Abstract
There is widespread interest in the use of bone marrow stromal cells (BMSC) for tissue reconstruction and repair and for gene therapy. BMSC represent the differentiated progeny of CFU-F, which however comprise a developmentally heterogeneous population as is reflected in the cellular heterogeneity of the cell populations to which they give rise. We have compared the efficacy of monoclonal antibodies recognising a series of stromal antigens, viz. STRO-1, HOP-26, CD49a and SB-10/CD166, as tools for the enrichment of CFU-F prior to culture and as developmental markers for culture-expanded BMSC. In freshly isolated bone marrow mononuclear cells (BMMNC), the proportion of antigen-positive cells was 27%, 46%, 5% and 19% for STRO-1, HOP-26, CD49a and CD166, respectively. All CD49a+ cells co-expressed STRO-1. The degree of CFU-F enrichment obtained with anti-CD49a (~18-fold) by a one-pass immunoselection strategy was significantly greater than that of all other antibodies tested. BMSC expressed higher levels of all antigens investigated (except for HOP-26) compared with BMMNC. Expression of STRO-1 and CD49a remained restricted to a subset of BMSC, whereas all BMSC were SB-10/CD166 positive. Treatment with dexamethasone (10 nM), which promotes the differentiation and further maturation of cells of the osteogenic lineage in this cell culture system, increased the expression of CD49a and HOP-26. The CD49a+ and HOP-26+ fractions of BMSC were further subdivided by dual-labelling with anti-STRO-1 and B4–78 (an antibody recognising the B/L/K isoform of the enzyme alkaline phosphatase), respectively. By using a variety of criteria, the HOP-26 antigen was identified as CD63, a member of the tetraspanin family of proteins thought to modulate integrin compartmentalisation and signalling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pioneering studies of Friedenstein and colleagues (Friedenstein et al. 1966, 1970; Friedenstein 1980) revealed that adult bone marrow contains rare (1 in 104–105 bone marrow mononuclear cells; BMMNC) clonogenic precursors (CFU-F) associated with the soft fibrous tissue of the marrow stroma. They revealed further that, within the CFU-F population, are cells capable of forming bone and reconstituting the haematopoietic microenvironment (Friedenstein et al. 1987). This observation, which has subsequently been confirmed by others (Kuznetsov et al. 1997a, 1997bb), has been cited as evidence for the existence of marrow stromal stem cells. In the absence of definitive proof, however, it has been suggested by some workers that these cells should be considered as multipotential progenitors rather than true stem cells (Bianco and Cossu 1999; Bianco et al. 2001).
When explanted in vitro, CFU-F give rise to adherent cultures of bone marrow stromal cells (BMSC). The results of studies to date suggest that appropriately primed BMSC may have a clinical application in the reconstruction and repair of cartilage and bone, in the haematopoietic microenvironment (Bruder et al. 1994, 1998a, 1998b; Connolly 1998; Krebsbach et al. 1998; Yoo and Johnstone 1998; Oreffo and Triffitt 1999; Nilsson et al. 1999; Petite et al. 2000; Kon et al. 2000; Koc et al. 2000) and in the correction of inherited disorders of the skeleton (Horowitz et al. 1999, 2001; McMahon et al. 2001). In addition, evidence is accumulating that a subset of BMSC has the capacity to differentiate into cells of a multiplicity of tissue types; these may represent a viable, and ethically less controversial, alternative to the use of human embryonic stem cells (Ferrari et al. 1998; Kopen et al. 1999; Makino et al. 1999; Woodbury et al. 2000; Mezey et al. 2000; Lagasse et al. 2000; Jiang et al. 2002).
CFU-F comprise a heterogeneous population of cells at different stages of differentiation and lineage commitment. In addition, little is known of the factors that regulate their numbers and promote the proliferation and further differentiation of their immediate progeny. This lack of knowledge presents a major obstacle to the widespread clinical exploitation of their developmental potential and, to a significant extent, reflects the current lack of a stromal CD repertoire analogous to that developed for cells of the haematopoietic system.
Of the available antibodies that appear to recognise stage- and/or lineage-specific stromal antigens, the most extensively used has been STRO-1. This recognises a trypsin-resistant cell-surface antigen expressed by a subset of freshly isolated BMMNC that includes essentially all assayable CFU-F (Simmons and Torok-Storb 1991; Gronthos et al. 1994). Other antibodies reported to recognise antigens expressed by the CFU-F population of human bone and marrow include HOP-26 (Joyner et al. 1997), SB-10 (subsequently shown to recognise the activated leukocyte adhesion molecule CD166: ALCAM; Bruder et al. 1997, 1998c) and, more recently, those recognising CD49a, the α1 integrin subunit (Deschaseaux and Charbord 2000). Each of these antibodies has been reported to facilitate the isolation of CFU-F from adult human bone marrow and to provide a means for assessing the developmental status of the cells to which they give rise, in particular those of the osteogenic (bone-forming) lineage.
In this investigation, the colony forming efficiency (CFE) of BMMNC subsets obtained by using monoclonal antibodies recognising STRO-1, CD166, HOP-26 and CD49a has been determined, as has the extent to which the expression of their cognate antigens remains developmentally regulated in culture. Our results show that, when used in isolation, the proportion of CFU-F recovered in the CD49a+ fraction is consistently greater than that obtained by using any of the other antibodies tested. In vitro, the expression of all antigens is up-regulated, although that of STRO-1, CD49a and HOP-26, which we identify here as the lysosomal membrane glycoprotein CD63, remains restricted to a subset of BMSC. The relative merits of these antibodies as a means of identifying and isolating CFU-F from adult human bone marrow is considered, as is their potential utility as developmental markers of cells of the osteoblast lineage.
Materials and methods
Subjects
Femoral reamings were obtained during routine surgery from osteoarthritic patients. Patients with a history of corticosterone treatment were excluded from this study. Samples were obtained according to procedures approved by the local ethics committee.
Cell preparation and culture
BMMNC were isolated by density gradient centrifugation over Ficoll (1.077 g/ml; Lymphoprep, Nycomed, Birmingham, UK), washed in serum-free Dulbecco's modified Eagles medium (DMEM; Life Technologies, Paisley, UK) and filtered through a 70-μΜ pore-size cell strainer (Becton Dickinson Labware, Fahrenheit, Milton Keynes, UK) to obtain a single cell suspension.
To obtain BMSC cultures, BMMNC were seeded at a density of 3×104 cells/cm2 in DMEM supplemented with 15% (v/v) fetal bovine serum (FBS; Sigma, Poole, UK) and 100 μM L-ascorbate-2-phosphate (Alpha Laboratories, Eastleigh, UK; Nomura et al. 1969). Non-adherent haematopoietic cells were removed after 5–7 days by washing twice with calcium/magnesium-free phosphate-buffered saline (PBS; Oxoid, Basingstoke, UK) and the cultures were fed twice weekly thereafter. To increase cell yields, BMSC were sub-cultured at day 14 as follows. Cultures were rinsed twice with PBS then incubated in serum-free DMEM containing 25 U/ml collagenase VII (Sigma) and an additional 2 mM CaCl2 for 90 min at 37°C. After being rinsed twice with PBS, cultures were incubated in 30 μl/cm2 trypsin-EDTA (Life Technologies) for 5–10 min at 37°C until the cells became detached. Medium was added and the cells were then grown until a confluent monolayer was formed (2–4 weeks; termed secondary cultures).
MG-63 and SaOS-2 osteosarcoma cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, Md., USA) and were grown in DMEM supplemented with 10% FBS. Prior to characterisation studies, cells were cultured until a confluent monolayer had formed and were then harvested following treatment with trypsin-EDTA. Rat basophilic leukaemia cells (RBL-2H3) transfected with human CD63 (RBL-CD63+; Smith et al. 1995) or CD9 (RBL-CD9+; Higginbottom et al. 2000) were grown in DMEM supplemented with 10% FBS and 400 μg/ml G418 (Sigma).
Antibodies
The antibody-producing hybridoma cell line STRO-1 (Simmons and Torok-Storb 1991) was obtained from Dr. P.J. Simmons, Peter MacCallum Cancer Institute, Melbourne, Australia. The B4-78 hybridoma (Lawson et al. 1985) was obtained from the Developmental Studies Hybridoma Bank, Iowa, USA. STRO-1 (IgM subclass) was used in the form of undiluted tissue culture supernatant and was routinely tested for giving more than 95% reactivity with the human osteosarcoma cell line MG-63, a cell line known to express high levels of the STRO-1 antigen (Stewart et al. 1999). Purified anti-alkaline phosphatase (AP) antibody from the B4-78 hybridoma (IgG1 subclass) was generated by using a protein G column (Pharmacia Biotech, St. Albans, UK). HOP-26 antibody (Joyner et al. 1997; IgM subclass) was obtained from Dr. J.T. Triffitt, Nuffield Orthopaedic Centre, Headington, Oxford, UK and used as undiluted tissue culture supernatant. An antibody raised against activated lymphocyte cellular adhesion molecule (ALCAM), CD166 (clone 18; IgG1 subclass) was obtained from Antigenix America, New York, USA. An antibody raised against the alpha 1 integrin subunit, CD49a (clone TS2/7; IgG1 subclass), was obtained from Serotec, Oxford, UK and an antibody raised against CD63 (clone H5C6; IgG1 subclass) was obtained from PharMingen, USA. Culture supernatant from an IgM-secreting hybridoma reactive with an irrelevant antigen (in-house) and non-immune mouse IgG1 obtained from Sigma (Poole, UK) were used as negative controls. Fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgM, FITC-conjugated anti-mouse IgG1 and R-phycoerythrin (RPE)-conjugated anti-mouse IgG1 antibodies were supplied by Cambridge Bioscience, Cambridge, UK.
Magnetic-activated cell sorting
Freshly isolated BMMNC were resuspended in Hanks' buffered saline solution (HBSS; Life Technologies) containing 10 mM HEPES (Sigma), 10% normal human AB serum (Blood Transfusion Service, Bristol, UK), 1% bovine serum albumin (BSA; Sigma) and 5% FBS (termed blocking buffer) at a concentration of 107/ml and incubated on ice for 20–30 min in order to block non-specific binding sites. Cells (108) were then pelleted and resuspended in 5 ml of either STRO-1 or HOP-26 supernatant or in 1 ml DMEM supplemented with 10% FBS containing 10 μg/ml CD166 or CD63 antibodies or 2 μg CD49a antibody. Cells were incubated on ice for 60 min, with regular mixing, washed in PBS supplemented with 2 mM EDTA (Sigma) and 0.5% (w/v) BSA (Sigma; MACS buffer, degassed according to instructions) and resuspended in 1 ml blocking buffer containing 200 μl rat anti-mouse IgM or IgG1 MicroBeads (Miltenyi Biotec, Surrey, UK). After 30 min on ice, FITC-conjugated anti-mouse IgM or IgG1 antibody was added (1/25 dilution) and incubation continued for a further 15 min. Cells were then washed and resuspended in 1 ml MACS buffer. Finally, cells were applied to MS separation columns (Miltenyi Biotec) and separated into positively- and negatively-labelled fractions by using a MiniMACS separation unit (Miltenyi Biotec) as outlined in the protocols supplied. The purity of recovered cell fractions was assessed by flow cytometry (range: 55%–95%). Remaining cells were seeded into 25-cm2 flasks at a density of 7×103/cm2 and cultured as described for BMSC, in triplicate or quadruplicate. Unseparated BMMNC were seeded at an equivalent density and grown under identical conditions for comparison.
Determination of the colony forming efficiency and number of colonies expressing AP
After 14–17 days of BMSC culture, the colonies formed were washed twice with PBS, fixed in methanol for 5 min at room temperature and allowed to air-dry. The number of colonies expressing AP was determined as previously described (Scutt and Bertram 1999). The total number, diameter and morphological characteristics of colonies present was assessed after staining with methylene blue (Scutt and Bertram 1999) by using an Olympus SZ-CTV microscope (×15 objective) and a measuring graticule.
Flow cytometry
Flow cytometric analysis was carried out as previously described (Stewart et al. 1999). The procedure for the single labelling of cells was as described below for dual labelling, with the exception that cells were incubated with either STRO-1, HOP-26, anti-CD166, anti-CD49a, B4–78 or isotype-matched control antibody alone and binding was detected by using either FITC-labelled goat anti-mouse IgM or goat anti-mouse IgG1 antibodies (1/25 dilution). Positivity was recorded as the percentage of labelled cells with fluorescence ≥95% control cells.
For competition binding experiments, cells were preincubated with either HOP-26, CD63 or equivalent concentrations of isotype-matched control antibodies for 2 h at 4°C and then washed once in HBSS supplemented with 10 mM HEPES and 5% FBS, pH 7.35 (HBSS/FBS) prior to labelling for either CD63 or HOP-26 expression, respectively, as described below for dual staining. Binding was detected by using either FITC-labelled goat anti-mouse IgM or goat anti-mouse IgG1 antibodies (1/25 dilution). Positivity was recorded as the percentage of labelled cells with fluorescence ≥95% control cells.
For dual staining, cells were resuspended in blocking buffer at a concentration of 106/ml and incubated for 20–30 min on ice. Cells (105) were pelleted and resuspended in 500 μl STRO-1 or HOP-26 supernatant, representing a saturating concentration of the antibodies and containing an optimised dilution of mouse IgG1 antibody directed against either AP or CD49a. Negative control mouse IgM and IgG1 antibodies were used under identical conditions. Cells were incubated for 60 min on ice and then washed in HBSS/FCS. Antibody binding was detected by resuspending cells in 100 μl blocking buffer containing FITC-labelled goat anti-mouse IgM (1/25 dilution) and RPE-labelled goat anti-mouse IgG1 (1/200 dilution) antibodies and incubating them for 45 min on ice. Finally, cells were washed once and resuspended in HBSS/FBS for analysis on a FACStarPLUS flow cytometer (Becton Dickinson, Oxford, UK). Control samples were used to align the cytometer and to set compensation for the interference between fluorescence signals; 10,000 events were collected for each sample and data was analysed by using the Cell Quest software package (Becton Dickinson).
Statistical analysis
Statistical analysis (ANOVA; Mann-Whitney U-test) was carried out by using version 4.5 of the StatView package (Abacus Concepts, Berkeley, Calif., USA).
Results
Enrichment for CFU-F in antigen-positive BMMNC fractions
A "one-pass" immunoselection strategy was used to isolate fractions of BMMNC either enriched or depleted in cells bearing the antigen of interest. This proved sufficient to obtain a substantial increase in the proportion of antigen-positive cells (mean fold enrichment of 2, 3, 7 and 10 for HOP-26, CD166, STRO-1 and CD49a respectively; Table 1) whilst ensuring their recovery in numbers sufficient for functional studies of colony-forming efficiency. The purity of the recovered fractions for STRO-1, HOP-26 and CD166 was typically ≥70%, whereas that for CD49a was appreciably less at 55% (Table 1). When the recoveries were examined, however, it was clear that, for each antibody, only a minor proportion of antigen-positive BMMNC was being retained on the column (≤5%; Table 1). The data for the mean fluorescence intensity (MFI) suggested that this "bound fraction" represented the antigen "bright" cell population, and that, by inference, the antigen "dim" cells were being lost in the washes (Table 1).
Functional analysis of the MACS-separated populations of BMMNC revealed that, for each of the antibodies investigated, CFU-F were recovered almost exclusively in the antigen-positive fractions. When compared with each other and with freshly isolated BMMNC, CFE was consistently greatest in the CD49a+ fraction and least in the CD166+ fraction (Table 1).
Antigen expression by culture-expanded BMSC
With the exception of CD166, which in all cases was expressed by the majority of cells, there was substantial inter-donor variation in the expression (% positive cells), by culture-expanded BMSC, of all the antigens investigated (Table 2). Analysis of the pooled data from multiple donors revealed that the proportion of cells expressing the HOP-26 antigen was similar in freshly isolated BMMNC and BMSC (40%–50%; Table 2). For the remaining antigens investigated, there was a tendency for the proportion of positive cells to increase with time in culture, although only in the cases of STRO-1 and CD166 were the increases of statistical significance (P<0.05 and 0.01 respectively; Table 2). The CD166-positive fraction of BMSC was also consistently brighter (greater MFI) than that of freshly isolated BMMNC (P<0.01; Table 2).
Co-expression of STRO-1 and CD49a
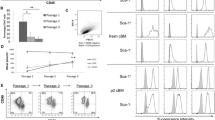
In contrast to the findings of Deschaseux and Charbord 2000, we found that essentially all CD49a+ BMMNC co-expressed the STRO-1 antigen (Fig. 1). In culture, however, CD49a+ BMSC were detected in the STRO-1+ and STRO-1- fractions (Fig. 1). Treatment with dexamethasone (Dx), which promotes the differentiation and further maturation of cells of the osteogenic lineage in this cell culture system (Stewart et al. 1998; Walsh et al. 2001), increased the proportion of BMSC expressing CD49a+ and the level (MFI) at which this antigen was expressed (P<0.05; Figs. 1, 2). The magnitude of this increase (approximately 1.8-fold) was similar in the STRO-1+ and STRO-1- fractions (Fig. 1). The Dx-induced increase in the proportion of BMSC with the surface phenotypes CD49a+/STRO-1+ and CD49a+/STRO-1− was associated with a decrease in the proportion of cells recovered in the STRO-1+/CD49a− fraction (Fig. 1).
Co-expression of STRO-1 and CD49a by BMMNC and confluent secondary cultures of BMSC with and without 10 nM dexamethasone (+Dx and −Dx, respectively). Flow cytometric data from representative experiments are shown as dot plots with CD49a fluorescence on the y-axis and STRO-1 fluorescence on the x-axis. Quadrants were set based on the fluorescence obtained with isotype-matched control antibodies. The percentage of positively labelled cells is given
Expression of CD49a in confluent secondary cultures of BMSC maintained in the absence (−Dx) or continuous presence (+Dx) of 10 nM Dx. Flow cytometric data is shown as mean±SEM percentage of positive cells with ≥95% control fluorescence or mean fluorescence intensity (MFI; n=3; *P<0.05 Dx treated vs untreated, Mann-Whitney U-test)
Co-expression of HOP-26 and AP
A significant proportion of HOP-26+ BMSC were found to co-express AP, an early developmental marker for cells of the osteoblast lineage (Fig. 3). Treatment with Dx increased the size of the HOP-26+/AP+ fraction without affecting that of the HOP-26+/AP− fraction (Fig. 3). Similarly, culture of BMSC in the continuous presence of 10 nM Dx resulted in an increase in the proportion of STRO-1+/AP+ cells (Fig. 3).
Co-expression of STRO-1 or HOP-26 and AP in confluent secondary cultures of BMSC maintained in the absence (−Dx) or continuous presence (+Dx) of 10 nM Dx. Flow cytometric data from representative experiments are shown as dot plots with AP fluorescence on the y-axis and STRO-1 or HOP-26 fluorescence on the x-axis. Quadrants were set based on the fluorescence obtained with isotype-matched control antibodies. The percentage of positively labelled cells is given
Identification of HOP-26 as CD63
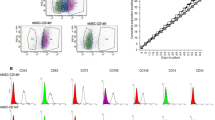
During the course of these studies, the antigen recognised by the antibody HOP-26 was identified as CD63. Preliminary screening with a panel of monoclonal antibodies recognising CD antigens revealed that the HOP-26 antigen and CD63 co-localised in freshly isolated BMMNC and in culture-expanded BMSC (data not shown). When saponin-permeabilised cells were analysed for HOP-26 binding by flow cytometry, high levels of intracellular binding sites were detected, an observation consistent with HOP-26 recognising CD63, a lysosomal membrane protein (data not shown). Subsequently, we found that CD63-transfected RBL-2H3 cells were both CD63+ and HOP-26+ (Fig. 4B). Neither HOP-26 nor H5C6 (anti-CD63) bound to untransfected cells (Fig. 4A), nor did cells transfected with the related tetraspanin CD9 (Fig. 4D). Cells transfected with a C-terminal deletion mutant of CD63 lacking the internalisation motif (CD63–7) showed an increased number of cell-surface binding sites (MFI) for both CD63 and HOP-26 (Fig. 4C). Competition studies revealed that H5C6 could inhibit the binding of HOP-26 to CD63-transfected RBL cells (Fig. 5A) and that the reverse was also true (Fig. 5B). Consistent with HOP-26 being of the IgM subclass, and therefore of lower affinity, the degree of inhibition obtained was less than that observed with H5C6, an antibody of the IgG1 subclass. Finally, the results of preliminary experiments showed that the CFE of the CD63+ and HOP-26+ fractions of BMMNC obtained from the same donor were essentially the same (data not shown).
Expression of HOP-26, CD63 and CD9 by untransfected RBL-2H3 cells (A), and CD63-transfected (B), CD63–7-transfected (C) or CD9-transfected (D) RBL-2H3 cells. Binding of the antibodies was detected by using an FITC-conjugated second antibody. Flow cytometric data are shown as frequency histograms of fluorescence intensity for representative experiments. The percentage of positively labelled cells with ≥95% control fluorescence and the mean fluorescence intensity are given. In immunohistochemical studies, cells were counterstained with ethidium bromide (ND not done). ×400
Competition of HOP-26 antibody (A) or CD63 antibody (B) binding to CD63-transfected RBL-2H3 cells with either CD63 or HOP-26 antibodies, respectively. Binding was detected by using an FITC-conjugated second antibody by flow cytometry. Data are shown as the percentage of inhibition based on the percentage of positively labelled cells recorded
Discussion
The bone marrow stroma comprises a developmentally heterogeneous population of cells that show a wide variation in their potential for proliferation and differentiation (Mankani and Robey 1998; Owen 1998; Phinney et al. 1999; Bianco et al. 2001). An antibody-based stromal classification system, akin to the CD nomenclature that exists for the haematopoietic system, would have a number of important benefits. It would lead to an improved understanding of the relationships that exist between the different lineages that form a part of this complex hierarchy. It would enable the effects of therapeutic agents (e.g. bone anabolics) or treatment regimens (chemotherapy, radiotherapy) on the cellular dynamics of the marrow stromal system to be assessed. Most importantly, it would provide a rational basis for realising the full clinical potential of ex vivo expanded subsets of BMSC of defined developmental potential in tissue reconstruction and repair, transplantation medicine and gene therapy.
The isolation of CFU-F from adult human bone marrow by using the monoclonal antibody STRO-1 was the first indication that this rare population of clonogenic precursors might be distinguishable from other BMMNC on the basis of their cell surface phenotype (Simmons and Torok-Storb 1991). It is important to appreciate, however, that, although relatively enriched in CFU-F, the STRO-1+ fraction also contains other cell types of both stromal and haematopoietic origin (Simmons and Torok-Storb 1991; Gronthos et al. 1994). Consequently, there is an urgent need for additional antibody reagents that recognise this clinically important cell population. Based on the literature, HOP-26 (Joyner et al. 1997) and those antibodies recognising CD166 (Bruder et al. 1997, 1998c) and CD49a (Deschaseaux and Charbord 2000) might satisfy this need. Prior to this investigation, however, no attempt had been made to compare directly their utility as reagents for the immunoselection of CFU-F or as developmental markers of culture-expanded BMSC.
In this investigation, in which a one-pass immunoselection strategy was employed, the incidence of CFU-F was consistently greatest in the CD49a+ fraction, although the degree of enrichment obtained was markedly donor-dependant. Absolute recovery of CFU-F, however, was consistently greatest in the STRO-1+ fraction (49% compared with 24% for the CD49a+ fraction). No correlation was observed between the degree of enrichment obtained and the purity of the antigen-positive fraction. Thus, the purity of the CD49a+ fraction was the least of all the antibodies investigated and yet it contained the highest proportion of CFU-F. Whereas the purity of this fraction could have been increased by passage over a second column, this, in our experience, would have been achieved only at the expense of a significant reduction in the absolute number of antigen-positive cells recovered (data not shown).
The relative enrichment of CFU-F in the CD49a+ fraction was approximately 18-fold, which was in good agreement with the findings of Deschaseux and Charbord (2000). In contrast to them, however, we found that essentially all CD49a+ BMMNC co-expressed STRO-1. This discrepancy may be related to the use of marrow sampled from different anatomical sites and from donors of different ages.
The recovery of CFU-F in the STRO-1, CD49a and HOP-26 antigen-positive fractions was considerably less than that reported previously by other investigators (Simmons and Torok-Storb 1991; Joyner et al. 1997; Deschaseaux and Charbord 2000). We suspect that this discrepancy relates to our use of the MACS protocol, which, based on a comparison of the pre- and post-selection values of MFI, appears to be selective for cells in the "antigen-bright" subpopulation. The absence of "antigen-dim" cells in the unbound fraction suggests that they are lost in the column washes. It will be important to establish whether this population contains additional CFU-F and, if so, whether their developmental potential differs from those recovered in the "antigen-bright" population.
The incidence of HOP-26+ BMMNC in this investigation (approximately 42%) is considerably greater than that reported by Joyner et al. (1997; ≤1%). This discrepancy most likely reflects the greater sensitivity of flow cytometry when compared with the immunostaining protocol used by those authors. A further factor is their use of fixed cell populations; we have found that the choice of fixative markedly alters the sensitivity of the subsequent staining procedure for a number of stromal antigens, including HOP-26 (data not shown). It seems reasonable to assume, therefore, that Joyner et al. (1997) detected only the "antigen-bright" subpopulation of HOP-26+ cells: a possibility supported by our observation that this fraction does indeed comprise only 1% of BMMNC.
There is widespread interest in the possibility of exploiting the developmental potential of CFU-F for tissue reconstruction and repair. The low incidence of this cell type, even in the bones of young healthy adults, has led many to propose the use of culture-expanded populations of BMSC in their stead. A major, and as yet unresolved, limitation of this approach is that, whilst capable of generating large numbers of cells, the cultures obtained remain poorly defined in terms of the extent and nature of their cellular heterogeneity. In consequence, the prospective evaluation of the likely clinical impact of the cultured cell population, although highly desirable, is currently not feasible. Establishing whether any of the antigens investigated has the potential to sub-type culture-expanded BMSC is considered, therefore, to have a high priority.
When cultured ex vivo, BMMNC gave rise consistently to populations of BMSC expressing the antigens STRO-1, CD49a, HOP-26 and CD166. Irrespective of the donor of origin, CD166 was expressed by ≥95% of BMMNC. A similar result was obtained by Bruder et al. (1997) and, on this basis, it was concluded that culture-expanded BMSC comprise an homogeneous population. The results presented here suggest that this conclusion was premature and illustrate the potential pitfalls of assigning identity on the basis of the expression of a single antigenic marker.
In contrast to that of CD166, the expression of STRO-1, CD49a and HOP-26 in vitro was markedly donor-dependent. We and others have shown that the potential of BMSC for proliferation and differentiation is similarly donor-dependent and that this variation is unrelated to differences in age or gender (Gundle and Beresford 1995; Phinney et al. 1999; Walsh et al. 2000). In future studies, it will be important to establish whether such differences in the developmental potential of BMSC can be linked to differences in the expression of these or other cell surface antigens.
In culture, the expression of STRO-1 and CD49a remains restricted to a subset of cells, albeit a higher proportion than are present in freshly isolated preparations of BMMNC. To assess whether the expression of these antigens by BMSC is developmentally regulated, cells were cultured in the absence or presence of the synthetic glucocorticoid Dx (10 nM), which, as we have shown previously, promotes the differentiation and further maturation of cells of the osteogenic lineage in this human cell culture system (Walsh et al. 2001). As expected, under these conditions, there was a marked increase in the proportion of BMSC present in the putative osteoprogenitor fraction (STRO-1+/AP+) and a decline in the proportion of cells present in the primitive precursor fraction (STRO-1+/AP−; Stewart et al. 1998; Walsh et al. 2001). This was associated with a significant increase in expression of CD49a+ (%-positive cells and MFI, an indirect measure of the number of binding sites/cell) in both the STRO-1+ and STRO-1- subpopulations. One interpretation of these findings is that the expression of CD49a in vitro, like that of STRO-1, is developmentally regulated and linked to the differentiation and/or further maturation of cells of the osteoblast lineage. Further experiments, including the co-localisation of CD49a and other osteoblast markers in vitro and in vivo, will be required to confirm or refute this interesting possibility.
Joyner and co-workers (1997) have reported that the expression of HOP-26 in culture is initially low (≤10%), reaches a maximum at day 3 (95% positive) but declines thereafter, such that, by day 28, less than 1% of BMSC are antigen-positive. Evidence for such temporal regulation of HOP-26 expression is lacking in our investigation. Indeed, its level of expression (%-positive cells and MFI) in BMMNC and BMSC is remarkably similar at 40%–50% of total. We suspect that this discrepancy relates to the use of different techniques (flow cytometry versus immunocytochemistry) to enumerate the fraction of antigen-positive cells.
Treatment with Dx had no consistent effect on the size of the HOP-26+ cell population but did alter the proportion of cells that co-expressed the developmental marker AP, the expression of which is widely accepted as an early indicator that a cell has entered the osteogenic pathway of differentiation. In vivo, the majority of cells, including osteoblasts, are HOP26−, but small numbers of HOP-26+ cells are located close to bone surfaces. On this basis, Joyner et al. (1997) have hypothesised that osteogenic precursors might be included within the HOP-26+ fraction but they did not explore this possibility further by attempting to co-localise the expression of HOP26 and AP. Our demonstration that HOP26+/AP+ BMSC are found in vitro and that their number is increased by treatment with Dx is consistent with the hypothesis of Joyner et al. (1997), but determining the exact developmental status of these cells will require their isolation and further characterisation.
In the course of this investigation, evidence was obtained to support the conclusion that HOP-26 recognises CD63, a lysosomal membrane glycoprotein that belongs to the tetraspanin or transmembrane 4 superfamily (TMS4; Metzelaar et al. 1991; Berditchevski 2001). Our observation of a high level of HOP-26 reactivity in freshly isolated BMMNC is consistent with this possibility, as CD63 is known to be expressed by cells of both haematopoietic and non-haematopoietic origin (Barclay et al. 1993). Molecular cloning of the cell surface antigen recognised by HOP-26 has since provided confirmation of its identity as CD63 (Zannettino et al. 2003).
The functional significance of CD63 expression by primitive cells of the osteoblast (bone-forming) lineage is currently unknown but merits further investigation. TMS4 proteins, including CD63, have been implicated in a wide range of cellular functions including migration, adhesion (both cell-cell and cell-matrix), proliferation and differentiation, possibly reflecting their ability to form complexes with integrins (Smith et al. 1995; McCullough et al. 1996; Berditchevski 2001). In adherent cells, integrin-TMS4 complexes are clustered into a distinct type of adhesion structure at the cell periphery. The presence of the TMS4 moiety is thought to modulate integrin signalling, compartmentalisation on the cell surface and intracellular trafficking and recycling (Berditchevski 2001). Nevertheless, the precise biochemical function of the TMS4 proteins remains undefined and no membrane or soluble protein that might serve as a physiological receptor/ligand has been identified.
Targeting of cell surface markers expressed selectively by primitive cells is potentially a fast and clinically efficient means of purifying a heterogeneous marrow sample. The results that we have presented here suggest that this is a realistic possibility but that further screening of antibodies characterised to date and the generation of antibodies recognising novel antigens is still required, as is an analysis of the antibody-defined cell sub-populations recovered both in vitro and in vivo. Identification of novel differentially expressed markers would further extend the phenotypic classification of the stromal cell lineage and hence enable greater standardisation of research carried out with these cells (Stewart et al. 1996, 2001; Letchford et al. 2001). The more that we learn of the complexities of the stromal system, the more likely it seems that the use of "cocktails" rather than single antibodies will provide the most effective means of isolating primitive marrow stromal cells and defining the developmental potential of the progeny that they give rise to when cultured ex vivo.
References
Barclay N, Birkeland ML, Brown MH, Beyers AD, Davis SJ, Samoza C, Williams AF (1993) The leukocyte antigen facts book. Academic Press, London San Diego
Berditchevski F (2001) Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114:4143–4151
Bianco P, Cossu G (1999) Uno, nessuno e centomila: searching for the identity of mesodermal progenitors. Exp Cell Res 251:257–263
Bianco P, Riminucci M, Gronthos S, Robey PG (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192
Bruder SP, Fink DJ, Caplan AI (1994) Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem 56:283–294
Bruder SP, Horowitz MC, Mosca JD, Haynesworth SE (1997) Monoclonal antibodies reactive with human osteogenic cell surface antigens. Bone 21:225–235
Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S (1998a) Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Rel Res 355S:S247-S256
Bruder SP, Kraus KH, Goldberg VM, Kadiyala S (1998b) The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg 80A:985–996
Bruder SP, Ricalton NS, Boynton RE, Connolly TJ, Jaiswal N, Zaia J, Barry FP (1998c) Mesenchymal stem cell surface antigen SB-10 corresponds to activated leukocyte cell adhesion molecule and is involved in osteogenic differentiation. J Bone Miner Res 13:655–663
Connolly JF (1998) Clinical use of marrow osteoprogenitor cells to stimulate osteogenesis. Clin Orthop Rel Res 355S:S257-S266
Deschaseaux F, Charbord P (2000) Human marrow stromal precursors are α1 intergrin subunit-positive. J Cell Physiol 184:319–325
Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavillo F (1998) Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279:1528–1530
Friedenstein AJ (1980) Stromal mechanocytes of bone marrow: Cloning in vitro and retransplantation in vivo. In: Thiernfelder S (ed) Immunology of bone marrow transplantation. Springer, Berlin Heidelberg New York, pp 19–29
Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16:381–390
Friedenstein AJ, Chailakhjan RK, Lalikina KS (1970) The development of fibroblast colonies in monolayer cultures of guinea pig marrow and spleen cells. Cell Tissue Kinet 3:393–403
Friedenstein AJ, Chailakhjan RK, Gerasimov UV (1987) Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet 20:263–272
Gronthos S, Graves SE, Ohta S, Simmons PJ (1994) The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood 84:4164–4173
Gundle R, Beresford JN (1995) Isolation and culture of cells from explants of human trabecular bone. Calcif Tissue Int 56 (Suppl 1):S8–S10
Higginbottom A, Wilkinson I, McCullough B, Lanza F, Azorsa DO, Partridge LJ, Monk PN (2000) Antibody cross-linking of human CD9 and high-affinity immunoglobulin E receptor stimulates secretion from transfected rat basophilic leukaemia cells. Immunology 99:546–552
Horowitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5:309–313
Horowitz EM, Prockop DJ, Gordon PL, Koo WWK, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK (2001) Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 97:1227–1231
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49
Joyner CJ, Bennett A, Triffitt JT (1997) Identification and enrichment of human osteoprogenitor cells by using differentiation stage-specific monoclonal antibodies. Bone 21:1–6
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM (2000) Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 18:307–316
Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giarino R, Cancedda R, Quarto R (2000) Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res 49:328–337
Kopen GC, Prockop DJ, Phinney DG (1999) Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 96:10711–10716
Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG (1998) Repair of craniotomy defects using bone marrow stromal cells. Transplantation 66:1272–1278
Kuznetsov SA, Friedenstein AJ, Robey PG (1997a) Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol 97:561–570
Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, Gehron Robey P (1997b) Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12:1335–1347
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6:1229–1234
Lawson GM, Katzmann JA, Kimlinger TK, O'Brien JF (1985) Isolation and preliminary characterization of a monoclonal antibody that interacts preferentially with the liver isoenzyme of human alkaline phosphatase. Clin Chem 31:381
Letchford J, Jefferiss C, Walsh S, Stewart K, Beresford JN (2001) Optimisation of selection procedures for the isolation of phage antibodies from STRO-1+ cell populations. J Bone Miner Res 16:P19
Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J-I, Umezawa A, Ogawa S (1999) Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103:697–705
Mankani MH, Robey PG (1998) Transplantation of bone-forming cells. Endocrinologist 8:459–467
McCullough B, Peppa D, Monk PN, Skubitz KM, Partridge LJ (1996) A role for CD63 in signal transduction. Immunology 89 (Suppl 1):OM114
McMahon C, Will A, Hu P, Shah GN, Sly WS, Smith OP (2001) Bone marrow transplantation corrects osteoporosis in the carbonic anhydrase II deficiency syndrome. Blood 97:1947–1950
Metzelaar MJ, Wijngaard PLJ, Peters PJ, Sixman JJ, Nieuwenhuis HK, Clevers HC (1991) CD63 antigen: a novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem 266:3239–3235
Mezey E, Chandross KJ, Harta G (2000) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290:1779–1782
Nilsson SK, Dooner MS, Weier H-U, Frenkel B, Lian JB, Stein GS, Quesenberry PJ (1999) Cells capable of bone production engraft from whole bone marrow transplants in nonablated mice. J Exp Med 189:729–734
Nomura H, Ishiguro T, Morimoto S (1969) Studies on L-ascorbic acid derivatives. III. Bis (L-ascorbic acid-3,3') phosphate and L-ascorbic acid 2-phosphate. Chem Pharm Bull 17:387–393
Oreffo ROC, Triffitt JT (1999) Future potentials for using osteogenic stem cells and biomaterials in orthopedics. Bone 25:5S–9S
Owen ME (1998) The marrow stromal cell system. In: Beresford JN, Owen ME (eds) Marrow stromal cell culture. Cambridge University Press, Cambridge, pp 1–9
Petite H, Viateau V, Bensaid W, Meunier A, Pollak C de, Bourguignon M, Oudina K, Sedel L, Guillemin G (2000) Tissue-engineered bone regeneration. Nat Biotech 18:959–963
Phinney DG, Kopen G, Isaacson RL, Prockop DJ (1999) Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem 72:570-585
Scutt A, Bertram P (1999) Basic fibroblast growth factor in the presence of dexamethasone stimulates colony formation, expansion, and osteoblastic differentiation by rat bone marrow stromal cells. Calcif Tissue Int 64:69–77
Simmons PJ, Torok-Storb B (1991) Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 78:55–62
Smith DA, Monk PA, Partridge LJ (1995) Antibodies against human CD63 activate transfected rat basophilic leukemia (RBL-2H3) cells. Mol Immunol 32:1339–1344
Stewart K, Gronthos S, Simmons PJ, Beresford JN (1996) H8G—a monoclonal antibody that subtypes the STRO-1+ CFU-F population of human bone and marrow. J Bone Miner Res 11:P19
Stewart K, Jefferiss C, Screen J, Walsh S, Beresford JN (1998) The identification and isolation of cells of the osteoblastic lineage in cultures of adult human bone-derived cells by dual labeling with monoclonal antibodies STRO-1 and B4–78. In: Russell RGG, Skerry TM, Kollenkirchen U (eds) Ernst Schering Research Foundation Workshop 25: novel approaches to treatment of osteoporosis. Springer, Berlin Heidelberg New York, pp 43–60
Stewart K, Walsh S, Screen J, Jefferiss C, Chainey J, Jordan GR, Beresford JN (1999) Further characterisation of cells expressing STRO-1 in cultures of adult human bone marrow stromal cells. J Bone Miner Res 14:1345–1356
Stewart K, Gronthos S, Bord S, Zannettino A, Simmons P, Beresford JN (2001) Further characterisation and molecular cloning of the antibody H8G. J Bone Miner Res 16:P14
Walsh S, Jefferiss C, Stewart K, Jordan GR, Screen J, Beresford JN (2000) Expression of the developmental markers STRO-1 and alkaline phosphatase in cultures of human marrow stromal cells: regulation by fibroblast growth factor (FGF)-2 and relationship to the expression of FGF receptors 1–4. Bone 27:185–195
Walsh S, Jordan GR, Jefferiss C, Stewart K, Beresford JN (2001) High concentrations of dexamethasone suppress the proliferation but not the differentiation or further maturation of osteoblast precursors in vitro: relevance to glucocorticoid-induced osteoporosis. Rheumatology 40:74–83
Woodbury D, Schwarz EJ, Prockop DJ (2000) Adult rat brain and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364–370
Yoo JU, Johnstone B (1998) The role of osteochondral progenitor cells in fracture repair. Clin Orthop Rel Res 355S:S73-S81
Zannettino ACW, Harrison K, Joyner CJ, Triffitt JT, Simmons PJ (2003) Molecular cloning of the cell surface antigen identified by the osteoprogenitor-specific monoclonal antibody, HOP-26. J Cell Biochem 89:56–66
Acknowledgements
The authors are grateful to the surgeons and theatre staff at The Royal Devon and Exeter Hospital, Exeter, without whom this work would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
K.S., S.W., C.M.J. and J.A.L. gratefully acknowledge the financial support of the University Bath, the Arthritis Research Campaign and the Wellcome Trust
Rights and permissions
About this article
Cite this article
Stewart, K., Monk, P., Walsh, S. et al. STRO-1, HOP-26 (CD63), CD49a and SB-10 (CD166) as markers of primitive human marrow stromal cells and their more differentiated progeny: a comparative investigation in vitro. Cell Tissue Res 313, 281–290 (2003). https://doi.org/10.1007/s00441-003-0762-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0762-9