (Langbein et al. 2003) in other kinds of skin tumors. Such regionalization and subtype differences might contribute to chemoresistancy, a notorious problem in the therapy of malignant melanomas. Hence, this is another reason for mentioning, in diagnostic evaluation, the degree of regionalization of cell junctions.
Conclusions and recommendations
All three forms, proliferative normal melanocytes growing in culture, certain cells of nevi in situ, and malignant melanoma cells, are highly proliferative and markedly heterogeneous with respect to their cell-cell adhesion molecule profiles, their junction assemblies, and in the variety of their surface cluster- and domain-forming regionalization patterns. Our findings reported here have made it clear that such heterogeneity patterns are not restricted to malignant melanomas, but can also be seen in normal, i.e., non-malignant melanocytes and in nevus cells. They also suggest that the emergence and widespread regeneration of heterogeneous cell-cell adhesion structures is a feature intrinsic to the proliferative melanocyte, and not a special feature of melanomas. As such diversities, subtypes, or special regional domains may be of general importance not only for tissue patterning, but also for pathogenic processes (notably in melanoma metastasis formation), we propose to characterize the specific adhesion molecule pattern of a given cell colony or tumor in initial diagnosis, in particular in cases in which this may be relevant for the metastatic process. We also postulate that potent “factors” exist that can interfere with the adhesion of melanocytes or melanocyte precursors with each other or to other kinds of cells, and that such factors obviously play important roles in normal development (Le Douarin 1984; see also Hari et al. 2002) and in melanoma metastasis.
References
Achtstaetter T, Moll R, Anderson A, Kuhn C, Pitz S, Schwechheimer K, Franke WW (1986) Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation 31:206–227
Aho S, Levänsuo L, Montonen O, Kari C, Rodeck U, Uitto J (2002) Specific sequences in p120ctn determine subcellular distribution of its multiple isoforms involved in cellular adhesion of normal and malignant epithelial cells. J Cell Sci 115:1391–1402
Alexander JS, Blaschuk OW, Haselton FR (1993) An N-cadherin-like protein contributes to solute barrier maintenance in cultured endothelium. J Cell Physiol 156:610–618
Andersen K, Nesland JM, Holm R, Florenes VA, Fodstad O, Maelandsmo GM (2004) Expression of S100A4 combined with reduced E-cadherin expression predicts patient outcome in malignant melanoma. Mod Pathol 17:990–997
Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA (2005) Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res 11:8606–8614
Bauer R, Wild PJ, Meyer S, Bataille F, Pauer A, Klinkhammer-Schalke M, Hofstaedter F, Bosserhoff AK (2006) Prognostic relevance of P-cadherin expression in melanocytic skin tumours analysed by high-throughput tissue microarrays. J Clin Pathol 59:699–705
Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771
Breathnach AS (1974) An atlas of the ultrastructure of human skin. Churchill, London
Buxton RS, Cowin P, Franke WW, Garrod DR, Green KJ, King IA, Koch PJ, Magee AI, Rees DA, Stanley JR, Steinberg MS (1993) Nomenclature of the desmosomal cadherins. J Cell Biol 121:481–483
Cavallaro U, Christofori G (2004) Multitasking in tumor progression: signaling functions of cell adhesion molecules. Ann N Y Acad Sci 1014:58–66
Chitaev NA, Troyanovsky SM (1997) Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion. J Cell Biol 138:193–201
Christofori G (2003) Changing neighbours, changing behaviour: cell adhesion molecule-mediated signalling during tumour progression. EMBO J 22:2318–2323
Cowin P, Mattey D, Garrod D (1984) Identification of desmosomal surface components (desmocollins) and inhibition of desmosome formation by specific Fab’. J Cell Sci 70:41–60
Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO (1986) Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 46:1063–1073
Danen EH, Vries TJ de, Morandini R, Ghanem GG, Ruiter DJ, Muijen GN van (1996) E-cadherin expression in human melanoma. Melanoma Res 6:127–131
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Demlehner MP, Schaefer S, Grund C, Franke WW (1995) Continual assembly of half-desmosomal structures in the absence of cell contacts and their frustrated endocytosis: a coordinated Sisyphus cycle. J Cell Biol 131:745–760
Duden R, Franke WW (1988) Organization of desmosomal plaque proteins in cells growing at low calcium concentrations. J Cell Biol 107:1049–1063
Duguay D, Foty RA, Steinberg MS (2003) Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol 253:309–323
Foty RA, Steinberg MS (2005) The differential adhesion hypothesis: a direct evaluation. Dev Biol 278:255–263
Franke WW, Heid H (1989) Desmosomal proteins and cytokeratins in the hair follicle. In: Rogers GE, Reis PJ, Ward KA, Marshall RC (eds) The biology of wool and hair. Chapman and Hall, London New York, pp 403–416
Franke WW, Schmid E, Winter S, Osborn M, Weber K (1979) Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res 123:25–46
Franke WW, Schumacher H, Borrmann CM, Grund C, Winter-Simanowski S, Schlechter T, Pieperhoff S, Hofmann I (2007) The area composita of adhering junctions connecting heart muscle cells of vertebrates. III. Assembly and disintegration of intercalated disks in rat cardiomyocytes growing in culture. Eur J Cell Biol 86:127–142
Godsel LM, Getsios S, Huen AC, Green KJ (2004) The molecular composition and function of desmosomes. In: Behrens J, Nelson WJ (eds) Cell adhesion. Handbook of experimental pharmacology. Springer, Berlin Heidelberg New York, pp 137–193
Goodwin M, Yap AS (2004) Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Histol 35:839–844
Haass NK, Smalley KS, Herlyn M (2004) The role of altered cell-cell communication in melanoma progression. J Mol Histol 35:309–318
Haass NK, Smalley KS, Li L, Herlyn M (2005) Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res 18:150–159
Haemmerling B, Grund C, Boda-Heggemann J, Moll R, Franke WW (2006) The complexus adhaerens of mammalian lymphatic endothelia revisited: a junction even more complex than hitherto thought. Cell Tissue Res 324:55–67
Hari L, Brault V, Kléber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L (2002) Lineage-specific requirements of β-catenin in neural crest development. J Cell Biol 159:867–880
Hatta K, Takeichi M (1986) Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320:447–449
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA (2000) Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 148:779–790
Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE (2001) Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA 98:8018–8023
Hendrix MJ, Seftor EA, Hess AR, Seftor RE (2003) Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 3:411–421
Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ (2006) VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther 5:228–233
Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ (2004) Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell 15:4310–4320
Hsu MY, Wheelock MJ, Johnson KR, Herlyn M (1996) Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc 1:188–194
Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M (2000a) E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol 156:1515–1525
Hsu MY, Andl T, Li G, Meinkoth JL, Herlyn M (2000b) Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. J Cell Sci 113:1535–1542
Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S (1993) The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol 121:491–502
Itoh M, Morita K, Tsukita S (1999) Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem 274:5981–5986
Jaggi M, Wheelock MJ, Johnson KR (2002) Differential displacement of classical cadherins by VE-cadherin. Cell Commun Adhes 9:103–115
Jamal S, Schneider RJ (2002) UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J Clin Invest 110:443–452
Jimbow K, Fitzpatrick TB, Quevedo WC Jr (1986) Formation, chemical composition and function of melanin pigments. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the integument 2. Springer, Berlin Heidelberg New York, pp 278–292
Jouneau A, Yu YQ, Pasdar M, Larue L (2000) Plasticity of cadherin-catenin expression in the melanocyte lineage. Pigment Cell Res 13:260–272
Klingelhoefer J, Troyanovsky RB, Laur OY, Troyanovsky S (2000) Amino-terminal domain of classic cadherins determines the specificity of the adhesive interactions. J Cell Sci 113:2829–2836
Koch PJ, Walsh MJ, Schmelz M, Goldschmidt MD, Zimbelmann R, Franke WW (1990) Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol 53:1–12
Koch PJ, Goldschmidt MD, Walsh MJ, Zimbelmann R, Franke WW (1991) Complete amino acid sequence of the epidermal desmoglein precursor polypeptide and identification of a second type of desmoglein gene. Eur J Cell Biol 55:200–208
Koch PJ, Goldschmidt MD, Zimbelmann R, Troyanovsky R, Franke WW (1992) Complexity and expression patterns of the desmosomal cadherins. Proc Natl Acad Sci USA 89:353–357
Koeser J, Troyanovsky SM, Grund C, Franke WW (2003) De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp Cell Res 285:114–130
Krengel S, Grotelueschen F, Bartsch S, Tronnier M (2004) Cadherin expression pattern in melanocytic tumors more likely depends on the melanocyte environment than on tumor cell progression. J Cutan Pathol 31:1–7
Kuphal S, Bosserhoff AK (2006) Influence of the cytoplasmic domain of E-cadherin on endogenous N-cadherin expression in malignant melanoma. Oncogene 25:248–259
Kuphal S, Poser I, Jobin C, Hellerbrand C, Bosserhoff AK (2004) Loss of E-cadherin leads to upregulation of NFkappaB activity in malignant melanoma. Oncogene 23:8509–8519
Kurzen H, Moll I, Moll R, Schaefer S, Simics E, Amagai M, Wheelock MJ, Franke WW (1998) Compositionally different desmosomes in the various compartments of the human hair follicle. Differentiation 63:295–304
Kurzen H, Munzing I, Hartschuh W (2003) Expression of desmosomal proteins in squamous cell carcinomas of the skin. J Cutan Pathol 30:621–630
Lampugnani MG, Resnati M, Reiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E (1992) A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol 118:1511–1522
Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW (2002) Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol 81:419–435
Langbein L, Pape UF, Grund C, Kuhn C, Praetzel S, Moll I, Moll R, Franke WW (2003) Tight junction-related structures in the absence of a lumen: occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol 82:385–400
Le Douarin N (1984) Pigment cells. In: Le Douarin N (ed) The neural crest. Cambridge University Press, Cambridge, pp 108–133
Li G, Satyamoorthy K, Herlyn M (2001a) N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res 61:3819–3825
Li G, Schaider H, Satyamoorthy K, Hanakawa Y, Hashimoto K, Herlyn M (2001b) Downregulation of E-cadherin and desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene 20:8125–8135
Li G, Fukunaga M, Herlyn M (2004) Reversal of melanocytic malignancy by keratinocytes is an E-cadherin-mediated process overriding beta-catenin signaling. Exp Cell Res 297:142–151
Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M (2006) Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res 66:4182–4190
Luo Y, Radice GL (2005) N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol 169:29–34
Moll I, Kurzen H, Langbein L, Franke WW (1997) The distribution of the desmosomal protein, plakophilin 1, in human skin and skin tumors. J Invest Dermatol 108:139–146
Montagna W, Parakkal PF (1974) The structure and function of skin. Academic Press, New York London
Moore R, Champeval D, Denat L, Tan SS, Faure F, Julien-Grille S, Larue L (2004) Involvement of cadherins 7 and 20 in mouse embryogenesis and melanocyte transformation. Oncogene 23:6726–6735
Nakagawa S, Takeichi M (1995) Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development 121:1321–1332
Nakagawa S, Takeichi M (1998) Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125:2963–2971
Navarro P, Ruco L, Decana E (1998) Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 140:1475–1484
Niehrs C, Huttner WB, Ruether U (1992) In vivo expression and stoichiometric sulfation of the artificial protein sulfophilin, a polymer of tyrosine sulfation sites. J Biol Chem 267:15938–15942
Niessen CM, Gumbiner BM (2002) Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol 156:389–399
Nishimura EK, Yoshida H, Kunisada T, Nishikawa SI (1999) Regulation of E- and P-cadherin expression correlated with melanocyte migration and diversification. Dev Biol 215:155–166
Omelchenko T, Fetisova E, Ivanova O, Bonder EM, Feder H, Vasiliev JM, Gelfand IM (2001) Contact interactions between epitheliocytes and fibroblasts: formation of heterotypic cadherin-containing adhesion sites is accompanied by local cytoskeletal reorganization. Proc Natl Acad Sci USA 98:8632–8637
Onken MD, Ehlers JP, Worley LA, Makita J, Yokota Y, Harbour JW (2006) Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res 66:4602–4609
Paffenholz R, Kuhn C, Grund C, Stehr S, Franke WW (1999) The arm-repeat protein NPRAP (neurojungin) is a constituent of the plaques of the outer limiting zone in the retina, defining a novel type of adhering junction. Exp Cell Res 250:452–464
Patel SD, Chen CP, Bahna F, Honig B, Shapiro L (2003) Cadherin-mediated cell-cell adhesion: sticking together as a family. Curr Opin Struct Biol 13:690–698
Peitsch WK, Grund C, Kuhn C, Schnoelzer M, Spring H, Schmelz M, Franke WW (1999) Drebrin is a widespread actin-associating protein enriched at junctional plaques, defining a specific microfilament anchorage system in polar epithelial cells. Eur J Cell Biol 78:767–778
Peitsch WK, Hofmann I, Praetzel S, Grund C, Kuhn C, Moll I, Langbein L, Franke WW (2001) Drebrin particles: components in the ensemble of proteins regulating actin dynamics of lamellipodia and filopodia. Eur J Cell Biol 80:567–579
Peitsch WK, Hofmann I, Bulkescher J, Hergt M, Spring H, Bleyl U, Goerdt S, Franke WW (2005) Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes. J Invest Dermatol 125:761–774
Perlis C, Herlyn M (2004) Recent advances in melanoma biology. Oncologist 9:182–187
Pieperhoff S, Schumacher H, Franke WW (2008) The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol 87:399–411
Qi J, Chen N, Wang J, Siu CH (2005) Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Mol Biol Cell 16:4386–4397
Qi J, Wang J, Romanyuk O, Siu CH (2006) Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell 17:1261–1272
Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B (1992) Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci 102:7–17
Sanders DS, Blessing K, Hassan GA, Bruton R, Marsden JR, Jankowski J (1999) Alterations in cadherin and catenin expression during the biological progression of melanocytic tumours. Mol Pathol 52:151–157
Sandig M, Voura EB, Kalnins VI, Siu CH (1997) Role of cadherins in the transendothelial migration of melanoma cells in culture. Cell Motil Cytoskeleton 38:351–364
Schaefer S, Koch PJ, Franke WW (1994) Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res 211:391–399
Schaefer S, Stumpp S, Franke WW (1996) Immunological identification and characterization of the desmosomal cadherin Dsg2 in coupled and uncoupled epithelial cells and in human tissues. Differentiation 60:99–108
Schmitt CJ, Franke WW, Goerdt S, Falkowska-Hansen B, Rickelt S, Peitsch WK (2007) Homo- and heterotypic cell-cell contacts in malignant melanoma cells and desmoglein 2 as a novel solitary surface glycoprotein. J Invest Dermatol 127:2191–2206
Schulze C, Firth JA (1993) Immunohistochemical localization of adherens junction components in blood-brain barrier microvessels of the rat. J Cell Sci 104:773–782
Seftor EA, Meltzer PS, Schatteman GC, Gruman LM, Hess AR, Kirschmann DA, Seftor RE, Hendrix MJ (2002) Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol 44:17–27
Shan WS, Tanaka H, Phillips GR, Arndt K, Yoshida M, Colman DR, Shapiro L (2000) Functional cis-heterodimers of N- and R-cadherins. J Cell Biol 148:579–590
Shimoyama Y, Tsujimoto G, Kitajima M, Natori M (2000) Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J 349:159–167
Silye R, Karayiannakis AJ, Syrigos KN, Poole S, Noorden S van, Batchelor W, Regele H, Sega W, Boesmueller H, Krausz T, Pignatelli M (1998) E-cadherin/catenin complex in benign and malignant melanocytic lesions. J Pathol 186:350–355
Simonneau L, Kitagawa M, Suzuki S, Thiery JP (1995) Cadherin 11 expression marks the mesenchymal phenotype: towards new functions for cadherins? Cell Adhes Commun 3:115–130
Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M (2005) Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol 166:1541–1554
Tang A, Eller MS, Hara M, Yaar M, Hirohashi S, Gilchrest BA (1994) E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci 107:983–992
Troyanovsky S (2005) Cadherin dimers in cell-cell adhesion. Eur J Cell Biol 84:225–233
Van Marck V, Stove C, Van Den Bossche K, Stove V, Paredes J, Vander Haeghen Y, Bracke M (2005) P-cadherin promotes cell-cell adhesion and counteracts invasion in human melanoma. Cancer Res 65:8774–8783
Volk T, Cohen O, Geiger B (1987) Formation of heterotypic adherens-type junctions between L-CAM-containing liver cells and A-CAM-containing lens cells. Cell 50:987–994
Wuchter P, Boda-Heggemann J, Straub BK, Grund C, Kuhn C, Krause U, Seckinger A, Peitsch WK, Ho AD, Franke WW (2007) Processus adhaerentes: giant cell processes studded with special adherens junctions attract and connect mesenchymal stem cells. Cell Tissue Res 328:499–514
Yin T, Green KJ (2004) Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol 15:665–677
Acknowledgements
We thank Martina Schnoelzer and Tore Kempf (Protein Analysis Facility, German Cancer Research Center) for performing the MALDI-TOF analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in parts by grants from the Deutsche Forschungsgemeinschaft to W. K. Peitsch (project PE 896/1) and the Deutsche Krebshilfe to W. W. Franke (project 10-2049).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Survey of the results of the tissue microarrays of human melanomas and nevi: clinical data and detailed immunofluorescence microscopical results (− few or no reactive tumor cells, + 5%–24% reactive cells, ++ 25%–49% reactive cells, +++ 50%–74% reactive cells, ++++ more than 75% reactive cells, n.d. not done) (DOC 161 KB)
Rights and permissions
About this article
Cite this article
Rickelt, S., Franke, W.W., Doerflinger, Y. et al. Subtypes of melanocytes and melanoma cells distinguished by their intercellular contacts: heterotypic adherens junctions, adhesive associations, and dispersed desmoglein 2 glycoproteins. Cell Tissue Res 334, 401–422 (2008). https://doi.org/10.1007/s00441-008-0704-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-008-0704-7