Abstract
Botrytis cinerea is a non-host-specific phytopathogenic fungus capable of infecting numerous cash crops. Here, we analyzed the functions of the Bcb1 gene in B. cinerea, which encodes a membrane protein belonging to the acyl-coenzyme A synthase family. Compared to the wild type, Bcb1-deletion mutants exhibited obvious morphological abnormalities, including slower vegetative growth and reduced melanin production. The absence of Bcb1 causes B. cinerea to form only small and incompletely developed infection cushions and fail to produce spores. The Bcb1 mutants displayed hypersensitivity to the membrane stressor SDS, the cell wall stressor Congo red, and the oxidative stressor H2O2 and increased resistance to intracellular osmotic stress caused by KCl compared to the wild-type strain. However, there were no differences in tolerance to extracellular osmotic stress caused by NaCl. The deletion of Bcb1 also caused a reduction in pathogenicity. The qRT‒PCR results showed that the genes Bcpks12 and Bcpks13, which are related to melanin biosynthesis, and Bcpg2, BcBOT2, and cutA, which are related to virulence, were downregulated in ∆Bcb1. These data suggest that BCB1 is important for conidial morphogenesis, and pathogenesis in B. cinerea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botrytis cinerea is an aggressive plant pathogen that causes gray mold disease in over 500 plant species, including fruits, vegetables, flowers, medicinal herbs, and other critical economic crops (Schumacher et al. 2017; Zhang et al. 2014; Yu et al. 2014; Moretti et al. 2015; Jin et al. 2021; Wang et al. 2011). It is difficult to control because the pathogen is characterized by abundant conidial production, high field adaptability, and diverse virulence factors and is also prone to chemical resistance (Petrasch et al. 2019). Hence, there is an urgent need to study the sporulation and pathogenesis mechanism of B. cinerea.
Membrane proteins are the bridge between the external environment and intracellular signal transduction, and play a key role as receptors and transporters in organisms. Many membrane proteins are potential drug targets. In this study, we compared the specificity of 12 pairs of primers reported for B. cinerea detection. The results showed that the primer pair UTAS-F/UTAS-R had the best specificity (Fig. S1, Table S1). Bioinformatics analysis indicated that the gene marked by the primer was a new membrane protein of the acyl-CoA synthetase (ACS) family. ACSs belong to the second subclass (Ib) of the first class of the adenylate forming enzyme superfamily, which is characterized by a variety of AMP-binding domains (Wang et al. 2016; Schmelz and Naismith 2009). The leading role of ACSs is to catalyze the thioesterification reaction of fatty acids with coenzyme A to form activated intermediates, a critical step for fatty acid activation and plays a vital role in the dynamic balance of energy supply, lipid metabolism, and lipid-related processes (Black and DiRusso 2007). In the maize pathogen Cochliobolus heterostrophus, knocking out of the gene encoding the ACS protein CPS1 decreased pathogenicity by almost 60% in leaves (Lu et al. 2003). The deletion of MoCPS1, a homologous gene of CPS1 in Magnaporthe oryzae, leads to multiple phenotypic changes, such as a reduction in conidial production, alteration in conidial morphology, defects in penetration pegs formation and a significant reduction in the pathogenicity (Wang et al. 2016). It was demonstrated in a recent report that the ACS protein BbFaa1 in Beauveria bassiana was associated with cell membrane function, conidial production and pathogenicity in a recent report (Li et al. 2022).
To date, there are no reports on functional studies of ACSs in B. cinerea. In this study, we identified and functionally characterized a gene in B. cinerea marked by primer pair UTAS-F/UTAS-R and named Bcb1. Bioinformatic analysis of the BCB1 protein suggested that it is a membrane protein with an AMP-binding domain belonging to the ACSs. ∆Bcb1 mutants exhibited defects in mycelial growth, infection cushion formation, melanin production, conidia formation and virulence. The qRT‒PCR result showed that genes related to the biosynthesis of melanin and plant toxin BOT biosynthesis were downregulated in ∆Bcb1. These results suggest that the BCB1 protein is involved in the regulation of development and virulence in B. cinerea.
Materials and methods
Fungal strains and culture conditions
The wild-type B. cinerea strain (ACCC37712) was purchased from the Agricultural Culture Collection of China. All strains were regularly incubated on potato dextrose agar (PDA, 200 g potato, 20 g glucose, 15 g agar, and 1 L water) plates at 20 °C. Regeneration medium (0.7 M sucrose, 0.5 g/l yeast extract, and 15 g/l agar) was used to regenerate B. cinerea protoplasts. The derived ΔBcb1-2 and ΔBcb1-16 strains were maintained on PDA amended with 100 µg/ml G418. Mycelial growth assays for each strain under different environmental stresses were performed on PDA containing 0.05% Congo red, 0.05% sodium dodecyl sulfate (SDS) or 1.2 M NaCl or 1.2 M KCl or 0.08% H2O2. Each plate was inoculated with a 5 mm-diameter mycelial plug taken from the edge of a 4-day-old colony grown on PDA. The percentage of inhibition of mycelial radial growth (PIMG) was calculated using the formula, PIMG = [(C − N)/(C − 5)] × 100, where C is the colony diameter of the untreated control and N is that of the treatment. Each experiment was repeated three times independently.
Identification of BCB1 protein
SignalP-5.0 (https://services.healthtech.dtu.dk/service.php?SignalP-5.0) was used to predict whether the BCB1 protein contains a signal peptide. Interpro (https://www.ebi.ac.uk/interpro/) and Pfam (https://pfam.xfam.org/) were used to predicted the conserved domain of the protein and the classification of this domain was predict by BLASTP. InterPro, PRED-TMR2 (http://athina.biol.uoa.gr/PRED-TMR2/) and TMHMM-2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/) were used to predict whether the protein contained a transmembrane region.
Construction of Bcb1-deletion strains
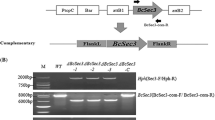
The gene Bcb1 was deleted by split-marker cassette technology based on PCR fusion as summarized in Fig. S2 A (Catlett et al. 2003). All the primers used in this study are listed in Table S1. The 1.4-kb upstream of Bcb1 “UP” and the 1.2-kb downstream “DOWN” were amplified from genomic DNA of the WT strain by primers UP-F/UP-R and DOWN-F/DOWN-R, respectively. Overlapping marker fragments “NE” and “EO” of the Neo cassette were amplified from the PKN plasmid vector using the NE-F/NE-R and EO-F/EO-R primers, respectively. Fragment “UP-NE” and “EO-DOWN” were amplified by primer pairs UP-NE-F/UP-NE-R and EO-DOWN-F/EO-DOWN-R, respectively. The fragments were purified for transformation.
Protoplasts were prepared as follows. Approximately 108 conidia of the WT strain were transferred to a flask containing 150 ml of potato glucose liquid medium (PDB) incubated in a rotary shaker (22 °C, 160 rpm) for 24 h. Mycelia were collected by filtration and was washed once with sterile water and twice with KC buffer (0.6 M KCl, 50 mM CaCl2). The mycelium was suspended in 20 ml of 0.5% Glucanex (Sigma, St. Louis) solution (dissolved in KC buffer) and incubated at 30 °C and 100 rpm for 2 ~ 3 h to generate protoplasts. Protoplasts were concentrated by centrifugation at 6000 rpm, and 4 °C for 10 min, washed twice and resuspended in STC buffer to a final concentration of 108 protoplasts per milliliter. Protocols for transformation were performed as described previously (Xue et al. 2016). Finally, the transformation mixture was mixed gently with melted RM agar medium (approximately 45 °C), and poured into 6-cm Petri dishes. After incubating at 20 °C for 20 h, each Petri dish with regenerating protoplasts was overlaid with 10 ml of RM agar medium containing 250 µg/ml G418. After incubating for 3–5 days at 20 °C, transformants were transferred to PDA containing 100 µg/ml G418 for further selection. The purified transformants were analyzed by PCR, Southern blot (validation strategy, Fig. S2D), and RT‒PCR (GAPDH gene as positive control) to ensure that the gene Bcb1 was completely knocked out.
Mycelial growth and conidiation
For vegetative growth assays, a 5-mm-diameter mycelial plug of wild-type or mutant strains was transferred onto the center of the PDA plates incubated in the dark at 20 °C. The colony diameter was measured every 24 h. For the conidial yield assay, the method described by Ma was performed (Ma et al. 2020). Three replicates were set for each strain treatment and the experiment was repeated three times.
Pathogenicity assays
Protocols for the pathogenicity assay were slightly modified from an established procedure (Feng et al. 2017). A 5-mm diameter mycelial plug of WT or a mutant was transferred to the surface of strawberry fruits and the underside side of strawberry leaves. Then fruits and leaves were incubated in artificial climate incubator at 20 °C with constant humidity and a 12 h light/12 h dark cycle environment. Three replicates were set for each strain treatment and the experiment was repeated three times.
Observation of infection cushions
Referring to a previous study (Liu et al. 2020), we used an optical microscope to observe the formation of infection cushions (ICs) of ∆Bcb1 mutants and the WT strain on microslides and the onion inner epidermis.
Quantitative RT-PCR assay
All the primers for the qRT‒PCR assay are listed in Table S1. Total RNA was extracted from the mycelial samples of mutants and WT cultured in liquid YEPD (10 mg/ml peptone, 3 mg/ml yeast extract, 20 mg/ml glucose) (Shao et al. 2017), excepted for the samples for detecting the expression of Bcpg1, Bcpg2, BcBOT2, or CutA which were cultured with polygalacturonic acid medium (0.2% (NH4)2SO4, 0.05% MgSO4, 0.1% K2HPO4, 0.05% NaCl, 1% polygalacturonic acid) (Liu et al. 2007).
RNA was isolated from mycelia with the RNA-Easy Isolation Regent (Vazyme., Nanjing, China). First-strand cDNA was synthesized with the HiScript II1st Strand cDNA Synthesis Kit (+ gDNA) (Vazyme., Nanjing, China). The reagents used for real-time PCR were ChamQ SYBR qPCR Master Mix (Vazyme., Nanjing, China). For each sample, the reference sequences were the genes encoding GAPDH and β-tubulin in B. cinerea. The analysis of relative gene expression levels using the qRT–PCR data was performed according to the 2−ΔΔCT method described previously (Livak and Schmittgen 2001). Data from three biological replicates were analyzed.
Results
Analysis of Bcb1 in B. cinerea
The gene Bcb1 (Gene ID: BCIN_13g02260) encodes a protein of 541 aa, which has a transmembrane region (226 aa–246 aa) but no signal peptide. Interpro and Pfam analyses show that it has a 306 aa adenosine monophosphate binding (AMP) domain (42 aa–347 aa) that belongs to ACSs (Fig. S3). It is a number of prokaryotic and eukaryotic enzymes, which appear to act via an ATP-dependent covalent binding of AMP to their substrate, share a region of sequence similarity. This region is a Ser/Thr/Gly-rich domain that is further characterised by a conserved Pro-Lys-Gly triplet (Jackson et al. 2016).
Bcb1 disruption resulted in abnormal colony morphology and a reduction in melanin and conidial formation
To investigate the function of the Bcb1 gene, we obtained Bcb1-deletion mutants by replacing the gene with the neomycin resistance gene (Neo). After PCR screening two stable mutants (Fig. S2B), ΔBcb1-2 and ΔBcb1-16, were confirmed by RT–PCR (Fig. S2E) and Southern blotting analysis (Fig. S2C) and used for further analysis.
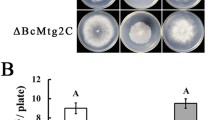
When cultured on PDA medium at 20 °C, the colony morphology of the two independent mutants was significantly different from that of the wild-type. The ∆Bcb1 mutants presented as a flat colony due to the reduced aerial hyphal growth and were whiter in color (Fig. 1A). After incubation on PDA for 25 days, the colonies of the WT strain turned gray and lots of melanin deposits appeared at the bottom of plates, while the colonies of mutants remained white and without melanin deposit at the bottom of plates (Fig. 4S). Compared with the WT strain, the colony growth rates of mutants ΔBcb1-2 and ΔBcb1-16 were significantly reduced by 67.56% and 67.07% at 96 h, respectively (Fig. 1B). After incubation on PDA for 10 days, the conidia of wild-type and mutants on each plate were collected by double-distilled water and filtered. The conidia number was quantitatively compared between wild-type and mutant strains using a hematocytometer. A large number of spores could be observed for the wild-type strain, while no conidia could be observed with the mutants (Fig. 1C). Melanin formation of all strains on sterilized carrots was observed for 4 weeks, and the expression levels of melanin biosynthesis related genes in wild-type and mutants were measured after 10 days on carrots (Zhang et al. 2015; Schumacher et al. 2016). The qRT-PCR results showed that the expression levels of the melanin biosynthesis-related genes, Bcpks12 and Bcpks13 were significantly downregulated in ∆Bcb1 mutants compared to the wild-type strain (Fig. 1D). After cultured on carrots for one week, the colonies of the WT strain began to be gray, while the colonies of ΔBcb1-2 and ΔBcb1-16 were white. Even four weeks after cultured on carrots, the colonies of two mutants remained white (Fig. 1E). These results indicate that Bcb1 is essential for hyphal growth, conidia and melanin formation.
Bcb1 disruption resulted in abnormal colony morphology and a reduction in melanin and conidial formation. A Phenotypes of the WT strain and ∆Bcb1 mutants after 10 days on PDA. B Radial growth rate of each strain. C Conidia number of the WT strain and ∆Bcb1 mutants after 10 days on PDA. D Relative expression levels of Bcpks12 and Bcpks13 in each strain after 10 days on carrots. Bars denote standard deviation from three experiments (*p < 0.05; **p < 0.01). E Processes of melanin accumulation in each strain on fresh carrots for 4 weeks.
Bcb1 is required for infection cushions and conidial formation
B. cinerea produces multicellular appressoria dedicated to plant penetration, named infection cushions (ICs). ICs develop into a haptophores from which conidiophores differentiate (Backhouse and Willetts 1987). We compared the ability of the WT strain and ∆Bcb1 mutants to form ICs and conidiation. After 3 days of incubation on PDA, visible ICs formed from the tip of the hyphae of the wild type. After 5 days, the ICs of the WT became larger and more complex and some of them developed into haptophores, while the ΔBcb1-2 and ΔBcb1-16 strains formed only small and incompletely developed ICs. (Fig. 2A). After 7 days, conidiophores differentiated from haptophores of the wild-type strain and produced large amounts of conidia, and the ICs of the mutants became larger and more complex; however, no conidium was produced. After 45 days, ∆Bcb1 mutants could form few haptophores but still could not form conidiophores and produce conidia. The same result was observed on the inner epidermis of the onion (Fig. 2B). The above results suggest that the Bcb1 gene is closely related to the development of ICs and conidia in B. cinerea.
Bcb1 contributes to the stress response in B. cinerea
Congo red binds to nascent chitin chains, inhibiting the assembly enzymes connecting chitin to 1,3-glucan and 1,6-glucan, resulting in a weakened cell wall (Bulawa 1993; Ram and Klis 2006). SDS acts as an antimicrobial membrane-active compound, that attacks cellular membranes and can therefore be used to determine the integrity of the cell membrane (Jiménez-Munguía et al. 2019). Four days after inoculation, the relative growth inhibition of ∆Bcb1 mutants on PDA containing 0.05% Congo red and 0.05% SDS was significantly higher than that of the wild type (p < 0.01) (Fig. 3A, B). These two results suggest that Bcb1 affects the cell wall and cell membrane integrity of the strain.
Growth and relative growth inhibition of each strain under different abiotic stresses. A Relative growth inhibition rates of wild-type and mutants under different abiotic stresses. B Phenotypes of the wild-type strain and ∆Bcb1 mutants after 4 days on PDA subjected to different abiotic stresses. Bars denote the standard deviation from three experiments (*p < 0.05; **p < 0.01)
Osmotic stress was triggered by both 1.2 M NaCl and 1.2 M KCl. There were no significant growth differences between the WT strain and ∆Bcb1 mutants on PDA supplemented with 1.2 M NaCl which caused extracellular osmotic stress. However, the relative inhibition of ∆Bcb1 mutants by KCl-generated intracellular osmotic stress was lower than that of the WT strain (p < 0.01) (Fig. 3A, B). Additionally, ΔBcb1-2 and ΔBcb1-16 exhibited significantly increased sensitivity to oxidative stress generated by 0.08% H2O2. The relative growth inhibition of ∆Bcb1 mutants on this medium was substantially higher than that of the WT strain (p < 0.01) (Fig. 3A, B). These results indicate that Bcb1 affects the resistance of B. cinerea to intracellular osmotic stress and oxidative stress.
BCB1 is essential for the full pathogenicity of B. cinerea
Apparent disease lesions could be observed on the fruits and leaves inoculated with WT or mutant strains. However, lesions caused by the WT strain expanded much more rapidly (Fig. 4A, B). To explore whether there were other reasons for decreased pathogenicity in mutants addition the defects in vegetable growth and conidia production, at 6 days after inoculation on leaves, we examined the expression of genes associated with pathogenicity in B. cinerea, including two genes encoding the polygalacturonases Bcpg1 and Bcpg2 (Kars et al. 2005); a gene related to the production of botrydial, BcBOT2 (Pinedo et al. 2008); and a gene encoding cutinase, cutA (Wang et al. 2013). The expression of all genes was downregulated, except for the Bcpg1 gene. The upregulation of Bcpg1 gene expression may be related to the functional complementarity between Bcpg1 and Bcpg2. (Fig. 4C). All results indicated that the Bcb1 gene plays a significant role in the pathogenicity of B. cinerea.
Knockout of the Bcb1 gene affects the pathogenicity of B. cinerea. A Disease symptoms of fruits 5 days after inoculation with a sterile PDA plug as CK and the statistical analysis of disease lesions on intact strawberry fruit 3 days or 5 days after inoculation. B Disease symptoms of leaves with a sterile PDA plug as CK and statistical analysis of disease lesions on intact strawberry leaves 3 days or 5 days after inoculation. C Expression levels of selected genes associated with pathogenicity in each strain. Bars denote standard deviation from three experiments (“*” means p < 0.05; “**” means p < 0.01)
Discussion
In this study, we identified and functionally characterized the gene Bcb1, which encodes a membrane protein that contains an AMP-binding domain. BCB1 belongs to the ACSs, which play important roles in fatty acid activation and lipid-related metabolism processes.
Loss of Bcb1 in B.cinerea affected the cell integrity. The Bcb1-mutants displayed decreased resistance to membrane (SDS) stress and cell wall (CR) stress and increased resistance to intracellular osmotic (KCl) stress compared to the wild-type strain. However, no differences in tolerance to extracellular osmotic (NaCl) stress were identified. The results were slightly different from the deletion of the ACS protein CPS1 in M. oryzae: Cps1 mutants were inhibited under extracellular osmotic (NaCl) stress but were unaffected by membrane (SDS) stress (Wang et al. 2016). In B. bassiana, the deletion of the ACS protein Faa1 also impaired cytomembrane integrity (Li et al. 2022).
Lacking Bcb1 seriously delayed the growth and morphogenetic development of B. cinerea. ∆Bcb1-mutants could only form small and incompletely developed ICs and failed to produce spores. This result agrees with what has been observed with the mutants of Cps1 genes encoding ACSs protein in M. oryzae. The deletion of MoCPS1 leads to a decrease in conidial numbers and impaired conidial morphology as well as infection hyphal development (Wang et al. 2016). At present, many genes related to sporulation have been studied in B. cinerea, such as two genes encoding hypothetical proteins, BC1G_12707.1 (Wang et al. 2013) and BcPDR1 (Zheng 2013); a gene encoding a subtilisin-like serine protease, Bcser2 (Liu et al. 2020); the autophagy-related gene BcATG14 (Yu 2017); the mitogen-activated protein kinase encoding gene BcSAK1 (Segmüller et al. 2007); the putative transcriptional regulator encoding gene Bcreg1 (Michielse et al. 2011), and the aquaporin encoding gene AQP8 (An et al. 2016). In our study, B. cinerea lost sporulation capacity after the deletion of the Bcb1 gene, which means that BCB1 may function upstream of these signal transduction pathways. The relationship between BCB1 and the above genes needs further study.
The ∆Bcb1 mutants presented as a white colony due to the reduction in pigment produced by the conidia and sclerotia. The main component of pigment was 1,8-dihydroxynaphthalene (DHN) melanin, which is an important part of the extracellular matrix. DHN imparts environmental tolerance to fungi, and is essential for the normal growth and pathogenicity of some filamentous fungi (Jia et al. 2021; Potisek et al. 2021). Although DHN does not seem necessary for the pathogenicity of B. cinerea, it is indispensable for B. cinerea to resist adverse external environments. In addition, this pigment may play a role in forming its asexual reproductive structure (Chen et al. 2021). The production of DHN in B. cinerea requires two key polyketide synthases, BcPKS12 and BcPKS13 (Schumacher 2016), which are closely related to melanin formation in the sclerotia and conidia, respectively. In our study, after the deletion of the Bcb1 gene, the expression of genes encoding these two enzymes was downregulated, especially Bcpks13, which means that BCB1 functions upstream of these signal transduction pathways.
Compared to the WT, the virulence of ∆Bcb1-mutants decreased dramatically. The affected virulence caused by the deletion of genes encoding ACS proteins has also been reported in the pathogens C. heterostrophus, M. oryzae and B. bassiana (Lu et al. 2003; Wang et al. 2016; Li et al. 2022). ICs are a special infection structure that is necessary for successful infection of mycelia. ICs of B. cinerea also secrete many phytotoxins, ROS, hydrolases, and plant cell death-inducing proteins that facilitate colonization (Choquer et al. 2021). During the infection cycle of B. cinerea, maintaining large numbers of conidia is a crucial part of successful primary and secondary infection (Carisse et al. 2018). Spore density was believed to play a major role during the first plant–fungus encounter and to determine whether B. cinerea could inhibit the plant's immune response to successfully invade the plant and cause gray mold (Veloso and Kan 2018). The decrease in virulence in the ∆Bcb1-mutant is most likely due to its IC malformation and failure to sporulate.
In addition to affecting the virulence of B. cinerea by affecting the production of ICs and conidia, BCB1 affects the expression of genes directly related to pathogenicity. Polygalacturonase plays a vital role in the infection process of plant pathogens. There are six polygalacturonases (BCPG1-BCPG6) in B. cinerea, among which BCPG2 plays a key role in its infection process (Have et al. 1998; Kars et al. 2005). Botrydial is produced by B. cinerea during plant infection and induces chlorosis and cell collapse. The BcBOT2 gene encodes a putative sesquiterpene cyclase that is responsible for the committed step of botrydial biosynthesis. Botrytis cinerea can infect undamaged plant tissue directly by penetration of the cuticle. Cutinase plays an important role in the infection process (van Kan et al. 1997). The expression levels of Bcpg2, BcBOT2, and the cutinase-encoding gene cutA were significantly downregulated in ∆Bcb1 mutants. In addition, BCB1 is involved in the resistance of B. cinerea to H2O2 which is produced by plants at the invasion site of pathogens and can kill pathogens or inhibit the growth of pathogens (Wang et al. 2022).
Fatty acids synthesized by plants can be transported to pathogenic fungi and are required for pathogen colonization and successful host infection (Jiang et al. 2017). ACSs are critical enzymes that activate fatty acids before they can be utilized for subsequent metabolism. The ACS protein BCB1 is specific to B. cinerea and plays vital roles in the development and pathogenicity of B. cinerea and. this result suggested that BCB1 has the potential to become a target for developing fungicides for B. cinerea.
References
An B, Li B, Li H et al (2016) Aquaporin8 regulates cellular development and reactive oxygen species production, a critical component of virulence in Botrytis cinerea. New Phytol 209:1668–1680
Backhouse D, Willetts HJ (1987) Development and structure of infection cushions of Botrytis cinerea. Trans Br Mycol 89:89–95
Black PN, DiRusso CC (2007) Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta 1771:286–298
Bulawa CE (1993) Genetics and molecular biology of chitin synthesis in fungi. Annu Rev Microbiol 47:505–534
Carisse O, McNealis V, Kriss A (2018) Association between weather variables, airborne inoculum concentration and raspberry fruit rot caused by Botrytis cinerea. Phytopathology 108:70–82
Catlett NL, Lee B, Yoder OC et al (2003) Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet Rep 50:Article 4
Chen X, Zhu C, Na Y et al (2021) Compartmentalization of melanin biosynthetic enzymes contributes to self-defense against intermediate compound scytalone in Botrytis cinerea. Mbio 12:e00007-21
Choquer M, Rascle C, Gonçalves IR et al (2021) The infection cushion of Botrytis cinerea: a fungal “weapon” of plant-biomass destruction. Environ Microbiol 23:2293–2314
Feng HQ, Li GH, Du SW et al (2017) The septin protein Sep4 facilitates host infection by plant fungal pathogens via mediating initiation of infection structure formation. Environ Microbiol 19:1730–1749
Grevengoed TJ, Klett EL, Coleman RA (2014) Acyl-CoA metabolism and partitioning. Annu Rev Nutr 34:1–30
Have AT, Mulder W, Visser J et al (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant Microbe Interact 11:1009–1016
Jackson DR, Tu SS, Nguyen M, Barajas JF, Schaub AJ, Krug D, Pistorius D, Luo R, Müller R, Tsai SC (2016) Structural insights into anthranilate priming during type II polyketide biosynthesis. ACS Chem Biol 11(1):95–103
Jia SL, Chi Z, Chen L, Liu GL, Hu Z, Chi ZM (2021) Molecular evolution and regulation of DHN melanin-related gene clusters are closely related to adaptation of different melanin-producing fungi. Genomics 113(4):1962–1975
Jiang Y, Wang W, Xie Q et al (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356:1172–1175
Jiménez-Munguía I, Volynsky PE, Batishchev OV et al (2019) Effects of sterols on the interaction of SDS, benzalkonium chloride, and a novel compound, kor105, with membranes. Biomolecules 9:627
Jin M, Yang C, Wei L, Cui L et al (2021) First report of Botrytis cinerea causing gray mold on Astragalus membranaceus in China. Plant Dis
Kars I, Krooshof GH, Wagemakers L et al (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J 43:213–125
Li XH, Peng YJ, Ding JL et al (2022) A homologue of yeast acyl-CoA synthetase Faa1 contributes to cytomembrane functionality involved in development and virulence in the insect pathogenic fungus Beauveria bassiana. Microb Pathog 164:105419
Liu QL, Zhang JX, Xu RF et al (2007) The influence aspect on using cellulose culture medium containing congo red differential medium to screen microorganism producing celluse. Acta Agric Boreali Occident Sin 16:279–281
Liu X, Xie J, Fu Y et al (2020) The subtilisin-Like protease Bcser2 affects the sclerotial formation, conidiation and virulence of Botrytis cinerea. Int J Mol Sci 21:603
Livak KJ, Schmittgen TD (2001)Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lu SW, Kroken S, Lee BN et al (2003) A novel class of gene controlling virulence in plant pathogenic ascomycete fungi. Proc Natl Acad Sci USA 100:5980–5985
Ma Z, Chen Z, Wang W et al (2020) Exocyst subunit BcSec3 regulates growth, development and pathogenicity in Botrytis cinerea. J Biosci 45:125
Michielse CB, Becker M, Heller J et al (2011) The Botrytis cinerea Reg1 protein, a putative transcriptional regulator, is required for pathogenicity, conidiogenesis, and the production of secondary metabolites. Mol Plant Microbe Interact 24:1074–1085
Moretti C, Quaglia M, Cerri M et al (2015) A real-time PCR assay for detection and quantification of Botrytis cinerea in Pelargonium × hortorum plants and its use for evaluation of plant resistance. Eur J Plant Pathol 143:159–171
Petrasch S, Knapp SJ, van Kan JAL et al (2019) Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol Plant Pathol 20:877–892
Pinedo C, Wang CM, Pradier JM et al (2008) Sesquiterpene synthase from the botrydial biosynthetic gene cluster of the phytopathogen Botrytis cinerea. ACS Chem Biol 19:791–801
Potisek M, Likar M, Vogel-Mikus K, Arcon I, Grdadolnik J, Regvar M (2021) 1,8-Dihydroxy naphthalene (DHN)-melanin confers tolerance to cadmium in isolates of melanised dark septate endophytes. Ecotoxicol Environ Saf 222:112493
Ram AF, Klis F (2006) Model organisms identification of fungal cell wall mutants using susceptibility assays based on calcofluor white and Congo red. Nat Protoc 1:2253–2256
Schmelz S, Naismith JH (2009) Adenylate-forming enzymes. Curr Opin Struct Biol 19:666–671
Schumacher J (2016) DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes. Mol Microbiol 99:729–748
Schumacher J (2017) How light affects the life of Botrytis. Fungal Genet Biol 106:26–41
Segmüller N, Ellendorf U, Tudzynski B et al (2007) BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot Cell 6:211–221
Shao W, Lv C, Zhang Y et al (2017) Involvement of BcElp4 in vegetative development, various environmental stress response and virulence of Botrytis cinerea. Microb Biotechnol 10:886–895
van Kan JA, van't Klooster JW, Wagemakers CA, Dees DC, van der Vlugt-Bergmans CJ (1997) Cutinase A of Botrytis cinerea is expressed, but not essential, during penetration of gerbera and tomato. Mol Plant Microbe Interact 10(1):30–38
Veloso J, van Kan JAL (2018) Many shades of grey in botrytis-host plant interactions. Trends Plant Sci 23:613–622
Wang HC, Li WH, Wang MS et al (2011) First report of Botrytis cinerea causing gray mold of tobacco in Guizhou Province of China. Plant Dis 95:612
Wang X, Xing JH, Zhao B et al (2013) Cloning and functional analysis of a gene related to conidiospore formation in Botrytis cinerea. Microbiol China 40:533–543
Wang Y, He D, Chu Y et al (2016) MoCps1 is important for conidiation, conidial morphology and virulence in Magnaporthe oryzae. Curr Genet 62:861–871
Wang Y, Li G, Chen T, Tian S (2022) Protein sulfenylation contributes to oxidative burst-triggered responses during the interaction between Botrytis cinerea and Nicotiana benthamiana. J Proteom 251:104423
Xue XM (2016) Research of pathogenicity and sclerotia related genes in Botrytis cinerea. Master’s thesis, Huazhong Agricultural University, Wu Han
Yu QY (2017) Functional analysis of autophagy-related genes BcATG26, BcATG17 and BcATG14 in Botrytis cinerea. Master’s thesis, Huazhong Agricultural University, Wu Han
Yu JR, Zhao SG, Xu YS (2014) First report of gray mold on Amorphophallus muelleri caused by Botrytis cinerea in China. Plant Dis 98:652
Zhang M, Wu HY, Wang XJ (2014) First report of Botrytis cinerea causing Eruit rot of Pyrus sinkiangensis in China. Plant Dis 98:281
Zhang C, He Y, Zhu P et al (2015) Loss of bcbrn1 and bcpks13 in Botrytis cinerea not only blocks melanization but also increases vegetative growth and virulence. Mol Plant Microbe Interact 28:1091–1101
Zheng HX (2013) Pathogenicity regulatory function of BcPDR1 gene in Botrytis cinerea. Master’s thesis, Heibei agricultural university, Heibei
Acknowledgements
This work was supported by Grants from the National Key R&D Program of China (2019YFD1002002), the Agricultural Science and Technology Innovation Fund of Hunan Province (2022CX72), the National Key Research and Development Program of Hunan (2021NK2003), Hunan Agriculture Research System (2022-31).
Author information
Authors and Affiliations
Contributions
Jiling Xiao and Ke Yang wrote the main manuscript text and Zhihuai Liang performed the data analysis. Yi Zhang and Lin Wei prepared all the figures.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, J., Yang, K., Liang, Z. et al. BCB1, a member of the acyl-coenzyme A synthetase family, regulates the morphogenesis and pathogenicity of Botrytis cinerea. Arch Microbiol 205, 206 (2023). https://doi.org/10.1007/s00203-023-03540-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03540-w