Abstract
Charcot-Marie-Tooth (CMT) disease is the most common inherited motor and sensory neuropathy. We have previously described a large Chinese CMT family and assigned the locus underlying the disease (CMT2L; OMIM 608673) to chromosome 12q24. Here, we report a novel c.423G→T (Lys141Asn) missense mutation of small heat-shock protein 22-kDa protein 8 (encoded by HSPB8), which is also responsible for distal hereditary motor neuropathy type (dHMN) II. No disease-causing mutations have been identified in another 114 CMT families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Charcot-Marie-Tooth (CMT) is the most common inherited peripheral neuropathy and is further subdivided into CMT1 or CMT2, the demyelinating and axonal forms respectively (De Jonghe et al. 1997). Eight loci and six genes have been reported to cause dominant inherited CMT2 (Inherited Peripheral Neuropathies Mutation Database, http://molgen-www.uia.ac.be/CMTMutations/). We have previously mapped CMT2L to a 6.8-cM candidate region at 12q24 in a Chinese family (Tang et al. 2004). In this study, we report a novel missense mutation (c.423G→T) in HSPB8, which is also the gene responsible for distal hereditary motor neuropathy type (dHMN) II (Irobi et al. 2004).
Patients and methods
Patients
A large Chinese CMT2L family was ascertained (Tang et al. 2004). Another 114 unrelated families, all diagnosed as exhibiting CMT by two or more neurologists according to generally accepted diagnosis criteria (De Jonghe et al. 1998), and 200 control individuals were included in this study.
Mutation analysis
Genomic DNA was extracted from peripheral blood leukocytes by standard extraction methods. All known exons and intron-exon boundaries of the selected 26 known candidate genes (KSR2, RFC5, JIK, HSPB8, RAB35, RPLP0, PXN, SIRT4, PLA2G1B, MSI1, SFRS9, RNF10, CABP1, P2RX7, RNF34, ARHF, MGC33630, MONDOA, VPS33A RSN, HM74, DENR, ABCB, RNP24, EIF2B1, and GTF2H3) were sequenced in the CMT2L family.
Results
Clinical information
The clinical information of the CMT2L family was as described previously (Tang et al. 2004). Motor nerve conduction velocities of the median nerves in the six patients subjected to electrophysiological examinations were normal, ranging from 56.7 to 69.2 m/s. Compound muscle action potentials (CMAPs) and sensory nerve action potentials (SNAPs) were decreased or absent in all six patients. A superficial peroneal nerve biopsy of the proband (IV:13) confirmed the presence of axonal neuropathy with an important loss of large myelinating fibers and a large number of clusters with mostly thinly myelinated axons.
Genetic analysis
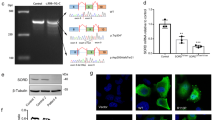
A novel c.423G→T transition (Lys141Asn; Fig. 1) was identified in small heat-shock 22-kDa protein 8 (encoded by HSPB8; also called HSP22) co-segregating perfectly with the CMT2 phenotype, but not in 200 normal control individuals. No disease-causing mutations were identified in another 114 unrelated patients diagnosed as having the CMT1 or CMT2 phenotype.
Discussion
We have shown here that the c.423G→T mutation of HSPB8 is involved in CMT2L. Recently, two different mutations, viz., c.423G→C (Lys141Asn) and c.421A→G (Lys141Glu), of HSPB8 were identified in four dHMN II families (Irobi et al. 2004). Our CMT2L family shares the Lys141Asn substitution but appears to have a distinct phenotype. The changes of CMAPs, SNAPs and pathology in the CMT2L family showed that the CMT2L family was different from the dHMN II family previously reported (Timmerman et al. 1992) according to the classification and diagnostic guidelines for CMT2 and dHMN (De Jonghe et al. 1998).
This is the third time that mutations in the same genes have been shown possibly to cause both CMT and dHMN, as dominant mutations in GARS are associated with CMT2D and dHMN V (Antonellis et al. 2003) and dominant mutations in HSPB1 are associated with CMT2F and a form of dHMN (Evgrafov et al. 2004). The reason that the HSPB8 gene mutations cause different diseases remains unclear. We suggest that the dysfunction of HSPB8 leads to the formation of aggregates that may block axonal transport. The different regions, such as the anterior horn cells or peripheral nerves, in which the aggregates are deposited may be associated with the different phenotypes, i.e., CMT and dHMN.
References
Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ, Jordanova A, Kremensky I, Christodoulou K, Middleton LT, Sivakumar K, Ionasescu V, Funalot B, Vance JM, Goldfarb LG, Fischbeck KH, Green ED (2003) Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet 72:1293–1299
De Jonghe P, Timmerman V, Nelis E, Martin JJ, Van Broeckhoven C (1997) Charcot-Marie-Tooth disease and related peripheral neuropathies. J Peripher Nerv Syst 2:370–387
De Jonghe P, Timmerman V, Van Broeckhoven C (1998) Second workshop of the European CMT consortium: 53rd ENMC international workshop on classification and diagnostic guidelines for Charcot-Marie-Tooth type 2 (CMT2-HMSN II) and distal hereditary motor neuropathy (Distal HMN-Spinal CMT): Naarden, The Netherlands, 26–28 September 1997. Neuromuscul Disord 8:426–431
Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E, Auer-Grumbach M, Windpassinger C, Wagner K, Mitrovic Z, Hilton-Jones D, Talbot K, Martin JJ, Vasserman N, Tverskaya S, Polyakov A, Liem RK, Gettemans J, Robberecht W, De Jonghe P, Timmerman V (2004) Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet 36:602–606
Irobi J, Impe KV, Seeman P, Jordanova A, Dierick I, Verpoorten N, Michalik A, Vriendt ED, Jacobs A, Gerwen VV, Vennekens K, Mazanec R, Tournev I, Hilton-Jones D, Talbot K, Kremensky I, Bosch LV, Robberecht W, Vandekerckhove J, Broeckhoven CV, Gettemans J, Jonghe PD, Timmerman V (2004) Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet 36:597–601
Tang B, Luo W, Xia K, Xiao J, Jiang H, Shen L, Tang J, Zhao G, Cai F, Pan Q, Dai H, Yang Q, Xia J, Evgrafov OV (2004) A new locus for autosomal dominant Charcot-Marie-Tooth Disease type 2 (CMT2L) maps to chromosome 12q24. Hum Genet 114:527–533
Timmerman V, Raeymaekers P, Nelis E, De Jonghe P, Muylle L, Ceuterick C, Martin JJ, Van Broeckhoven C (1992) Linkage analysis of distal hereditary motor neuropathy type II (distal HMN II) in a single pedigree. J Neurol Sci 109:41–48
Acknowledgements
We thank the participating families for their cooperation throughout the investigation. This study was supported by grants from the National 863 High-tech Project (2001AA227011 and 2004AA227040) and National Natural Science Foundation of China (30300199, 30300200 and 30370515). We also thank Dr. Zheng-mao Hu for skillful technical assistance and critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Bs., Zhao, Gh., Luo, W. et al. Small heat-shock protein 22 mutated in autosomal dominant Charcot-Marie-Tooth disease type 2L. Hum Genet 116, 222–224 (2005). https://doi.org/10.1007/s00439-004-1218-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-004-1218-3