Abstract
The subcommissural organ (SCO) is an ependymal differentiation located in the dorsal midline of the caudal diencephalon under the posterior commissure. SCO cells synthesize and release glycoproteins into the cerebrospinal fluid (CSF) forming a threadlike structure known as Reissner’s fiber (RF), which runs caudally along the ventricular cavities and the central canal of the spinal cord. Numerous monoclonal antibodies have been raised against bovine RF and the secretory material of the SCO. For this study, we selected the 4F7 monoclonal antibody based on its cross-reactivity with chick embryo SCO glycoproteins in vivo. E4 chick embryos were injected with 4F7 hybridoma cells or with the purified monoclonal antibody into the ventricular cavity of the optic tectum. The hybridoma cells survived, synthesized and released antibody into the CSF for at least 13 days after the injection. E5 embryos injected with 4F7 antibody displayed precipitates in the CSF comprising both the monoclonal antibody and anti-RF-positive material. Such aggregates were never observed in control embryos injected with other monoclonal antibodies used as controls. Western blot analysis of CSF from E4-E6 embryos revealed several immunoreactive bands to anti-RF (AFRU) antibody. We also found AFRU-positive material bound to the apical surface of the choroid plexus primordia in E5 embryos. These and other ultrastructural evidence suggest the existence of soluble SCO-related molecules in the CSF of early chick embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The subcommissural organ (SCO) is an ependymal differentiation located in the roof plate of the caudalmost portion of the diencephalon (prosomere 1), under the posterior commissure. The SCO is an ancient and phylogenetically conserved structure, present throughout the vertebrate phylum (Oksche 1961). SCO ependymal cells are specialized in the synthesis and secretion of high-molecular-mass glycoproteins that are released into the cerebrospinal fluid (CSF), where they aggregate to form a threadlike supramolecular structure known as Reissner’s fiber (RF; Oksche 1969; E.M. Rodriguez et al. 1992, 1998; A. Meiniel 2001; Nualart and Hein 2001). In the encephalon, the glycoproteins forming the RF can be found in two conformations: (1) aggregated fibrillar material on top of SCO cilia, the so called pre-RF and (2) the RF itself, which is a cylindrical regular structure. RF grows caudally by the addition of SCO-glycoproteins at its cephalic end and extends along the brain aqueduct, the fourth ventricle and the central canal of the spinal cord (Sargent 1900; Sterba et al. 1967; Leonhardt 1980; E.M. Rodriguez et al. 1992). The SCO develops early during ontogeny (Oksche 1956; Naumann 1986; Schoebitz et al. 1986; Naumann et al. 1987) and the embryonic development of the SCO-RF complex has been studied in rat (Castaneyra-Perdomo et al. 1983; Marcinkiewicz and Bouchaud 1983, 1986; Naumann et al. 1987), rabbit (Kimble and Mollgard 1975), chicken (Wingstrand 1953; Schoebitz et al. 1986; Naumann et al. 1987; Karoumi et al. 1990; R. Meiniel et al. 1993; Didier et al. 1995; Cifuentes et al. 1996) and humans (Oksche 1956; Wislocki and Roth 1958; Oksche 1961; Olsson 1961; Mollgard 1972; E.M. Rodriguez et al. 2001). By using antibodies that were raised against bovine RF (named AFRU; E.M. Rodriguez et al. 1984) and that recognize chick SCO secretion, Schoebitz et al. (1986, 1993) performed an immunocytochemical study of chick SCO development. With respect to secretory material synthesis, ventricular release, assembly and RF formation, these authors have established that, in the chick embryo, the SCO secretion is first synthesized at embryonic day 3 (E3) by morphologically undifferentiated neuroepithelial cells of the dorsal diencephalon. At E7, the SCO starts to release glycoproteins into the CSF; these aggregate as pre-RF onto the surface of the neuroepithelium. The RF itself is not present until E11, when it appears in the cerebral aqueduct. At E12, RF is found in the lumbar spinal cord. Some authors have postulated that a part of the SCO secretion remains soluble in the CSF, under both physiological and experimental conditions (E.M. Rodriguez et al. 1993) and have found RF-immunoreactive material in non-competitive solid-phase enzyme immunoassay of cisternal and ventricular CSF from adult rabbit. The amount of soluble material detected increases notably in rabbits injected with the anti-RF serum, AFRU. These authors have identified three AFRU-immunoreactive bands in rabbit CSF analysed by Western blot; these bands might correspond to CSF-soluble polypeptides secreted by the SCO. During embryonic development, the release of SCO secretory material occurs several days before RF formation. Accordingly, Schoebitz et al. (1986) state that, for 4 days, the SCO of the embryonic chick secretes material that does not aggregate in a RF and remains soluble or forms small fibrils. In the rat, Schoebitz et al. (1993) have found that the secretory material can remain soluble for 5 days after the first evidence of ventricular release on the first postnatal day. Furthermore, in the last six embryonic days, the rat SCO displays abundant apical secretory granules, suggesting a release towards the CSF of compounds that do not aggregate in a pre-RF (Schoebitz et al. 1993). A parallel situation may occur in the chick embryo. As described previously, immunocytochemical and lectin studies of chick embryo SCO have suggested that the ependymal cells start to secrete into the ventricle at E7 (Schoebitz et al. 1986; Karoumi et al. 1990). Such a conclusion is based on the presence of AFRU-positive fibrilar aggregates on top of the SCO apical surface. However, the elapsed time between the first evidence of synthesis of secretory material (E3) and the formation of such ventricular aggregates (E7) suggests that, during this time, some soluble secretion might be released into the CSF.
The present investigation has been designed to gain new insights into the existence of soluble SCO secretions in the CSF at early developmental stages (E4-E6). To achieve this objective, we have used three approaches: (1) the injection, into the CSF of E4 embryos, of antibodies that recognized the chick SCO secretion; ii) the analysis, by Western blot, of E4-E6 chick embryo CSF and (3) the ultrastructural and immunocytochemical study of E4-E6 embryos. Our findings from these studies suggest that a SCO-related secretory material is present in the CSF at early (E4-E6) stages of chick development.
Materials and methods
Animals
Fertilized eggs from White Leghorn chicken (Gallus domesticus) were obtained from a local farm (Granja Santa Isabel, Córdoba) and incubated in a forced-draft incubator at 38.5°C with high humidity. Embryos were staged according to Hamburger and Hamilton (1951). The animals were handled in conformity with current national legislation (Royal Decree 223/1988, BOE no. 67) and guidelines from the Council of the European Communities (86/609/CEE).
Monoclonal antibody selection
Twenty-three monoclonal antibodies (MAbs) against bovine RF (2A5, 2A8, 3B1, 3E6, 3F1, 1F4, 1F12, 5G4, 1H3) and SCO secretory material (4A6, 5B9, 4B11, 2B12, 2C2, 1C12, 5D8, 1D9, 5E2, 4F7, 3F8, 5G7, 3G12, 4H6; Perez et al. 1995, 1996; Fernandez-Llebrez et al. 2001b) were screened to find those that immunoreacted with chick SCO secretory material. SCO explants at E13 were dissected out under a dissecting microscope (MZ6, Leica Microsystems, Wetzlar, Germany) under sterile conditions, maintained in culture (DMEM-F12 supplemented with 10% fetal calf serum; FCS) for 2 h (see Hoyo-Becerra et al. 2005 for details) and then incubated with various MAbs (hybridoma culture supernatant, concentration 50 μg/ml) for 1 h followed by Alexa-568-labelled anti-mouse IgG for 30 min. After two extensive washes in 0.1 M phosphate-buffered saline pH 7.3 (PBS), they were fixed with 4% PFA, mounted in glycerol-PBS (1:1) and observed by fluorescence microscopy. Incubation with antibodies that were against bovine RF (AFRU and AFRA, see below for description) and that recognize chick SCO (E.M. Rodriguez et al. 1984) and antibodies against a non-related antigen were used as positive and negative controls respectively.
Three out of 23 MAbs recognized chick SCO secretory material in vivo. However, only 4F7 was selected on the basis of: (1) its stronger reaction with chick SCO secretion in vivo and (2) its ability to recognize chick SCO in tissue sections, albeit only when the brains were fixed with a mixture of ethanol-acetic acid (3:1) and embedded in paraffin. As a control, we used a MAb that was developed in our laboratory against adult bovine ependymal cells (3E5; F. J. Bermúdez-Silva and J. Pérez, personal communication) and that did not recognize SCO secretory material. Either purified MAb or MAb-producing hybridoma cells were injected into the CSF of chick embryos used as controls.
Hybridoma cell culture and injection into the CSF
Hybridoma cells producing 4F7 were rapidly thawed and culture in DMEM-F12 supplemented with 20% FCS (hybridoma tested; Sigma, St. Louis, Mo., USA). After 2 days of culture, the cell density was 8×105 cells/ml. Cells were pelleted and resuspended in Hanks’ solution (Sigma) to reach a density of 105 cells/μl. By using a glass needle, 1 μl hybridoma cell suspension (105 cells) was injected into the ventricular cavity of the optic tectum of each of 15 E4 chick embryos. After surgery, the eggs were sealed with adhesive tape and kept in the incubator for various time periods. In some experiments, purified 4F7 MAb was injected instead of MAb-producing hybridoma cells. Purification of 4F7 MAb was performed by affinity chromatography in a protein G column according to Perez et al. (1996).
Collection of embryos, histology and immunocytochemistry
Most of the injected embryos survived the manipulation. They were collected at E5, E6, E7, E11, E13 and E17 and fixed with: (1) Bouin’s fluid or (2) a mixture of ethanol-acetic acid (3:1). From E5 to E7, complete embryos were fixed, whereas from E11 to E17, brains were removed from the skull for fixation. In both cases, tissues were fixed by immersion for 48 h at room temperature, dehydrated and embedded in paraffin wax. Sections (10 μm thick) were obtained on a microtome (RM2125, Leica Microsystems, Wetzlar, Germany). Double-immunofluorescence was used to localize simultaneously the SCO secretory material and the 4F7 antibody injected into the CSF or produced by CSF-injected hybridoma cells. The sections were hydrated, rinsed with PBS with 0.05% Tween-20 (Sigma) and incubated in the primary antibody for 18 h at room temperature. The primary antibodies used in this study were: (1) a rabbit polyclonal antiserum that was raised against bovine RF (AFRU, 1:1,000; E.M. Rodriguez et al. 1984; kindly provided by Prof. Rodriguez, Valdivia, Chile) and that recognizes chick SCO secretory material (previously used to study embryonic development of chick; Schoebitz et al. 1986); (2) a mouse polyclonal antibody against bovine RF raised in our laboratory (AFRA); (3) a rabbit polyclonal antibody against Ng-CAM (kindly provided by Prof. de la Rosa, Madrid, Spain) used to reveal the posterior commissure. In order to demonstrate the presence of antibodies against the SCO in the CSF of hybridoma-injected embryos, several microlitres of CSF were obtained from these embryos at 1 or 2 days after the injection and used as primary antibody on SCO sections. The CSF was centrifuged to discard hybridoma cells (5 min at 1,000g) and the supernatant was used as primary antibody (diluted 1:5).
To localize the 4F7 MAb, incomplete immunocytochemistry (S. Rodriguez et al. 1990; Cifuentes et al. 1994) was employed with an anti-mouse IgG labelled with Alexa 568. A mixture of Alexa-labelled secondary antibodies (Fab fragments; antimouse IgG Alexa 568 and anti-rabbit IgG Alexa 488, diluted 1:1,000) was applied for 30 min to reveal mouse and rabbit primary antibodies, respectively. Some sections were immunostained according to the immunoperoxidase method of Sternberger et al. (1970) with some modifications (for details, see Hoyo-Becerra et al. 2005). All antisera (primary and secondary) were diluted in PBS, pH 7.2, containing 10% normal goat serum and 0.3% Triton X-100 (Sigma). All incubations with antisera were performed in a moist chamber at room temperature. Negative controls for immunostaining included incubation of sections with (1) non-immune rabbit serum or (2) diluent buffer, instead of the primary antiserum. The sections were then mounted in SlowFade Light anti-fading reagent (Molecular Probes, Invitrogen) and examined with a Nikon fluorescence microscope (Eclipse E800). Digitized images were captured with ACT-1 software and processed with image-editing software (Adobe Photoshop 5.0). Some double-stained tissue sections were analysed by confocal laser scanning microscopy (Leica, TCS NT).
Collection of chick embryo CSF and tissue extracts
Non-injected chick embryos from E4 to E7 were used to obtain CSF, and amniotic fluid was used as a negative control. A volume of 5–10 μl was obtained through a fine glass needle inserted into the ventricular cavity of the optic tectum of each embryo. Amniotic fluid was collected from the amniotic cavity by using the same procedure. Samples were centrifuged and the supernatant was frozen. To prepare tissue extracts, SCO and optic tectum from E5 and E13 embryos were dissected out from surrounding tissue by using thin tweezers and scissors under a dissecting microscope (MZ6, Leica Microsystems); the posterior commissure could not be completely removed during the SCO dissection. The protein extraction medium was composed of 50 mM ammonium bicarbonate pH 8, containing the following protease inhibitors: 1 mM EDTA (Sigma), 1 mM phenylmethylsulfonyl fluoride (Merck, Darmstadt, Germany), 1 mM pepstatin (Sigma), and 1 mM leupeptin (Sigma). The protein content of the extracts was determined by the bicinchoninic acid method (Sigma) according to the manufacturer’s instructions. Homogenization, sonication and centrifugation were performed according to a procedure described previously (Perez et al. 1993; Grondona et al. 1994).
Western blot
SDS polyacrylamide gel electrophoresis was performed through 5%–15% gradients gels prepared in the Mini-protean II system (Bio-Rad). Samples of CSF, SCO and optic tectum (25–30 μg total protein) were mixed with loading buffer (65 mM TRIS-HCl, pH 6.8, 2% SDS, 2% dithiothreitole and 29% glycerol; all from Sigma), heated at 95°C for 2 min and loaded. Electrophoresis was performed at 30 mA for 1 h. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, Mass., USA) at 125 mA for 3 h. Molecular-weight standards were myosin (200.0 kDa), β-galactosidase (116.2 kDa), phosphorylase b (97.4 kDa), serum albumin (66.2 kDa), ovalbumin (45.0 kDa), carbonic anhydrase (31.0 kDa), soybean trypsin inhibitor (21.5 kDa), lysozyme (14.4 kDa) and aprotinin (6.5 kDa; Bio-Rad). For immunostaining, the PVDF membranes were: (1) blocked with 2% non-fat milk in TTBS (100 mM TRIS-HCl, pH 7.5, 0.9% NaCl, 0.05% Tween-20) for 2 h; (2) incubated with primary antibody, diluted in the blocking solution, for 18 h at 4°C and (3) incubated with goat anti-rabbit IgG peroxidase conjugated (Sigma; diluted 1:5,000). Two primary antibodies were used: AFRU diluted 1:1,000 and 4F7 MAb at 50 μg/ml. Immunoreactive bands were visualized by a chemiluminescence system (ECL Western Blotting Detection Reagents RPN2109, Amersham, UK) following the instructions provided by the manufacturer.
Lectin-binding analysis
Blots parallel to those used for immunostaining were used for lectin binding. The blots were sequentially incubated with oxidized bovine serum albumin (Sigma) and with peroxidase-labelled lectins for 30 min. The lectins used were (1) concanavalin A (Con A; affinity for mannose, glucose; Sigma; 0.5 μg/ml); (2) wheat germ agglutinin (WGA; affinity for N-acetyl-glucosamine, sialic acid; Sigma; 0,5 μg/ml). Peroxidase was detected by the enhanced chemiluminescence system (see above).
Electron microscopy
Four E5 chick embryos and three E13 brain fragments containing the SCO were fixed in 1% paraformaldehyde and 1% glutaraldehyde in 0.12 M phosphate buffer (PB), pH 7.4, for 2 h at room temperature. The embryos were then dissected out and immersed in the same fixative for 1 h at 4°C. After being washed in PB with 0.2 M glucose, tissue blocks containing the SCO were postfixed in 1% osmium tetroxide in PB for 2 h at 4°C, dehydrated in a graded series of ethanol and embeded in 512 Araldite resin. Resin blocks were sectioned at 70–80 nm with a Reichert ultramicrotome. Semithin sections were stained with toluidine blue. Ultrathin sections were stained with uranyl acetate and lead citrate and observed in a Philips CM100 transmission electron microscope. All the reagents used for electron microscopy were from Electron Microscopy Sciences (Fort Washington, Pa., USA).
Lectin histochemistry
Double-fluorescent labelling with the lectin WGA (Sigma) and the AFRA antiserum (see above) was performed on paraffin sections. Slides were hydrated and sequentially incubated in: (1) 3 μg/ml WGA-peroxidase conjugated (Sigma) in PBS, pH 7.3, for 1 h; (2) a mixture of anti-peroxidase antiserum raised in rabbit (obtained in our laboratory) and the AFRA antiserum raised in mouse, both diluted 1:1,000 in PBS containing 10% normal goat serum and 0.3% Triton X-100 (Sigma) for 18 h; (3) a mixture of Alexa-labelled secondary antibodies (Fab fragments; anti-mouse IgG Alexa 568 and antirabbit IgG Alexa 488, diluted 1:1,000) for 30 min. The remainder of the protocol was performed as described in the immunocytochemistry section (see above).
Lipid histochemistry with Oil red-O
Non-fixed E5 embryos and brains from E13 embryos were quickly embedded in OCT compound and frozen in liquid nitrogen vapor. Cryosections (16 μm) were prepared in a cryostat (Frigocut, Leica), fixed in 4% paraformaldehyde for 10 min, washed in distilled water, dipped in 60% isopropyl alcohol, stained for 10 min with Oil red-O (1.8 mg/ml; Hartman Leddon Company, Philadelphia, Pa., USA), washed with 60% isopropyl alcohol, washed in several changes of water, mounted in glycerol/PBS (1:1) and observed by Nomarski interference contrast microscopy (Leica DMLB photomicroscope).
Results
Monoclonal antibodies against bovine RF recognize chick SCO secretory material in vitro
Twenty-three MAbs developed against bovine RF and SCO secretory material were tested for recognition of chick SCO secretion in vitro. Chick SCO explants from E13 embryos were incubated with the MAbs; only three of them (4F7, 4B11 and 1C12) reacted with the secretory material located in the apical surface of the SCO. The 4F7 MAb gave the strongest reaction, comparable to the signal displayed by two polyclonal antibodies against bovine RF, AFRU and AFRA, used as positive controls (compare Fig. 1d with Fig. 1b,c). Moreover, the 4F7 MAb immunostained the chick SCO when fixed with an ethanol-acetic acid mixture and embedded in paraffin but not when fixed with Bouin (data not shown). This MAb recognized the SCO secretory material at every embryonic stage studied here, from E5 (Fig. 1e and f) to E17 (data not shown).
a Sagittal section through the subcommissural organ (SCO) of an E13 chick embryo immunostained with an anti-Reissner’s fiber serum (AFRU). The dotted line indicates the dissection plane used to perform the explants in the subsequent figures (CAq cerebral aqueduct, LS leptomeningeal space, PC posterior commissure). b–d Confocal images of cultured SCO explants obtained from E13 chick embryos and incubated in AFRU (b), anti-bovine Reissner’s fiber raised in mouse (AFRA; c) and 4F7 monoclonal antibody (d). The antibodies bind to the apical surface of SCO cells (arrows) but not to other portions of the explants (dotted lines regions of explants devoid of SCO secretory cells, SCOe subcommissural organ explant). e,f Sagittal and transverse sections, respectively, through the diencephalic roof of an E5 chick embryo immunostained with 4F7 MAb (red) and anti-Ng-CAM (polyclonal raised in rabbit, green). 4F7 labels the secretion of SCO cells in the soma and in the basal processes (arrows). Anti-Ng-CAM labels the axon bundles of the developing posterior commissure, among the basal portion of the SCO cells (CAq cerebral aqueduct, SCO subcommissural organ). Bars 100 μm (a–d), 50 μm (e–f)
Hybridoma cells survive and release MAbs into the chick embryo CSF
Hybridoma cells producing 4F7 MAb were injected into the ventricular cavity of the optic tectum of E4 chick embryos. They survived for 13 days after the injection (until E17), although some apoptotic hybridoma cells were also observed, mainly during the first 48 h after injection (not shown). At E15 and E17, the number of antibody-synthesizing hybridoma cells diminished, with some appearing to be embedded into the nervous tissue (Fig. 2c and inset). The cytoplasm of hybridoma cells found in the ventricular cavities contained mouse IgG (MAb), as demonstrated by incomplete immunocytochemistry with anti-mouse IgG (Fig. 2a, g).
a,b Details of a transverse section through the fourth ventricle (IVv) of an E11 chick embryo injected with 4F7-producing hybridoma cells at E4 and immunostained with an anti-mouse IgG (CP choroid plexus). Numerous immunoreactive MAb-producing hybridoma cells (a) and immunoreactive flocculent materials (asterisk in b) are present in the CSF. c The same region of an E17 embryo, showing hybridoma cell clusters penetrating the nervous tissue (arrowheads). Inset: Detail of one of these penetrating clusters. d Transverse section through the SCO of an E6 chick embryo immunostained by using the CSF (as first antibody; dilution 1:10) from an E6 embryo injected with 4F7-producing hybridoma cells at E4. Note the positive reaction in the SCO cells (CAq, cerebral aqueduct, LS leptomeningeal space, SCO subcommissural organ). e Detail of the tectal neuroepithelium of an 4F7-injected E6 chick embryo immunostained with anti-mouse IgG (LS leptomeningeal space, OT optic tectum). The MAb did not reach the nervous tissue and was confined to the apical surface of the neuroepithelium (arrowheads), although some neuroepithelial cells were filled with immunopositive material (arrows). f–h Confocal images of a paraffin section through the IV ventricle (IVv) of an E11 chick embryo injected with 4F7-producing hybridoma cells at E4 and double-immunostained with AFRU and anti-mouse IgG (arrows/green/red fibrillar AFRU-positive extracellular SCO secretory material that binds 4F7 MAb). Anti-mouse IgG also labels hybridoma cells (arrowheads). Bars 50 μm (c,d), 20 μm (a,b,e–h), 10 μm (inset)
Two days after the hybridoma injection, aggregated material positive to anti-mouse IgG was found in the CSF of all ventricular cavities (asterisk in Fig. 2b), thus revealing the presence of 4F7 MAb in such aggregates and also in the ventricular cavities. To confirm this point, chick CSF from E6-E7 embryos injected with hybridoma cells was collected and used as a primary antibody on sections of E13 chick SCO. The positive reaction observed in the chick SCO (Fig. 2d) confirmed the presence of the 4F7 MAb in the CSF. Although the MAb was present in the CSF, it never appeared in the extracellular space of the nervous tissue, being confined to the ventricular cavities (arrowheads in Fig. 2e). However, some neuroepithelial cells appeared positive to the anti-mouse IgG (arrows in Fig. 2e) suggesting intake of the MAb from the CSF.
4F7 MAb recognizes chick SCO secretory material in vivo
As mentioned above, the 4F7 clone was selected because of its ability to recognize the secretory material of chick SCO explants. Similarly, this MAb, either secreted by injected hybridoma cells or directly injected into the CSF, immunoreacted with aggregated fibrillar SCO secretory material throughout the ventricular cavities in E11 embryos. The antigen-antibody complexes were revealed by double-immunofluorescence with AFRU, which recognized the SCO secretory material, and an anti-mouse IgG that bound to 4F7 MAb. With this approach, AFRU-positive fibrillar structures (green) were strongly labelled with the anti-mouse IgG (red; Fig. 2f–h). In control chick embryos injected with a non-related MAb, AFRU-positive material was only slightly labelled with anti-mouse IgG (data not shown). We considered this faint staining to be non-specific and attributable to the binding of the injected MAb to all surfaces of the ventricular system.
4F7 MAb immunoreacts with SCO-related material in early chick embryos
E5 chick embryos injected either with 4F7-producing hybridoma cells or with purified 4F7 MAb exhibited aggregated material in the CSF; this material was positive to both AFRU and anti-mouse IgG (Fig. 3a–f), indicating that such precipitates contained SCO-related compounds and 4F7 MAb. AFRU-positive aggregates were not observed either in non-injected embryos or in control embryos injected with a non-related MAb (3E5), suggesting that such precipitates were indeed the result of specific antigen-antibody reaction between the 4F7 MAb and the SCO-related material (AFRU-positive), since it depended on the presence of the 4F7 MAb in the CSF and was not the result of fixative-induced precipitation. In addition, the amount of precipitates was higher when the purified 4F7 MAb was injected directly into the CSF, probably because the MAb could reach the optimal concentration for producing aggregates in the CSF.
a–f Midline sagittal section through the diencephalic roof of an E5 chick embryo (CAq cerebral aqueduct, LS leptomeningeal space, SCO subcommissural organ) injected with purified 4F7 at E4 and double-immunostained with AFRU (a) and anti-mouse IgG (b). d–f Higher magnification of boxed area in a. Aggregated material in the CSF of the third ventricle contains AFRU-positive material and 4F7 MAb (see merged images in c and f). g,h Midline sagittal section of an E5 non-treated embryo (IIIv third ventricle) immunostained with AFRU (green). Note positive SCO (arrow). h Higher magnification view of boxed area in g showing AFRU-positive material on the apical surface of the choroid plexus primordium. Inset in h: Detailed view of boxed area in h (arrowheads AFRU-immunoreactive material on the apical membrane of choroid cells, CP choroid plexus). i Choroid plexus (CP) of the third ventricle (IIIv) of an E13 chick embryo. Note binding of AFRU-immunoreactive material to choroidal cell surface (arrowheads). Bars 250 μm (g), 100 μm (a–c), 50 μm (d–f,h,i), 20 μm (inset)
AFRU-positive material binds to choroid plexus primordia in early chick embryos
Control non-injected E5 embryos displayed an anti-RF-positive fibrillar material attached to the apical surface of choroid plexus primordia (Fig. 3g–i). The cytoplasm of the epithelial cells forming the choroid plexus was devoid of label, suggesting the non-choroidal source of this material. In this case, the AFRU-positive material should reach the apical surface through the CSF and, therefore, the presence of such material in non-treated embryos suggests that SCO-related compound(s) are present in the CSF.
Western blot analysis of CSF at early stages of chick embryo development
In order to determine whether AFRU-positive compounds were present in the CSF of chick embryos, we analysed both CSF and SCO extracts from various stages by Western blot with AFRU (lanes 1, 2 and 3 in Fig. 4). Four immunoreactive low-molecular-weight bands (66, 39, 34 and 6 kDa, arrows in lanes 2 and 3, Fig. 4) were identified in the CSF from E5 (lane 3) to E7 (lane 2) embryos. However, such bands were missing in SCO extracts from both E5 (not shown) and E13 (lane 1, Fig. 4) embryos and from the amniotic fluid of the same stages used as a negative control (not shown). A high-molecular-weight band (540 kDa) was present in E5 SCO extracts (not shown) and was missing in optic tectum extracts used as a negative control (not shown). This high-molecular-weight band was also present in E13 SCO extracts (small arrow in lane 1, Fig. 4) and, interestingly, in CSF from E7 embryos (small arrows in lane 2, Fig. 4). The 4F7 MAb did not recognize any compound in immunoblots (not shown); denaturing conditions or SDS might have masked the epitope recognized in vivo by 4F7 MAb.
Western blot and lectin-binding analysis of SCO extract of E13 chick embryo (lanes 1, 4, 8), CSF of E5 (lanes 3, 5, 9) and of E7 (lanes 2, 6, 10) and amniotic fluid of E5 (lane 7). AFRU antiserum was used as primary antibody in the Western blot (AFRU). Concanavalin A (Con A) and wheat germ agglutinin (WGA) lectins were used to study the carbohydrate moiety. In E13 chick embryos, SCO extracts displayed a high-molecular-weight band of 540 kDa (small arrow in lane 1), which had affinity for both lectins (Con A, small arrow in lane 4; WGA, small arrow in lane 8). CSF possessed four AFRU-immunoreactive bands of 66, 39, 34 and 6 kDa at E5 and E7 stages (arrows in lanes 3, 8, 10). The 66-kDa band was WGA-negative and faintly Con-A-positive. Bands of 39 and 34 kDa were WGA-positive and Con-A-negative. The 6-kDa band had neither affinity for WGA nor Con A lectins
Lectin-binding analysis of CSF
The AFRU-immunoreactive 66-kDa compound had faintly affinity for Con A lectin (lanes 9 and 10, Fig. 4) but no affinity for WGA (lanes 5 and 6, Fig. 4). AFRU-immunoreactive compounds of 39 and 34 kDa had no affinity for Con A lectin but strongly reacted with WGA. Neither Con A nor WGA bound to the compound at 6 kDa. The 540-kDa compound found in E13 SCO extracts displayed affinity for both lectins (small arrow in lanes 4 and 6, Fig. 4).
Ultrastructural analysis of chick embryo SCO at early stages of development
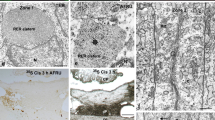
A detailed ultrastructural study of E5 SCO was performed to determine the secretory features of this specialized ependyma. We distinguished four regions in the SCO ependymal cells: apical, intermediate cytoplasm, perinuclear-basal cytoplasm and basal process with its leptomeningeal ending (Fig. 5a,b). In all these areas, the most prominent secretory-related structure was the rough endoplasmic reticulum (RER), which appeared as dilated cisternae in the basal cytoplasm (Fig. 5d). Typical secretory apical granules were absent and the Golgi apparatus was poorly developed. However, large electron-dense inclusions of various sizes (ranging from 300 to 1,000 nm) were found in all compartments of the cell, mainly in the apical and intermediate cytoplasm (Fig. 5e). These inclusions were not surrounded by membrane and were strongly osmiophilic, thus suggesting a lipidic nature. To clarify this point, we stained SCO-containing cryostat sections from E5 embryos with oil red-O (Fig. 5h). Many stained lipid droplets was located mainly in the apical and intermediate cytoplasm of the SCO. Similar lipid droplets were also present in the apical cytoplasm of all neuroepithelial cells (NE area in Fig. 5h). We also studied the ultrastructure of E13 chick SCO. At this stage, the typical features of an adult SCO were present: dilated perinuclear RER cisternae (Fig. 6a,b), a highly developed Golgi apparatus in the intermediate cytoplasm (Fig. 6c) and secretory granules at the apical cytoplasm (Fig. 6d and inset). Apical tight junctions were found among SCO epithelial cells (arrows in Fig. 6d).
a Transverse semithin section through E5 chick embryo SCO (boxed areas various regions analysed by electron microscopy: leptomeningeal endings in c, perinuclear-basal cytoplasm in d, intermediate cytoplasm in e and apical cytoplasm in f). b Similar transverse paraffin section immunostained with AFRU antiserum and counterstained with haematoxylin. The four boxed areas correspond to those in a; all contain immunoreactive secretion (CAq cerebral aqueduct, PC posterior commissure, LS leptomeningeal space). c Leptomeningeal endings display a few organelles, such as mitochondria (M) and rough endoplasmic reticulum (RER). The basal membrane (BM), leptomeningeal space (LS) and extracellular space (ECS) are indicated. d Perinuclear region contains mainly dilated RER cisternae (RER; N nucleus). e Intermediate cytoplasm displays many RER cisternae (RER) and a few profiles resembling Golgi cisternae. Heterogeneous osmiophilic droplets devoid of cell membrane are also present (L). f, g Apical cytoplasm of SCO cells contains RER cisternae (RER) and osmiophilic droplets (L). The apical surface is irregular with images suggestive of openings of RER cisternae (arrowheads). Note the intercellular junctions between the two SCO cells in f. h Detail of a cryostat section of an E5 chick embryo SCO stained with oil red-O to reveal lipid (CAq cerebral aqueduct). Numerous lipid droplets are located in the apical and intermediate cytoplasm of the SCO and in undifferentiated neuroepithelial cells (NE). Bars 200 μm (a,b,h), 1 μm (e), 500 nm (c,d), 250 nm (f), 100 nm (g)
Electron micrographs of SCO of E13 chick embryo (M mitochondria, N nucleus). a Basal and perinuclear cytoplasm display many dilated RER cisternae, mainly located at the infranuclear region. b Higher magnification of boxed area in a. Both flat and dilated cisternae are present. c The intermediate cytoplasm contains numerous dilated RER cisternae (RER), osmiophilic droplets of variable size and devoid of cell membrane (L) and abundant Golgi cisternae (GA). d Apical cytoplasm displays many secretory granules (SG in inset) of irregular shape and size (range: 50–200 nm). At E13, SCO cells display cilia on their apical surface (arrowheads basal body of cilia). Tight junctions are present between the SCO cells (arrows). The cerebral aqueduct (CAq) lies bottom right. Bars 2 μm (a), 1 μm (d), 500 nm (b,c), 200 nm (inset in d)
Lectin histochemistry of chick embryo SCO
WGA-binding sites were virtually absent in the SCO of E5 chick embryos. At this stage, the WGA lectin displayed faint staining in the supranuclear cytoplasm and in the apical portion of the SCO cells (asterisk in Fig. 7b). The remaining secretory structures were WGA-negative, including the AFRU-positive leptomeningeal endings of the basal processes (arrows in Fig. 7a). The apical surface of all neuroepithelial cells was strongly stained by WGA (arrowheads in Fig. 7b), probably corresponding to the glycocalyx. At E13, the SCO cells displayed a strong positive WGA reaction in the supranuclear and apical cytoplasm (Fig. 7d,f), a region more weakly immunostained by AFRA (Fig. 7c,e). Conversely, the basal cytoplasm of the SCO cells showed higher affinity for AFRA, whereas the WGA reaction was weaker (small arrows in Fig. 7e). The extracellular AFRA-positive secretory material (large arrow in Fig. 7e) forming the pre-RF was also stained with the WGA lectin. Midline AFRA-negative SCO cells (arrowhead in Fig. 7e) displayed WGA-positive material in their supranuclear and apical regions, suggesting secretory activity by these AFRU-negative cells.
a,b Sagittal section through E5 chick embryo SCO double-labelled with an anti-RF antibody (AFRA) raised in mouse (a) and the wheat germ agglutinin (WGA) lectin (b). AFRA-positive leptomeningeal endings are WGA-negative (arrows). The apical region of the SCO cells are WGA-positive (arrowheads in b). The cytoplasmic region is faintly positive to WGA (asterisk in b). c,d Transverse section of the SCO chick embryo at E13 labelled with AFRA (c) and WGA (d). e,f Higher magnification of c and d, respectively. At E13, the intermediate and apical cytoplasm contain many WGA-positive structures (large arrow extracellular SCO secretory material positive to both AFRA and WGA and corresponding to a fragment of pre-RF). Strongly AFRA-positive basal structures are located at the infranuclear region (small arrows in e). AFRA-negative and WGA-positive cells are located in the midline of the SCO (arrowheads in c–f (CAq cerebral aqueduct, LS leptomeningeal space, SCO subcommissural organ). Bars 50 μm (c,d), 20 μm (a,b,e,f)
Discussion
We have injected MAb-producing hybridoma cells into the CSF of E4 chick embryos. The 4F7 MAb released into the CSF by hybridoma cells binds to AFRU-positive material and induces its immunoprecipitation in the CSF of early (E5) chick embryos. The existence of CSF-soluble AFRU-immunoreactive material at this early stage has been confirmed by (1) AFRU immunocytochemistry revealing attached immunoreactive material on the apical surface of the choroid plexus epithelium in control (non-injected) E5 embryos and (2) Western blot analysis with AFRU of early chick embryo CSF. Our results strongly suggest that CSF-soluble SCO-related compounds are present in E5 chick embryos. The source and functional meaning of such compounds is discussed below.
CSF-injected hybridoma cells synthesize and release antibodies that bind SCO secretory material in vivo
Function-blocking antibodies have been thoroughly used to unravel protein function both in vitro and in vivo (Ziyadeh and Sharma 1996; Crowe et al. 2001; Orgiazzi et al. 2003; Ando and Davies 2005; Robert et al. 2005). However, the injection of MAb-producing hybridoma cells into the CSF has seldom been reported and then only in rats (Molnar et al. 1997; Wenk et al. 1999; Oudega et al. 2000; Fouad et al. 2004). In the present work, we have used this approach to achieve the continuous delivery of MAbs into the CSF of chick embryos. Interestingly, the MAbs do not pass from the CSF to the brain tissue, because of the presence of a barrier at the inner neuroepithelial surface. Such a barrier consisting of strap junctions has been described in the developing brain of mammals (Saunders 1992; Saunders et al. 2000; Dziegielewska and Saunders 2002) and, according to our results, seems also to be present in chick embryos. This finding should be taken into account in experiments involving function-blocking antibodies delivered via the CSF and directed against molecules located in the nervous tissue parenchyma.
The immunological blockade of the extracellular SCO secretory material has previously been achieved in rat embryos by maternal delivery of specific anti-RF antibodies through transplacental transport (S. Rodriguez et al. 1999; Vio et al. 2000; S. Rodriguez and Caprile 2001) and in adult rats by a single injection of anti-RF antibody into the CSF (S. Rodriguez et al. 1990). In both cases, effects related to CSF hydrodynamics have been reported: (1) disturbances in CSF circulation in adult rats (Cifuentes et al. 1994) and (2) development of hydrocephalus in fetuses (Vio et al. 2000). In our chick embryos, the 4F7 MAb produces immunoprecipitation of the SCO secretory material, but no morphological changes in brain development or in the volume of the ventricular cavities have been observed during the experimental period studied (E5–E17). The only alterations that have been indentified occur in RF aggregation in E11 embryos (not shown). We cannot exclude the possibility that pathological effects appear at a more advanced stage.
CSF-soluble SCO secretory material
Several previous studies have reported the possibility that a part of the secretory material released by the SCO remains soluble in the CSF. Thus, the release of CSF-soluble material has been described in induced hydrocephalus (Irigoin et al. 1990), in SCO transplants under the kidney capsule (E.M. Rodriguez et al. 1989) and in SCO transplanted into the ventricular cavities of chick embryos (Hoyo-Becerra et al. 2005). In vitro experiments performed by Lehmann and Sterba (1993) have demonstrated the presence of soluble AFRU-positive compounds in the culture medium of bovine SCO explants analysed by enzyme-linked immunosorbent assay (ELISA). In the CSF of adult rabbits, AFRU-positive soluble compounds have been detected by both ELISA and immunoblot (E.M. Rodriguez et al. 1993). The same authors have produced antibodies against various compounds isolated from the CSF of hydrocephalic children, some of which recognize the embryonic SCO, demonstrating the presence of SCO soluble compounds in the CSF (E.M. Rodriguez et al. 1993). Based on morphological evidence, Schoebitz et al. (1986, 1993) have also proposed that some compounds secreted by chick and rat embryonic SCO remain soluble in the CSF. Thus, the finding that the chick SCO starts to release secretory material several days before the formation of the RF indicates that the first SCO secretion remains soluble in the CSF (Schoebitz et al. 1986). A similar situation has been reported in rats, since the first signs of ventricular release are observed in fetuses of 21 days and a proper RF has not been described until postnatal day 6 (Schoebitz et al. 1993). The present work provides new evidence for the existence of CSF-soluble SCO-related compounds: (1) the formation of aggregates immunoreactive to 4F7 and AFRU in the ventricles of chick embryos injected with 4F7 MAb, (2) the presence of various AFRU-immunoreactive bands in the CSF analysed by Western blot and (3) the existence of AFRU-positive material attached to the choroid plexus as early as E5.
Source of CSF-soluble SCO-immunorelated material
According to our data, CSF-soluble AFRU-immunoreactive material is present in chick embryos as early as E5. Two sources of such AFRU-positive material are possible: the SCO and the floor plate (FP) cells. However, several points argue against the E5 SCO as being the source of such compounds. (1) The first sign of ventricular release is reported to occur at E7 (Schoebitz et al. 1986) based on the presence of fibrillar immunoreactive material on top of the SCO microvilli. (2) By using lectin histochemistry, we have shown that E5 SCO has no affinity for WGA lectin, whereas two of the CSF compounds (at 39 kDa and 34 kDa) are strongly positive to this lectin. In addition, ultrastructural studies of E5 SCO have confirmed the absence of Golgi apparatus and apical secretory granules; this agrees with the lectin histochemistry results. (3) Classical SCO glycoproteins secreted into CSF (i.e. pre-RF or RF proper) has been reported as being Con-A-negative (Hein et al. 1993; Nualart and Hein 2001). However, the AFRU-positive CSF compound of 66 kDa has moderate affinity for this lectin, thereby indicating a source different to SCO cells. Concerning this point, previous studies have suggested the possibility of SCO secretion being released directly from RER cisternae into the ventricle (Oksche 1969; E.M. Rodriguez 1970; Chen et al. 1973). For this reason, we cannot exclude completely the possibility that this Con-A-positive compound is synthesized and released by the SCO. Our ultrastructural results in the apical portion of E5 SCO cells support this possibility.
An alternative source of soluble secretory SCO-related material could be the floor plate (FP) cells. Numerous authors have reported that FP cells, particularly those located in the rostralmost region (the so-called flexural organ), contain AFRU-immunorelated compounds at early stages of brain development (Olsson 1958; Schoebitz et al. 1993; E.M. Rodriguez et al. 1996; Lopez-Avalos et al. 1997; Yulis et al. 1998; Lichtenfeld et al. 1999; del Brio et al. 2000, 2001; Fernandez-Llebrez et al. 2001a; Richter et al. 2001; Yulis and Munoz 2001; Guiñazú et al. 2002; our present results). Indeed, Olsson (1956, 1958) considers the flexural organ as a transient source of RF material preceding the onset of SCO secretory activity; this has been confirmed in several vertebrate species by Yulis et al. (1998). Several authors have even suggested that such material is released into the CSF (Schoebitz et al. 1993; E.M. Rodriguez et al. 1996; Yulis et al. 1998; del Brio et al. 2000). Some results are in favour of the FP as the alternative source of at least part of the CSF AFRU-positive compounds in E5 chick embryos. (1) Two of the CSF compounds (39 kDa and 34 kDa) are WGA-positive and the capacity to release WGA compounds during early development has been reported in FP explants of bovine embryos (Guiñazú et al. 2002). However, the SCO at this stage is negative to this lectin. (2) The existence of an AFRU-positive Con-A-positive WGA-negative CSF compound (66 kDa) suggests a putative secretory route bypassing the Golgi apparatus. A similar biosynthetic pathway has been proposed to occur in the FP cells of rats (del Brio et al. 2001) and in bovine FP explants, which release a Con-A-positive compound into the culture medium (Guiñazú et al. 2002). (3) Bovine FP explants release AFRU-immunoreactive compounds that remain soluble in the culture medium (Guiñazú et al. 2002). Conversely, the secretion released by SCO explants aggregates in the form of clusters on the surface of the explants (Schoebitz et al. 2001). Thus, CSF-soluble AFRU-positive material found in E5 chick embryos might be more closely related to FP secretion than to SCO secretion. (4) The FP of chick embryos displays AFRU-positive material from HH29 (E6) to HH36 (E10; del Brio et al. 2000), stages close to those when the CSF-soluble compounds appear in our Western blots. In this scenario, both the SCO and the FP appear as putative sources of the AFRU-positive CSF-soluble secretory material at E5. However, low-mass bands are missing in both SCO (Cifuentes et al. 1996; del Brio et al. 2000; our present results) and FP extracts (del Brio et al. 2000; Guiñazú et al. 2002). Two non-excluding possibilities may account for this finding: (1) “classical” high-molecular-weight compounds from E5 SCO might undergo post-release processing rendering low-mass compounds and (2) low-molecular-mass CSF-soluble compounds may be present in SCO cells at low concentrations because of fast synthesis and release turnover.
Functional considerations regarding CSF-soluble SCO-related compounds
The participation of SCO secretory material in developmental events in the nervous system has been proposed by numerous authors on the basis of findings from (1) experimental embryology (Winkelmann 1960; Rühle 1971; Hauser 1972, 1976), (2) in vitro biological assays of SCO secretory material on the differentiation of neuronal cells (Monnerie et al. 1995, 1996, 1997, 1998), (3) the molecular properties of the SCO/RF secretion (Gobron et al. 1996, 1999; Creveaux et al. 1998; A. Meiniel et al. 2003). The SCO secretion has been proposed to participate in ontogenetic processes in the CNS, such as neuronal differentiation, neuronal aggregation and axonal pathfinding (for reviews, see A. Meiniel 2001; A. Meiniel et al. 2003). The existence of CSF-soluble SCO secretion supports the idea of a functional role of the SCO in CNS development and increases the variety of the so-far-unknown targets for such secretion (E.M. Rodriguez et al. 1993, 2001; Schoebitz et al. 1993; del Brio et al. 2000). In the current report, we present three findings with functional implications. First, the presence of a barrier at the interface between the CSF and brain tissue during early developmental stages (previously reported in mammals; for reviews, see Saunders et al. 2000; Dziegielewska and Saunders 2002) would confine the SCO secretion to the ventricular cavities and the meningeal spaces and, therefore, the target cells would of necessity be in such compartments. Second, the existence of CSF-soluble material as early as E5 in chick embryos (2 days before the stages reported in previous studies) supports a putative functional role early in development. Third, the appearance of AFRU-positive material bound to the apical surface of the choroid plexus primordium in E5 chick embryos indicates that it participates in the differentiation of the choroid plexus. Similarly, binding of SCO secretion to the choroid plexus has been found in older chick embryos (Hoyo-Becerra et al. 2005). On the basis of various experimental approaches, Miranda et al. (2001) have proposed that the choroid plexus cells are targets for SCO secretory compounds in bovine fetuses, but at stages when the choroid plexus is differentiated. The above results suggest that SCO-related CSF-soluble compounds are involved in the differentiation of the choroid plexus at early stages of development and later in the regulation of choroidal function.
References
Ando T, Davies TF (2005) Monoclonal antibodies to the thyrotropin receptor. Clin Dev Immunol 12:137–143
Brio MA del, Riera P, Munoz RI, Montecinos H, Rodriguez EM (2000) The metencephalic floor plate of chick embryos expresses two secretory glycoproteins homologous with the two glycoproteins secreted by the subcommissural organ. Histochem Cell Biol 113:415–426
Brio MA del, Riera P, Peruzzo B, Rodriguez EM (2001) Hindbrain floor plate of the rat: ultrastructural changes occurring during development. Microsc Res Tech 52:615–626
Castaneyra-Perdomo A, Meyer G, Ferres-Torres R (1983) Development of the subcommissural organ in the albino mouse (a Golgi study). J Hirnforsch 24:363–370
Chen IL, Lu KS, Lin HS (1973) Electron microscopic and cytochemical studies of the mouse subcommissural organ. Z Zellforsch Mikrosk Anat 139:217–236
Cifuentes M, Rodriguez S, Perez J, Grondona JM, Rodriguez EM, Fernandez-Llebrez P (1994) Decreased cerebrospinal fluid flow through the central canal of the spinal cord of rats immunologically deprived of Reissner’s fibre. Exp Brain Res 98:431–440
Cifuentes M, Lopez-Avalos MD, Perez J, Grondona JM, Fernandez-Llebrez P (1996) Identification of a high molecular weight polypeptide in the subcommissural organ of the chick embryo. Cell Tissue Res 286:543–546
Creveaux I, Gobron S, Meiniel R, Dastugue B, Meiniel A (1998) Complex expression pattern of the SCO-spondin gene in the bovine subcommissural organ: toward an explanation for Reissner’s fiber complexity? Brain Res Mol Brain Res 55:45–53
Crowe JE Jr, Suara RO, Brock S, Kallewaard N, House F, Weitkamp JH (2001) Genetic and structural determinants of virus neutralizing antibodies. Immunol Res 23:135–145
Didier R, Dastugue B, Meiniel A (1995) The secretory material of the subcommissural organ of the chick embryo. Characterization of a specific polypeptide by two-dimensional electrophoresis. Int J Dev Biol 39:493–499
Dziegielewska KM, Saunders NR (2002) The ins and outs of brain-barrier mechanisms. Trends Neurosci 25:69–71
Fernandez-Llebrez P, Hernandez S, Andrades JA (2001a) Immunocytochemical detection of Reissner’s fiber-like glycoproteins in the subcommissural organ and the floor plate of wildtype and cyclops mutant zebrafish larvae. Cell Tissue Res 305:115–120
Fernandez-Llebrez P, Miranda E, Estivill-Torrus G, Cifuentes M, Grondona JM, Lopez-Avalos MD, Perez-Martin M, Perez J (2001b) Analysis and quantification of the secretory products of the subcommissural organ by use of monoclonal antibodies. Microsc Res Tech 52:510–519
Fouad K, Klusman I, Schwab ME (2004) Regenerating corticospinal fibers in the marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur J Neurosci 20:2479–2482
Gobron S, Monnerie H, Meiniel R, Creveaux I, Lehmann W, Lamalle D, Dastugue B, Meiniel A (1996) SCO-spondin: a new member of the thrombospondin family secreted by the subcommissural organ is a candidate in the modulation of neuronal aggregation. J Cell Sci 109:1053–1061
Gobron S, Creveaux I, Meiniel R, Didier R, Dastugue B, Meiniel A (1999) SCO spondin is evolutionarily conserved in the central nervous system of the chordate phylum. Neuroscience 88:655–664
Grondona JM, Perez J, Cifuentes M, Lopez-Avalos MD, Nualart FJ, Peruzzo B, Fernandez LP, Rodriguez EM (1994) Analysis of the secretory glycoproteins of the subcommissural organ of the dogfish (Scyliorhinus canicula). Brain Res Mol Brain Res 26:299–308
Guiñazú MF, Richter HG, Rodriguez EM (2002) Bovine floor plate explants secrete SCO-spondin. Cell Tissue Res 308:177–191
Hamburger V, Hamilton H (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–91
Hauser R (1972) Morphogenetic action of the subcommissural organ on tail regeneration in Xenopus larvae. Wilhelm Roux Archiv 169:70–184
Hauser R (1976) Distortion of body axis in young minnows (Phoxinus laevis) following destruction of the subcommissural organ. Rev Suisse Zool 83:898–903
Hein S, Nualart F, Rodriguez EM, Oksche A (1993) Partial characterization of the secretory products of the subcommissural organ. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 79–88
Hoyo-Becerra C, Lopez-Avalos MD, Alcaide-Gavilan M, Gomez-Roldan MC, Perez J, Fernandez-Llebrez P, Grondona JM (2005) Reissner’s fiber formation depends on developmentally regulated factors extrinsic to the subcommissural organ. Cell Tissue Res 321:429–441
Irigoin C, Rodriguez EM, Heinrichs M, Frese K, Herzog S, Oksche A, Rott R (1990) Immunocytochemical study of the subcommissural organ of rats with induced postnatal hydrocephalus. Exp Brain Res 82:384–392
Karoumi A, Croisille Y, Croisille F, Meiniel R, Belin MF, Meiniel A (1990) Glycoprotein synthesis in the subcommissural organ of the chick embryo. II. An immunochemical study. J Neural Transm Gen Sect 80:203–212
Kimble JE, Mollgard K (1975) Subcommissural organ-associated neurons in fetal and neonatal rabbit. Cell Tissue Res 159:195–204
Lehmann W, Sterba G (1993) The subcommissural organ in vitro. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 133–140
Leonhardt H (1980) Ependym und circumventrikuläre Organe. In: Oksche A, Vollrath L (eds) Handbuch der Mikroskopischen Anatomie des Menschen, Part IV, vol 10. Neuroglia I. Springer, Berlin Heidelberg New York, pp 176–665
Lichtenfeld J, Viehweg J, Schutzenmeister J, Naumann WW (1999) Reissner’s substance expressed as a transient pattern in vertebrate floor plate. Anat Embryol (Berl) 200:161–174
Lopez-Avalos MD, Cifuentes M, Grondona JM, Miranda E, Perez J, Fernandez-Llebrez P (1997) Rostral floor plate (flexural organ) secretes glycoproteins immunologically similar to subcommissural organ glycoproteins in dogfish (Scyliorhinus canicula) embryos. Brain Res Dev Brain Res 102:69–75
Marcinkiewicz M, Bouchaud C (1983) The ependymal secretion of the fetal and adult rat subcommissural organ. Morphological aspects linked to the synthesis, storage and release of the secretory products. Biol Cell 48:47–52
Marcinkiewicz M, Bouchaud C (1986) Formation and maturation of axo-glandular synapses and concomitant changes in the target cells of the rat subcommissural organ. Biol Cell 56:57–65
Meiniel A (2001) SCO-spondin, a glycoprotein of the subcommissural organ/Reissner’s fiber complex: evidence of a potent activity on neuronal development in primary cell cultures. Microsc Res Tech 52:484–495
Meiniel A, Meiniel R, Goncalves-Mendes N, Creveaux I, Didier R, Dastugue B (2003) The thrombospondin type 1 repeat (TSR) and neuronal differentiation: roles of SCO-spondin oligopeptides on neuronal cell types and cell lines. Int Rev Cytol 230:1–39
Meiniel R, Didier R, Molat JL, Meiniel A (1993) Developmental aspects of the subcommissural organ: an approach using lectins and monoclonal antibodies. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 51–59
Miranda E, Almonacid JA, Rodriguez S, Perez J, Hein S, Cifuentes M, Fernandez-Llebrez P, Rodriguez EM (2001) Searching for specific binding sites of the secretory glycoproteins of the subcommissural organ. Microsc Res Tech 52:541–551
Molnar M, Ruberti F, Cozzari C, Domenici L, Cattaneo A (1997) A critical period in the sensitivity of basal forebrain cholinergic neurones to NGF deprivation. Neuroreport 8:575–579
Mollgard K (1972) Histochemical investigations on the human foetal subcommissural organ. I. Carbohydrates and mucosubstances, proteins and nucleoproteins, esterase, acid and alkaline phosphatase. Histochemie 32:31–48
Monnerie H, Boespflug-Tanguy O, Dastugue B, Meiniel A (1995) Reissner’s fibre supports the survival of chick cortical neurons in primary mixed cultures. Cell Tissue Res 282:1–91
Monnerie H, Boespflug-Tanguy O, Dastugue B, Meiniel A (1996) Soluble material from Reissner’s fiber displays anti-aggregative activity in primary cultures of chick cortical neurons. Brain Res Dev Brain Res 96:120–129
Monnerie H, Dastugue B, Meiniel A (1997) In vitro differentiation of chick spinal cord neurons in the presence of Reissner’s fibre, an ependymal brain secretion. Brain Res Dev Brain Res 102:167–176
Monnerie H, Dastugue B, Meiniel A (1998) Effect of synthetic peptides derived from SCO-spondin conserved domains on chick cortical and spinal-cord neurons in cell cultures. Cell Tissue Res 293:407–418
Naumann W (1986) Immunhistochemische Untersuchungen zur Ontogenese des Subcommissuralorgans. Acta Histochem Suppl 33:265–272
Naumann W, Muller G, Kloss P (1987) Immunoreactive glycoproteins of the subcommissural organ in the embryonic stages of the vertebrate brain. Wiss Z Karl-Marx-Univ Leipzig Math-Naturwiss R 36:17–20
Nualart F, Hein S (2001) Biosynthesis and molecular biology of the secretory proteins of the subcommissural organ. Microsc Res Tech 52:468–483
Oksche A (1956) Funktionelle histologische Untersuchungen über die Organe des Zwischenhirndaches der Chordaten. Anat Anz 102:404–419
Oksche A (1961) Vergleichende Untersuchungen über die sekretorische Aktivität der Subkommissuralorgans und den Gliacharakter seiner Zellen. Z Zellforsch Mikrosk Anat 54:549–612
Oksche A (1969) The subcommissural organ. J Neurovisc Relat Suppl 9:111–139
Olsson R (1956) The development of Reissner’s fibre in the brain of the salmon. Acta Zool 37:1–16
Olsson R (1958) Studies on the subcommissural organ. Acta Zool 39:71–102
Olsson R (1961) Subcommissural ependyma and pineal organ development in human fetuses. Gen Comp Endocrinol 1:117–123
Orgiazzi J, Madec AM, Ducottet X (2003) The role of stimulating, function-blocking and growth-blocking anti-TSH receptor antibodies (TRAbs) in GD, Hashimoto’s disease and in atrophic thyroiditis. Ann Endocrinol (Paris) 64:31–36
Oudega M, Rosano C, Sadi D, Wood PM, Schwab ME, Hagg T (2000) Neutralizing antibodies against neurite growth inhibitor NI-35/250 do not promote regeneration of sensory axons in the adult rat spinal cord. Neuroscience 100:873–883
Perez J, Grondona JM, Cifuentes M, Nualart FJ, Fernández-Llebrez P, Rodriguez EM (1993) Immunochemical analysis of the dogfish subcommissural organ.In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 99–107
Perez J, Peruzzo B, Estivill-Torrus G, Cifuentes M, Schoebitz K, Rodriguez E, Fernandez-Llebrez P (1995) Light- and electron-microscopic immunocytochemical investigation of the subcommissural organ using a set of monoclonal antibodies against the bovine Reissner’s fiber. Histochem Cell Biol 104:221–232
Perez J, Garrido O, Cifuentes M, Alonso FJ, Estivill-Torrus G, Eller G, Nualart F, Lopez-Avalos MD, Fernandez-Llebrez P, Rodriguez EM (1996) Bovine Reissner’s fiber (RF) and the central canal of the spinal cord: an immunocytochemical study using a set of monoclonal antibodies against the RF-glycoproteins. Cell Tissue Res 286:33–42
Richter HG, Munoz RI, Millan CS, Guinazu MF, Yulis CR, Rodriguez EM (2001) The floor plate cells from bovine express the mRNA encoding for SCO-spondin and its translation products. Brain Res Mol Brain Res 93:137–147
Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T (2005) Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6:491–500
Rodriguez EM (1970) Ependymal specializations. II. Ultrastructural aspects of the apical secretion of the toad subcommissural organ. Z Zellforsch Mikrosk Anat 111:15–31
Rodriguez EM, Oksche A, Hein S, Rodriguez S, Yulis R (1984) Comparative immunocytochemical study of the subcommissural organ. Cell Tissue Res 237:427–441
Rodriguez EM, Rodriguez S, Schoebitz K, Yulis CR, Hoffmann P, Manns V, Oksche A (1989) Light- and electron-microscopic investigation of the rat subcommissural organ grafted under the kidney capsule, with particular reference to immunocytochemistry and lectin histochemistry. Cell Tissue Res 258:499–514
Rodriguez EM, Oksche A, Hein S, Yulis CR (1992) Cell biology of the subcommissural organ. Int Rev Cytol 135:39–121
Rodriguez EM, Jara P, Richter H, Montecinos H, Flandez B, Wiegand R, Oksche A (1993) Evidence for the release of CSF-soluble secretory material from the subcommissural organ, with particular reference to the situation in the human. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 121–131
Rodriguez EM, Brio Leon MA del, Riera P, Menendez J, Schoebitz K (1996) The floor plate of the hindbrain is a highly specialized gland. Immunocytochemical and ultrastructural characteristics. Brain Res Dev Brain Res 97:153–168
Rodriguez EM, Rodriguez S, Hein S (1998) The subcommissural organ. Microsc Res Tech 41:98–123
Rodriguez EM, Oksche A, Montecinos H (2001) Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc Res Tech 52:573–590
Rodriguez S, Caprile T (2001) Functional aspects of the subcommissural organ-Reissner’s fiber complex with emphasis in the clearance of brain monoamines. Microsc Res Tech 52:564–572
Rodriguez S, Rodriguez EM, Jara P, Peruzzo B, Oksche A (1990) Single injection into the cerebrospinal fluid of antibodies against the secretory material of the subcommissural organ reversibly blocks formation of Reissner’s fiber: immunocytochemical investigations in the rat. Exp Brain Res 81:113–124
Rodriguez S, Vio K, Wagner C, Barria M, Navarrete EH, Ramirez VD, Perez-Figares JM, Rodriguez EM (1999) Changes in the cerebrospinal-fluid monoamines in rats with an immunoneutralization of the subcommissural organ-Reissner’s fiber complex by maternal delivery of antibodies. Exp Brain Res 128:278–290
Rühle HJ (1971) Anomalien im Wachstum der Achsenorgane nach experimenteller Ausschaltung des Komplexes Subcommissuralorgan-Reissnerscher Faden. Untersuchungen am Rippenmolch (Pleurodeles waltii). Acta Zool 52:23–68
Sargent PE (1900) Reissner`s fibre in the canalis centralis of vertebrates. Anat Anz 17:33–44
Saunders NR (1992) Ontogenetic development of brain barrier mechanism. In: Bradbury MWB (ed) Handbook of experimental pharmacology. Springer, Berlin Heidelberg New York, pp 327–369
Saunders NR, Knott GW, Dziegielewska KM (2000) Barriers in the immature brain. Cell Mol Neurobiol 20:29–40
Schoebitz K, Garrido O, Heinrichs M, Speer L, Rodriguez EM (1986) Ontogenical development of the chick and duck subcommissural organ. An immunocytochemical study. Histochemistry 84:1–40
Schoebitz K, Rodriguez EM, Garrido O, del Brio MA (1993) Ontogenetic development of the subcommissural organ with reference to the flexural organ. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 41–49
Schoebitz K, Gonzalez C, Peruzzo B, Yulis CR, Rodriguez EM (2001) Organ culture of the bovine subcommissural organ: evidence for synthesis and release of the secretory material. Microsc Res Tech 52:496–509
Sterba G, Ermisch A, Freyer K, Hartmann G (1967) Incorporation of sulphur-35 into the subcommissural organ and Reissner’s fibre. Nature 216:504
Sternberger LA, Hardy PH, Jr., Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Vio K, Rodriguez S, Navarrete EH, Perez-Figares JM, Jimenez AJ, Rodriguez EM (2000) Hydrocephalus induced by immunological blockage of the subcommissural organ-Reissner’s fiber (RF) complex by maternal transfer of anti-RF antibodies. Exp Brain Res 135:41–52
Wenk CA, Thallmair M, Kartje GL, Schwab ME (1999) Increased corticofugal plasticity after unilateral cortical lesions combined with neutralization of the IN-1 antigen in adult rats. J Comp Neurol 410:143–157
Wingstrand KG (1953) Neurosecretion and antidiuretic activity in chick embryos with remarks on the subcommissural organ. Arkh Zool (Stockholm) 6:41–67
Winkelmann E (1960) Experimental studies on the regeneration of the spinal cord of Amblystoma mexicanum after extirpation of a small section. Z Mikrosk Anat Forsch 66:147–176
Wislocki GB, Roth WD (1958) Selective staining of the human subcommissural organ. Anat Rec 130:125–133
Yulis CR, Munoz RI (2001) Vertebrate floor plate transiently expresses a compound recognized by antisera raised against subcommissural organ secretion. Microsc Res Tech 52:608–614
Yulis CR, Mota MD, Andrades JA, Rodriguez S, Peruzzo B, Mancera JM, Ramirez P, Garrido M, Perez-Figarez JM, Fernandez-Llebrez P, Rodriguez EM (1998) Floor plate and the subcommissural organ are the source of secretory compounds of related nature: comparative immunocytochemical study. J Comp Neurol 392:19–34
Ziyadeh FN, Sharma K (1996) The use of neutralizing antibodies to demonstrate the role of transforming growth factor-beta and Amadori-glycated albumin as mediators of experimental diabetic kidney disease. Contrib Nephrol 118:188–194
Acknowledgements
The authors are grateful to Dr. de la Rosa (Centro de Investigaciones Biológicas, CSIC, Madrid, Spain) for providing rabbit polyclonal antibody against Ng-CAM, José Esteban Casares Mira and Pedro Jiménez Palomo for valuable technical assistance, David Navas Fernández for technical assistance in the confocal microscopy study and Gregorio Martín Caballero for technical assistance with transmission electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Hoyo-Becerra and M.D. López-Ávalos contributed equally to this study and should be considered as first authors.
C. Hoyo-Becerra was the recipient of a predoctoral fellowship (PFPI) from the Ministerio de Educacion y Cultura (Spain). This work was supported by grants from DGICYT (BFI2003-03348; Spain) and FIS (01/0948; Spain), FIS (01-0948, PI021517; Spain) and ISCIII (red CIEN, nodo Fundación Carlos Haya).
Rights and permissions
About this article
Cite this article
Hoyo-Becerra, C., López-Ávalos, M.D., Pérez, J. et al. Continuous delivery of a monoclonal antibody against Reissner’s fiber into CSF reveals CSF-soluble material immunorelated to the subcommissural organ in early chick embryos. Cell Tissue Res 326, 771–786 (2006). https://doi.org/10.1007/s00441-006-0231-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0231-3