Abstract
WRKY proteins constitute one of the largest transcription factor families in higher plants, and they are involved in multiple biological processes such as plant development, metabolism, and responses to biotic and abiotic stresses. Genes of this family have been well documented in response to many abiotic and biotic stresses in many plant species, but not yet against Pectobacterium carotovorum subsp. carotovorum and Fusarium oxysporum f.sp. conglutinans in any of the plants. Moreover, potentiality of a specific gene may vary depending on stress conditions and genotypes. To identify stress resistance-related potential WRKY genes of Brassica rapa, we analyzed their expressions against above-mentioned pathogens and cold, salt, and drought stresses in B. rapa. Stress resistance-related functions of all Brassica rapa WRKY (BrWRKY) genes were firstly analyzed through homology study with existing biotic and abiotic stress resistance-related WRKY genes of other plant species and found a high degree of homology. We then identified all BrWRKY genes in a Br135K microarray dataset, which was created by applying low-temperature stresses to two contrasting Chinese cabbage doubled haploid (DH) lines, Chiifu and Kenshin, and selected 41 BrWRKY genes with high and differential transcript abundance levels. These selected genes were further investigated under cold, salt, and drought stresses as well as after infection with P. carotovorum subsp. carotovorum and F. oxysporum f.sp. conglutinans in B. rapa. The selected genes showed an organ-specific expression, and 22 BrWRKY genes were differentially expressed in Chiifu compared to Kenshin under cold and drought stresses. Six BrWRKY genes were more responsive in Kenshin compared to Chiffu under salt stress. In addition, eight BrWRKY genes showed differential expression after P. carotovorum subsp. carotovorum infection and five genes after F. oxysporum f.sp. conglutinans infection in B. rapa. Thus, the differentially expressed BrWRKY genes might be potential resources for molecular breeding of Brassica crops against abiotic and biotic stresses and several genes, which showed differential expressions commonly in response to several stresses, might be useful for multiple stress resistance. These findings would also be helpful in resolving the complex regulatory mechanism of WRKY genes in stress resistance and for this further functional genomics study of these potential genes in different Brassica crops is essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The WRKY transcription factor family is one of the 10 largest gene families in higher plants and extends all over the green lineage (Cormack et al. 2002). WRKY is a recently identified transcription factor family, and its name is derived from the most prominent amino acid sequence feature within the domain. WRKY proteins play key regulatory roles in developmental processes, such as trichome initiation (Johnson et al. 2002), embryo morphogenesis (Lagace and Matton 2004), senescence (Robatzek and Somssich 2002), and some signal transduction processes mediated by plant hormones such as gibberellic acid (Zhang et al. 2004), abscisic acid (Xie et al. 2006), or salicylic acid (Du and Chen 2002). As a transcription factor, WRKY genes act in concert with other components of the transcriptional machinery and play a vital role in plant defense against internal and external stimuli (Ciolkowski et al. 2008; Eulgem and Somssich 2007). These stimuli may be abiotic stresses, such as cold, salt, heat, UV-B, drought, wounding, etc., or biotic stresses, such as bacteria, fungi, and viruses. In Arabidopsis thaliana, microarray analyses have shown a number of WRKY proteins to be involved in the response to abiotic stresses such as salinity, drought, and cold (Karam et al. 2002; Kilian et al. 2007). In Jatropha curcas, 47 WRKY genes have been shown to be responsive to at least one abiotic stress (drought, salinity, phosphate starvation, and nitrogen starvation) in individual tissues (leaf, root, and/or shoot cortex) (Xiong et al. 2013). On the other hand, AtWRKY25 are involved in responses to plant defense against the bacterial pathogen Pseudomonas syringae (Zheng et al. 2007). Additionally, AtWRKY3, AtWRKY4 and AtWRKY8 can increase defense to the necrotrophic fungal pathogens Botrytis cinerea (Chen et al. 2010; Lai 2008). In Potato plants, S1-9D (WRKY-box transcription factor-like) protein was induced 1–2 days after Phytophthora infestans infection (Beyer et al. 2001). Thus, it has been well documented that WRKY proteins play a key role in plant defense against various biotic stresses including bacterial, fungal and viral pathogens (Zheng et al. 2006). But it is also well known that specific gene plays role against specific stresses at a varying degree of potentiality depending on genotypes. On the other hand, Pactobacterium carotovorum subsp. carotovorum and Fusarium oxysporum f.sp. conglutinans are very serious pathogens for Brassica plants and function of WRKY genes against these pathogens has not yet been studied in Brassica crops.
Proteins in this family possess one or two unique DNA-binding domains consisting of about 60 amino acids, which include the highly conserved WRKYGQK sequence followed by a zinc-finger motif. WRKY proteins are classified into three groups based on the number of WRKY domains as well as the type of zinc-finger motif. Group I proteins possess two WRKY domains, including a C2H2 zinc-finger motif. Group II proteins typically contain a single WRKY domain and a C2H2 zinc-finger motif, and this group can be further divided into five distinct subgroups (IIa–IIe) based on the type of conserved motif (Eulgem et al. 2000). Finally, group III proteins have a single WRKY domain and a C2HC zinc-finger motif. Since the first WRKY cDNA was cloned from sweet potato (Ipomoea batatas) (Ishiguro and Nakamura 1994), many WRKY genes have been extensively identified and characterized in various plant species (Eulgem et al. 2000; Wu et al. 2005; Li et al. 2012; Tripathi et al. 2012; Xiong et al. 2013). In the Arabidopsis genome, at least 72 WRKY family members have been identified (Eulgem et al. 2000), 102 in rice (Wu et al. 2005), 55 in cucumber (Ling et al. 2011), and 105 in poplar (He et al. 2012). In addition, several WRKY-type genes were recently detected in the lineage of non-plant eukaryotes (Giardia lamblia; Dictyostelium discoideum) and green alga (Chlamydomonas reinhardtii), implying that the WRKY transcription factor family has its origin in eukaryotes and is largely amplified in plants (Zhang and Wang 2005). Gene duplication events can lead to the generation of new WRKY genes. A previous evolutionary study showed that the different WRKY gene groups originated at different times. The majority of WRKY gene members in groups 1 and 2 emerged before the divergence of monocots and dicots plants, whereas group 3 genes appeared relatively later (Wu et al. 2005).
The genus Brassica is an important group of crops for vegetable production and is normally grown worldwide. Well known crop species are included in Brassica that belongs mainly to the species Brassica rapa, as well as B. oleracea and B. napus (Collinge and Slusarenko 1987). Chinese cabbage (B. rapa) is widely recognized for its economic importance and contribution to human nutrition (Salunkhe and Kadam 1998), but its yield is severely affected by various biotic and abiotic stresses. Each type of stress functions through different types of molecular mechanisms that affect plants and eventually cause damage. In the current study, we determined the WRKY gene expression pattern in B. rapa doubled haploid (DH) lines, Chiifu and Kenshin, in response to abiotic (cold, salt, and drought) stresses. B. rapa ‘SUN-3061’ plants were used for biotic stress treatments (P. carotovorum subsp. carotovorum and F. oxysporum f.sp. conglutinans), and their homology with other plant species stress response-related genes was measured. We also investigated a considerable number of biotic and abiotic stress-responsive WRKY genes in B. rapa. Our results may provide a solid foundation for further plant breeding studies.
Materials and methods
Plant materials
Two Chinese cabbage (Brassica rapa ssp. pekinensis) doubled haploid (DH) lines, Chiifu and Kenshin, were grown for approximately 4 weeks in a growth chamber at 22 °C under a 16 h light/8 h dark photoperiod with a photon flux density of 140 μmol m−2 s−1. Fresh roots and leaves (3rd and 4th leaves) of B. rapa plants were harvested, immediately frozen in liquid nitrogen, and then stored at −80 °C for RNA isolation.
Abiotic stress treatments
Chiifu and Kenshin seeds were aseptically germination on Murashige and Skoog (MS) agar medium in a culture room under a 16-h light photoperiod at 25 °C. After 10 days of germination, the seedlings were carefully uprooted and transferred into fresh liquid MSH (half-strength MS medium) medium to ensure good growth conditions. After 4 weeks, liquid media were changed using the same media along with 200 mM NaCl to induce salt stress. To induce cold stress, the seedlings were maintained at 4 °C. Drought treatment was applied by keeping the seedlings on filter paper at 25 °C. The samples were treated with all stresses for 0 h, 30 m, 1, 4, 8, 12, 24, and 48 h, after which samples were collected, frozen immediately in liquid nitrogen, and stored at −80 °C for RNA isolation.
Biotic stress treatments
B. rapa plants (SUN-3061) were used for biotic stress (F. oxysporum f.sp. conglutinans) treatment, and plants were inoculated with the fungus according to the methods described by Ahmed et al. (2013). After infection, samples were collected from infected and mock-infected plants at 0, 3, 6, 24 h, 6 and 11 days. The local (4th) and systemic (5th) leaves were harvested as samples. After harvesting, the samples were immediately frozen in liquid nitrogen and stored at −80 °C until RNA isolation.
For P. carotovorum subsp. carotovorum infection, B. rapa (SUN-3061) plants were grown in soil under culture room conditions with a 16 h light/8 h dark for 6 weeks, and the bacterial culture and infection were carried out according to the methods described by Ahmed et al. (2013). Inoculations were performed three times, and infection was confirmed by detecting disease lesions on plants leaves. Approximately one-third of each infected leaf was harvested at 0, 6, 12, 24 h, 3 and 7 days after inoculation, frozen in liquid nitrogen, and stored at −80 °C for RNA extraction.
RNA extraction
Total RNA was extracted from roots and leaves of frozen samples using an RNeasy mini kit (Qiagen, USA). RNA was treated with RNase-free DNase (Promega, USA) to remove genomic DNA contaminants. RNA quantification was done by NanoDrop machine and the acceptable A260/A280 ratio of the samples was maintained from 1.90 to 2.08 at neutral pH.
Sequence analysis of BrWRKY genes
The name search method using the keyword “WRKY” was employed in the SWISSPORT of the B. rapa database (http://brassicadb.org/brad/index.php). We also searched the microarray annotated database in cold-treated B. rapa two doubled haploid (DH) lines, Chiifu and Kenshin, using the keyword “WRKY”. Genomic and protein sequences of the identified WRKY genes of B. rapa were processed or deduced using the B. rapa database website (http://brassicadb.org/brad/index.php) (Cheng et al. 2011). Subsequently, BrWRKY protein sequences were analyzed to confirm the presence of the WRKY domain using the SMART program (http://smart.embl-heidelberg.de/) (Letunic et al. 2009). Additionally, primary structure parameters of genes (length, molecular weight, and isoelectric point) were analyzed using the ExPasy website (http://au.expasy.org/tools/pi_tool.html), whereas protein homology study was done using the Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST/) in order to confirm the identified domain less WRKY genes. Positional information of all candidate WRKY genes along the ten (10) chromosomes of B. rapa was obtained from the Brassica database (http://brassicadb.org/brad/) (Cheng et al. 2011), and the locations of the BrWRKY genes were drafted by MapChart version 2.2 (http://www.wageningenur.nl/en/show/Mapchart.htm).
Microarray expression analysis
For microarray expression analysis, the Br135K microarray chip (Brapa_V3_microarray, 3′-Tiling microarray) was used, which is a high-density DNA array prepared with Maskless Array Synthesizer (MAS) technology by NimbleGen (http://www.nimblegen.com/). Probes were designed based on 41,173 genes of B. rapa accession Chiifu-401-42, a Chinese cabbage (Wang et al. 2011). Total and polysomal RNAs were extracted from two B. rapa doubled haploid (DH) lines, Chiifu and Kenshin, after applying low-temperature (4, 0, −2 and −4 °C) stress using an RNeasy Mini kit (Qiagen, USA). The RNA protection reagent (Qiagen) and DNA were removed by on-column DNase digestion with the RNase-Free DNase set (Qiagen). Labeling was performed by NimbleGen Systems Inc. (Madison, WI, USA), following their standard operating protocol (see http://www.nimblegen.com). The raw data (pair file) were subjected to RMA (Robust Multi-Array Analysis) (Irizarry et al. 2003), quantile normalization (Bolstad et al. 2003), and background correction as implemented in the NimbleScan software package, version 2.4.27 (Roche NimbleGen, Inc.). RMA normalized and averaged gene-level signal intensity. The complete microarray data have been deposited in the Omics database of NABIC (http://nabic.rda.go.kr) as enrolled number, NC-0024-000001-NC-0024-000012.
qRT-PCR expression analysis
Real-time quantitative PCR was performed using 1 µl of cDNA in a 10-µl reaction volume employing 2× Quanti speed SYBR mix (Korea). The specific primers used for real-time PCR are listed in Supplementary Table 2. The conditions for real-time PCR were as follows: 5 min at 95 °C, followed by 40 cycles at 95 °C for 10 s, 58 °C for 10 s, and 72 °C for 15 s. The fluorescence was measured following the last step of each cycle, and three replications were used for each sample. Amplification detection and data analysis were conducted using LightCycler96 (Roche, Germany). The Br-Actin was used as the internal reference in all analyses and relative gene expression level was calculated on the basis of the 2−ΔΔCt method (Livak and Schmittgen 2001).
Results
In silico functional analysis of BrWRKY proteins
WRKY genes constitute a large and important gene family in higher plants. Complete and accurate annotation of genes is an essential starting point for further functional study of a gene family. We identified a total of 145 gene sequences as members of the WRKY family in B. rapa plants, and Tang et al. (2014) found the same result. A homology search was carried out using BLAST from NCBI, and all WRKY proteins showed a high level of sequence identity with WRKY proteins from different plant species. In a functional study, a majority of BrWRKY proteins showed a high degree of similarity with biotic and abiotic stress-related WRKY proteins from different plant species (Supplementary Table 1). Specifically, 51 BrWRKY proteins out of 145 total proteins showed a high level of identity with biotic (especially fungal) stress-related WRKY proteins from different plant species. On the other hand, 42 BrWRKY proteins showed identity with abiotic stress-related WRKY proteins from different plant species. In addition, 48 BrWRKY proteins showed a high degree of similarity with both biotic and abiotic stress-related WRKY proteins from different plant species. Lastly, only four homolog genes of BrWRKY46, 85, 129, 138 showed no biotic or abiotic stress-related function. Thus, we speculated that BrWRKY proteins are involved in the response to various biotic and abiotic stresses.
Microarray expression analysis
Microarray is one of the most useful global transcriptome analysis technologies which provide an opportunity to understand patterns of gene expression. To study the expression patterns of the 145 BrWRKY genes in two contrasting B. rapa doubled haploid (DH) lines, Chiifu and Kenshin, systematic analyses of the microarray dataset were carried out at different temperatures (22, 4, 0, −2, and −4 °C) (unpublished). In a previous study, Tang et al. (2014) classified BrWRKY genes into three large groups (i.e., I, II, III), with group II further into five subgroups (IIa–e). The microarray results are presented in a group-wise fashion showing the gene expression level of B. rapa (Supplementary Fig. 1). Genes from groups I, IIa, and IId showed very high and differential expression. In group I, 13 BrWRKY genes showed no expression or very low expression. On the other hand, BrWRKY10, 46, 48, 85, 87, 94, 105, 105, and 111 showed higher expression but not differential expression. In subgroup IId, only BrWRKY8 showed very low expression. In subgroup IIb, BrWRKY5, 45, 102, 114, 117, 124, and 141 showed low-to-medium differential expression in both lines of B. rapa, whereas the remaining members of subgroup IIb showed no or very low expression.
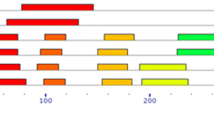
In subgroup IIc, a large number of genes showed no expression while other genes showed low-to-medium expression. BrWRKY20, 29, 31, 32, 49, 63, 74, 75, 78, 91, 93, 99, 101, 118, 119, and 121 showed differential expression within and between the lines, whereas BrWRKY143 showed differential expression only between the lines. Most members of subgroup IIe showed low or no expression, although BrWRKY18, 60′ 113, 116, and 132 exhibited low-to-medium and differential expression within and between the Chiifu and Kenshin lines. In group III, the majority of genes showed medium-to-high and differential expression, although 8 genes (BrWRKY23, 25, 44, 62, 71, 90, 120, and 138) out of the 25 genes displayed the lowest expression at all temperatures treatment courses. Particularly, BrWRKY16 and 17 showed no expression in the Kenshin, whereas BrWRKY23, 25, and 62 simply showed no differential expression within or between the lines. These results demonstrate that a majority of the selected genes exhibited higher expression in Chiifu than in Kenshin. Previous studies have showed that the Chiifu and Kenshin lines have different geographic origins; Chiifu originated in temperate regions while Kenshin originated in subtropical and tropical regions. As such, these two lines are expected to respond differently to cold and freezing temperatures (Lee et al. 2013). In nature, Kenshin exhibits severe damage upon exposure to cold and freezing temperatures, whereas Chiifu does not. In this study, we selected 41 BrWRKY genes considering two points; first, the genes which showed variation in transcript abundance level among the temperature treatments compared to control and second, the genes which showed variation in transcript abundance level in between two contrasting B. rapa lines (Chiifu and Kenshin) (Fig. 1). We thoroughly studied all the BrWRKY genes, after which their organ-specific expression and responses to biotic and abiotic stress conditions were determined.
Microarray expression analysis of 41BrWRKY genes in B. rapa under various temperature treatments. Here, C and K indicates two inbred lines Chiifu and Kenshin of B. rapa, respectively, were treated under five (5) temperatures; control (C1 and K1), 4 °C (C2 and K2), 0 °C (C3 and K3), −2 °C (C4 and K4), and −4 °C (C5 and K5). Responsive genes under different temperatures from different BrWRKY groups have been shown on the left side. Color bar at the right side represents differential expression (color figure online)
Organ-specific expression analysis
Organ-specific expression analysis was carried out by RT-PCR with equal amounts of cDNA templates prepared from the mRNAs of roots, stems, leaves, and flower buds of B. rapa (SUN-3061). Expression of all BrWRKY genes occurred in an organ-specific manner. All genes were expressed in at least one of the four organs (Fig. 2). Fourteen genes (BrWRKY7, 30, 40, 42, 49, 51, 57, 65, 66, 72, 92, 97, 104, and 116) were expressed in all tested organs. Most of the genes showed higher expression in flower buds, whereas BrWRKY30, 42, 49, 66, and 97 showed relatively higher expression in stems. On the other hand, BrWRKY5, 41, 44, 51, 59, 77, 98, 101, and 115 were expressed at very low levels in the tested organs. However, four genes (BrWRKY5, 101, 113, and 121) were expressed only in roots while BrWRKY59 was expressed in flower buds only. Particularly, BrWRKY55, 118, and 128 were expressed in all tested organs except flower buds, which indicates that these three genes do not play a role in flower development. Our RT-PCR findings showed that BrWRKY49 was expressed in all tested organs at relatively high levels, which indicates that BrWRKY49 could play a role not only in development of roots and shoots but also in male and female organ development. Moreover, BrWRKY30, 42, 57, 66, and 97 also showed relative high expression levels in all tested organs. WRKY genes that are highly expressed in plant organs often play key roles in plant development (Ramamoorthy et al. 2008). Specifically, WKRY genes participate in the transcriptional regulation of expression of target genes that are involved in physiological pathways (Sun et al. 2003). Thus, we concluded that the highly expressed BrWRKY genes in our study may play regulatory roles in the growth and development of B. rapa plants. However, more research is needed to determine the developmental functions of these BrWRKY genes.
Expression analysis of BrWRKY genes against abiotic stresses
WRKY genes have already been identified as playing a significant role in plant responses against abiotic stresses (cold, salt, and drought). These responses suggest that BrWRKY genes are involved in defense-related processes or stress responses. To investigate these responses, we analyzed the expression patterns of 41 BrWRKY genes using specific primers after applying abiotic stress treatments; including cold, salt, and drought, in the B. rapa DH lines Chiifu and Kenshin. Particularly, in Chiifu, BrWRKY22, 26, 42, 44, 59, 65, 66, 70, 72, 77, 79, 92, 109, 116, and 141 showed differential expression in response to cold stress with most of the genes being up-regulated from 0 to 1 h, down-regulated up to 12 h, and then again up-regulated with time courses (Fig. 3a). In addition, BrWRKY70, 72, and 77 were up-regulated from 0 to 4 h and than down-regulated, although BrWRKY70 and 77 again were up-regulated at 12 and 24 h, respectively. BrWRKY22, 44, and 79 were up-regulated from 0 to 8 h and then drastically down-regulated. On the other hand, BrWRKY65 and 66 showed gradually elevated expression up to 12 and 24 h, respectively, followed by a slow decrease in expression. BrWRKY92 and 141 were up-regulated from 0 to 1 h, gradually down-regulated up to 12 h, and again up-regulated to the end of the period. On the other hand, BrWRKY26, 42, 59, 109, and 116 in Chiifu showed up-regulation within 1 h, down-regulation up to 8 h, up-regulation again up to 12 h, and then down-regulation again until the end of the period. However, five genes (BrWRKY22, 44, 70, 72, and 77) in Chiifu showed about 175-, 32-, 54-, 42-, and 24-fold higher expression, respectively, compared to the control. In contrast, most of the genes in Kenshin did not show significant up-regulation during cold stress. Very few genes in Kenshin showed low expression at 4 and 8 h compared to Chiifu, after which they were down-regulated during rest of the time courses. However, BrWRKY92 and 141 were significantly down-regulated throughout the total time period. BrWRKY79 was the only gene to show rapid expression within 30 m, after which it was down-regulated up to 12 h and then again activated and showed to its highest expression level at 24 h. Remarkably, BrWRKY26 and 79 showed higher expressions in Kenshin throughout the whole time course.
Expression analysis of BrWRKY genes under biotic and abiotic stresses using real-time quantitative RT-PCR. The relative expression levels of BrWRKY genes under a cold b salt c drought d Fusarium oxysporum f.sp. conglutinans and e Pectobacterium carotovorum subsp. carotovorum stress treatments. The error bars represent the standard error of the means of three independent replicates
All of the selected WRKY genes were rapidly expressed after salt stress treatment in Chiifu and then down-regulated from 4 to 24 h. However, most genes were again up-regulated at 48 h, with BrWRKY17, 57, and 58 showing about 5-, 2-, and 3-fold higher expression, respectively, compared to control at 48 h (Fig. 3b). BrWRKY22 showed early expression that remained constant up to 1 h, after which it was down-regulated to a constant level up to 24 h and then quickly up-regulated at 48 h. BrWRKY65 showed up-regulation within 30 m in Chiifu, after which its expression remained the same up to 12 h, was down-regulated at 24 h, and then up-regulated again by about threefold at 48 h compared to control. On the other hand, BrWRKY40, 49, 58, 72, and 97 showed high and early expression (30 m) in Kenshin, followed by gradual down-regulation to the end of the time courses. In addition, BrWRKY57 and 113 were up-regulated from 0 to 1 h and then gradually down-regulated until the end. BrWRKY65 showed its highest expression at 1 h, after which its expression level remained the same until 12 h and then decreased. However, BrWRKY17 showed its highest expression at 4 h, after which its expression level suddenly decreased.
Except BrWRKY7, all of the selected genes were significantly up-regulated in Chiifu during drought stress treatment. However, BrWRKY14, 72, 66, 97, and 131 were up-regulated from 0 to 1 h, whereas BrWRKY40, 57, and 113 were up-regulated up to 4 h and then gradually down-regulated to the end of the time courses (Fig. 3c). In addition, five genes (BrWRKY51, 65, 98, 104, and 109) showed their highest expression very rapidly within 30 m, whereas BrWRKY49 was up-regulated up to 8 h and then slowly down-regulated to the end of the period. In Chiifu, BrWRKY51, 65, 98 and 104 showed their highest expression within 30 m after drought stress treatment and were about 85-, 15-, 18-, and 38-fold higher than the control, respectively. Only BrWRKY7 was down-regulated through the entire time course. In contrast, the majority of BrWRKY genes in Kenshin showed no or very little expression within 30 m. BrWRKY109 and 113 showed up-regulation from 0 to 4 h and then down-regulation until the end of the period in Kenshin, although their expression level was several fold lower than in Chiifu. Interestingly, BrWRKY7 and 14 showed about 45- and 7-fold higher expression, respectively, in Kenshin at 4 h than in Chiifu after drought stress treatment. During drought stress, BrWRKY51, 72, 97, and 131 showed the opposite expression pattern in Chiifu and Kenshin.
Expression of BrWRKY genes against biotic stresses
WRKY genes have already been shown to play an important role in plant responses to biotic stresses. In this study, the responses of WRKY genes to biotic stress treatment were investigated by measuring the responses of selected BrWRKY genes to infection with F. oxysporum f.sp. conglutinans and P. carotovorum subsp. carotovorum. For this, specific primers were used in real-time PCR (Supplementary Table 2). First, we examined the expression levels of BrWRKY genes in response to F. oxysporum f.sp. conglutinans, which specifically attacks Brassica species and causes wilt and root rot diseases. Six genes (BrWRKY4, 65, 72, 97, 133, and 141) showed significantly higher expression after F. oxysporum f.sp. conglutinans infection (Fig. 3d). The highest expression levels of BrWRKY4, 72, 97, 133, and 141 were observed at 6 days after infection and were about 8-, 6-, 6-, 3-, and 5-fold higher, respectively, compared to levels in uninfected plants, which were very low. After infection, all genes were up-regulated and showed their highest expression at 6 days, followed by a drastic reduction in expression. Therefore, it is evident that these genes may be involved in resistance against F. oxysporum f.sp. conglutinans.
We also analyzed BrWRKY gene expression profiles after infection with P. carotovorum subsp. carotovorum, which is a serious pathogen affecting Brassica and causes soft rot disease. All selected genes were expressed differentially after infection and showed higher expression at 7 days (Fig. 3e). Most of the genes showed very low expression from 0 to 3 days, followed by a sudden increase in expression at 7 days. Only three genes (BrWRKY4, 79, and 141) showed increased expression at 6 h, which remained constant up to 3 days, followed by a drastic increase in expression at 7 days. Particularly, BrWRKY141 showed about 180-fold higher expression at 7 days after infection compared to mock treatment. On the other hand, BrWRKY65 was down-regulated from 0 to 12 h until its expression dipped below that of control until 3 days, after which it was weakly up-regulated. Only one gene, BrWRKY133, was down-regulated early on, after which its expression increased radically, decreased gradually, and then increased again thereafter. However, BrWRKY72 and 97 showed decreased expression immediately after infection, which gradually increased until the end of the time period. All genes showed very low expression in uninfected plants.
Discussion
Transcriptional control is a major mechanism by which a cell or organism regulates its gene expression and WRKY proteins are recently identified transcriptional regulators comprising a large gene family in higher plants. Complete and accurate annotation of genes is an essential starting point for further functional study of a gene family. We identified 145 gene sequences as members of the WRKY family in B. rapa and an in silico functional study was carried out using BLAST of NCBI, where a majority of BrWRKY proteins showed a high degree of similarity with biotic and abiotic stress-related WRKY proteins from different plant species. Thus, we speculated that BrWRKY proteins might be involved in resistance activities against various biotic and abiotic stresses. Subsequently, expression patterns of 145 BrWRKY genes were analyzed through a whole genome microarray dataset, carried out at different temperatures (22, 4, 0, −2, and −4 °C) in two contrasting B. rapa doubled haploid (DH) lines, Chiifu and Kenshin (Unpublished). We selected 41 BrWRKY genes considering their variation in transcript abundance level among the temperature treatments compared to control and in between two contrasting B. rapa lines (Chiifu and Kenshin) (Fig. 1). From our homology study, 15 out of 41 selected BrWRKY genes showed a high degree of similarity with biotic stress-related WRKY genes from different plant species, whereas 11 showed a high degree of similarity with abiotic stress-related ones. However, 15 out of 41 selected BrWRKY genes showed a high degree of similarity with both biotic and abiotic stress-related WRKY genes from other plant species. Among the 41 selected genes, 22 genes were obtained from group II, 13 genes from group III, and six genes from group I. Thus, the results indicate that these genes might play an important role against low-temperature stresses in Chinese cabbage. Although selection of these genes was based on the different temperature stresses, it is important to study the expression of these selected genes under cold stresses for various time spans.
During cold stress treatment at various time courses, 15 BrWRKY genes showed highly differential expression in two lines of B. rapa, cold-tolerant Chiifu and cold-susceptible Kenshin (Lee et al. 2013). Most of the genes (13 out of 15 genes) showed several fold higher expression in the Chiifu than the Kenshin, which indicates that these 13 BrWRKY genes might be responsible for cold resistance. Alongside, BrWRKY26 and 79 expressed highly in Kenshin, suggesting that these two genes might be responsible for cold susceptibility. In previous studies, WRKY proteins were also shown to respond to cold stress. A cold-induced WRKY transcription factor has been characterized in Solanum dulcamara (Huang and Duman 2002). The PtrWRKY2 gene was shown to be expressed early in response to cold treatment, but then repressed at the end (Sahin-Çevik 2012). Likewise, Mare et al. (2004) showed increased expression of the Hv-WRKY38 gene when exposed to 2 °C temperature, after which its expression level decreased in barley plants.
Accordingly, ten BrWRKY genes showed deferential expression under salt treatment, where the expression was higher in the Kenshin than the Chiifu in most cases. Furthermore, early response of BrWRKY genes was observed in Kenshin after salt stress treatment, while in Chiifu at the end of the time courses. Accumulating previous evidences also show that the WRKY proteins respond to salt stress in various plants. BcWRKY46, a novel cold-inducible gene from Pak-choi, enhances cold, salt, and dehydration stress tolerance abilities in transgenic tobacco (Wang et al. 2012). Further, over expression of AtWRKY33 enhances thermo- and salt tolerance in A. thaliana (Jiang and Deyholos 2009). In case of drought stress treatment, 13 out of 15 BrWRKY genes showed several fold higher expression in Chiifu compared to Kenshin at certain period of drought stress treatment. Two genes (BrWRKY7 and 14) showed higher expression in Kenshin than in Chiifu, which indicates their probable involvement against drought stress.
Thus, expression results under abiotic stress treatment showed that most of the BrWRKY genes showed several fold higher expression in the Chiifu than the Kenshin subjected to cold and drought stresses under same conditions. During salt stress, the majority of BrWRKY genes showed higher expression in Kenshin at comparatively earlier stages of treatment than in Chiifu. In fact, previous studies indicated that several WRKY transcription factors have been shown to participate in the abiotic stress responses. For instance, AtWRKY63 is involved in the responses to ABA and drought stresses (Ren et al. 2010). Ling et al. (2011) showed that 23 CsWRKY genes were differentially expressed in response to at least one abiotic stress (cold, drought, or salinity). At least 54 OsWRKY genes from rice and 26 GmWRKY genes from soybean were found to be differentially expressed under abiotic stresses (Ramamoorthy et al. 2008). In the current study, BrWRKY65 and 72 were expressed in response to all abiotic (cold, salt, and drought) stresses, whereas BrWRKY66 and 109 showed expression in response to cold and drought stresses. On the other hand, BrWRKY40, 49, 57, 97, and 131 showed expression in response to drought and salt stresses while only BrWRKY22 was expressed in response to cold and salt stresses. Therefore, the highly expressed BrWRKY genes reported here might play an important role against abiotic stresses that might facilitate the development of stress resistance in B. rapa.
According to previous reports, WRKY transcription factors not only play a vital role against abiotic stresses, but also against biotic stresses in plant. The majority of AtWRKY genes were induced by pathogen infection or SA treatment (Dong et al. 2003). Wei et al. (2012) reported that ZmWRKY115 apparently increased upon fungal infection, implying a parallel function in the defense response. Numerous plant defense or defense-related genes, including pathogenesis-related (PR) genes and the regulatory NPR1 gene, contain W-box sequences in their promoters, which are recognized by WRKY proteins (Yu et al. 2001). Further, studies have shown that these W-box sequences are necessary for inducible expression of these defense genes. In this study, eight BrWRKY genes showed differential expression after P. carotovorum subsp. carotovorum infection and five genes after F. oxysporum f.sp. conglutinans infection in B. rapa. Among them, BrWRKY4, 72, 97, 133 and 141 showed expression against both the infectious organisms. Thus, the highly expressed BrWRKY genes against biotic stresses reported here might play a vital role against these two pathogens and other genes might be useful against other biotic stresses.
Finally, it can be concluded that the selected BrWRKY genes showed an organ-specific expression and might play a regulatory role in growth and developmental processes of B. rapa. Accordingly, five BrWRKY genes showed responses after infection with F. oxysporum f.sp. conglutinans and eight BrWRKY genes showed responses after infection with P. carotovorum subsp. carotovorum in B. rapa plants, while BrWRKY4, 72, 97, 133, and 141 expressed under both the infections. Further, BrWRKY22, 40, 42, 44, 49, 51, 57, 59, 65, 66, 70, 72, 77, 92, 97, 98, 104, 109, 113, 116, 131, and 141 showed several fold greater expression in Chiifu than in Kenshin under cold and drought stresses. Subsequently, BrWRKY17, 58, 65, 72, 97, and 113 showed higher expressions in Kenshin compared to Chiffu under salt stress. Interestingly, BrWRKY65 and 72 showed responses to all biotic and abiotic stresses tested here. Thus, the highly induced BrWRKY genes might be potential resources for developing resistant variety of Brassica crops applying molecular breeding techniques against abiotic and biotic stresses and several genes of them might be useful against multiple stresses. These findings also suggest that the stress-induced BrWRKYs would be new factors in enlightening the complex regulatory network of stress resistance mechanism of WRKY genes and further functional genomics study of these potential genes in different Brassica crops would be helpful to achieve this.
References
Ahmed NU, Park JI, Jung HJ, Kang KK, Lim YP, Hur Y, Nou IS (2013) Molecular characterization of thaumatin family genes related to stresses in Brassica rapa. Sci Hortic 152:26–34
Beyer K, Binder A, Boller T, Colling M (2001) Identification of potato genes induced during colonization by Phytophthora infestans. Mol Plant Pathol 2:125–134
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193
Chen L, Zhang L, Yu D (2010) Wounding-induced WRKY8 is involved in basal defense in Arabidopsis. Mol Plant-Microbe Interact 23:558–565
Cheng F, Liu S, Wu J, Fang L, Sun S, Liu B, Wang X (2011) BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biol 11:136
Ciolkowski I, Wanke D, Birkenbihl RP, Somssich IE (2008) Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol 68:81–92
Collinge DB, Slusarenko AJ (1987) Plant gene expression in response to pathogens. Plant Mol Biol 9:389–410
Cormack RS, Eulgem T, Rushton PJ, Kochner P, Hahlbrock K, Somssich IE (2002) Leucine zipper containing WRKY proteins widen the spectrum of immediate early elicitor-induced WRKY transcription factors in parsley. Biochem Biophys Acta 1576:92–100
Dong J, Chen C, Chen Z (2003) Expression profile of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51:21–37
Du L, Chen Z (2002) Identification of genes encoding receptor like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J 24:837–847
Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10:366–371
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
He H, Dong Q, Shao Y, Jiang H, Zhu S, Cheng B, Xiang Y (2012) Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep 31:1199–1217
Huang T, Duman JG (2002) Cloning and characterization of a thermal hysteresis (antifreeze) protein with DNA-binding activity from winter bittersweet nightshade, Solanum dulcamara. Plant Mol Biol 48:339–350
Irizarry RA, Hobbs B, Collin F, Beazer-barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Ishiguro S, Nakamura K (1994) Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 50 upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol Gen Genet 244:563–571
Jiang Y, Deyholos MK (2009) Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol Biol 69:91–105
Johnson SC, Kolevski B, Smyth DR (2002) TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 14:1359–1375
Karam BS, Rhonda CF, Luis OS (2002) Transcription factors in plant defense and stress response. Curr Opin Plant Biol 5:430–436
Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50:347–363
Lagace M, Matton DP (2004) Characterization of a WRKY transcription factor expressed in late torpedo-stage embryos of Solanum chacoense. Planta 219:185–189
Lai Z (2008) Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol 8:68
Lee J, Lim YP, Han CT, Nou IS, Hur Y (2013) Genome-wide expression profiles of contrasting inbred lines of Chinese cabbage, Chiifu and Kenshin, under temperature stress. Genes Genom 35:273–288
Letunic I, Doerks T, Bork P (2009) SMART 6: recent updates and new developments. Nucleic Acids Res 37:D229–D232 (database issue)
Li HL, Zhang LB, Guo D, Li CZ, Peng SQ (2012) Identification and expression profiles of the WRKY transcription factor family in Ricinus communis. Gene 503:248–253
Ling J, Jiang W, Zhang Y, Yu H, Mao Z, Gu X, Huang S, Xie B (2011) Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genom 12:471
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Mare C, Mazzucotelli E, Crosatti C, Francia E, Stanca AM, Cattivelli L (2004) Hv-WRKY38: a new transcription factor involved in cold- and drought-response in barley. Plant Mol Biol 55:399–416
Ramamoorthy R, Jiang SY, Kumar N, Venkatesh PN, Ramachandran S (2008) A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol 49:865–879
Ren X, Chen Z, Liu Y, Zhang H, Zhang M, Liu Q, Hong X, Zhu JK, Gong Z (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J 63:417–429
Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16:1139–1149
Şahin-Çevik M (2012) A WRKY transcription factor gene isolated from Poncirus trifoliata shows differential responses to cold and drought stresses. Plant Omics J 5:438–445
Salunkhe DK, Kadam SS (1998) Handbook of vegetable science technology: production, composition, storage, and processing. Marcel Dekker Inc., New York
Sun C, Palmqvist S, Olsson H, Boren M, Ahlandsberg S, Jansson C (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar responsive elements of the iso1 promoter. Plant Cell 15:2076–2092
Tang J, Wang F, Hou X, Wang Z, Huang Z (2014) Genome-wide fractionation and identification of WRKY transcription factors in Chinese cabbage (Brassica rapa ssp. pekinensis) reveals collinearity and their expression patterns under abiotic and biotic stresses. Plant Mol Biol Rep 32:781–795
Tripathi P, Rabara RC, Langum TJ, Boken AK, Rushton DL, Boomsma DD, Rinerson CI, Rabara J, Reese RN, Chen X, Rohila JS, Rushton PJ (2012) The WRKY transcription factor family in Brachypodium distachyon. BMC Genom 13:270
Wang X, Wang H, Wang J, Sun R, Wu J, Liu S (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1049
Wang F, Hou X, Tang J, Wang Z, Wang S, Jiang F, Li Y (2012) A novel cold inducible gene from Pak-choi (Brassica campestris ssp. chinensis), BcWRKY46, enhances the cold, salt and dehydration stress tolerance in transgenic tobacco. Mol Biol Rep 39:4553–4564
Wei K, Chen J, Chen Y, Wu L, Xie D (2012) Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in Maize. DNA Res 19:153–164
Wu KL, Guo ZJ, Wang HH, Li J (2005) The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res 12:9–26
Xie Z, Zhang ZL, Zou X, Yang G, Komatsu S, Shen QJ (2006) Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J 46:231–242
Xiong W, Xu X, Zhang L, Wu P, Chen Y, Li M, Jiang H, Wu G (2013) Genome-wide analysis of the WRKY gene family in physic nut (Jatropha curcas L.). Gene 524:124–132
Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13:1527–1540
Zhang Y, Wang L (2005) The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol 5:1
Zhang ZL, Xie Z, Zou X, Casaretto J, David TH, Zhen QJ (2004) A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol 134:1500–1513
Zheng Z, Qamar SA, Chen Z, Mengiste T (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48:592–605
Zheng Z, Mosher SL, Fan B, Klessig DF, Chen Z (2007) Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol 7:2
Acknowledgments
This research was supported by Golden Seed Project (Center for Horticultural Seed Development, No. 213003-04-2-CG100), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Service (KFS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. K. Varshney.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kayum, M.A., Jung, HJ., Park, JI. et al. Identification and expression analysis of WRKY family genes under biotic and abiotic stresses in Brassica rapa . Mol Genet Genomics 290, 79–95 (2015). https://doi.org/10.1007/s00438-014-0898-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-014-0898-1