Abstract
Copper is an essential trace element that serves as a cofactor for numerous enzymes. In eukaryotes, copper-transporting ATPases deliver copper to various copper-containing proteins in the trans-golgi network. This study identified a copper-transporting ATPase gene BcCcc2 in a fungus pathogenic to plants, Botrytis cinerea. We investigated the biological roles of BcCCC2 by generating null mutants for BcCcc2. Melanization, conidiation and the formation of sclerotia were severely affected in ∆BcCcc2 mutants. Moreover, a pathogenicity assay using tomato leaves and carnation petals revealed the mutants to be nonpathogenic. Further analysis indicated that they formed fewer appressoria and infection cushions than the wild-type. These structures were aberrant in morphology and in many cases had a significantly reduced ability to penetrate the plant epidermis. An assay also indicated that ∆BcCcc2 mutants were defective in infection through wounds. BcCCC2 is necessary not only for penetrating a host but also for fungal growth within plant tissues. Our results also imply that B. cinerea requires copper-containing proteins for infection that are inactive in the absence of the copper-transporting ATPase BcCCC2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper is an essential transition metal in all aerobic organisms where it serves as a cofactor for numerous enzymes (copper-containing proteins). Copper-transporting ATPases are P-type ATPases, conserved from bacteria to mammals (Hirayama et al. 1999; Hung et al. 1997; Rensing and Grass 2003; Wakabayashi et al. 1998). In eukaryotic cells, they supply copper to various copper-containing proteins in the trans-golgi network. Most copper-containing proteins accept copper from these ATPases in eukaryotes (Culotta and Gitlin 2001).
In the budding yeast Saccharomyces cerevisiae, a copper-transporting ATPase, CCC2, appears to transport copper to a multicopper oxidase, FET3, that is necessary for high-affinity iron uptake in the trans-golgi network. Mutation of the Ccc2 gene causes the inactivation of FET3, resulting in an iron deficiency (Yuan et al. 1995). In filamentous fungi, Parisot et al. (2002) characterized a copper-transporting ATPase, CLAP1, from a hemi-biotrophic plant pathogen, Colletotrichum lindemuthianum. They revealed that the clap1 mutant is impaired in melanization. Moreover, the mutant failed to cause disease in the host plant, suggesting that CLAP1 is necessary for the pathogenicity of this hemi-biotrophic pathogen. However, there is little information about copper-transporting ATPases in other filamentous fungi. To gain further insight into the roles of copper-transporting ATPases in fungal pathogenicity, analyses of this protein in necrotrophic fungi are needed.
This study characterized a copper-transporting ATPase gene (BcCcc2) of the filamentous fungus Botrytis cinerea. B. cinerea is a fungal plant pathogen with a wide range of hosts. It infects a large number of plant species, including many commercially important vegetables, fruits, and ornamentals, both in the field or greenhouse and during storage, causing important economic losses worldwide (Elad et al. 2004). Our results showed melanization, conidiation and the formation of sclerotia to be severely impaired in null mutants for the BcCcc2 gene (∆BcCcc2 mutants). Moreover, an inoculation assay revealed that ∆BcCcc2 mutants were nonpathogenic to host plants, suggesting BcCCC2 to be necessary for the pathogenicity. This study indicates that copper-containing proteins are required for the pathogenicity of a wide range of fungal pathogens.
Materials and methods
Strains and growth condition
The B. cinerea HYOGO11 was used as a wild-type strain in this study. This strain was obtained from a stock culture from the Pesticide Research Institute, Graduate School of Agriculture, Kyoto University, and was originally isolated from grey-mold rot symptom of strawberry in Hyogo Pref., Japan by C. T. All B. cinerea cultures were maintained on complete medium agar (CMA; Tanaka et al. 1992) at 25 ± 2°C. Liquid complete medium (CM: CMA minus agar) was used to extract DNA.
Construction of the gene deletion cassette for BcCcc2 and fungal transformation
The sequence of a B. cinerea copper-transporting ATPase, BC1G_10836, was obtained from databases maintained by the Broad Institute’s Fungal Genome Initiative (FGI) (http://www.broad.mit.edu/annotation/fgi/) by the blastP search with S. cerevisiae SCRG_00256 (CCC2). This gene was named BcCcc2. Sequences of other copper-transporting ATPases in B. cinerea, Candida albicans, Magnaporthe grisea, Neurospora crassa, S. cerevisiae and Stagonospora nodorum were also obtained from the FGI website. Sequences in Aspergillus fumigatus were retrieved from the NCBI gene bank. The E1-E2 ATPase (PF00122) and hydrolase (PF00702) domains were identified within the retrieved sequences using Pfam 22.0 software (http://pfam.jouy.inra.fr/index.html). Alignments of the E1-E2 ATPase domain sequences and hydrolase domain sequences were performed using the ClustalX 2.0 program (http://www.clustal.org/), with the default settings for multiple alignments. Alignments were adjusted manually and combined to one. A phylogenetic tree was constructed using the neighbor-joining function of the ClustalX 2.0 program. The tree was drawn using the PhyloDraw version 0.8 program (http://pearl.cs.pusan.ac.kr/phylodraw/).
The BcCcc2 gene of the wild-type strain was deleted using the targeted replacement method by double crossover events in homologous recombination. The deletion cassettes were constructed by PCR fusion with a strategy similar to that used by Izumitsu et al. (2009). The construction of the deletion cassette is illustrated in Fig. S1 of Electronic Supplementary Materials (ESM). To delete the BcCcc2 gene from the wild-type strain, we replaced it with a hygromycin B phosphotransferase (Hph) gene cassette obtained from the plasmid pCB1004 (Carroll et al. 1994). In the first round of PCR, the 5′untranslated region (UTR) (amplicon 1) and 3′UTR (amplicon 2) of BcCcc2 gene fragments, and the hygromycin B resistance cassette (amplicon 3) were amplified from wild-type genomic DNA and pCB1004 templates, respectively. Amplicon 1 was amplified using the primers CCC2-5UTR-FW (5′-TCGAACATCTTTCTTGTCTTGA-3′) and CCC2-5UTR-REV (5′-CTGAGCAAACTGGCCTCAGGCATTTGAGAAGCACATATTTCGGAGCCATTTCTAC-3′), amplicon 2 with CCC2-3UTR-FW (5′-GATCAAAAGTGCTCATCATTGGAAAACGTTCTTCGGATGTGTTGATTTGGCTTCTCTTA-3′) and CCC2-3UTR-REV (5′-GCAACTTCTTCTCCCACTATTAC-3′), and amplicon 3 with Hph-FW (5′-GTGCTTCTCAAATGCCTGAG-3′) and Hph-REV (5′-CGAAGAACGTTTTCCAATG-3′). The primers CCC2-5UTR-REV and CCC2-3UTR-FW were 34- to 35-bp chimeric oligonucleotides, containing a reverse complement sequence (i.e., 5′-CTGAGCAAACTGGCCTCAGGCATTTGAGAAGCAC-3′ and 5′-GATCAAAAGTGCTCATCATTGGAAAACGTTCTTCG-3′) for PCR fusion. The three resulting PCR products were gel-purified and used for a second round of PCR in order to fuse these three separate fragments into a deletion cassette using the primers CCC2-5UTR-FW and CCC2-3UTR-REV. The resulting major PCR product (approximately 2.5 kb) was purified from gel and used to transform the wild-type strain.
Transformation experiments were performed using the method described by ten Have et al. (1998) and Izumitsu et al. (2007) with modifications. Briefly, a DNA fragment of the BcCcc2 deletion cassette (5 μg) was added to a protoplast suspension (5 × 107 protoplasts/100 μl) in STC [1.2 M sorbitol, 10 mM CaCl2, 10 mM Tris–HCl (pH 7.5)] and incubated for 20 min at room temperature. Subsequently, 1 ml of PTC solution [60% (w/v) polyethylene glycol dissolved in STC] was added to the suspension and incubated for 20 min at room temperature. After removing the PTC solution through centrifugation, protoplasts were resuspended in 100 μl of STC and mixed with 10 ml of regeneration medium (1 g tryptone, 1 g yeast extracts, 15 g agar, and 1.2 M sucrose, per liter). After regeneration and germination of the resulting protoplasts, the solution was overloaded with 10 ml of selection medium (1 g tryptone, 1 g yeast extract, 15 g agar, and 200 mg hygromycine B, per liter). The resultant transformants were isolated and reinoculated onto CMA containing 100 μg/ml hygromycin B.
Integration of the deletion cassette was confirmed to be correct by PCR methods using a primer, CCC2-5UTR-268 (5′-CTAATAATTGCTTCTCGTACGTACAAC-3′), that annealed to a region outside the deletion cassette, and a primer, Hph-r2 (5′-TGTAGAAGTACTCGCCGATAGTGG-3′), that annealed to a sequence within the Hph gene. The deletion of BcCcc2 was confirmed using CCC2-5UTR-268 and a primer, CCC2-ORF79 (5′-ATACATTTCCAATGCCATCTAC-3′), that annealed to a region within the BcCcc2 ORF.
Southern blot analysis
Genomic DNA was isolated using the method described by Nakada et al. (1994). DNA digestion and gel electrophoresis were performed using standard methods (Sambrook et al. 1989). The probe consisted of a DNA fragment obtained through PCR amplification using CCC2-3UTR-FW as a forward primer and CCC2-3UTR-REV as a reverse primer. The DNA probe was labeled using the AlkPhos Direct labeling kit (GE healthcare). Hybridization and detection were conducted using the AlkPhos Direct detection system (GE healthcare) and a lumino-imager (LAS-1000, Fujifilm), according to the manufacturer’s instructions.
Complementation of the null mutant strain
For functional complementation of the null mutant, we amplified the complete gene of BcCcc2 between −1497 and +936 with primers CCC2-f0 (GGATCCTGGTGATCAATCGATAGTG) and CCC2-r0 (TGGGAAGTCGAGTGGATGTTG), using the PrimeStar GXL DNA-polymerase (Takara). The amplified fragment was cloned into the EcoRV site of the plasmid pZNat1 containing a nourseothricin-resistance (Nat) gene cassette (Izumitsu et al. 2009). The resulting plasmid pZN1CCC2 was used for transformation of the ΔBcCcc2 mutant DBC2-1. Transformation experiments were performed using a method described above. Nourseothricin-resistant transformants were selected on regeneration medium containing nourseothricin (200 μg/ml) (Werner Bioagents). Integration of the cassettes was confirmed by PCR methods using primers, CCC2-5UTR-268 and CCC2-ORF79.
Assays for the formation and function of appressoria and infection cushions
Mycelial disks (5 mm in diam.) were cut out from margins of 3-day-old colonies of the wild-type and BcCcc2 null (∆BcCcc2) mutant strains on CMA, and were placed upside down onto bottoms of plastic Petri dishes (35 mm in diam.) filled with sterile water. After 24 h, cultures were inspected microscopically using an inverted microscope (Labovert, Wild-Leitz), and the numbers of appressoria and infection cushions formed on the bottoms were counted. To investigate the function of these structures, mycelial blocks (2 × 2 mm) cut from margins of 3-day-old colonies on CMA were placed on onion epidermis and incubated in moist conditions at 25°C (Gourgues et al. 2004; Ishihara et al. 2008). After 24 h, cultures were inspected microscopically using a microscope (DMLB DIC, Leica Microsystems) and the invasions of mycelia from appressoria and infection cushions of the wild-type strain and ΔBcCcc2 mutants were observed. The rate of appressoria and infection cushions forming invasive mycelia (i.e., penetration rate) was calculated as follows. In each experiment, at least 30 of these structures were examined and counted to calculate the percentage of structures forming invasive mycelia. The means and standard errors were calculated from three independent experiments. Statistical analyses in penetration rate between the wild-type strain, ΔBcCcc2 mutants and the BcCcc2-reconstituted strain were determined by Tukey’s multiple comparison test (Tamhane 2009).
Inoculation test
Mycelial disks were cut out from the margins of 14-day-old colonies using a sterilized cork borer, and placed upside down on leaves and artificially wounded leaves detached from 1-month-old tomato plants. Mycerial disks were also placed on intact or wounded petals of carnation flowers. Inoculated plants were incubated for 4 days in moist conditions at 25°C.
Results
Identification of a copper-transporting ATPase gene of B. cinerea
The sequence of B. cinerea BC1G_10836 was obtained from the FGI website by the blastP search with the sequence of S. cerevisiae CCC2. We named this gene BcCcc2. BcCcc2 is composed of a single open reading frame (ORF) containing no introns, and encodes a protein of 1,157 aa. Previously, we revealed that copper-transporting ATPase genes could be divided into three conserved classes (Saitoh et al. 2009). The results of a phylogenetic analysis confirmed that this gene encodes a CCC2 orthologue (Fig. 1). A program for predicting transmembrane helices, TMHMM server 2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/), indicated BcCCC2 to have eight transmembrane helices (H1 to H8, Fig. S2 in ESM). Three putative copper-binding motifs (i.e., GMTCGAC) were identified at the N terminus. A putative Golgi signal (i.e., MDVLVVL) that was similar to amino acid consensus sequence of human copper-transporting ATPase ATP7A for a signal involved in retention in the Golgi (Francis et al. 1998) was found near the N terminus of H3. A phosphatase motif (i.e., TGEA) was identified between H4 and H5. A cation-transducing domain (i.e., CPC), which is highly conserved in copper-transporting ATPases, was located in H6. A phosphorylation site (i.e., DKTGT), a HP motif (i.e., SEHP), an ATP-binding motif (i.e., TGD), and a hinge motif (i.e., VGDGIND) were located between H6 and H7. The degree of overall sequence identity between BcCCC2 and CLAP1 of C. lindemuthianum, CCC2 of S. cerevisiae, and CaCCC2 of C. albicans was approximately 71, 37 and 39%, respectively.
Unrooted neighbor-joining (NJ) tree of copper-transporting ATPases in ascomycetous fungi. Bootstrap values are from 1,000 replications. Species code: Af, Aspergillus fumigatus; Bc, Botrytis cinerea; Ca, Candida albicans; Cl, Colletotrichum lindemuthianum; Mg, Magnaporthe grisea; Nc, Neurospora crassa; Sc, Saccharomyces cerevisiae; Sn, Stagonospora nodorum
Generation of null mutants for BcCcc2
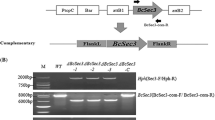
To investigate the role of BcCCC2, we generated null mutants. The BcCcc2 gene of the wild-type strain was deleted using a targeted replacement method by a double crossover event in homologous recombination. The deletion cassette containing the hygromycin B phosphotransferase gene (Hph) cassette flanked by upstream and downstream sequences of the BcCcc2 ORF was constructed by the PCR fusion method and used for transformation of the B. cinerea wild-type strain HYOGO11 (Fig. 2a). We obtained nine transformants that showed resistance to hygromycin B. Subsequently, all hygromycin B-resistant transformants were screened by the PCR method described in “Materials and methods”, and three strains, DBC2-1, DBC2-2, and DBC2-3, were isolated as homokaryotic BcCcc2 null (∆BcCcc2) mutants. The other six strains were heterokaryotic, in which nuclei containing the integrated deletion cassette and wild-type alleles were detected (data not shown). The result of Southern blot analysis showed that there were no ectopic insertions in genomes of the ∆BcCcc2 mutants DBC2-1, DBC2-2 and DBC2-3 (Fig. 2b). To confirm function of BcCCC2, we also generated a reconstituted strain by reintroduction of BcCcc2 gene of the wild-type strain into the ΔBcCcc2 strain DBC2-1, and obtained a reconstituted strain CBC2-1 (Fig. S3 in ESM).
Gene deletion of BcCcc2. a Scheme used to delete the BcCcc2 gene with the deletion cassette containing a 1.5 kb hygromycin B phosphotransferase gene (Hph) cassette as a selection marker between 0.5 kb upstream and downstream of the BcCcc2 open reading frame. The construction of the deletion cassette for BcCcc2 using the fusion PCR method is illustrated in Fig. S1. b Southern blot analysis of Xho I digests of genomic DNA from ΔBcCcc2 mutants DBC2-1, DBC2-2, DBC2-3 and the wild-type strain HYOGO11. Lanes M, λ DNA Sty I digest (size marker); 1 DBC2-1, 2 DBC2-2, 3 DBC2-3, 4 HYOGO11
BcCCC2 is required for melanization, conidiation and sclerotium formation
Colonies of the wild-type strain and ∆BcCcc2 mutants are portrayed in Fig. 3. There was no clear difference in the radial growth rate between the wild-type strain and ∆BcCcc2 mutants (data not shown). Melanization of the mutants was, however, severely impaired on CMA compared with that of the wild-type. We also found that the culture media of ∆BcCcc2 mutants stained yellow (Fig. 3). ∆BcCcc2 mutants have poorly developed aerial hyphae and were markedly impaired in conidiation. The number of conidia formed on colonies was 1,000-fold less than that of the wild-type strain (Table 1). ∆BcCcc2 mutants also showed a defect in the formation of sclerotia. After cultivation on CMA at 16°C for 4 weeks, the wild-type formed numerous sclerotia, whereas the mutants formed none (Fig. 4). The melanization, conidiation and sclerotium formation were normal in the BcCcc2 reconstituted strain (Figs. 3, 4). These results suggest that BcCCC2 is required for melanization and morphogenesis in B. cinerea. The impairment of both melanization and sclerotium formation was also reversed when ∆BcCcc2 mutants were grown in medium supplemented with 100 μM CuSO4 (Figs. 3, 4). Conidiation of ∆BcCcc2 mutants was also restored partially on copper-supplemented media (Table 1). In S. cerevisiae, a copper-transporting ATPase, CCC2, was also required for uptake of iron (i.e., high-affinity iron uptake: Askwith et al. 1994; Eide 1998). However, the addition of FeSO4 or FeCl3 did not restore impairments in the ∆BcCcc2 mutants (Figs. 5, S4 in ESM).
∆BcCcc2 mutants are impaired in the ability to penetrate plant cells
B. cinerea is known to form appressoria and infection cushions to penetrate host plant tissues (Choquer et al. 2007). We investigated whether BcCCC2 was necessary for the formation of these structures (Fig. 6). In the standard assay for the formation of appressoria, conidia are allowed to germinate on a glass slide or plant epidermis (Coertze et al. 2001; Segmüller et al. 2008). Because ∆BcCcc2 mutants formed very few conidia, we adopted mycelial disks (5 mm in diam.) from 3-day-old colonies for the assay. After 24 h of incubation, the sums of appressoria and infection cushions generated from a mycelial disk were 32.0 ± 4.0 in the wild-type strain while 10.1 ± 1.3 in ∆BcCcc2 mutants. We also observed that appressoria of ∆BcCcc2 mutants tended to be small when compared with those of the wild-type strain. ∆BcCcc2 mutants also formed small appressoria on onion epidermis (Fig. 6). The diameters of appressoria were 1.72 ± 0.23 μm in the wild-type strain and 1.07 ± 0.25 μm, in the mutants on onion epidermis. The infection cushions of ∆BcCcc2 mutants were also aberrant in morphology. Infection cushions of the wild-type strain were composed of cells with globular shape, while those of the mutants consisted of elongated and winding cells, and in some cases, secondary vegetative mycelia proliferated from these structures (Fig. 6). The rate of penetration on onion epidermis (i.e., the number of appressoria and infection cushions that produced invasive mycelia per the total number of these structures formed on onion epidermis) was 78.1% in the wild-type strain and 11.7–13.6% in ∆BcCcc2 mutants (Table 2). ∆BcCcc2 mutants clearly showed reduced rates of penetration in plant cells. The penetration rate was regained by BcCCC2 reconstitution (66.2%). Moreover, there were no clear differences in formation number and morphology of appressoria and infection cushions between the wild-type strain and the BcCcc2-reconstituted strain (data not shown). These results suggest that BcCCC2 plays important roles in the formation and function of appressoria and infection cushions in B. cinerea.
Appressoria and infection cushions of the wild-type strain HYOGO11 and the ∆BcCcc2 mutant DBC2-1 on an onion epidermis. Mycelia on the surface of the onion epidermis were stained with cotton blue while mycelia invading onion cells were not stained. AP appressorium, IC infection cushion, IM invasive mycelium, VM vegetative mycelium
∆BcCcc2 mutants were not able to cause disease in host plants
To investigate whether BcCCC2 is necessary for the pathogenicity of B. cinerea, tomato leaves were inoculated with the mycelial disks from colonies of the wild-type and ∆BcCcc2 mutant strains (Fig. 7a). The wild-type produced well-developed lesions, whereas the mutants did not produce lesions on tomato leaves even 4 days after the inoculation. ∆BcCcc2 mutants failed to form lesions on carnation petals either (Fig. 7b). The BcCcc2-reconstituted strain formed lesions on the plant as the wild-type strain did (Fig. 7c). These results suggest that BcCCC2 is necessary for the infection of a wide range of host plants in B. cinerea. The assay using onion epidermis indicated that ∆BcCcc2 mutants were severely perturbed in their ability to penetrate plant tissue. Only few appressoria and infection cushions could successfully penetrate the onion epidermis and develop invasive mycelium. To determine whether ∆BcCcc2 mutants can grow within host plants after their penetration, tomato leaves and carnation petals were wounded with a needle and inoculated. The mutants failed to develop lesions even at wounded sites (Fig. 7a, b). These results suggest that BcCCC2 is also necessary for fungal growth after penetration by B. cinerea.
Inoculation assay for the wild-type strain and ∆BcCcc2 mutants. a Inoculation of tomato leaves with the wild-type strain HYOGO11 (labeled as 1 Wild-type), ∆BcCcc2 mutants DBC2-1 (2 ∆BcCcc2) and DBC2-2 (3 ∆BcCcc2); and wound inoculation with DBC2-1 (4 ∆BcCcc2), DBC2-2 (5 ∆BcCcc2). b Inoculation of carnation petals with HYOGO11 (1 Wild-type), DBC2-1 (2 ∆BcCcc2) and DBC2-2 (3 ∆BcCcc2). c Inoculation of tomato leaves with the wild-type strain HYOGO11 (1 Wild-type), ∆BcCcc2 mutant DBC2-1 (2 ∆BcCcc2) and the BcCcc2-reconstituted strain CBC2-1 (3 ∆BcCcc2 + BcCcc2). The photographs were taken after 4 days
Discussion
In fungi, copper-transporting ATPases are known to supply copper to various copper-containing proteins such as multicopper oxidases (MCOs), tyrosinases and amine oxidases (AMOs) (Laliberté and Labbé 2006; Parisot et al. 2002; Petris et al. 2000; Yuan et al. 1995). Filamentous fungi appear to have many genes encoding these copper-containing proteins (Hoegger et al. 2006), suggesting the possibility that copper-transporting ATPases play important roles in various aspects of fungal life including pathogenicity by activating a wide range of copper-containing proteins, although there little information about this ATPase in filamentous fungi.
This study characterized a copper-transporting ATPase, BcCCC2, from B. cinerea. Clear difference was not found in growth between the wild-type strain and ∆BcCcc2 mutants, suggesting that BcCCC2 is not necessary for saprophytic growth. On the other hand, the results revealed ∆BcCcc2 mutants to be severely impaired in melanization. This has also been reported in fungal copper-transporting ATPase mutants of Cryptococcus neoformans and C. lindemuthianum (Parisot et al. 2002; Walton et al. 2005). Our results confirm and extend these findings to B. cinerea ∆BcCcc2 mutants. Among proteins targeted by copper-transporting ATPases, tyrosinases, laccases and metallo-oxidase are known to play important roles in melanization (Petris et al. 2000; Saitoh et al. 2010; Tanaka et al. 1992). Inactivation of these proteins is thought to be a main cause of the abnormal melanization in copper-transporting ATPase mutants. Our results also revealed that the cultured medium of ∆BcCcc2 mutants stained yellow. The substances responsible for this staining remain to be determined. Intermediates or shunt-products of metabolites produced in a pathway involving BcCCC2-targeted proteins might be released in the medium by ∆BcCcc2 cells.
Our results also revealed that conidiation and sclerotium formation were severely impaired in ∆BcCcc2 mutants, suggesting that a copper-transporting ATPase is required for fungal morphogenesis. Impairments in morphogenesis are also described in fungal mutants with defects in copper uptake or intracellular distribution (Borghouts et al. 2002; Marbach et al. 1994; Tucker et al. 2004). Some proteins might be inactivated by the reduced availability of copper, which affects morphogenesis in copper-transporting ATPase mutants.
Impaired functions in ∆BcCcc2 mutants were restored when the cells were grown in a copper-supplemented medium. Similar phenomena are also described in other fungal copper-transporting ATPase mutants (Parisot et al. 2002; Saitoh et al. 2009; Walton et al. 2005; Yuan et al. 1995). The phenotypic restoration of ∆BcCcc2 mutants on copper-supplemented media would result from the reactivation of BcCCC2-targeted proteins. In S. cerevisiae, a copper-transporting ATPase CCC2 is also required for high-affinity iron uptake (Askwith et al. 1994; Eide 1998). FET3, one of MCO in the yeast, is essential for high-affinity iron uptake. In this organism, mutations of Ccc2 gene resulted in a FET3 inactivation, inducing an iron starvation in the mutant. Fungal genome databases indicate that homologues of FET3 and other components of the high-affinity iron uptake system are conserved in most fungi (data not shown). However, an external supply of ferrous or ferric iron to the growth media did not restore the impairments in ΔBcCcc2 mutants (Figs. 5, S4 in ESM), unlike the case of the S. cerevisiae Ccc2 mutant (Yuan et al. 1995). Iron starvation was therefore not responsible for the impairments observed in ΔBcCcc2 mutants. Similar results are also reported in the copper-transporting ATPase mutant of C. lindemuthianum (Parisot et al. 2002). In fungi, iron-specific chelators (i.e., siderophores) mediate iron uptake (Hissen et al. 2005; Haas 2003; Oide et al. 2006). Such chelators are not present in S. cerevisiae (Haas 2003), introducing the possibility that siderophores may have enabled ΔBcCcc2 mutants to import iron despite defects in the high-affinity iron uptake system.
B. cinerea is a pathogen that causes disease in over 200 plant species. Our assay revealed that ∆BcCcc2 mutants did not cause lesions on tomato leaves or carnation petals, demonstrating that BcCCC2 plays an important role in the pathogenicity of B. cinerea, which is required for the infection of a wide range of host plants. A copper-containing protein, copper-zinc superoxide dismutase (Cu/Zn SOD) which mediates the cellular adaptation against oxidative stress, appears to be essential for pathogenicity of B. cinerea (Rolke et al. 2004). In addition, a cytochrome c oxidase (COX) which is essential for respiration in eukaryotes is also known to require copper as a cofactor (Steffens et al. 1987). However, it has been shown that both Cu/Zn SOD and COX act independently of the copper pathway involving copper-transporting ATPases in eukaryotes (Huffman and O’Halloran 2001). On the other hand, there is little evidence that proteins targeted by copper-transporting ATPases, such as AMOs, MCOs and tyrosinases, play important roles during infection in plant pathogenic fungi. In B. cinerea, a laccase BcLCC2 is strongly expressed during infection, although Bclcc2 replacement mutants were as virulent as the wild-type strain on various hosts (Schouten et al. 2002). AMOs are known to produce H2O2 through the oxidation of primary amines (Li et al. 1998), which might be important for virulence, because significant amounts of H2O2 are produced, most probably of fungal origin, during the infection by B. cinerea (Tenberge et al. 2002). However, to our knowledge, roles of fungal AMOs have not been experimentally confirmed during infection by B. cinerea and other fungal pathogens. Our results provide strong evidence that these and other proteins targeted by copper-transporting ATPases are necessary for pathogenicity by B. cinerea.
Our results suggest that BcCCC2 is necessary for penetration of host plant in B. cinerea. ∆BcCcc2 mutants formed fewer appressoria and infection cushions. The results also showed that these structures of ∆BcCcc2 mutants were aberrant in morphology and significantly less capable of penetrating the plant epidermis. Parisot et al. (2002) described that a copper-transporting ATPase, CLAP1, is necessary for pathogenicity in the fungus C. lindemuthianum. They also mentioned that the clap1 mutant formed few appressoria, similar to the ∆BcCcc2 mutants in our experiments. In addition, the authors reported that the clap1 mutant produced less melanized and misshapen appressoria, and failed to penetrate the host plant cells. In Colletotrichum spp. and Magnaporthe spp., appressorial melanization is essential for maintaining the content of the appresorium to generate a turgor pressure suitable for penetration of the cuticle (Balhadère and Talbot 2001; Kubo et al. 1991; Talbot 2003). The impaired appressorial penetration by the clap1 mutant would be due to the defect in appressorial melanization. Eventually, an improper turgor pressure would result in a misshapen appresorium. The ∆BcCcc2 mutants produce nonfunctional appressoria that cannot penetrate plant tissue. This defect is not caused by the inability of ∆BcCcc2 mutants to produce melanin, since appressoria of wild-type B. cinerea are not melanized and do not rely on turgor pressure for penetration (Choquer et al. 2007; van Kan 2006). These observations imply that B. cinerea appressorium function is likely to require critical (as yet unknown) copper-containing protein(s) that is (are) inactive in the absence of the copper-transporting ATPase BcCCC2. Moreover, ∆BcCcc2 mutants did not form lesions even in wounded host plant tissues. Parisot et al. (2002) reported a similar trait for the clap1 mutant, suggesting that a copper-transporting ATPases itself and its target proteins are necessary not only for penetration, but also for pathogenic growth within host plant tissues in both B. cinerea and C. lindemuthianum. B. cinerea and C. lindemuthianum are plant pathogens that employ different infection strategies (Choquer et al. 2007; Mendgen and Hahn 2002). Therefore, our results, combined with the study of the clap1 mutant, demonstrate that copper-transporting ATPases are commonly required for pathogenicity in a wide range of phytopathogenic fungi.
References
Askwith C, Eide D, Ho AV, Bernard PS, Li L, Davis-Kaplan S, Sipe DM, Kaplan J (1994) The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403–410
Balhadère PV, Talbot NJ (2001) PDE1 encodes a P-Type ATPase involved in appressorium-mediated plant infection by the rice blast fungus Magnaporthe grisea. Plant Cell 13:1987–2004
Borghouts C, Scheckhuber CQ, Stephan O, Osiewacz HD (2002) Copper homeostasis and aging in the fungal model system Podospora anserina: differential expression of PaCtr3 encoding a copper transporter. Int J Biochem Cell Biol 34:1355–1371
Carroll AM, Sweigard JA, Valent B (1994) Improved vectors for selecting resistance to hygromycin. Fungal Genet Newsl 41:22
Choquer M, Fournier E, Kunz C, Levis C, Pradier J-M, Simon A, Viaud M (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol Lett 277:1–10
Coertze S, Holz G, Sadie A (2001) Germination and establishment of Infection on grape berries by single airborne conidia of Botrytis cinerea. Plant Dis 85:668–677
Culotta VC, Gitlin JD (2001) Disorders of copper transport. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The molecular and metabolic basis of inherited disease 3. McGraw-Hill, New York, pp 3105–3136
Eide DJ (1998) The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu Rev Nutr 18:441–469
Elad Y, Williamson B, Tudzynski P, Delen N (2004) Botrytis spp. and diseases they cause in agricultural systems—an introduction. In: Elad Y, Williamson B, Tudzynski P, Delen N (eds) Botrytis: biology, pathology and control. Kluwer, Dordrecht, pp 1–8
Francis MJ, Jones EM, Levy ER, Ponnambalam S, Chelly J, Monaco AP (1998) A Golgi localization signal identified in the Menkes recombinant protein. Hum Mol Genet 7:1245–1252
Gourgues M, Brunet-Simon A, Lebrun M-H, Levis C (2004) The tetraspanin BcPls1 is required for appressorium-mediated penetration of Botrytis cinerea into host plant leaves. Mol Microbiol 51:619–629
Haas H (2003) Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl Microbiol Biotechnol 62:316–330
Hirayama T, Kieber JJ, Hirayama N, Kogan M, Guzman P, Nourizadeh S, Alonso JM, Dailey WP, Dancis A, Ecker JR (1999) Responsive-to-antagonisti, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 97:383–393
Hissen AHT, Wan ANC, Warwas ML, Pinto LJ, Moore MM (2005) The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding l-ornithine N 5-oxygenase, is required for virulence. Infect Immun 73:5493–5503
Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273:2308–2326
Huffman DL, O’Halloran TV (2001) Function, structure, and mechanism of intracellular copper trafficking proteins. Ann Rev Biochem 70:677–701
Hung IH, Suzuki M, Yamaguchi Y, Yuan DS, Klausner RD, Gitlin JD (1997) Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J Biol Chem 272:21461–21466
Ishihara A, Hashimoto Y, Tanaka C, Dubouzet JG, Nakao T, Matsuda F, Nishioka T, Miyagawa H, Wakasa K (2008) The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J 54:481–495
Izumitsu K, Yoshimi A, Tanaka C (2007) Two-component response regulators Ssk1p and Skn7p additively regulate high-osmolarity adaptation and fungicide sensitivity in Cochliobolus heterostrophus. Eukaryot Cell 6:171–181
Izumitsu K, Yoshimi A, Kubo D, Morita A, Saitoh Y, Tanaka C (2009) The MAPKK kinase ChSte11 regulates sexual/asexual development, melanization, pathogenicity, and adaptation to oxidative stress in Cochliobolus heterostrophus. Curr Genet 55:439–448
Kubo Y, Nakamura H, Kobayashi K, Okuno T, Furusawa I (1991) Cloning of a melanin biosynthetic gene essential for appressorial penetration of Colletotrichum lagenarium. Mol Plant-Microbe Interact 4:440–445
Laliberté J, Labbé S (2006) Mechanisms of copper loading on the Schizosaccharomyces pombe copper amine oxidase 1 expressed in Saccharomyces cerevisiae. Microbiology 152:2819–2830
Li R, Klinman JP, Mathews FS (1998) Copper amine oxidase from Hansenula polymorpha: the crystal structure determined at 2.4 Å resolution reveals the active conformation. Structure 6:293–307
Marbach K, Fernández-Larrea J, Stahl U (1994) Reversion of a long-living, undifferentiated mutant of Podospora anserina by copper. Curr Genet 26:184–186
Mendgen K, Hahn M (2002) Plant infection and the establishment of fungal biotrophy. Trends Plant Sci 7:352–356
Nakada M, Tanaka C, Tsunewaki K, Tsuda M (1994) RFLP analysis for species separation in genera Bipolaris and Curvularia. Mycoscience 35:271–278
Oide S, Moeder W, Krasnoff S, Gibson D, Haas H, Yoshioka K, Turgeon BG (2006) NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell 18:2836–2853
Parisot D, Dufresne M, Veneault C, Laugé R, Langin T (2002) clap1, a gene encoding a copper-transporting ATPase involved in the process of infection by the phytopathogenic fungus Colletotrichum lindemuthianum. Mol Genet Genomics 268:139–151
Petris MJ, Strausak D, Mercer JFB (2000) The Menkes copper transporter is required for the activation of tyrosinase. Hum Mol Genet 9:2845–2851
Rensing C, Grass G (2003) Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev 27:197–213
Rolke Y, Liu S, Quidde T, Williamson B, Schouten A, Weltring K-M, Siewers V, Tenberge KB, Tudzynski B, Tudzynski P (2004) Functional analysis of H2O2-generating systems in Botrytis cinerea: the major Cu–Zn-superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol Plant Pathol 5:17–27
Saitoh Y, Izumitsu K, Tanaka C (2009) Phylogenetic analysis of heavy-metal ATPases in fungi and characterization of the copper-transporting ATPase of Cochliobolus heterostrophus. Mycol Res 113:737–745
Saitoh Y, Izumitsu K, Morita A, Shimizu K, Tanaka C (2010) ChMCO1 of Cochliobolus heterostrophus is a new class of metallo-oxidase, playing an important role in DHN-melanization. Mycoscience. doi:10.1007/s10267-010-0043-x
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schouten A, Wagemakers L, Stefanato FL, van der Kaaij RM, van Kan JAL (2002) Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Mol Microbiol 43:883–894
Segmüller N, Kokkelink L, Giesbert S, Odinius D, van Kan J, Tudzynski P (2008) NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol Plant Microbe Interact 21:808–819
Steffens GCM, Biewald R, Buse G (1987) Cytochrome c oxidase is three-copper, two-heme-A protein. Eur J Biochem 164:295–300
Talbot NJ (2003) On the trail of cereal killer: exploring the biology of Magnaporthe grisea. Ann Rev Microbiol 57:177–202
Tamhane AC (2009) Statistical analysis of designed experiments: theory and applications. Wiley, Hoboken
Tanaka C, Tajima S, Furusawa I, Tsuda M (1992) The pgr1 mutant of Cochliobolus heterostrophus lacks a p-diphenol oxidase involved in naphthalenediol melanin synthesis. Mycol Res 96:959–964
ten Have A, Mulder W, Visser J, van Kan JAL (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant Microbe Interact 11:1009–1016
Tenberge KB, Beckedorf M, Hoppe B, Schouten A, Solf M, von den Driesch M (2002) In situ localization of AOS in host–pathogen interactions. Microsc Microanal 8:250–251
Tucker SL, Thornton CR, Tasker K, Jacob C, Giles G, Egan M, Talbot NJ (2004) A fungal metallothionein is required for pathogenicity of Magnaporthe grisea. Plant Cell 16:1575–1588
van Kan JAL (2006) Licensed to kill: the life style of a necrotrophic plant pathogen. Trends Plant Sci 11:247–253
Wakabayashi T, Nakamura N, Sambongi Y, Wada Y, Oka T, Futai M (1998) Identification of the copper chaperone, CUC-1, in Caenorhabditis elegans: tissue specific coexpression with the copper transporting ATPase, CUA-1. FEBS Lett 440:141–146
Walton FJ, Idnurm A, Heitman J (2005) Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol Microbiol 57:1381–1396
Yuan DS, Stearman R, Dancis A, Dunn T, Beeler T, Klausner RD (1995) The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc Natl Acad Sci USA 92:2632–2636
Acknowledgments
We thank Drs. Jean-Michel Fustin and Abdul Gafur for critical reading of the manuscript and valuable comments. We also thank two anonymous reviewers for their kind suggestions and comments on the revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Fischer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saitoh, Y., Izumitsu, K., Morita, A. et al. A copper-transporting ATPase BcCCC2 is necessary for pathogenicity of Botrytis cinerea . Mol Genet Genomics 284, 33–43 (2010). https://doi.org/10.1007/s00438-010-0545-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-010-0545-4