Abstract
Observational studies have shown an association between obesity and venous thromboembolism (VTE) but it is not known if observed associations are causal, due to reverse causation or confounding bias. We conducted a Mendelian Randomization study of body mass index (BMI) and VTE. We identified 95 single nucleotide polymorphisms (SNPs) that have been previously associated with BMI and assessed the association between genetically predicted high BMI and VTE leveraging data from a previously conducted GWAS within the INVENT consortium comprising a total of 7507 VTE cases and 52,632 controls of European ancestry. Five BMI SNPs were associated with VTE at P < 0.05, with the strongest association seen for the FTO SNP rs1558902 (OR 1.07, 95% CI 1.02–1.12, P = 0.005). In addition, we observed a significant association between genetically predicted BMI and VTE (OR = 1.59, 95% CI 1.30–1.93 per standard deviation increase in BMI, P = 5.8 × 10−6). Our study provides evidence for a causal relationship between high BMI and risk of VTE. Reducing obesity levels will likely result in lower incidence in VTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
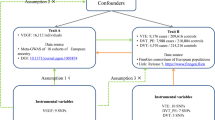
Venous thromboembolism (VTE) is the collective term for deep vein thrombosis (DVT) and its life-threatening complication pulmonary embolism (PE). Between 300,000 and 600,000 Americans are diagnosed with VTE every year, though VTE is under-diagnosed and accurate incidence data are difficult to estimate (Anderson et al. 2007; Heit et al. 2001; Hirsh and Hoak 1996). Further, it has been suggested that 60,000–100,000 Americans die of VTE annually. In addition, approximately a third of all VTE cases experience recurrence within 10 years (Beckman et al. 2010). The underlying mechanisms causing VTE remain, to a large extent, elusive. This is especially true for idiopathic (unprovoked) VTE. Known risk factors include oral contraception, menopausal hormone therapy, smoking, family history of VTE, and high body mass index (BMI) (Heit 2015). Data based on the National Hospital Discharge Survey between 1979 and 1999 showed that obese patients had a 2.5-fold [95% confidence interval (CI) = 2.49–2.51] risk to develop a DVT and 2.2-fold (95% CI = 2.20–2.23) risk to develop PE. Risks associated with obesity were highest in obese individuals less than 40 years old (Stein et al. 2005). Using data from the Nurses’ Health Study, we previously showed that the association between BMI and VTE is linear and apparent even across a modest range (22.5–25 kg/m2) (Kabrhel et al. 2009). A drawback with observational studies is their limited ability to provide insights into causality. Establishing a causal relationship between a risk factor and disease requires circumventing issues of confounding, bias and reverse causation. Indeed, it is possible that observed BMI–VTE associations have been confounded by other risk factors that were not captured appropriately, including smoking habits, dietary habits, physical ability/activity or other underlying medical conditions. Further, observed BMI–VTE associations might also have been subject to reverse causation if individuals diagnosed with VTE have less physical ability leading to increased BMI. Mendelian Randomization (MR) is an approach that utilizes robust genetic predictors of risk factors to assess causal associations between risk factors and diseases (Davey Smith and Hemani 2014). As inherited genetic variation is determined at conception and is in general not vulnerable to confounding, it represents a powerful tool for assessing causal relationships. Here, we leverage information from a recent genome-wide association study (GWAS) of BMI (Locke et al. 2015) to explore the causal relationship between obesity and VTE. We assessed the associations of 95 BMI single nucleotide polymorphisms (SNPs) in a VTE study comprising 7507 cases and 52,632 controls (Germain et al. 2015).
Materials and methods
GWAS of VTE
To assess whether a genetically predicted BMI is associated with VTE, we used data from a recent GWAS of VTE within the INVENT consortium. Details about the GWAS design and participating studies have been published previously (Germain et al. 2015) and can be found in Supplementary Table 1. In total, 7507 VTE cases and 52,632 controls from 12 studies were included. Study participants were European-ancestry adults in two French case–control studies, two Dutch case–control studies, and four cohort and four case–control studies from the United States. In all studies, VTE (PE or DVT) was objectively diagnosed by physicians using different techniques including compression venous duplex ultrasonography, computed tomography, Doppler ultrasound, impedance plethysmography, magnetic resonance, venography, pulmonary angiography, and ventilation/perfusion lung scan. VTE events related to cancer, autoimmune disorders, or natural anticoagulant inhibitor deficiencies (protein C, protein S, antithrombin) were excluded in most studies. All participating studies were approved by their respective institutional review board and informed consent was obtained from studied individuals. Genotyping arrays differed between studies (Germain et al. 2015). Each study was imputed using the 1000 Genomes phase I, version 3 reference dataset. Association analyses were performed separately in each study using logistic or Cox-proportional regression analyses adjusted for study-specific covariates. Results were then combined using fixed-effects meta-analysis. We only included SNPs with a minor allele frequency (MAF) >0.005 and with an imputation quality score (r 2) >0.3 in all 12 studies.
Identification of SNPs associated with BMI
We conducted a literature search to identify and extract information for SNPs that were associated with BMI on a genome-wide significant level (P < 5 × 10−8) in the largest GWAS to date. We identified 97 SNPs associated with BMI (Locke et al. 2015) (Supplementary Table 2). Of those 97 SNPs, 77 reached genome-wide significance in European ancestry populations only (European ancestry-specific P values for the 20 remaining SNPs ranged between 6.0 × 10−8 and 6.1 × 10−6). BMI SNPs rs11057405, rs2245368, and rs7239883 were not assessed in the INVENT VTE GWAS. We replaced rs7239883 with rs4569374 (r 2 = 1.0 in 1000 Genomes CEU population), but we could not identify any proxies for the other two SNPs and they were, therefore, excluded from the analysis. For all identified SNPs, we obtained information about the effect allele, trait-specific association estimates, and standard errors from the original publications. We then extracted VTE-specific effect estimates and P values from the INVENT GWAS for each of the 95 SNPs.
Statistical analysis
Since we do not have access to individual-level GWAS data in the VTE GWAS, we leverage recently developed methods to obtain an estimate of the causal effect of BMI on VTE. Specifically, we conducted 2-sample MR analyses to estimate the association between BMI and VTE using summary genetic association statistics, as described previously (Burgess et al. 2013). The ratio estimate (\(\hat{\beta }\)) of the effect of BMI (X) on VTE (Y) using genetic variants k = 1, …, K (here, K = 95) can be calculated as \(\hat{\beta } = \frac{{\mathop \sum \nolimits_{k} X_{k} Y_{k} \sigma_{{Y_{k} }}^{ - 2} }}{{\mathop \sum \nolimits_{k} X_{k}^{2} \sigma_{{Y_{k} }}^{ - 2} }},\) where X k is the per-allele effect of SNP k on BMI, Y k is the per-allele change in the log odds ratio for VTE for SNP k, and \(\sigma_{{Y_{k} }}\) is the standard error for Y k . The standard error for \(\hat{\beta }\) is given by: \({\text{se}}\left( {\hat{\beta }} \right) = \sqrt {\frac{1}{{\mathop \sum \nolimits_{k} X_{k}^{2} \sigma_{{Y_{k} }}^{ - 2} }}}\). Under certain assumptions (VanderWeele et al. 2014), \(\hat{\beta }\) can be interpreted as the causal log odds ratio of VTE associated with one standard deviation (SD) unit change in BMI. We conducted two sets of analyses, the first analysis including all 95 SNPs for which we had data and the second analysis only including SNPs (n = 75) that had been found associated on genome-wide significant levels with adult BMI in European ancestry populations only. For both SNP sets, effect estimates for BMI were extracted from a European ancestry population. To explore if the effect of BMI SNPs on VTE is independent of known VTE genetic risk factors, we reran the individual SNP analysis conditioning on the known VTE SNPs including F5 rs6025, F5 rs4524, F11 rs2289252, F11 rs2036914, FGG rs2066865, ABO rs8176645, ABO rs8176746, ABO rs2519093, F2 rs1799963, PROCR rs867186, and PROCR rs6088735 (Germain et al. 2015). We then reran the MR analysis including the 95 SNPs that have been shown to be associated with BMI. A key assumption in MR analysis is that the instrumental variables (genetic variants) can only be associated with the outcome through the risk factor of interest (BMI) and not through some other pathway (so called pleiotropy). Therefore, we conducted sensitivity analyses using MR Egger regression (Bowden et al. 2015) to assess bias from directional pleiotropy. All P values are unadjusted for multiple testing.
Results
We selected 97 SNPs that have been found to be associated with BMI on a genome-wide significant level in a meta-analysis including all ethnicities (Locke et al. 2015). Together these SNPs explain ~2.7% of the variation in BMI. We were able to include 95 SNPs in our MR analysis. Five SNPs were associated with VTE at P < 0.05, with the strongest association seen for the FTO SNP rs1558902 (OR 1.07, 95% CI 1.02–1.12, P = 0.005, Supplementary Table 2). The plot of VTE effect sizes vs. BMI effect sizes is seen in Fig. 1. The overall association between genetically predicted BMI and VTE was 1.59 per SD increase in BMI (95% CI 1.30–1.93, P = 5.8 × 10−6; Table 1). We reran the analysis including only the 75 SNPs found to be genome-wide significant in European ancestry only and observed a similar association (OR = 1.58, 95% CI 1.28–1.95 per SD increase in BMI, P = 2.02 × 10−5; Table 1). To explore if the effect of BMI SNPs on VTE is independent of known VTE genetic risk factors, we reran the analysis conditioning on 11 known VTE SNPs. The overall association between genetically predicted BMI (based on 95 BMI-related SNPs) and VTE remained unchanged (OR = 1.62 per SD increase in BMI, 95% CI 1.31, 2.00; P = 7.2 × 10−6). We further explored the potential impact of directional pleiotropy by conducting MR Egger regression to estimate the average directional pleiotropic effect across the SNPs and to estimate the causal effect of BMI after adjusting for potential directional pleiotropic effects. We found that the intercept from MR Egger regression was not significantly different from zero (−0.005, 95% CI −0.02, 0.01; P = 0.43) suggesting no directional pleiotropy. Further, the estimated causal effect estimated by MR Egger regression was larger in magnitude compared to the MR analysis (OR = 1.90, 95% CI 1.17, 3.08; P = 0.01; Table 1).
Discussion
We used a Mendelian Randomization approach to assess the causal relationship between BMI and VTE in a European ancestry population. Our results showed evidence for a causal association between higher BMI and VTE, lending support to previous observational studies. Among the SNPs we assessed, we observed the strongest association for the FTO locus. SNP rs1558902 has been estimated to explain 0.33% of the variation in adult BMI, which is almost three times as much as any other single SNP and interestingly, this is also the SNP that showed strongest association with VTE in this analysis. Due to its association with BMI, SNP rs1558902 has been assessed for association with multiple other traits and has been found to be associated with C-reactive protein (CRP) levels and HDL cholesterol (Ligthart et al. 2016). A correlated SNP, rs9939609 (r-sq = 0.92), has been found to be associated with a plethora of traits including heart failure, type II diabetes, dyslipidemia, hypertension, metabolic syndrome, liver enzymes, fasting insulin, CRP, and triglycerides (Fall et al. 2013).
In addition to its association with environmental risk factors like obesity, VTE has a strong genetic basis following a multifactorial inheritance model (Crous-Bou et al. 2016). Most identified genetic risk factors involve mutations in the clotting system, including: variants in factor V (e.g. the Factor V Leiden mutation), prothrombin (e.g. prothrombin 20210-A), fibrinogen gamma, antithrombin, protein C, protein S as well as genetic variations coding for blood group non-O. To explore if the observed association between genetically predicted BMI and VTE is independent of known VTE genetic risk factors, we reran the analysis using VTE SNP effects conditioned on 11 known VTE genetic variants. The association between genetically predicted BMI and VTE remained unchanged, arguing that the causal effect of BMI on VTE is independent from known VTE genetic risk markers.
There are several plausible biological mechanisms by which obesity causes VTE. At one level, the development of VTE depends on mechanical factors such as venous stasis. Obesity may decrease venous return of blood from the lower extremities thereby increasing the risk of both VTE and chronic venous insufficiency (Willenberg et al. 2010). Obesity is also correlated with low-grade chronic inflammation (Blokhin and Lentz 2013), and inflammatory markers (e.g. CRP and albumin) have been shown to modulate the relationship between BMI and VTE (Olson et al. 2014). Finally, obesity may lead to impaired fibrinolysis, as obese subjects have increased circulating levels of plasminogen activator inhibitor-1 (PAI-1) which inhibits the breakdown of clot (Ouchi et al. 2011; Shimomura et al. 1996). Based on recent estimates, more than one-third of Americans are obese, and the population prevalence of obesity in western countries continues to rise. The most recent data reported by the Centers for Disease Control and Prevention suggest that 37.7% of adults in the United States were obese in 2013–2014. This is up from 30.5% in 1999–2000 (Ogden et al. 2015). Simultaneously, the incidence of VTE has also been increasing. In 2009, the annual incidence of first-time VTE diagnosis (DVT or PE or both) was 133/100,000 individuals. This is up from 95/100,000 individuals in 1999 (Heit et al. 2016). The increased incidence of VTE is multifactorial, and likely related to both increasing disease occurrence in the population and increased detection due to improvements in diagnostic technology. By demonstrating a causal relationship between obesity and VTE, our data provide support that rising prevalence of obesity may help explain the high prevalence of VTE. Moreover, to the extent that public health interventions can reduce the incidence of obesity, there may be an accompanying decrease in the incidence of VTE.
MR analysis is based on a few key assumptions: (1) valid associations between SNPs and risk factors; (2) SNPs are not associated with any confounders between the risk factors and outcome; and (3) SNPs only affect the outcome through the risk factor of interest (no pleiotropic effects). For the first assumption, we only used SNPs that have shown to be associated with BMI on a genome-wide significant level. For the second assumption, the major potential confounder is population stratification. We believe this to be small since the VTE GWAS was conducted in European ancestry populations only and analyses were adjusted for population stratification through principal components as appropriate. Although we included BMI SNPs that reached genome-wide significance in a multi-ethnic meta-analysis, our results did not change when we restricted our analysis to SNPs that reached genome-wide significance in European ancestry populations. We assessed the validity of the third assumption by performing MR Egger regression and observed no evidence of directional pleiotropic effects that would have influenced our results. However, we note that MR Egger regression is valid under the assumption that the association between genetic variants and the exposure (here BMI) is independent of the direct effects the genetic variants have on the outcome (here VTE). This assumption is also known as the InSIDE assumption and would thus be violated if any pleiotropic effects act through a confounder of the BMI–VTE association. Further, we note that MR Egger regression is only sensitive to directional pleiotropy, thus it will not detect situations where several SNPs exhibit pleiotropy but in different directions, canceling out the “overall effect” of pleiotropy. In addition, compared to the 2-sample MR analysis that was primarily used here, MR Egger regression has less power to detect a true causal effect in the presence of balanced pleiotropy (Bowden et al. 2016). Since our outcome was binary, there is a possibility that adjustment for covariates in the VTE analysis (in our case, primarily sex and age) will affect the causal estimate asymptotically as the coefficients from logistic regression are non‐collapsible (Burgess et al. 2016). However, this should not affect the validity of causal findings, provided that the instrumental variables are valid both marginally and conditionally on the covariates. A potential limitation of our study is the use of summary-level statistics rather than individual-level data which would have allowed us to explore casual relationships in subgroups such as PE vs. DVT, smokers vs. non-smokers or women vs. men.
In conclusion, using data from the largest GWAS of VTE to date, we found evidence of a causal relationship between high BMI and risk of VTE. Fighting current increasing trends in obesity will most likely lead to a reduction of VTE incidence.
Change history
19 May 2018
The co-author name Immaculata DeVivo was incorrectly published. The correct name is given below:
References
Anderson FA Jr, Zayaruzny M, Heit JA, Fidan D, Cohen AT (2007) Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol 82:777–782. doi:10.1002/ajh.20983
Beckman MG, Hooper WC, Critchley SE, Ortel TL (2010) Venous thromboembolism: a public health concern. Am J Prev Med 38:S495–S501. doi:10.1016/j.amepre.2009.12.017
Blokhin IO, Lentz SR (2013) Mechanisms of thrombosis in obesity. Curr Opin Hematol 20:437–444. doi:10.1097/MOH.0b013e3283634443
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525. doi:10.1093/ije/dyv080
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR (2016) Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. doi:10.1093/ije/dyw220
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665. doi:10.1002/gepi.21758
Burgess S, Dudbridge F, Thompson SG (2016) Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med 35:1880–1906. doi:10.1002/sim.6835
Crous-Bou M, Harrington LB, Kabrhel C (2016) Environmental and genetic risk factors associated with venous thromboembolism. Semin Thromb Hemost 42:808–820. doi:10.1055/s-0036-1592333
Davey Smith G, Hemani G (2014) Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23:R89–R98. doi:10.1093/hmg/ddu328
Fall T, Hagg S, Magi R, Ploner A, Fischer K, Horikoshi M, Sarin AP, Thorleifsson G, Ladenvall C, Kals M, Kuningas M, Draisma HH, Ried JS, van Zuydam NR, Huikari V, Mangino M, Sonestedt E, Benyamin B, Nelson CP, Rivera NV, Kristiansson K, Shen HY, Havulinna AS, Dehghan A, Donnelly LA, Kaakinen M, Nuotio ML, Robertson N, de Bruijn RF, Ikram MA, Amin N, Balmforth AJ, Braund PS, Doney AS, Doring A, Elliott P, Esko T, Franco OH, Gretarsdottir S, Hartikainen AL, Heikkila K, Herzig KH, Holm H, Hottenga JJ, Hypponen E, Illig T, Isaacs A, Isomaa B, Karssen LC, Kettunen J, Koenig W, Kuulasmaa K, Laatikainen T, Laitinen J, Lindgren C, Lyssenko V, Laara E, Rayner NW, Mannisto S, Pouta A, Rathmann W, Rivadeneira F, Ruokonen A, Savolainen MJ, Sijbrands EJ, Small KS, Smit JH, Steinthorsdottir V, Syvanen AC, Taanila A, Tobin MD, Uitterlinden AG, Willems SM, Willemsen G, Witteman J, Perola M, Evans A, Ferrieres J, Virtamo J, Kee F, Tregouet DA, Arveiler D, Amouyel P, Ferrario MM, Brambilla P, Hall AS, Heath AC, Madden PA, Martin NG, Montgomery GW, Whitfield JB, Jula A, Knekt P, Oostra B, van Duijn CM, Penninx BW, Smith GD, Kaprio J, Samani NJ, Gieger C et al (2013) The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med 10:e1001474. doi:10.1371/journal.pmed.1001474
Germain M, Chasman DI, de Haan H, Tang W, Lindstrom S, Weng LC, de Andrade M, de Visser MC, Wiggins KL, Suchon P, Saut N, Smadja DM, Le Gal G, van Hylckama Vlieg A, Di Narzo A, Hao K, Nelson CP, Rocanin-Arjo A, Folkersen L, Monajemi R, Rose LM, Brody JA, Slagboom E, Aissi D, Gagnon F, Deleuze JF, Deloukas P, Tzourio C, Dartigues JF, Berr C, Taylor KD, Civelek M, Eriksson P, Cardiogenics C, Psaty BM, Houwing-Duitermaat J, Goodall AH, Cambien F, Kraft P, Amouyel P, Samani NJ, Basu S, Ridker PM, Rosendaal FR, Kabrhel C, Folsom AR, Heit J, Reitsma PH, Tregouet DA, Smith NL, Morange PE (2015) Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet 96:532–542. doi:10.1016/j.ajhg.2015.01.019
Heit JA (2015) Epidemiology of venous thromboembolism. Nat Rev Cardiol 12:464–474. doi:10.1038/nrcardio.2015.83
Heit JA, Silverstein MD, Mohr DN, Petterson TM, Lohse CM, O’Fallon WM, Melton LJ 3rd (2001) The epidemiology of venous thromboembolism in the community. Thromb Haemost 86:452–463
Heit JA, Spencer FA, White RH (2016) The epidemiology of venous thromboembolism. J Thromb Thrombolysis 41:3–14. doi:10.1007/s11239-015-1311-6
Hirsh J, Hoak J (1996) Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 93:2212–2245
Kabrhel C, Varraso R, Goldhaber SZ, Rimm EB, Camargo CA (2009) Prospective study of BMI and the risk of pulmonary embolism in women. Obesity (Silver Spring) 17:2040–2046. doi:10.1038/oby.2009.92
Ligthart S, Vaez A, Hsu YH, Inflammation Working Group of the CC, Pmi Wg XCP, LifeLines Cohort S, Stolk R, Uitterlinden AG, Hofman A, Alizadeh BZ, Franco OH, Dehghan A (2016) Bivariate genome-wide association study identifies novel pleiotropic loci for lipids and inflammation. BMC Genomics 17:443. doi:10.1186/s12864-016-2712-4
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Magi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Hua Zhao J, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Mateo Leach I, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stancakova A, Strawbridge RJ, Ju Sung Y, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Arnlov J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Bluher M, Bohringer S, Bonnycastle LL, Bottcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Ida Chen YD, Clarke R, Daw EW, de Craen AJ, Delgado G, Dimitriou M et al (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518:197–206. doi:10.1038/nature14177
Ogden CL, Carroll MD, Fryar CD, Flegal KM (2015) Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 219:1–8
Olson NC, Cushman M, Lutsey PL, McClure LA, Judd S, Tracy RP, Folsom AR, Zakai NA (2014) Inflammation markers and incident venous thromboembolism: the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. J Thromb Haemost 12:1993–2001. doi:10.1111/jth.12742
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97. doi:10.1038/nri2921
Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y (1996) Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 2:800–803
Stein PD, Beemath A, Olson RE (2005) Obesity as a risk factor in venous thromboembolism. Am J Med 118:978–980. doi:10.1016/j.amjmed.2005.03.012
VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P (2014) Methodological challenges in mendelian randomization. Epidemiology 25:427–435. doi:10.1097/EDE.0000000000000081
Willenberg T, Schumacher A, Amann-Vesti B, Jacomella V, Thalhammer C, Diehm N, Baumgartner I, Husmann M (2010) Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg 52:664–668. doi:10.1016/j.jvs.2010.04.023
Acknowledgements
This work was supported by National Health Lung and Blood Institute Grants HL116854, HL43201, HL60739, HL68986, HL73410, HL74745, HL85251, HL107442, HL66261, HL83141, HL59367 and HL95080 and the National Human Genome Research Institute (HG04735). The Nurses’ Health Study, Nurses’ Health Study II and Health Professionals Follow up Study are supported by the National Cancer Institute (CA186107, CA176726, CA167552). The WGHS is supported by the National Heart, Lung, and Blood Institute (HL043851 and HL080467) and the National Cancer Institute (CA047988 and CA182913). This work was partially supported by the GenMed LABEX (ANR-10-LABX-0013) and the ICAN Institute for Cardiometabolism and Nutrition (ANR-10-IAHU-05) and an independent grant from the K.G. Jebsen Foundation in Norway.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All participating studies were approved by their respective institutional review board and informed consent was obtained from all individual participants in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lindström, S., Germain, M., Crous-Bou, M. et al. Assessing the causal relationship between obesity and venous thromboembolism through a Mendelian Randomization study. Hum Genet 136, 897–902 (2017). https://doi.org/10.1007/s00439-017-1811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-017-1811-x