Abstract
A detailed account is given by the octopaminergic innervation of the antennal heart in Schistocerca gregaria using various immunohistochemical methods. Anterograde axonal filling illustrates the unilateral innervation on the medial ventral surface of the pumping muscle of the antennal heart via the paired corpora cardiaca nerve III. In addition, antibody staining revealed that ascending axons of this nerve terminate at the ampullae of the antennal heart forming synaptoid structures and extensive neurohaemal release sites. Due to the innervation by two dorsal unpaired median neurons, the presence of the biogenic amines octopamine and tyramine could be visualized by immunocytochemistry in an insect antennal heart for the first time. The data suggest that tyramine acts as a precursor and not purely as an independent transmitter. While the octopaminergic fibers innervating the pumping muscle of the antennal heart indicate a cardioregulatory role, we conclude that octopamine released from the neurohaemal area is pumped into the antennae and an involvement in the modulation of the antennal sensory sensitivity is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects possess accessory pulsatile organs to facilitate haemolymph circulation through long body appendages (reviews: Pass 1998, 2000; Pass et al. 2006; Wirkner et al. 2013). These auxiliary hearts are autonomous pumps, which work independently from the dorsal vessel, the primary pumping organ of the insect circulatory system. Accessory pulsatile organs, which supply haemolymph to the antennae, were initially discovered in cockroaches by Pawlowa (1895) and later described for numerous other insects (review: Pass et al. 2006). The most detailed analysis of the functional anatomy and physiology of an antennal heart is that for the cockroach, Periplaneta americana (Hertel et al. 1985, 1988, 2012; Pass 1985; Pass et al. 1988a, b; Hertel and Penzlin 1992; Richter and Hertel 1997).
Likewise, antennal heart innervation was first investigated in detail in P. americana (Pass et al. 1988a). Retrograde cobalt fills of the antennal heart nerve showed that the somata of the innervating neurons are located in the suboesophageal ganglion (SOG). These neurons include dorsal unpaired median (DUM) neurons and a pair of contralateral neurons whose axons run together via an anterior branch of the paired nervus corporis cardiaci III (NCC III) into the corpora cardiaca and from there to the antennal heart. Numerous peripheral branches of this nerve ramify at the surface of the pumping muscle, and a large number of axon terminals are found within the ampulla wall. Transmission electron microscopy revealed that these terminals contain neurosecretory granules indicating that the antennal heart has a neurohaemal function, in addition to that of a circulatory motor (Beattie 1976; Pass et al. 1988a). Studies in imagines of Locusta migratoria (Bräunig 1990) revealed almost the same innervation pattern of the antennal heart as in the cockroach. It is innervated by a branch of the NCCIII which contains the axons of two DUM neurons and a pair of contralateral motor neurons (CN3) located in the suboesophageal ganglion.
DUM neurons are present in insects in all segmental ganglia of the ventral nerve cord (reviews: Stevenson and Spörhase-Eichmann 1995; Bräunig and Pflüger 2001). They possess somata in the dorsal area of these ganglia in a medial position and have bifurcating axons projecting bilaterally to the periphery (Duch et al. 1999; Watson 1984). Earlier biochemical or immunohistochemical studies suggest that all DUM neurons contain octopamine (OA; Crossman et al. 1971; Duch et al. 1999; Evans 1985; Hoyle 1975; Orchard and Lange 1985; Kononenko et al. 2009). The enzyme tyramine-β-hydroxylase (TβH) converts tyramine to OA through the addition of a hydroxyl group in β-position, which makes tyramine the biological precursor of OA (Roeder 2005). OA is recognized as one of the major modulators within the nervous system of invertebrates and is from its chemical structure and biological function related to noradrenaline in vertebrates (reviews: Roeder 1999; Verlinden et al. 2010). Centrally applied OA can elicit rhythmic behaviors in locusts, such as flight, running, and swimming (Ramirez and Pearson 1991; Sombati and Hoyle 1984; Stevenson and Kutsch 1986; Rillich et al. 2013). In addition, it modulates visceral muscle contractions of the female reproductive tract of locusts and cockroaches (review: Lange 2009). In particular, it was shown that OA decreases the frequency and amplitude of myogenic contractions in oviducts (Lange and Orchard 1986; Orchard and Lange 1985, 1986, 1987; Kalogianni and Pflüger 1992) and spermathecae (Clark and Lange 2003). Furthermore, a modulatory effect of OA has been reported for insect circulatory organs (Papaefthimiou and Theophilidis 2011; reviews: Miller 1985; Neckameyer and Leal 2009).
The presence of OA in the antennal heart of P. americana could be demonstrated by a radioenzymatic assay (Pass et al. 1988b). The measured OA concentrations are with 9500 pg mg−1 wet mass in male antennal hearts extraordinarily high compared to other insect tissues (see tables in Evans 1985; Roeder 1999). Remarkably, more than 90% of the OA is concentrated in the outermost lateral part of this organ consisting primarily of the ampullae. This strongly indicates that the bulk of OA in the antennal heart is localized in the neurohaemal area associated with the ampullae. All hormones and other substances released from this site into the haemolymph are further pumped into the antennae. Since the circulation time within the antennae is considerable, it is evident that the target of quickly degraded hormones, such as OA (Goosey and Candy 1982), must be located within the antennae. In ectognathan insects, only the basal segment, the scape, contains muscles (Imms 1939) which are innervated aside from several motor neurons also by DUM neurons of the SOG (Honegger et al. 1990). Most of the antenna length is, however, formed by the flagellum that, aside from the extensive sensory apparatus, contains only epidermis and a few tracheae. In view of this fact, it appears most likely that the sensory receptors in the flagellum are the target of hormones released from the antennal heart.
The antennal sensory apparatus comprises a multitude of sensory organs for various modalities, although olfactory sensilla are prevailing (reviews: Schneider 1964; Keil 1999). Olfactory guided behavior exhibits a great plasticity, and a large number of investigations show that insects modulate their olfactory system according to their physiological state upon interaction with their environment (review: Dukas 2008; Gadenne et al. 2016). In particular, OA has proven to be involved as a modulator that enhances the responsiveness to olfactory and other sensory stimuli in insects (Mercer and Menzel 1982; Linn and Roelofs 1986; Linn et al. 1992, 1996; Matheson 1997, Pophof 2000; Grosmaitre et al. 2001; Flecke and Stengl 2009; Jung et al. 2013; Hillier and Kavanagh 2015; review: Farooqui 2007). Any of the various levels of the olfactory pathway are possible targets of the modulatory interference by OA, including peripheral receptors, as well as the central processing centers in the brain (reviews: Hansson and Christensen 1999; Stengl 2010; Galizia 2014; Gadenne et al. 2016).
Recently, tyramine (TA) was suggested to act as an individual transmitter with a possible antagonistic function to OA (Downer et al. 1993; Fox et al. 2006; Fussnecker et al. 2006; Lange 2009; Roeder 2005). In Drosophila melanogaster, it was shown that TA antagonizes the effect of OA at the neuromuscular junctions (Nagaya et al. 2002) and functions synergistically on oviducts and the spermatheca in locusts (da Silva and Lange 2008; Donini and Lange 2004). Furthermore, Kononenko et al. (2009) showed that purely tyraminergic cells exist in the locust brain, SOG, and abdominal fused ganglia.
The aim of the present study was to investigate for the first time the extent of the innervation and the distribution of varicosities and synapses in the antennal heart of Schistocerca gregaria by several neuronal and synaptic markers. We used synapsin to label areas with high synaptic density (see Kurylas et al. 2008; Michels et al. 2005) and to indicate synaptic release sites (see Benfenati et al. 1989). The neuroanatomical tracer neurobiotin was applied in anterograde tracing and, thus, synaptic structures and varicosities could be visualized (see Heinrich et al. 1998). Neurobiotin, together with synapsin, enabled the visualization of axonal varicosities and likely presynaptic release sites. Furthermore, the octopaminergic and tyraminergic innervation of the antennal heart of S. gregaria was visualized by applying respective antibodies.
Materials and methods
Animals
For the present study, antennal hearts of both sexes of S. gregaria were dissected and examined. The locust specimens were taken from our crowded laboratory colony that is maintained under a constant light regime (12:12/h—light:dark) at appr. 30 °C, and with a relative humidity of 40–60%.
All experiments adhere to the Principles of Laboratory Animal Care and the German Law on the Protection of Animals (Deutsches Tierschutzgesetz).
Dissection of S. gregaria
Prior to the experiments, the animals were stored overnight in a refrigerator at approximately 4 °C to slow metabolism, in particular, neuronal activities. Heads were separated and prefixed in the respective fixative solution for 90 min. To facilitate dissection of the antennal hearts, the heads were stabilized in the preparation dish by melted beeswax. The location of the antennal heart can be easily recognized from the exterior by a slight bulge of the cuticle close to the medial margin of each antennal base (Fig. 1a). To prepare an antennal heart for the labeling experiments, a rectangular-shaped window was cut between the two antennae. Once the antennal heart dilator muscle was located, the surrounding tracheae and fatty tissue were carefully removed and the antennal heart was dissected out of the head capsule together with parts of the frontal cuticle and then pinned to a small Sylgard dish. To avoid damage to the antennal heart, the remaining fatty tissue around it was initially left undisturbed. After fixation with the respective fixative solution, the preparations were cleaned of the remaining fatty tissue and tracheae during the ascending dehydration series with ethanol (50, 70, 90, and 100%).
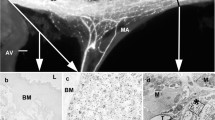
Anatomy of the antennal heart in S. gregaria. a Frontal view of a locust head with the antennal heart drawn in by red lines. An ampulla is located at the base of each antenna to which a long antennal vessel is connected; the two ampullae are joined by an ampulla dilator muscle. b Horizontal semi-thin section through right half of the antennal heart. The ampulla is attached to the frontal cuticle with the dilator muscle inserting at the inner ampulla wall. Slit-like valved ostium located at the frontal side of the ampulla, scale bar 100 µm. ant anterior, lat lateral, DAmp dilator of ampulla, Ost ostium, Amp ampulla, AV antennal vessel
Semi-thin sections
To produce serial semi-thin sections, the heads were fixed in a formaldehyde–acetic acid–ethanol mixture (FAE, see Beutel et al. 2014). After dehydration in an ascending acetone series, the objects were embedded under vacuum impregnation in Agar low viscosity resin (Agar Scientific). Following the procedure of Blumer et al. (2002), serial semi-thin sections (thickness 1 µm) were cut with a Leica EM UC6 microtome using a Histo Jumbo Diatome diamond knife. The sections were stained with a mixture of 1% azure and 1% methylene blue in a 1% aqueous borax solution, diluted 1:20 with distilled water, for approximately 30 s at 60–70 °C.
Anterograde nerve staining with neurobiotin
Anterograde staining (axonal fill) of the circumoesophageal connective was conducted to elucidate the innervation of the antennal heart via the NCC III. First, the locusts were stored in a refrigerator for 30–60 min. Afterward, the heads were severed posterior to the compound eyes and stabilized with minute pins in Sylgard dishes. After carefully removing fatty tissue and tracheae, the circumoesophageal connectives were severed just anterior to the SOG and the anterior end of one connective was embedded in a vaseline pool first filled with distilled water to osmotically widen the axons and to test for leakiness of the vaseline pool. After 10 min, the distilled water was removed and replaced with a 3% neurobiotin solution (Neurobiotin tracer, Vector Laboratories). The pool was sealed airtight and the preparation placed in a moist chamber. The chamber was stored in a cool room (approximately 4 °C) for 2–4 days to enable diffusion of the neurobiotin dye throughout the nerves.
Neurobiotin/streptavidin–fluorochrome labelling and immunohistochemistry on whole-mount dissections
To use neurobiotin/streptavidin and synapsin as labels, the dissections of the antennal heart were fixed in a 4% paraformaldehyde fixative solution (PFA) for 1 h and then washed in 0.1 M phosphate buffered saline (PBS) for 3 × 10 min. The preparations were dehydrated in an ascending ethanol series (50, 70, 90, and 100%) for 10 min each and then degreased in xylene for 2 × 5 min. Subsequently, they were rehydrated in a descending ethanol series (100, 90, 70, 50%; each 10 min) and then washed in 0.1 M PBS for 1 × 15 min. Following this procedure, the dissections were washed in 1% Triton X-100 in 0.1 M PBS (PBS-TX) for 4 × 15 min and preincubation was conducted for 1 h in a solution containing 10% normal goat serum (NGS) in PBS-TX.
Neurobiotin/streptavidin labeling
The dissected antennal hearts, prepared according to the aforementioned procedure, were incubated with Cy2 or Cy5 conjugated biotin-binding protein Streptavidin (dissolved 1:100 in PBS-TX) for 1 day at room temperature. After 24 h, the preparations were washed in 0.1 M PBS for 3 × 10 min and dehydrated in an ascending ethanol series (50, 70, 90, and 100%) for 10 min each. They were permeabilized in a 1:1 mixture of 100% ethanol and methyl salicylate for 10 min and then transferred into pure methyl salicylate (for detailed protocol, see online resource 1).
Synapsin labeling
To visualize synaptic terminals, we used an established primary antibody, anti-Synorf1 (monoclonal anti-mouse, DSHB, Würzburg, Germany), that recognizes the vesicular protein synapsin. The marker was dissolved 1:10 in a prepared buffer solution, containing PBS-TX, 1% NGS, and 1% sodium azide (NaN3). After storing the incubating dissections in a cool room (approximately 4 °C) for 4–5 days, they were washed in PBS-TX for 6 × 20 min. The dissections were incubated in a Cy2 or Cy5 conjugated antibody (goat-anti-mouse; 1:100) for 1 day at room temperature. The following day, the preparations were washed in 0.1 M PBS for 3 × 20 min and dehydrated with an ascending ethanol series (50, 70, 90, and 100%, each for 10 min). They were permeabilized in a 1:1 mixture of 100% ethanol and methyl salicylate for 10 min and then transferred into pure methyl salicylate (for detailed protocol, see online resource 2).
Octopamine and tyramine labeling
To label TA and OA, the dissected antennal hearts were fixed in a picric acid fixative mixture [6.25% glutaraldehyde, 75% picric acid, 5% (glacial) acetic acid, and 1% sodium metabisulfite (SMBS)] for 1 h. Then, they were washed for 2 × 10 min in 0.1 M Tris–HCl–SMBS (0.45%, pH 7.6) and kept in the same buffer solution overnight. The following day, they were washed again for 10 min with the above-mentioned solution, dehydrated in an ascending ethanol series (50, 70, 90, 100%; each for 10 min), and degreased in xylene for 2 × 5 min. The dissections were dehydrated in a descending ethanol series (100, 90, 70, 50; each 10 min) and then washed in 0.1 M Tris–HCl–SMBS (0.45%, pH 7.6) for 15 min. To break up the glutaraldehyde bonds, they were washed in 1% sodium borohydride (0.04 g sodium borohydride in 4 ml Tris–HCl) for 10 min and then 15 min in 0.1 M Tris–HCl–SMBS (0.45%, pH 7.6). The dissections were washed in 1% Triton X-100 in 0.1 M Tris–HCl–SMBS (0.45%, pH 7.6) to increase permeability and subsequently treated with 10% NGS in 1% Triton X-100 in 0.1 M Tris–HCl–SMBS (0.45%, pH 7.6) for 1 h. Following preincubation, incubation with primary antibodies was applied following largely the procedures developed for these two antibodies by Kononenko et al. (2009). The primary anti-tyramine antibody (polyclonal anti-rabbit, Chemicon, Temecula, CA, USA) was dissolved 1:200 in a prepared buffer solution, containing 1% Triton X-100 in 0.1 M Tris–HCl–SMBS (0.45%, pH 7.6), 1% NGS, and 1% sodium azide (NaN3). The primary anti-OA antibody (monoclonal mouse anti-OA, Jena, Bioscience) was dissolved 1:1000 in the same buffer solution. After storing the incubating dissections in a cool room (approximately 4 °C) for 4–5 days, they were washed 6 × 20 min in 1% Triton X-100 in 0.1 M Tris–HCl–SMBS (0.45%, pH 7.6). Thereafter, the dissections were incubated in Cy2- or Cy5-conjugated antibodies [goat-anti-rabbit for anti-tyramine (conjugated to Cy2) and goat-anti-mouse for anti-OA (conjugated to Cy5), both 1:100)] for 1 day at room temperature. The following day, the preparations were washed in 0.1 M Tris–HCl for 4 × 20 min and subsequently dehydrated with an ascending ethanol series (50, 70, 90, and 100%, each for 10 min). The specimens were permeabilized in a 1:1 mixture of 100% ethanol and methyl salicylate for 10 min and then transferred into pure methyl salicylate (for detailed protocol see online resource 3).
Image acquisition
The staining of the antennal hearts was initially assessed using a fluorescence microscope (Zeiss Photomicroscope Axiophot). More than 120 antennal hearts treated with different staining methods were evaluated in this way, and preparations with the most intensive staining were used for further processing by confocal scanning microscopes (TCS SP2, Leica, Germany; TCS SP8, Leica, Germany). Different objectives such as HC PL 5×/0.15, HC PL 10×/0.5, and HC PL APO CS2 20×/0.7 mm were used for scanning. The optical zoom factor varied from 1 to 1.5 for an overview and from 2.5 to 6 for a detailed scan. Each antennal heart was scanned with a resolution of 2048 × 2048 pixels. Depending on the thickness of the preparation, the appropriate slice numbers of the serial stacks were scanned with step sizes of 1.04 µm and an average from 2 to 3 times. Two different lasers, an Argon/Krypton laser at 488 nm and a Helium/Neon laser at 633 nm, were used to scan the images simultaneously. Images were analyzed using the open source software ImageJ 1.48r (Java 1.6.0_65, National Institutes of Health, USA). For detailed image acquisition, see online resource 4.
Results
Anatomy of the antennal heart
The functional anatomy of the antennal heart in S. gregaria resembles that described for other species of locust (Bayer 1968; Pass 1991). It consists of pulsatile ampullae, which are oval-shaped vesicles that are attached to the frontal cuticle medially of each antenna base (Fig. 1). Each ampulla is connected to a vessel, which extends into the antenna up to the apex. The two ampullae are linked by a transverse muscle, which inserts at their inner walls. Contraction of this muscle results in a simultaneous diastole of both ampullae, and haemolymph enters through slit-like valved ostia (Fig. 1b). The systole phase follows when the transverse dilator muscle relaxes, obviously due to the elasticity of the ampulla walls (see Pass 1985). Haemolymph is thus forced through the antennal vessel up to the apex where it emanates through a terminal pore into the antennal haemocoel where it flows in the opposite direction back to the head capsule. Therefore, there is a clearly a directed haemolymph flow through the antennae. The dimensions of the transverse dilator muscle are: 1350 µm (mean ± SD 162 µm, n = 20) in length, 175 ± 43 µm width, with a mean diameter of 26 ± 9 µm.
Ramification of NCC III terminals
Axonal tracing with neurobiotin
The dilator muscle of the ampullae receives innervation from four neurons (two DUMs and a pair of motor neurons CN3) located in the SOG. Among them are DUM neurons, whose axons run through the circumoesophageal connectives and the nerve NCC III, which extends to the corpora cardiaca and from there to the antennal heart (Bräunig 1990). In the present study, the connectives of all specimens were unilaterally stained by neurobiotin to determine whether the antennal heart receives lateral innervation from each NCC III nerve. Figure 2 shows a clearly labeled octopaminergic fiber that innervates the antennal heart via the medial ventral surface (Fig. 2b, dashed white circle) and separates unilaterally into collaterals, which run towards the ampulla (Fig. 2, white arrows).
Labeled fibers on the antennal heart as a result of anterograde staining of one circumoesophageal connective with antibodies against neurobiotin/streptavidin. a Neurobiotin-labeled structures and varicosities run towards the ampulla (white arrow), scale bar 200 µm. b Enlarged section of the neurobiotin-labeled varicosities. The axon collaterals (white arrow) and nerve entrance (dashed white circle) are visible, scale bar 50 µm. c Neurobiotin-labeled beaded fibers and varicosities near the ampulla wall (dashed line), scale bar 20 µm
At the point where nerve NCC III joins the dilator muscle, some minor beaded fibers continue for a short distance in the contralateral direction, whereas all main collaterals appearing as beaded fibers of various thicknesses extend in the opposite direction towards the ampulla where they form a dense, plexus-like network of terminal fibers. Especially at the wall of the ampulla, vast amounts of beaded fibers and thick profiles are visible (see Figs. 2c, 3).
Immunoreactive varicosities and beaded fibers on the antennal heart stained with antibodies against neurobiotin/streptavidin and synapsin. a Neurobiotin labeling reveals the nerve entrance (dashed white circle), axon collaterals, and ramifications (white arrows). b The same stack of images with anti-synapsin staining of neuronal structures reveals the same nerve entrance (dashed white circle) and axon collaterals (white arrows). c Double labeling of neurobiotin (green) and anti-synapsin (magenta) shows co-localization of the nerve entrance (dashed white circle), beaded fibers, and axon collaterals (white arrows), scale bar 100 µm (see Fig. 4 for dashed square)
Neurobiotin/streptavidin and synapsin labeled structures
Synaptic zones were visualized using anti-synapsin simultaneously with neurobiotin to determine presynaptic release sites. The preparations show numerous active zones near the ampulla wall and in the varicosities of the beaded fibers on the antennal heart (Fig. 3). Neurobiotin reveals axonal structures, which ramify from the stained nerve NCC III, and synapsin illustrates presynaptic sites and, thus most likely, the locations of vesicle release sites such as in neuromuscular junctions or neurohaemal terminals.
The innervation pattern shown in Fig. 3a once again reveals the nerve supplying the antennal heart on the medial ventral surface (Fig. 3a, white circle) and the ramifications of one labeled fiber into axon collaterals (Fig. 3, white arrows). The synapsin staining reveals synaptic sites, which are clearly located on beaded fibers and mainly label varicosities proceeding towards the ampullae. The ramifications of the axon collaterals are more widely distributed in the median section of the muscle and become particularly dense near the wall of the ampulla (Fig. 3a–c). Double labeling of synapsin and neurobiotin (Fig. 3c) shows the varicosities of the beaded fibers stained by neurobiotin co-localizing with the synapsin immunoreactivity.
The enlarged section of the ampulla (dashed square in Fig. 3c) reveals a closer aspect of the dense area mentioned above (Fig. 4). The co-localizations are visible in Fig. 4bi–iii (white arrows). The synaptic active zones appeared as loosely arranged boutons of a “chain” (beaded fibers) in all preparations.
Enlarged view of the dense area near the ampulla wall (dashed square in c) a Neurobiotin-labeled beaded fibers and varicosities near the ampulla wall (ai). Anti-synapsin staining reveals a strong labeling of active zones (aii). Double labeling with neurobiotin (green) and anti-synapsin (magenta) reveals a co-localization of a vast amount of fibers and varicosities (aiii), scale bar 50 µm. b Enlarged view of dashed square in Fig. 5aiii. A group of neurobiotin-labeled beaded fibers (bi, white arrow) are revealed. The labeling of anti-synapsin shows the same group of beaded fibers (bii, white arrow). Double labeling of neurobiotin (green) and anti-synapsin (magenta) shows co-localizations of the before mentioned group of beaded fibers (biii, white arrow), scale bar 10 µm
Allocation of octopaminergic and tyraminergic structures
To reveal a possible co-localization of labeled neuromodulatory structures with TA/OA, a polyclonal antibody against TA was applied simultaneously to anti-OA.
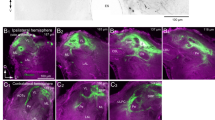
Figure 5ai displays the pattern of OA immunoreactivity on the antennal heart and near the ampulla wall. Each half of this visceral muscle receives separate innervation and both ends located towards the ampulla were densely covered by neuromodulatory varicosities and structures. Anti-tyramine staining reveals similar structures (Fig. 5aii). Again, these varicosities are densely distributed near the ampulla wall, similar to the octopaminergic structures. Figure 5aiii demonstrates that tyramine immunoreactivity on the antennal muscle is exclusively limited to neuromodulatory structures also labeled by anti-OA. This co-localization is clearly visible, especially close to the ampulla wall. The double-labeled octopaminergic and tyraminergic structures appear as chains of loosely arranged varicosities and, thus, each contains both transmitters (white arrows in Fig. 5bi–iii).
Octopaminergic and tyraminergic fibers appear mostly in immediate vicinity of the ampullae and are co-labeled on the antennal heart muscle. a Octopamine immunoreactivity reveals the distribution of varicosities on the muscle and near the ampullae (ai). The same stack of images reveals tyraminergic fibers with the similar distribution (aii). The co-localization of octopamine and tyramine is shown in chains of loosely arranged varicosities on the antennal heart muscle (aiii), scale bar 50 µm. b Detailed view of octopaminergic (bi) and tyraminergic (bii) fibers (dashed square in aiii). Co-localized fibers of tyramine and octopamine staining (biii, white arrows), scale bar 20 µm
Co-localization of anti-OA and anti-tyramine is presented for a detailed part of the visceral muscle (Fig. 5bi–iii). Figure 5ai, bi reveals the parts of beaded fibers labeled by anti-OA. Anti-tyramine staining reveals a similar pattern of varicosities (Fig. 5aii, bii).
Discussion
In this study, we present data showing the extent of innervation at the antennal heart in S. gregaria. For the first time in insects, the distribution of the synapses, varicosities, and neurohaemal terminal on this organ was visualized using several neuronal and synaptic markers. With respect to the biological role of the octopaminergic fibers innervating the antennal heart, we must differentiate between (1) the innervation of the dilator muscle and (2) the supply of the neurohaemal release sites in the ampulla area of this organ.
Nerve branching, synapses, and neurohaemal release sites
The previous studies in P. americana (Pass et al. 1988a) and L. migratoria (Bräunig 1990) revealed that the neurons that are associated with their antennal hearts are located in the SOG. Axons of contralateral neurons and DUM neurons located there run via the paired NCC III into the CC and further to the antennal heart. Since the innervation proceeds unilaterally via the circumoesophageal connectives (Bräunig 1990; Pass et al. 1988a), only one connective was stained with neurobiotin to visualize the lateral innervation of this visceral muscle. This tracer distributes quickly through nerves and has proved to be best suited for axonal tracing (Heinrich et al. 1998). The staining revealed specific innervation via the medial ventral surface. Axon collaterals of the NCC III axon ramify towards the ampulla, where the axons develop into a dense meshwork of arborizations and then terminate at the ampulla wall.
Another staining technique comprises simultaneous anti-synapsin labeling. It is well known that synapsin labels synaptic release sites and active zones (Benfenati et al. 1989). Simultaneous staining with anti-synapsin and neurobiotin shows a similar density of arborizations near the ampulla and confirms the lateral innervation. It also shows a major overlap in the region of the ampulla, which indicates synaptic release sites. This result of S. gregaria reiterates the condition in P. americana where numerous neurosecretory terminals form a neurohaemal area (Beattie 1976; Pass et al. 1988a).
Octopamine and tyramine immunoreactivity
Since all efferent DUM neurons biochemically and immunohistochemically examined contain OA (cf. Evans 1985; Orchard and Lange 1985, Stevenson and Spörhase-Eichmann 1995; Kononenko et al. 2009), this biogenic amine was suspected to occur in the nerve fibers associated with the antennal heart. In accordance with earlier studies that used a radioenzymatic assay and that demonstrated a high content of OA in the antennal heart of the cockroach, especially in the region of the ampullae (Pass et al. 1988b), both octopaminergic and tyraminergic fibers were shown in S. gregaria to be highly prominent near the area of the ampulla (Fig. 5).
It is possible that TA acts only as a precursor for OA, because we have no evidence, suggesting that OA’s precursor tyramine could act as an independent transmitter in this system. The immunoreactivity provides further evidence that the described innervation of the antennal heart belongs to the SOG DUM neurons. In addition, the occurrence and prominent distribution of loosely arranged boutons near the ampulla resembled in some aspect that of type II synapses described for vertebrates (e.g. Hassler and Chung 1976) and of type II terminals described for insect muscle in D. melanogaster (Atwood et al. 1993). The tyraminergic beaded fibers proved to be exclusively limited to the octopaminergic fibers, which leads us to conclude that tyramine is not an independent transmitter in the antennal heart. In addition, the previous studies also identified varicosities with a co-localization of TA and OA in skeletal muscles (Kononenko et al. 2009; Stocker 2011). Tyraminergic innervation has also been shown in visceral muscles (da Silva and Lange 2008; Donini and Lange 2004). Orchard and Lange (1985) examined the functionality of TA and in connection with the results of the latter study, they showed that a purely tyraminergic innervation of visceral muscle is not present. As far as known, TA exclusively binds to octopaminergic receptors in muscle, and it is unlikely that TA acts as an independent neuromodulator in the antennal heart, as Roeder (2005) and Kononenko et al. (2009) showed for the central nervous system in insects. Studies investigating TA at the spermatheca of L. migratoria showed that both transmitters act on OA receptors (da Silva and Lange 2008).
Cardiomodulatory function of octopamine
The physiology of antennal hearts has been investigated so far only in cockroaches (Hertel et al. 1985, 1988; Hertel and Richter 1997; Hertel and Penzlin 1992; Lange et al. 1993; Richter and Hertel 1997; Predel et al. 2004) and mosquitoes (Boppana and Hillyer 2014; Suggs et al. 2016). In Periplaneta, it was shown that contractions of this circulatory organ are based on a myogenic automatism, while frequency and amplitude of the muscle contractions are regulated by the antennal heart nerve (Hertel et al. 1988). In most cases, OA applied to isolated antennal hearts causes a short transient cardiac block followed by a decreased beating frequency (Hertel et al. 1988; Hertel and Penzlin 1992).
OA also affects the pumping frequency of the primary circulatory pump of insects, the dorsal vessel (“heart”), although the results achieved from different species provide no clear picture (Zornik et al. 1999; Tsai et al. 2004; Papaefthimiou and Theophilidis 2011; reviews: Miller 1985; Neckameyer and Leal 2009). This could be due to different species-specific effects, and eventually also to biphasic effects at various concentration levels as described by Papaefthimiou and Theophilidis (2011). In L. migratoria, rhythmically active DUM neurons innervate the dorsal vessel (Ferber and Pflüger 1990, 1992). These two so-called “DUM-heart neurons” were studied in great detail, and a co-localization of OA (Stevenson and Pflüger 1994) together with three other transmitters was determined (RFamide-like peptide: Ferber and Pflüger 1992; taurine: Stevenson 1999; nitric oxide: Bullerjahn et al. 2006).
Earlier studies on the innervation of visceral muscles, for example the oviduct muscle of L. migratoria, investigated both the release of OA and the neuropeptide proctolin (Orchard and Lange 1987). It turned out that the release of both transmitters was frequency-dependent, with the maximum release of OA at 5 Hz and maximum release of proctolin at 30 Hz. This strengthened the argument that OA is one of the natural regulators of insect visceral muscles and that it modulates muscle contractions (Orchard and Lange 1985, 1986). In the antennal heart of Periplaneta, the presence of proctolin has been demonstrated by immunohistochemistry, high-performance liquid chromatography, and mass spectrometry (Hertel et al. 1995, 2012; Predel 2001). Moreover, proctolin was shown to have a strong excitatory effect on the beating frequency of this circulatory pump (Lange et al. 1993; Hertel et al. 1995). In addition, a strong allatostatin immunoreactivity has been demonstrated in nerve fibers innervating the antennal heart of the cockroach Diploptera punctata (Woodring et al. 1992).
In summary, for at least the cockroach and the locust, it was ascertained that DUM neurons are involved in the innervation of the antennal heart, as well as the dorsal vessel. OA is suggested to act as a modulator of their beating frequency. A concerted action together with proctolin and other neuropeptides is probable.
Octopamine in the neurohaemal area of the antennal heart
Of particular interest is the well-developed neurohaemal release site in the ampulla region of the antennal heart. The anatomical condition in Schistocerca closely resembles that in Periplaneta (see: Fig. 15 in Pass et al. 1988a). In the cockroach, the determination of OA was performed with a radioenzymatic assay, which permitted only an indirect conclusion that it is localized in the neurosecretory terminals of the ampullae (Pass et al. 1988b). The present results in Schistocerca now clearly demonstrate that there are octopaminergic terminals in the region of the ampullae. Therefore, we can assume that OA is released there and pumped into the antennae. In Schistocerca, the cuticle of the antennae is non-transparent, and therefore, the circulation time cannot be calculated by observation of the haemocyte flow. However, in Periplaneta, it was possible to determine the velocity of haemocyte movement by visual observation, which led to a rough estimate of 10 min for a full circulation cycle through the antenna (Pass et al. 1988b). This time span corresponds approximately to the half-life of OA in insect haemolymph (Goosey and Candy 1982) which clearly indicates that the target of OA must be located within the antennae. As previously noted, based on the anatomy of the antenna, we further concluded that the sensory apparatus located there is the most probable target site of OA and other substances released from neurohaemal release sites in the antennal heart. Since the antennae in Schistocerca are much shorter than in Periplaneta, the circulation time is certainly significantly shorter. Therefore, it seems conceivable that there are additional targets of the OA in the lateral region of the head capsule.
OA has been shown to be involved in the sensitivity modulation of the olfactory receptor neurons (ORNs) in the antennae of a number of insect species (Periplaneta: Zhukovskaya and Kapitsky 2006; Zhukovskaya 2007, 2008, 2012; Jung et al. 2013; several species of Lepidoptera: Pophof 2000, 2002; Grosmaitre et al. 2001; Dolzer et al. 2001; Flecke and Stengl 2009; Hillier and Kavanagh 2015; Schendzielorz et al. 2015; Apis: Vergoz et al. 2009). All these studies refer to pheromone communication and a few papers consider non-pheromone odorants (Pophof 2002; Stelinski et al. 2003; Zhukovskaya 2012). OA was shown to increase the firing rate of pheromone-sensitive sensilla in various insect species (Pophof 2000; Grosmaitre et al. 2001; Zhukovskaya and Kapitsky 2006; Zhukovskaya 2007; Flecke and Stengl 2009; Vergoz et al. 2009; Jung et al. 2013; Hillier and Kavanagh 2015). Different receptor cells of the same sensillum were shown to be modulated independently from each other, indicating that the OA receptors reside in the receptor neurons and not in the accessory cells (Zhukovskaya 2007). Evidence for OA receptors located in the antennal receptor cells has been presented for several species using in situ hybridization (von Nickisch-Rosenegk et al. 1996; Dacks et al. 2006; Brigaud et al. 2009; Jung et al. 2013; Lam et al. 2013). Experiments with injection of dsRNAs of OA receptors in Periplaneta provide additional support for the modulation of ORNs in the antennae (Jung et al. 2013). Furthermore, antennal ORNs have been shown to represent endogenous circadian oscillators (Merlin et al. 2007; review: Gadenne et al. 2016), and experiments indicate that these rhythms are modulated by OA in correlation with the insect’s activity cycle (Flecke and Stengl 2009). Schendzielorz et al. (2015) demonstrated day-time-dependent changes in the concentration of OA in the antennae of Manduca sexta, which corresponds to the observation that changes in OA concentrations in the haemolymph of this moth are subject to a circadian rhythm (Lehman 1990). Furthermore, a tyramine-like immunoreactive fiber was described in each fascicle of the antennal nerve in the flagellum region of Manduca (Schendzielorz et al. 2015). The authors speculated that these fibers might originate from neurons that mediate more rapid stimulus-dependent OA actions, thereby overruling the basic circadian rhythms of OA concentration changes in the haemolymph.
In conclusion, there is increasing evidence for a peripheral sensitivity modulation of the antennal ORNs by OA. For the task of long-term control via a hormonal pathway, the antennal heart represents the most suitable release site, since all haemolymph, which enters the antennae, must pass through this organ. Moreover, due to these anatomical constraints and conditions, a local regulation of the concentration of OA in the haemolymph within the antenna compartment is conceivable, which could be quite different from that of the general body cavity.
References
Atwood HL, Govind CK, Wu CF (1993) Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol 24:1008–1024
Bayer R (1968) Untersuchungen am Kreislaufsystem der Wanderheuschrecke (Locusta migratoria migratorioides R. et F., Orthopteroidea) mit besonderer Berücksichtigung des Blutdruckes. Z vgl Physiol 58:76–135
Beattie TM (1976) Autolysis in axon terminals of a new neurohaemal organ in the cockroach Periplaneta americana. Tissue Cell 8:305–310
Benfenati F, Bähler M, Jahn R, Greengard P (1989) Interactions of synapsin I with small synaptic vesicles. J Cell Biol 108:1863–1872
Beutel RG, Friedrich F, Ge S-Q, Yang X-K (2014) Insect morphology and phylogeny. De Gruyter, Berlin
Blumer MJ, Gahleitner P, Narzt T, Handl C, Ruthensteiner B (2002) Ribbons of semithin sections: an advanced method with a new type of diamond knife. J Neurosci Methods 120:11–16
Boppana S, Hillyer JF (2014) Hemolymph circulation in insect sensory appendages: functional mechanics of antennal accessory pulsatile organs (auxiliary hearts) in the mosquito Anopheles gambiae. J Exp Biol 217:3006–3014. doi:10.1242/jeb.106708
Bräunig P (1990) The morphology of suboesophageal ganglion ceils innervating the nervus corporis cardiaci III of the locust. Cell Tissue Res 260:95–108
Bräunig P, Pflüger HJ (2001) The unpaired median neurons of insects. Adv Insect Physiol 28:185–266
Brigaud I, Grosmaitre X, François MC, Jacquin-Joly E (2009) Cloning and expression pattern of a putative octopamine/tyramine receptor in antennae of the noctuid moth Mamestra brassicae. Cell Tissue Res 335:455–463. doi:10.1007/s00441-008-0722-5
Bullerjahn A, Mentel T, Pflüger HJ, Stevenson PA (2006) Nitric oxide: a co-modulator of efferent peptidergic neurosecretory cells including a unique octopaminergic neuron innervating locust heart. Cell Tissue Res 325:345–360. doi:10.1007/s00441-006-0188-2
Clark J, Lange AB (2003) Octopamine modulates spermathecal muscle contractions in Locusta migratoria. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 189:105–114. doi:10.1007/s00359-002-0375-x
Crossman AR, Kerkut GA, Pitman RM, Walker RJ (1971) Electrically excitable nerve cell bodies in the central ganglion of two insect species Periplaneta americana and Schistocerca gregaria. Investigation of cell geometry and morphology by intracellular dye injection. Comp Biochem Physiol A 40:579–594
da Silva R, Lange AB (2008) Tyramine as a possible neurotransmitter/neuromodulator at the spermatheca of the African migratory locust, Locusta migratoria. J Insect Physiol 54:1306–1313. doi:10.1016/j.jinsphys.2008.07.001
Dacks AM, Dacks JB, Christensen TA, Nighorn AJ (2006) The cloning of one putative octopamine receptor and two putative serotonin receptors from the tobacco hawkmoth, Manduca sexta. Insect Biochem Mol Biol 36:741–747. doi:10.1016/j.ibmb.2006.07.002
Dolzer J, Krannich S, Fischer K, Stengl M (2001) Oscillations of the transepithelial potential of moth olfactory sensilla are influenced by octopamine and serotonin. J Exp Biol 204:2781–2794
Donini A, Lange AB (2004) Evidence for a possible neurotransmitter/neuromodulator role of tyramine on the locust oviducts. J Insect Physiol 50:351–361. doi:10.1016/j.jinsphys.2004.02.005
Downer RGH, Hiripi L, Juhos S (1993) Characterization of the tyraminergic system in the central nervous system of the locust, Locusta migratoria migratoides. Neurochem Res 18:1245–1248
Duch C, Mentel T, Pflüger HJ (1999) Distribution and activation of different types of octopaminergic DUM neurons in the locust. J Comp Neurol 403(1):119–134
Dukas R (2008) Evolutionary biology of insect learning. Annu Rev Entomol 53:145–160. doi:10.1146/annurev.ento.53.103106.093343
Evans PD (1985) Octopamine. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 11. Pergamon Press, Oxford UK, pp 499–530
Farooqui T (2007) Octopamine-mediated neuromodulation of insect senses. Neurochem Res 32:1511–1529. doi:10.1007/s11064-007-9344-7
Ferber M, Pflüger HJ (1990) Bilaterally projecting neurons in pregenital abdominal ganglia of the locust: anatomy and peripheral targets. J Comp Neurol 302:447–460. doi:10.1002/cne.903020303
Ferber M, Pflüger HJ (1992) An identified dorsal unpaired median neuron and bilaterally projecting neurons exhibiting bovine pancreatic polypeptide/FMRFamide-like immunoreactivity in abdominal ganglia of the migratory locust. Cell Tissue Res 267:85–98
Flecke C, Stengl M (2009) Octopamine and tyramine modulate pheromone-sensitive olfactory sensilla of the hawkmoth Manduca sexta in a time-dependent manner. J Comp Physiol A 195:529–545. doi:10.1007/s00359-009-0429-4
Fox LE, Soll DR, Wu CF (2006) Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxylase mutation. J Neurosci 26:1486–1498. doi:10.1523/JNEUROSCI.4749-05.2006
Fussnecker BL, Smith BH, Mustar JA (2006) Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera). J Insect Physiol 52:1083–1092
Gadenne C, Barrozo RB, Anton S (2016) Plasticity in insect olfaction: to smell or not to smell? Annu Rev Entomol 61:317–333. doi:10.1146/annurev-ento-010715-023523
Galizia CG (2014) Olfactory coding in the insect brain: data and conjectures. Eur J Neurosci 39:1784–1795. doi:10.1111/ejn.12558
Goosey MW, Candy DJ (1982) The release and removal of octopamine by tissues of the locust Schistocerca americana gregaria. Insect Biochem 12:681–685. doi:10.1016/0020-1790(82)90057-9
Grosmaitre X, Marion-Poll F, Renou M (2001) Biogenic amines modulate olfactory receptor neurons firing activity Mamestra brassicae. Chem Senses 26:653–661
Hansson BS, Christensen TA (1999) Functional characteristics of the antennal lobe. In: Hansson BS (ed) Insect olfaction. Springer, Berlin, pp 125–161
Hassler R, Chung JW (1976) The discrimination of nine different types of synaptic boutons in the fundus striati (nucleus accumbens septi). Cell Tissue Res 168:489–505. doi:10.1007/BF00215999
Heinrich R, Jacobs K, Lakes-Harlan R (1998) Tracing of a neuronal network in the locust by pressure injection of markers into a synaptic neuropil. J Neurosci Methods 80:81–89
Hertel W, Penzlin H (1992) Function and modulation of the antennal heart of Periplaneta americana (L.). Acta Biol Hung 43:113–125
Hertel W, Richter M (1997) Contributions to physiology of the antenna-heart in Periplaneta americana (L.) (Blattodea: Blattidae). J Insect Physiol 43:1015–1021. doi:10.1016/S0022-1910(97)00073-5
Hertel W, Pass G, Penzlin H (1985) Electrophysiological investigation of the antennal heart of Periplaneta americana and its reaction to proctolin. J Insect Physiol 31:563–572
Hertel W, Pass G, Penzlin H (1988) The effects of the neuropeptide proctolin and of octopamine on the antennal heart of Periplaneta americana. Symp Biol Hung 36:351–362
Hertel W, Richter M, Rapus J, Eckert M, Penzlin H (1995) The role of proctolin in the antenna-heart beat acceleration of Periplaneta americana (L.). Acta Biol Hung 46:491–506
Hertel W, Neupert S, Eckert M (2012) Proctolin in the antennal circulatory system of lower Neoptera: a comparative pharmacological and immunohistochemical study. Physiol Entomol 37:160–170
Hillier NK, Kavanagh RMB (2015) Differential octopaminergic modulation of olfactory receptor neuron responses to sex pheromones in Heliothis virescens. PLoS One 10:17. doi:10.1371/journal.pone.0143179
Honegger HW, Allgäuer C, Klepsch U, Welker J (1990) Morphology of antennal motoneurons in the brains of two crickets, Gryllus bimaculatus and Gryllus campestris. J Comp Neurol 291:256–268
Hoyle G (1975) Evidence that insect dorsal unpaired median (DUM) neurons are octopaminergic. J Exp Zool 193(3):425–431
Imms AD (1939) On the antennal musculature in insects and other arthropods. Q J Microsc Sci 81:273–320
Jung JW, Kim JH, Pfeiffer R, Ahn YJ, Page TL, Kwon HW (2013) Neuromodulation of olfactory sensitivity in the peripheral olfactory organs of the American cockroach, Periplaneta americana. PLoS One 8(11):e81361
Kalogianni E, Pflüger HJ (1992) The identification of motor and unpaired median neurons innervating the locust oviduct. J Exp Biol 168:177–198
Keil TA (1999) Morphology and development of the peripheral olfactory organs. In: Hansson BS (ed) Insect olfaction. Springer, Berlin, pp 5–47
Kononenko NL, Wolfenberg H, Pflüger HJ (2009) Tyramine as an independent transmitter and a precursor of octopamine in the locust central nervous system: an immunocytochemical study. J Comp Neurol 512(4):433–452. doi:10.1002/cne.21911
Kurylas AE, Rohlfing T, Krofczik S, Jenett A, Homberg U (2008) Standardized atlas of the brain of the desert locust, Schistocerca gregaria. Cell Tissue Res 333(1):125–145
Lam F, Mcneil JN, Cn Donly (2013) Octopamine receptor gene expression in three lepidopteran species of insect. Peptides 41:66–73. doi:10.1016/j.peptides.2012.03.034
Lange AB (2009) Tyramine: from octopamine precursor to neuroactive chemical in insects. Gen Comp Endocrinol 162:18–26. doi:10.1016/j.ygcen.2008.05.021
Lange AB, Orchard I (1986) Identified octopaminergic neurons modulate contractions of locust visceral muscle via adenosine 3′,5′-monophosphate (cyclic AMP). Brain Res 363:340–349
Lange AB, Chan KK, Stay B (1993) Effect of allatostatin and proctolin on antennal pulsatile organ and hindgut muscle in the cockroach, Diploptera punctata. Arch Insect Biochem Physiol 24:79–92. doi:10.1002/arch.940240203
Lehman HK (1990) Circadian control of Manduca sexta flight. Soc Neurosci Abs 16:1334
Linn CE, Roelofs WL (1986) Modulatory effects of octopamine and serotonin on male sensitivity and periodicity of response to sex pheromone in the cabbage looper moth, Trichoplusia ni. Arch Insect Biochem Physiol 3:161–171
Linn CE, Campbell MG, Roelofs WL (1992) Photoperiod cues and the modulatory action of octopamine and 5-Hydroxytryptamine on locomotor and pheromone response in male gypsy moth, Lymantria dispar. Arch Insect Biochem 20:265–284
Linn CE, Campell MG, Poole KR, Wu WQ, Roelofs WL (1996) Effects of photoperiod on the circadian timing of pheromone response in male Trichoplusia ni: relationship to the modulatory action of octopamine. J Insect Physiol 42:881–891
Matheson T (1997) Octopamine modulates the responses and presynaptic inhibition of proprioreceptive sensory neurones in the locust Schistocerca gregaria. J Exp Biol 200:1317–1325
Mercer AR, Menzel R (1982) The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J Comp Physiol 145:363–368
Merlin C, Lucas P, Rochat D, François MC, Maïbèche-Coisne M, Jacquin-Joly E (2007) An antennal circadian clock and circadian rhythms in peripheral pheromone reception in the moth Spodoptera littoralis. J Biol Rhythms 22:502–514. doi:10.1177/0748730407307737
Michels B, Diegelmann S, Tanimoto H, Schwenkert I, Buchner E, Gerber B (2005) A role for synapsin in associative learning: the Drosophila larva as a study case. Learn Mem 12:224–231. doi:10.1101/lm.92805
Miller TA (1985) Structure and physiology of the circulatory system. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry, and pharmacology, vol 3. Pergamon Press, Oxford, pp 289–353
Nagaya Y, Kutsukake M, Chigusb SI, Komatsu A (2002) A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett 329:324–328
Neckameyer WS, Leal SM (2009) Biogenic amines as circulating hormones in insects. In: Pfaff DW et al (eds) Hormones, brain and behavior, 2nd edn, part 2. Academic Press, Amsterdam, pp 967–1003. doi:10.1016/B978-008088783-8.00028-0
Orchard I, Lange AB (1985) Evidence for octopaminergic modulation of an insect visceral muscle. J Neurobiol 16:171–181. doi:10.1002/neu.480160303
Orchard I, Lange AB (1986) Neuromuscular transmission in an insect visceral muscle. J Neurobiol 17:359–372
Orchard I, Lange AB (1987) The release of octopamine and proctolin from an insect visceral muscle: effects of high-potassium saline and neural stimulation. Brain Res 413:251–258
Papaefthimiou C, Theophilidis G (2011) Octopamine—a single modulator with double action on the heart of two insect species (Apis mellifera macedonica and Bactrocera oleae): acceleration vs. inhibition. J Insect Physiol 57:316–325. doi:10.1016/j.jinsphys.2010.11.022
Pass G (1985) Gross and fine structure of the antennal circulatory organ in cockroaches (Blattodea, Insecta). J Morphol 185:255–268
Pass G (1991) Antennal circulatory organs in Onychophora, Myriapoda and Hexapoda: functional morphology and evolutionary implications. Zoomorphology 110:145–164
Pass G (1998) Accessory pulsatile organs. In: Harrison F, Locke M (eds) Microscopic anatomy of invertebrates, vol 11B. Wiley, New York, pp 621–640
Pass G (2000) Accessory pulsatile organs: evolutionary innovations in insects. Annu Rev Entomol 45:495–518
Pass G, Agricola H, Birkenbeil H, Penzlin H (1988a) Morphology of neurons associated with the antennal heart of Periplaneta americana (Blattodea, Insecta). Cell Tissue Res 253:319–326
Pass G, Sperk G, Agricola H, Baumann E, Penzlin H (1988b) Octopamine in a neurohaemal area within the antennal heart of the American cockroach. J Exp Biol 135:495–498
Pass G, Gereben-Krenn BA, Merl M, Plant J, Szucsich NU, Tögel M (2006) Phylogenetic relationships of the orders of Hexapoda: contributions from the circulatory organs for a morphological data matrix. Arthropod Syst Phylogeny 64:165–203
Pawlowa M (1895) Über ampullenartige Blutcirculationsorgane im Kopfe verschiedener Orthopteren. Zool Anz 18:7–13
Pophof B (2000) Octopamine modulates the sensitivity of silkmoth pheromone receptor neurons. J Comp Physiol A 186:307–313
Pophof B (2002) Octopamine enhances moth olfactory responses to pheromones, but not those to general odorants. J Comp Physiol A 188:659–662
Predel R (2001) Peptidergic neurohaemal system of an insect: mass spectrometric morphology. J Comp Neurol 436:363–375. doi:10.1002/cne.1073
Predel R, Neupert S, Wicher D, Gundel M, Roth S, Derst C (2004) Unique accumulation of neuropeptides in an insect: FMRFamide-related peptides in the cockroach Periplaneta americana. Eur J Neurosci 206:1499–1513. doi:10.1111/j.1460-9568.2004.03598.x
Ramirez JM, Pearson KG (1991) Octopamine induces bursting and plateau potentials in insect neurons. Brain Res 549:332–337
Richter M, Hertel W (1997) Contributions to the physiology of the antenna–heart in Periplaneta americana (L.) (Blattodea: Blattidae). J Insect Physiol 43:1015–1021
Rillich J, Stevenson PA, Pflüger HJ (2013) Flight and walking in locusts–cholinergic co-activation, temporal coupling and its modulation by biogenic amines. PLoS One 8:e62899
Roeder T (1999) Octopamine in invertebrates. Prog Neurobiol 59:533–561
Roeder T (2005) Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 50:447–477. doi:10.1146/annurev.ento.50.071803.130404
Schendzielorz T, Schirmer K, Stolte P, Stengl M (2015) Octopamine regulates antennal sensory neurons via daytime-dependent changes in cAMP and IP-3 levels in the hawkmoth Manduca sexta. PLoS One 10:e0121230
Schneider D (1964) Insect antennae. Annu Rev Entomol 9:103–122
Sombati S, Hoyle G (1984) Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol 15:481–506
Stelinski LL, Miller JR, Gut LJ (2003) Increased EAG responses of tortricid moths after prolonged exposure to plant volatiles: evidence for octopamine-mediated sensitization. J Insect Physiol 49:845–856. doi:10.1016/S0022-1910(03)00136-7
Stengl M (2010) Pheromone transduction in moths. Front Cell Neurosci 4:1–5. doi:10.3389/fncel.2010.00133
Stevenson PA (1999) Colocalisation of taurine- with transmitter-immunoreactivities in the nervous system of the migratory locust. J Comp Neurol 404:86–96
Stevenson PA, Kutsch W (1986) Basic circuitry of an adult-specific motor program completed with embryogenesis. Naturwissenschaften 71:741–743
Stevenson PA, Pflüger HJ (1994) Colocalisation of octopamine and FMRFamide related peptide in identified heart projecting (DUM) neurons in the locust revealed by immunocytochemistry. Brain Res 638:117–125
Stevenson PA, Spörhase-Eichmann U (1995) Localization of octopaminergic neurons in insects. Comp Biochem Phys A 110:203–215
Stocker B (2011) Locust thoracic dorsal unpaired median (DUM) neurons: differential activation and peripheral distribution of octopamine and tyramine, Dissertation. Freie Universität Berlin, Berlin
Suggs JM, Jones TH, Murphree SC, Hillyer JF (2016) CCAP and FMRFamide-like peptides accelerate the contraction rate of the antennal accessory pulsatile organs (auxiliary hearts) of mosquitoes. J Exp Biol 219:2388–2395. doi:10.1242/jeb.141655
Tsai JP, Tung LC, Lee MC, Lin JT (2004) The effects of octopamine on the cardiac output of cockroach by using computer-based video analysis on measuring stroke volume. Taiwana 49:7–15
Vergoz V, McQuillan HJ, Geddes LH, Pullar K, Nicholson BJ, Paulin MG, Mercer AR (2009) Peripheral modulation of worker bee responses to queen mandibular pheromone. Proc Natl Acad Sci USA 106:20930–20935. doi:10.1073/pnas.0907563106
Verlinden H, Vleugels R, Marchal E, Badisco L, Pflüger HJ, Blenau W, Broeck JV (2010) The role of octopamine in locusts and other arthropods. J Insect Physiol 56:854–867. doi:10.1016/j.jinsphys.2010.05.018
von Nickisch-Rosenegk E, Krieger J, Kubick S, Laage R, Strobel J, Strotmann J, Breer H (1996) Cloning of biogenic amine receptors from moths (Bombyx mori and Heliothis virescens). Insect Biochem Mol Biol 26:817–827
Watson AH (1984) The dorsal unpaired median neurons of the locust metathoracic ganglion: neuronal structure and diversity, and synapse distribution. J Neurocytol 13:303–327
Wirkner CS, Tögel M, Pass G (2013) The arthropod circulatory system. In: Minelli A, Boxshall G, Fusco G (eds) Arthropod biology and evolution, molecules, development, morphology. Springer, Berlin, pp 343–391
Woodring AP, Stoltzman CA, Stay B (1992) Allatostatins in the nerves of the antennal pulsatile organ muscle of the cockroach Diploptera punctata. Arch Insect Biochem Physiol 20:253–263
Zhukovskaya MI (2007) Aminergic regulation of pheromone sensillae in the cockroach Periplaneta americana. J Evol Biochem Physiol 43:318–326
Zhukovskaya MI (2008) Selective regulation of sensitivity to odors of different behavioral significance in the American cockroach, Periplaneta americana. Physiol Entomol 33:162–166
Zhukovskaya MI (2012) Modulation by octopamine of olfactory responses to nonpheromone odorants in the cockroach, Periplaneta americana L. Chem Senses 37:421–429
Zhukovskaya MI, Kapitsky SV (2006) Activity modulation in cockroach sensillum: the role of octopamine. J Insect Physiol 52:76–86
Zornik E, Paisley K, Nichols R (1999) Neural transmitters and a peptide modulate Drosophila heart rate. Peptides 20:45–51
Acknowledgements
We thank John Plant for linguistic assistance. The support of the DFG (DFG FOR 1363, Pf128/30-1) and of the Austrian Science Fund FWF P 23251 is gratefully acknowledged. Furthermore, special thanks go to Heike Wolfenberg, Konstantin Lehmann, and Leonard Nadler for their immense support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antemann, V., Pass, G. & Pflüger, HJ. Octopaminergic innervation and a neurohaemal release site in the antennal heart of the locust Schistocerca gregaria . J Comp Physiol A 204, 131–143 (2018). https://doi.org/10.1007/s00359-017-1213-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1213-5