Abstract
Immunocytochemistry with monoclonal antibodies was used to investigate the locations of muscarinic acetylcholine receptors (mAChR) and choline acetyltransferase (ChAT) in sections of the developing antennae of the moth Manduca sexta. The results were correlated with a previous morphological investigation in the developing antennae which allowed us to locate different cell types at various stages of development. Our findings indicated that the muscarinic cholinergic system was not restricted to the sensory neurons but was also present in glial and epidermal cells. By day 4–5 of adult development, immunoreactivity against both antibodies was present in the axons of the antennal nerve, and more intense labeling was present in sections from older pupae. At days 4–9, the cell bodies of the sensory neurons in the basal part of the epidermis were also intensely immunolabeled by the anti-mAChR antibody. In mature flagella, large numbers of cells, some with processes into hairs, were strongly labeled by both antibodies. Antennal glial cells were intensely immunolabeled with both antibodies by days 4–5, but in later stages, it was not possible to discriminate between glial and neural staining. At days 4–9, we observed a distinctly labeled layer of epidermal cells close to the developing cuticle. The expression of both ChAT and mAChRs by neurons in moth antennae may allow the regulation of excitability by endogenous ACh. Cholinergic communication between neurons and glia may be part of the system that guides axon elongation during development. The cholinergic system in the apical part of the developing epidermis could be involved in cuticle formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antennae of the sphinx moth Manduca sexta bear thousands of sensilla (Sanes and Hildebrand 1976a, 1976b; Lee and Strausfeld 1990). Most are chemosensory and are located in the long distal segment, or flagellum, that consists of ~80 annuli. The two basal segments (scape and pedicel) do not have chemosensory sensilla but have fields of mechanosensory hairs called Böhm’s bristles (Böhm 1911). The proprioceptive scolopidial Johnston’s organ is also located in the pedicel (Schneider 1964; Vande Berg 1971). In addition, each flagellar annulus houses mechano-, thermo-, and hygrosensory organs (Lee and Strausfeld 1990).
Adult M. sexta antennae develop during the pupal period that lasts for ~18 days. Insect antennal epidermal cells give rise to all of the various cell types that form the sensilla, including the sensory neurons, the glial cells surrounding the neurons, and the sheath cells that secrete the external processes such as hairs (Sanes and Hildebrand 1976a, 1976b). The sheath cells also secrete the cuticle and the receptor lymph space that surround the dendritic processes (McIver 1986; French 1988). Axons of the sensory neurons join the antennal nerve and extend their processes into the central nervous system. During development, bundles of axons become enwrapped with antennal glial cells that are initially only found in the external surface of the nerve trunk (Sanes and Hildebrand 1976b). These glial cells are believed to aid axonal elongation (Tucker and Tolbert 2003).
M. sexta antennal sensory neurons are believed to use acetylcholine (ACh) as their main transmitter. The antennae have been shown to synthesize and store ACh, and antennal extracts contain the ACh-synthesizing enzyme choline acetyltransferase (ChAT) and the ACh-degrading enzyme acetylcholine esterase (AChE) (Sanes and Hildebrand 1976c). In addition, the binding activity for α-bungarotoxin, an antagonist of nicotinic ACh receptors, has been detected in the antennal lobe (Hildebrand et al. 1979) indicating that ACh released from sensory afferent endings acts on nicotinic receptors. Cytochemical techniques have also shown strong AChE activity in antennal mechanosensory neurons (Stengl et al. 1990). ACh, ChAT, and AChE are present in the antennae from early pupal stages and increase exponentially to plateau levels within ~10 days after pupal ecdysis (Sanes and Hildebrand 1976c).
A recent study on cell cultures derived from the antennae of early stage M. sexta pupae has reported immunoreactivity against ChAT antibody in neurons in 1-day-old cultures (Torkkeli et al. 2005); in more mature cultures, ChAT labeling was not limited to the neurons, because a group of non-neural cells was also labeled by the same antibody. This study also provided evidence that cultured antennal neurons and non-neural cells had muscarinic ACh receptors (mAChR). Fluorescently labeled pirenzepine, an antagonist of mAChRs, bound to cultured cells, and these cells were also labeled by an antibody against mAChRs (Torkkeli et al. 2005). However, the identification of cultured non-neural cells is difficult, and it was not clear from the study of Torkkeli et al. (2005) whether the labeled non-neural cells were glial cells, some type of epidermal cell, or both. Here, we have performed an immunocytochemical study on M. sexta antennal sections at various stages of development to determine whether cells in the intact antennae also express ChAT and mAChR immunoreactivity, and in which cell types this labeling might be located.
Morphological aspects of the development of M. sexta antennae have previously been studied in detail from thin histological sections (Sanes and Hildebrand 1976a, 1976b). We have used information derived from these studies to locate immunoreactivity to antibody labeling against ChAT and mAChRs in sensory neurons, glial cells, and epidermal cells at different stages of development. To aid the identification of sensory neurons, we have also used specific methods to label these fibers in the antennal nerve and Johnston’s organ.
Materials and methods
Experimental animals
Manduca sexta (Lepidoptera: Sphingidae) were reared from eggs on an artificial diet at 25–28°C and 50%–60% humidity under a long-day photoperiod regimen (16 h light/8 h dark). When the larvae stopped feeding, the pupae were placed on wood chips and kept in the dark. Adult development began soon after the molt of the larvae to pupae and went through 18 stages corresponding roughly to days. Pupae of both sexes were staged as described by Sanes and Hildebrand (1976a). They were then chilled at 4°C for 10–15 min, and their antennae were removed according to a protocol approved by the Dalhousie University Committee of Laboratory Animals (03-001I).
Tissue preparation
All chemicals were purchased from Sigma (Oakville, ON, Canada) unless otherwise stated. The pupal cuticle from the dorsal side of the head was carefully removed with surgical scissors. The antennae were excised, placed into Manduca saline, cleaned, and cut into pieces of approximately four annuli each, which were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight at 4°C. The fixed tissue was rinsed three times in PBS, cryoprotected in 30% sucrose in PBS overnight at 4°C, and embedded in gelatin/albumin blocks (2.4 g gelatin, 15 g albumin in 50 ml distilled H2O). The blocks were fixed in 10% formaldehyde in PBS overnight at 4°C, washed three times in PBS followed by overnight incubation in 30% sucrose in PBS at 4°C. Blocks were mounted with Optimal Cutting Temperature (OCT) compound (Tissue-Tek, Sakura Finetek, Torrance, Calif.) on a freezing stage (BFS-3 MP; Physitemp, Clifton, N.J.) and frozen to −25 to −30°C. Frozen sections (20–30 μm thick) were cut on a sliding microtome (SM 2000R; Leica Microsystems, Vienna, Austria) and collected in tissue culture dishes on PBS.
Immunocytochemistry
Antennal sections were collected on Netwell mesh bottom inserts (Electron Microscopy Sciences, Hatfield, PA.) in multiwell tissue culture plates filled with PBS and stained by an immunoenzymatic technique. Three different primary antibodies were used. (1) The monoclonal antibody 4B1 against Drosophila ChAT (Takagawa and Salvaterra 1996) was initially obtained as a gift from Dr. Paul Salvaterra (Beckman Research Institute, Calif.) and used at a 1:250 dilution. Additional 4B1 antibody was obtained as a cell culture supernatant from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa Department of Biological Sciences (Iowa City, IA 52242) and used at a 1:20–1:50 dilution. This antibody was previously shown to be specific against Manduca antennal tissue in Western blot analysis in which a clear band was detected at the same 65-kDa molecular weight as for Drosophila head extracts (Torkkeli et al. 2005). (2) The monoclonal antibody against mAChR, M35 (Argene, Varilhes, France 10-217), which has been shown to recognize all known mAChR subtypes in a wide variety of species (Van der Zee and Luiten 1999), was used here at a 1:1,000 dilution. The specificity of the M35 antibody against Manduca antennal tissue was previously determined in Western blot analysis in which it labeled an 85-kDa band, which is the predicted molecular weight of cloned Drosophila muscarinic receptors (Torkkeli et al. 2005). (3) Mechanosensory-preferring monoclonal antibody (MPA), which is thought to label neurofilaments in M. sexta mechanosensory neurons (Hishinuma et al. 1988), was a gift from John G. Hildebrand and Lynne A. Oland (Arizona Research Laboratories, Tucson, Ariz.) and was used in a 1:100 dilution.
Sections were incubated for 45 min in 0.1% Triton X-100 in PBS followed by a 30-min incubation in 0.2% H2O2. In the 4B1 antibody protocol, a dehydration followed by a re-hydration step in an ethanol series was applied to improve antibody penetration. All sections were incubated for 2 h at room temperature in blocking solution (3% goat serum, 0.5% Triton X-100, 3% skimmed milk powder in PBS) followed by overnight incubation in the primary antibody in blocking solution at 4°C. Sections were incubated in a biotinylated goat-anti-mouse secondary antibody (BA-9200; Vector Laboratories, Burlingame, Calif.,) in blocking solution for 1 h at room temperature followed by incubation in Vectastain ABC-KIT (PK-6100; Vector laboratories) according to manufacturer’s instructions. The antibody labeling was visualized by using 0.06% 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma D5637) as the chromogen, intensified with 0.3% NiCl2 and 0.003% H2O2. The labeling was followed under a stereo microscope, and the reaction was stopped by a wash with PBS as soon as the dark deposit appeared. Control sections were prepared by using the same protocol, but omitting the primary antibody or replacing it with mouse ascites fluid.
Stained sections were collected on gelatin-coated microscope slides, dehydrated in an ethanol series and cleared with xylene. Coverslips were mounted with Entellan (Electron Microscopy Sciences). The sections were observed with a compound microscope (Olympus BH2; Carson Group, Markham, ON, Canada) or with an Axiovert 100 inverted microscope (Carl Zeiss, Oberkochen, Germany) under bright field optics. Photographs were taken with an Axiocam digital camera (Carl Zeiss). Final images were processed in Adobe Photoshop 7.
AChE staining
The direct method of Karnovsky and Roots (1964) was used for AChE staining of frozen antennal sections. Sections were washed in 0.1% Triton X-100 in maleic buffer three times, incubated in 0.1% hydrogen peroxide (H2O2) in PBS for 30 min, and washed in PBS followed by a 30-min incubation in reaction solution (100 μg/ml acetylthiocholine iodide, 100 μM sodium citrate, 80 μM CuSO4, 10 μM K3Fe(CN)6, in maleate buffer with 0.1% Triton X-100). Sections were then rinsed with 50 mM TRIS-HCl buffer, and staining was intensified by the technique of Tago et al. (1986). Sections were incubated in a solution containing 0.04% DAB, 0.3% nickel ammonium sulphate in 50 mM TRIS HCl, and 0.003% H2O2. Staining was stopped by washes with 50 mM TRIS HCl, and the sections were collected on gelatin-coated slides and dehydrated and embedded as described above.
Results
Frozen antennal sections were prepared from developing M. sexta antennae from day 4 to day 17 after pupation. To determine whether the antennal neurons and/or other cell types expressed ChAT immunoreactivity, the sections were immmunolabeled with 4B1 antibody (Takagawa and Salvaterra 1996). To locate mAChRs on antennal sections, we used M35 antibody. Mechanosensory neurons were identified by using MPA antibody, which detects neurofilaments in these neurons. In addition, AChE histochemistry was performed on mature antennal sections to clarify further the way in which the mechanosensory neurons were arranged in the antennal sections. Results from these different labeling methods were divided into three age groups: days 4–5, 7–9, and 15–17 after pupation (collected in Table 1). To correlate our results with morphological data from developing M. sexta antennae, schematic illustrations were drawn based on our longitudinal histological sections of the antennae. In addition, schematic illustrations of the cross sections of developing antennal nerve and flagellar epidermis, including the male-specific long sensilla trichodea, were drawn based on images from previously published thin (0.5–1 μm) sections of the antennae (Sanes and Hildebrand 1976a).
Days 4 and 5; 20%–30% of adult development
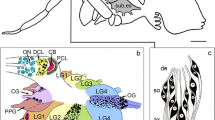
The M. sexta antenna retracts from the cuticle by the beginning of the fourth day of pupal development (Sanes and Hildebrand 1976a). At this stage, antennal sensilla have started to differentiate, and axons of the sensory neurons join the antennal nerve and extend toward the brain (Sanes and Hildebrand 1976b). At day 4–5, the flagella of both male and female M. sexta are nearly cylindrical in form, and annulation starts (Fig. 1). In the flagellar epidermis, clusters of cells that form the sensilla have developed and are surrounded by smaller epidermal cells. By day 5, the clusters span the entire epidermis, with the neurons being located in the most basal part and ensheathed by glial cells (Sanes and Hildebrand 1976b). Two nerve trunks are present in each antenna and contain about 300 axons immediately after pupation; this number increases to the final count of ~260,000 by 7–9 days after pupal ecdysis. At days 4–5, glial cells are only present in the external surface of the nerve (Fig. 1; Sanes and Hildebrand 1976a, 1976b).
Representation of the antenna of a 5-day-old male M. sexta pupa. A longitudinal section (basal antenna facing down) is shown right. One of the two antennal nerves is visible. A cross section of the nerve trunk (middle) shows the glial cells (Gl) that are confined to the external surface. The nerve axons (N) fill the central area. The antennal sensilla arise from the epidermis (cross section left, apical surface up), and clusters of cells that will form each sensillum can be distinguished from the surrounding epidermal cells. The most basal cells in each cluster are neurons (Ne) that are ensheathed by glial cells (Gl). Axons of the sensory neurons join the antennal nerve. The cross sections are based on images in Sanes and Hildebrand (1976b). Boxed areas in the longitudinal section indicate the locations of the histological sections presented in Fig. 2
MPA immunolabeling of antennal sections at days 4–5 produced dark deposits in the central part of the antennal nerve (Fig. 2a), but no labeling was detected in the external surface of the nerve or in any other parts of the antennae. Since this antibody preferentially recognizes mechanosensory neurons, the external non-labeled areas of the nerve may possess chemosensory axons. The glial cell layer in the external surface of the nerve was not visible with this antibody.
Immunolabeling of antennal sections from 4- and 5-day-old pupae. Locations of the sections are indicated in Fig. 1. a MPA immunoreactivity was intense in the central part of the antennal nerve (asterisk) by day 4. The external surface of the nerve trunk was not labeled (black arrows), and no labeling was seen in the epidermal layer of the flagellar annuli. b–d M35 immunoreactivity was strong in the outermost (gray arrow in b, white arrows in d) and innermost (white arrow in b) layers of the epidermis, and labeling was also present in the nerve (asterisks in b, c). Nerve labeling was intense on the external surface (black arrows in b) but, in some sections, it was also present more centrally (black arrow in c). Granular tissue between the nerve and epidermis was not labeled with M35 antibody, but strongly labeled areas of the nerve and epidermis surrounded this tissue. Small (2–5 μm in diameter) M35-immunoreactive cells in the apical part of the epidermis (white arrows in d) were especially dense in the intersegmental areas of the antennae. e–h In 5-day-old pupae, 4B1 immunoreactivity was seen in the apical part of the epidermis (white arrows in e, g, h). Faint 4B1 labeling was detectable in the antennal nerve (asterisks in e, f, h), but stronger labeling was concentrated in its external surface (black arrows in e). Dense immunoreactivity was also present in some cells in central areas of the nerve (black arrow in f). The granular tissue between the antennal nerve and epidermis was not labeled with 4B1 (black arrow in h), and there was no labeling in the basal epidermis. Bars 20 μm
M35 labeling was intense in the external surface of the antennal nerve (Fig. 2b), being especially intense close to the basal epidermis. Patches of labeling were also present in central areas of the nerve in some sections (Fig. 2c). In addition, the basal part of the flagellar epidermis was densely labeled (Fig. 2b). Since the cell bodies of sensory neurons were located in the basal epidermis at this stage of development and their axons ran into the antennal nerve, the M35 labeling was probably in the sensory neurons. However, the neurons and the nerve were ensheathed by glial cells (Fig. 1), making it possible that the glial cells were also labeled. M35 immunoreactivity was also strong in the apical parts of the epidermis (Fig. 2b, d) on all sides of the antennae. Immunolabeling was distinct in the intersegmental areas (Fig. 2d) in which small cell bodies (diameter: 2–5 μm) were clearly identifiable. Based on the location of these cells in the apical epidermis and especially in antennal areas not housing many sensilla, such as the intersegmental area, these cells could not be considered as neurons or other components of sensilla but must have been other types of epidermal cells.
The antennae of 4 to 5-day-old male and female M. sexta had fine 4B1-immunopositive fibers in the antennal nerve (Fig. 2e). The perimeter of the nerve was more strongly labeled, and 4B1-labeled cells were also present in the central part of some sections of the antennae (Fig. 2e, f). These cells were probably glial cells, since they often had a nucleus (e.g., arrow in Fig. 2f), and since glial cells are the only nucleated cells shown to be present in antennal nerve trunks (Sanes and Hildebrand 1976b). No clear 4B1 labeling was observed in the basal region of the epidermis, suggesting that cell bodies of sensory neurons were not labeled. However, the apical part of the antennal epidermis contained distinctly labeled, small cells, similar in appearance to those labeled with the M35 antibody (Fig. 2e, g, h). These labeled cells were present in the intersegmental areas, in all annuli, and in pupae of both sexes, suggesting that these putative cholinergic cells were not part of sensilla but were other types of epidermal cells.
In longitudinal sections, a chain of tissue with a granular structure was often present between the basal epidermis and the nerve (Fig. 2b, h). The nature of this tissue was not clear, but it was not labeled with any of the antibodies that we used here and was not present after day 6 of pupal development. This tissue may form the perineural sheath and/or neural lamella that surround the mature antennal nerve (Sanes and Hildebrand 1976b).
Days 7–9; 40%–50 % of adult development
By day 7, the annulation of antenna is complete, and its three parts can be clearly identified (Fig. 3). The scape is the most basal part, which eventually bears the muscles that move the antenna (Kloppenburg et al. 1997). However, at days 7–9, only small areas of developing muscles are seen next to, but not connected with, the thick epidermis that covers the scape walls. Two fields of mechanosensory Böhm’s bristles also develop into the basal region of the scape but are not clearly identifiable at this stage. The pedicel houses the mechanosensory Johnston’s organ that arises from the epidermis surrounding the antennal nerve into which its axons join. Longitudinal sections from the basal antennae at days 7–9 show parts of the Johnston’s organ on either side of the nerve (Fig. 3) or on top of the nerve. By day 8, the trichogen cells in each sensilla have pierced the apical margin and formed the setae surrounding the dendrites of the sensory neurons. Cell T2 appears only transiently in the sensilla partially surrounding the trichogen cell and participating in the formation of the setae. A tormogen cell encircles the apical part of the trichogen and the T2 cell. The cell bodies of the sensory neurons are located beneath the trichogen cell and are ensheathed by glial cells. The glial cells in the antennal nerve now extend from the periphery toward the center of the nerve.
Representation of the basal part of an antenna of 8-day-old male pupa. A longitudinal section through the scape, pedicel, and basal flagellum is shown below. One of the two antennal nerves (N) is visible, and a cross section through the nerve (above left) shows that the glial cells (Gl) extend from the external surface toward the center of the nerve. The developing Johnston’s organ (Jo) extends from the epidermis of the pedicel toward the antennal nerve. A cross section through the flagellar epidermis (above right) shows two groups of cells forming the sensilla trichodea (apical side up). The sensory neurons (Ne) are located beneath large trichogen cells (Tr) and ensheathed by glial cells (Gl). T2 and tormogen cells (To) surround the trichogen cells. The axons extend into the antennal nerve and toward the brain, and the dendrites run to the setae (Se) forming processes of trichogen cells. The cross sections are based on histological sections in Sanes and Hildebrand 1976b. The locations of the sections in Fig. 4 are indicated by boxed areas
At days 7–9, the antennal nerve expressed strong immunoreactivity to the MPA antibody (Fig. 4a), but no labeling was seen in any of the flagellar annuli. However, the Johnston’s organ had distinctly labeled areas in its central part (Fig. 4b) in which most of the mechanosensory neuron cell bodies were located (Vande Berg 1971). M35 labeling was also intense in the antennal nerve (Fig. 4c), and the labeling in Johnston’s organ was more extensive than with the MPA antibody, especially in its most apical and basal regions (Fig. 4d), suggesting that other components than the mechanosensory neurons express mAChRs. The 4B1 antibody produced uniform labeling in the antennal nerve (Fig. 4e). Labeling was also present in the cell bodies and axons of the developing Johnston’s organ, and some labeling was present in the distal region (Fig. 4f).
Immunostaining of antennal sections of 7 to 9-day-old pupae. Locations of the sections are indicated in Fig. 3. The antennal nerve (asterisk in a) expressed strong MPA immunoreactivity, and some labeling was also present in Johnston’s organ (b), but not in its distal dendritic region (white arrow in b). No MPA immunoreactivity was detected in the epidermal layer of any of the flagellar annuli. The antennal nerve was also strongly labeled with M35 and 4B1 antibodies (asterisks in c, e, respectively). Immunoreactivity against M35 and 4B1 was also clearly present in Johnston’s organ (d, f, respectively) including the distal region (white arrows in d, f). 4B1 immunoreactive fine fibers could be seen in the axons extending into the antennal nerve (black arrow in f). M35 labeling in the flagellar annuli was most intense in cells located in the apical (black arrow in g) and basal (white arrow in g) regions of the epidermis. 4B1 immunoreactivity was mainly found in the apical part of the epidermis (black arrow in h), whereas the basal part of the epidermis was not labeled (white arrow in h). However, strong 4B1 immunoreactivity was present between two flagellar annuli (gray arrow in h). The epidermis in the scape was thick at days 7–8, and a chain of cells that were clearly labeled with both M35 and 4B1 was present (black arrows in i, j). M35 immunoreactivity was also present in the most basal part of the scape epidermis (white arrow in i), but faint 4B1 labeling was present in this region (white arrow in j). A layer of small cells intensely labeled with M35 and 4B1 (white arrows in k, l, respectively) was present in the intersegmental area of the flagella. Bars 20 μm
In the epidermis of each flagellar annulus, M35 labeling was present in two locations: apically, close to the sites of setae, and basally, in the area in which the sensory neuron cell bodies and glial cells are located (Fig. 4g). M35-immunoreactive fibers were also present between these layers. 4B1 immunoreactivity in the epidermis was restricted to the apical margin and the sides of each annulus (Fig. 4h). In addition, strong labeling was present close to the cuticle between each annulus (gray arrow in Fig. 4h). At day 7, a chain of cells labeled with M35 (Fig. 4i) and 4B1 (Fig. 4j) was present in the thick epidermis lining the scape. The basal part of the epidermis here was also strongly stained with the M35 antibody, but only a thin line could be seen with the 4B1 antibody. At days 8 and 9, during intersegment formation (Sanes and Hildebrand 1976a), distinct M35 and 4B1 labeling were present in the buckling intersegmental areas of the flagella (Fig. 4k, l)
Days 15–17; 85%–95 % of adult development
A longitudinal section of the mature basal antenna is shown schematically in Fig. 5. The antennal muscles are now located in the scape, which also houses fields of mechanosensory Böhm’s bristles. The cuticle over the epidermis has thickened from ~0.5 μm at day 11 to ~20 μm by day 16 (Sanes and Hildebrand 1976a). In the sensillum trichodea, the trichogen cell has retracted from the growing seta, leaving it hollow. The neurons are now located close to the cuticle just beneath the base of the setae with their dendrites running into the setae (Sanes and Hildebrand 1976b). The neurons are surrounded by glial and trichogen cells. The axons run into the antennal nerve, which is now surrounded by the perineural sheath and neural lamella. Groups of axons in the nerve are segregated into pockets by glial processes (Sanes and Hildebrand 1976b).
Representation of antennal sections of male M. sexta 16 days after pupation. A longitudinal section through the basal antenna (below) shows the scape, pedicel, and basal part of the flagellum. One of the antennal nerves (N) is visible in this section. The muscles (M) that move the antenna and two fields of mechanosensory Böhm’s bristles (B) are located in the scape. Parts of the Johnston’s organ (Jo) that surrounds the nerve in the pedicel are visible. A cross section through the epidermis of a flagellar annulus (above) shows one sensillum trichodeum. The large trichogen cell (Tr) is surrounded by a tormogen cell (To). The two neurons (Ne) have migrated to the apical margin of the epidermis and are ensheathed by glial cells (Gl). The cross section is based on histological images in Sanes and Hildebrand 1976b. Boxed areas indicate the locations of the sections presented in Fig. 6
To clarify the location of the sensory neurons in the Johnston’s organ and to show the mechanosensory axons in the antennal nerve, we performed AChE staining of mature antennae in addition to the MPA labeling that was more effective in showing the axons than the cell bodies. Similar AChE staining has previously been performed by Stengl et al. (1990). Figure 6a shows AChE staining in Johnston’s organ with the darkly stained cell bodies concentrated at the center. The bundle of axons (facing upward in Fig. 6a) was also intensely stained, and labeling was also present in the fine dendritic fibers (white arrow in Fig. 6a). AChE also stained many of the axons in the antennal nerve (Fig. 6b). Antennal nerve fibers and Johnston’s organ were immunoreactive to MPA (Fig. 6c). In Johnston’s organ, MPA immunolabeling was concentrated in the same area in which AChE labeling was present, indicating the location of the cell bodies of the sensory neurons. Only the membranes of the cell bodies were immunoreactive to MPA. Similar areas were also labeled with the M35 antibody (Fig. 6d), which also labeled other parts of the organ, including those associated with the dendritic region. 4B1 immunoreactivity was also present in the Johnston’s organ, being most clearly visible in the cell bodies and axons (Fig. 6e). The antennal nerve was strongly labeled with both M35 and 4B1 antibodies (asterisks in Fig. 6d, e, respectively).
Immunolabeling of the antenna of male pupae 15–17 days after ecdysis. Locations of the sections are indicated in Fig. 5. a AChE activity in the Johnston’s organ (black arrow) was found in darkly stained cell bodies and axons. The thin dendrites of the sensory neurons were also stained (white arrow). b The antennal nerve (asterisk) had distinctly labeled fibers. c Anti-MPA immunoreactivity in the Johnston’s organ (black arrow) surrounded the cell bodies and extended to the axons. Clear MPA labeling was also present in fibers in the antennal nerve (asterisk). d M35 immunoreactivity in the Johnston’s organ (black arrow) was extensive, and the antennal nerve (asterisk) was also strongly labeled. e 4B1 immunoreactivity was also present in cell bodies and axons of the Johnston’s organ (arrow), and the antennal nerve was clearly labeled (asterisk). f–i M35-immunoreactive cells were present in the flagellar annuli of both female (f, g) and male (h, i) pupae. Large numbers of darkly labeled cell bodies (4–10 μm in diameter; black arrows in f–h), with or without processes, were visible in each annulus. In sections in which the hairs were visible, cells with processes lie close to the base of the hair (black arrows in g, h). The processes extended to an intensely labeled area close to the basal part of the epidermis (white arrow in g). j–m The 4B1 antibody also produced clear immunoreactivity in the flagellar annuli of both female (j) and male (k–m) antennae. Groups of cells in the apical part of the epidermis closely associated with the hairs were labeled (black arrows in j–m). Darkly labeled areas were also present in the basal part of the epidermis (white arrows in k, m). A few large cells were unstained (asterisk in j). Bars 20 μm
Longitudinal sections from different levels of flagellar annuli showed large numbers of cells that were intensely labeled with M35 (Fig.6f–i) and 4B1 (Fig.6j–m) in both male and female pupae. M35-immunoreactive cells could be seen close to the base of the setae with thin processes extending toward the basement membrane (Fig. 6g) and into the setae (Fig. 6h), suggesting that the sensory neurons were labeled. Similarly, 4B1 immunoreactivity was present in cells with processes into the setae (Fig.6k, l) and into the basal part of the epidermis (Fig. 6m). 4B1- or M35-immunoreactive cells that could be distinguished in these sections had a diameter of 4–10 μm, corresponding to the sizes of sensory neurons. Some larger nonlabeled cells were also visible (e.g., Fig.6j, k). Although the sexual dimorphism of the flagellum and absence of the long sensilla trichodea in female antennae were obvious in mature antennae, the pattern of labeling showed no differences. Most or all of the thousands of sensory neurons that occupied each annulus were immunoreactive to both M35 and 4B1 in the mature antennae.
Discussion
ChAT and mAChRs in the sensory neurons
Sanes and Hildebrand (1976b) have shown that, at days 4–10 after pupal ecdysis, the sensory neurons innervating M. sexta antennal hairs are located beneath the trichogen cells close to the basement membrane of the epidermis. They migrate apically and, by day 14, reside in their final locations beneath the setae under the cuticle. Although this information is based on research in the large male-specific sensilla trichodea, all cuticular sensilla derived from epidermal cells have similar cellular components (McIver 1986; French 1988; Keil 1997) and are likely to follow similar patterns of development.
The frozen antennal sections used here for immunocytochemistry were relatively thick (20–30 μm) and did not allow the identification of individual neurons. However, M35 immunoreactivity was intense in the basal parts of the epidermis at days 4–5 and 7–9, and large numbers of immunoreactive cell bodies with processes were found close to the cuticle in each flagellar annulus in the mature antenna. In addition, M35 labeling was present in the antennal nerve at day 4, and its intensity increased greatly by day 7. The Johnston’s organ was also intensely labeled as soon as it was identifiable. These findings indicate that the antennal sensory neurons express mAChRs and are in agreement with results from antennal cultures in which intense labeling with the M35 antibody has also been detected in sensory neurons (Torkkeli et al. 2005).
Immunoreactivity against 4B1 antibody was noticeable in the nerve trunk by day 4 of adult development. However, we detected no labeling at the basal part of the epidermis at days 4–5 and 7–9 indicating that the cell bodies of the sensory neurons were not stained. The Johnston’s organ exhibited clear immunoreactivity by day 7, but the labeled cells could not be identified. In the mature Johnston’s organ, the cell bodies of the mechanosensory neurons were labeled with 4B1 antibody. In addition, in the flagellar annuli of mature antennae, 4B1 immunoreactivity was found on cells close to the cuticle, and their darkly labeled processes could be seen extending into the antennal hairs. Previous immunocytochemical studies in Drosophila found ChAT immunoreactivity in only a small number of cell bodies, even though the neuropil region was clearly labeled (Gorczyca and Hall 1987; Yasuyama et al. 1995); other methods have indicated that all Drosophila antennal sensory neurons are cholinergic (reviewed by Yasuyama and Salvaterra 1999). Hence, the 4B1 labeling in M. sexta antennal nerve alone suggests that the sensory neurons are cholinergic as previously indicated (Sanes and Hildebrand 1976c) and may be able to produce ACh 4–5 days after pupal ecdysis. Dense labeling with the same antibody was also found in cultured M. sexta antennal neurons in 1-day-old cultures (Torkkeli et al. 2005).
These results provide evidence to support the hypothesis that the antennal sensory neurons of M. sexta are cholinergic, as previously suggested (Sanes and Hildebrand 1976c; Stengl et al. 1990). Similar findings have been obtained in other arthropod mechano- and chemoreceptor systems (e.g., Trimmer and Weeks 1989; Burrows 1996). Muscarinic activity has not been demonstrated in mature M. sexta chemo- or mechanoreceptor neurons, but excitability of larval proleg motoneurons has been shown to be regulated by muscarinic agonists; these motoneurons are directly innervated by mechanosensory afferents (Trimmer and Weeks 1989). This type of effect could be achieved by muscarinic receptors on the mechanosensory neurons or on inhibitory neurons presynaptic to them (Trimmer 1995). Our findings indicate that the mechanosensory neurons themselves have mAChRs suggesting that their excitability is regulated by ACh, possibly released from the same neurons.
ChAT and mAChRs in the antennal glial cells
Cell bodies of antennal sensory neurons are enwrapped by glial cells (Sanes and Hildebrand 1976b). Glial cells are also present in the antennal nerve in which they are initially confined to the external surface before growing toward the center and eventually surrounding bundles of axons (Sanes and Hildebrand 1976b). The functions of insect antennal glial cells are not well known, but these cells are believed to aid in axon elongation and to provide clues for pathfinding (Yonoussi-Hartenstein and Hartenstein 1993; Tucker and Tolbert 2003). The results here indicate that the antennal glial cells are able to synthesize ACh and that they have mAChRs. This is most clearly seen in the sections from 4 to 5-day-old pupae in which the external surface of the antennal nerve trunk is intensely stained with both antibodies. This region was not labeled with the MPA antibody, which preferably labeled mechanosensory neurons, presumably their neurofilaments (Hishinuma et al. 1988). We were not able to distinguish the nerve axons and glia in sections from older pupae in which the whole nerve was intensely labeled with both antibodies. Therefore, it is not clear whether the glial staining is a transient phenomenon or whether it also exists in mature antennae.
In some sections at days 4–5, intense immunoreactivity against both antibodies was found in more centrally located cells in the nerve trunk. Glial cells are the only cells in the nerve that have nuclei (Sanes and Hildebrand 1976b), and in some of the 4B1-labeled cells, a nucleus was visible. M35 labeling was more intense and widespread, and we did not detect associated nuclei. The M35-immunoreactive structures are possibly clusters of axons, or glial cells, or both.
Previously, 4B1 labeling has been found in M. sexta antennal cultures in lamellipodia-forming non-neural cells that create scaffold-like structures in older cultures (Torkkeli et al. 2005). These are morphologically similar cells to those identified as glial cells in antennal cultures by Tucker and Tolbert (2003). They also express M35 immunoreactivity, but only in the area around nuclei (Torkkeli et al. 2005), suggesting that, although the protein may have been present, it was not transported along the cell. Since cultured cells do not retain normal cellular interactions, it is possible that the cellular clues for mAChR location were missing in these cells.
These results suggest that, like the antennal sensory neurons, glial cells can synthesize ACh, and that they have mAChRs. Cholinergic communication between the neurons and glia may be part of the system that is used to guide the axons toward their target tissues in the central nervous system.
ChAT and mAChRs in epidermal cells
Small cells lining the apical portion of the epidermis were distinctly labeled with 4B1 and M35 antibodies at days 4–5 and 7–9 of pupal development. It was not clear whether the same cells were labeled with both antibodies, or whether they were different cells in close proximity to each other. Since both of the antibodies that were used here were monoclonal antibodies raised in the mouse, we could not perform double-labeling experiments. Immunoreactivity was present in all of the flagellar annuli, in the developing intersegmental area, in the pedicel, and in the scape. The labeled cells could not be neurons, since they were located in areas in which neurons are not present (Sanes and Hildebrand 1976b). These cells were also unlikely to be non-neural components of the sensilla, since they were found in areas containing few or no sensilla. The only common denominator was that they were located close to the developing cuticle (Sanes and Hildebrand 1976a). Filopodia- and lamellipodia-forming cells resembling cultured fibroblasts were intensely labeled with M35 but not by 4B1 antibody in antennal cultures (Torkkeli et al. 2005). These might have been the same cells that were labeled here in the apical epidermis with both antibodies, suggesting that their ability to synthesize ACh in cultures was lost.
Interestingly, several types of mAChRs are also expressed in human skin epidermal keratinocytes (Grando 1997) where they have functions in cell migration, adhesion, and differentiation that may aid in wound healing (Chernyavsky et al. 2004). Keratinocytes have also been demonstrated to possess enzymes for ACh synthesis and degradation (Grando et al. 1993). The current investigation suggests that an endogenous cholinergic muscarinic system is also present in developing insect antennal epidermis. This system does not seem to be connected to sensillar development but is more likely to be associated with cuticular development.
References
Böhm LK (1911) Die antennalen Sinnesorgane der Lepidopteren. Arb Zool Inst Wien 14:219–246
Burrows M (1996) The neurobiology of an insect brain. Oxford University Press, Oxford
Chernyavsky AI, Arredondo J, Wess J, Karlsson E, Grando SA (2004) Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J Cell Biol 166:261–272
French AS (1988) Transduction mechanisms of mechanosensilla. Annu Rev Entomol 33:39–58
Gorczyca MG, Hall JC (1987) Immunohistochemical localization of choline acetyltransferase during development and in ChaTS mutants of Drosophila melanogaster. J Neurosci 7:1361–1369
Grando SA (1997) Biological functions of keratinocyte cholinergic receptors. J Invest Dermatol Symp Proc 2:41–48
Grando SA, Kist DA, Qi M, Dahl MV (1993) Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol 101:32–36
Hildebrand JG, Hall LM, Osmond BC (1979) Distribution of binding sites for 125I-labeled α-bungarotoxin in normal and deafferented antennal lobes of Manduca sexta. Proc Natl Acad Sci USA 76:499–503
Hishinuma A, Hockfield S, McKay R, Hildebrand JG (1988) Monoclonal antibodies reveal cell-type-specific antigens in the sexually dimorphic olfactory system of Manduca sexta. II. Expression of antigens during postembryonic development. J Neurosci 8:308–315
Karnovsky MJ, Roots L (1964) A “direct-coloring” thiocholine method for cholinesterases. J Histochem Cytochem 12:219–221
Keil TA (1997) Comparative morphogenesis of sensilla: a review. Int J Insect Morphol Embryol 26:151–160
Kloppenburg P, Camazine SM, Sun XJ, Randolph P, Hildebrand JG (1997) Organization of the antennal motor system in the sphinx moth Manduca sexta. Cell Tissue Res 287:425–433
Lee JK, Strausfeld NJ (1990) Structure, distribution and number of surface sensilla and their receptor cells on the olfactory appendages of the male moth Manduca sexta. J Neurocytol 19:519–538
McIver SB (1986) Mechanoreception. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. Pergamon, Oxford, pp 71–132
Sanes JR, Hildebrand JG (1976a) Structure and development of antennae in a moth Manduca sexta. Dev Biol 51:282–299
Sanes JR, Hildebrand JG (1976b) Origin and morphogenesis of sensory neurons in an insect antenna. Dev Biol 51:300–319
Sanes JR, Hildebrand JG (1976c) Acetylcholine and its metabolic enzymes in developing antennae of the moth, Manduca sexta. Dev Biol 52:105–120
Schneider D (1964) Insect antennae. Annu Rev Entomol 9:103–122
Stengl M, Homberg U, Hildebrand JG (1990) Acetylcholinesterase activity in antennal receptor neurons of the sphinx moth Manduca sexta. Cell Tissue Res 262:245–252
Tago H, Kimura H, Maeda T (1986) Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by sensitive histochemical procedure. J Histochem Cytochem 34:1431–1438
Takagawa K, Salvaterra P (1996) Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci Res 24:237–243
Torkkeli PH, Widmer A, Meisner S (2005) Expression of muscarinic acetylcholine receptors and choline acetyltransferase enzyme in cultured antennal sensory neurons and non-neural cells of the developing moth Manduca sexta. J Neurobiol 62:316–329
Trimmer BA (1995) Current excitement from insect muscarinic receptors. Trends Neurosci 18:104–111
Trimmer BA, Weeks JC (1989) Effects of nicotinic and muscarinic agents on an identified motoneurone and its direct afferent inputs in larval Manduca sexta. J Exp Biol 144:303–337
Tucker ES, Tolbert LP (2003) Reciprocal interactions between olfactory receptor axons and olfactory nerve glia cultured from the developing moth Manduca sexta. Dev Biol 260:9–30
Vande Berg JS (1971) Fine structural studies of Johnston’s organ of the tobacco hornworm moth, Manduca sexta (Johansson). Morphology 133:439–456
Van der Zee EA, Luiten PG (1999) Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: a review of immunocytochemical localization in relation to learning and memory. Prog Neurobiol 58:409–471
Yasuyama K, Salvaterra PM (1999) Localization of choline acetyltransferase-expressing neurons in Drosophila nervous system. Microsc Res Tech 45:65–79
Yasuyama K, Kitamoto T, Salvaterra PM (1995) Localization of choline acetyltransferase-expressing neurons in the larval visual system of Drosophila melanogaster. Cell Tissue Res 282:193–202
Younossi-Hartenstein A, Hartenstein V (1993) The role of the tracheae and musculature during pathfinding of Drosophila embryonic sensory axons. Dev Biol 158:430–447
Acknowledgements
We thank Andrew S. French for critical reading of the manuscript. The original monoclonal antibody against ChAT (4B1) was a donation from Dr. Paul Salvaterra (Beckman Research Institute of the City of Hope, Calif.) and the monoclonal antibody against MPA was a gift from Drs. John G. Hildebrand and Lynne A. Oland (Arizona Research Laboratories, Tucson, Ariz.). We are grateful to Monika Stengl, Harjit Seyan, and Ian A. Meinertzhagen for advice regarding the initiation of the histological procedures.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Foundation for Innovation, and the Nova Scotia Research and Innovation Trust to P.H.T. and a NSERC postdoctoral fellowship to J.C.
Rights and permissions
About this article
Cite this article
Clark, J., Meisner, S. & Torkkeli, P.H. Immunocytochemical localization of choline acetyltransferase and muscarinic ACh receptors in the antenna during development of the sphinx moth Manduca sexta. Cell Tissue Res 320, 163–173 (2005). https://doi.org/10.1007/s00441-004-1039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1039-7