Abstract
Currently, the only genetic resistance against root-knot nematodes in the cultivated tomato Solanum lycopersicum (Lycopersicon esculentum) is due to the gene Mi-1. Another resistance gene, Mi-3, identified in the related wild species Solanum peruvianum (Lycopersicon peruvianum) confers resistance to nematodes that are virulent on tomato lines that carry Mi-1, and is effective at temperatures at which Mi-1 is not effective (above 30°C). Two S. peruvianum populations segregating for Mi-3 were used to develop a high-resolution map of the Mi-3 region of chromosome 12. S. lycopersicum BACs carrying flanking markers were identified and used to construct a contig spanning the Mi-3 region. Markers generated from BAC-end sequences were mapped in S. peruvianum plants in which recombination events had occurred near Mi-3. Comparison of the S. peruvianum genetic map with the physical map of S. lycopersicum indicated that marker order is conserved between S. lycopersicum and S. peruvianum. The 600 kb contig between Mi-3-flanking markers TG180 and NR18 corresponds to a genetic distance of about 7.2 cM in S. peruvianum. We have identified a marker that completely cosegregates with Mi-3, as well as flanking markers within 0.25 cM of the gene. These markers can be used to introduce Mi-3 into cultivated tomato, either by conventional breeding or cloning strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes are important pathogens of many cultivated crops, including tomato (Solanum lycopersicum). All resistance to root-knot nematodes in cultivated tomato is conditioned by the gene Mi-1, which was transferred from the related wild species S. peruvianum to S. lycopersicum by embryo rescue followed by traditional breeding (Smith 1944; Medina Filho and Stevens 1980). Mi-1 has been cloned and shown to encode a protein with a nucleotide-binding site and leucine-rich repeats, which are features characteristic of many other pathogen resistance proteins (Milligan et al. 1998; Vos et al. 1998). Mi-1 also confers resistance against the potato aphid, Macrosiphum euphorbiae (Rossi et al. 1998) and the white fly, Bemisia tabaci (Nombela et al. 2003).
Mi-1-mediated resistance is effective against the three most economically important root-knot nematode species, Meloidogyne incognita,M. javanica, and M. arenaria and is widely used in modern processing tomato cultivars to control nematodes (Roberts and Thomason 1986; Williamson 1998). However, there are natural isolates of each of these three nematode species that can overcome the resistance mediated by Mi-1 (Riggs and Winstead 1959; Castagnone-Sereno et al. 1994; Kaloshian et al. 1996). In addition, resistance mediated by the Mi-1 gene is compromised at soil temperatures above 30°C (Holzmann 1965; Dropkin 1969; Ammati et al. 1986). Due to the increasing importance of host resistance for nematode control, new sources of resistance are needed.
Root-knot nematode resistance has been reported in several accessions of the diverse wild species S. peruvianum (Cap et al. 1993; Yaghoobi et al. 1995; Veremis and Roberts 1996; Veremis et al. 1999). Some of these sources carry genes that confer resistance to nematodes that are virulent on strains carrying Mi-1. Other sources carry resistance that functions at temperatures above 30°C at which Mi-1 is no longer effective. However, few of these potential resistance genes have been mapped, let alone cloned. Mi-9, which confers heat-stable resistance to a similar set of nematode isolates as Mi-1, is located on the short arm of chromosome 6 in the same genetic interval as Mi-1 (Ammiraju et al. 2003). The resistance gene, Mi-3, which confers resistance to Mi-1-virulent root-knot nematode strains, has been mapped to the short arm of chromosome 12 (Yaghoobi et al. 1995; Huang et al. 2004). In addition, Mi-3, or a closely linked gene, confers resistance at temperatures above 30°C (Yaghoobi et al. 1995; Veremis and Roberts 1996).
Since Mi-3 confers resistance to Mi-1-virulent nematode isolates and may also confer heat-stable resistance, incorporation of this trait into cultivated tomato is desirable and is expected to complement the qualities of Mi-1. Attempts to introduce these traits via traditional breeding schemes are ongoing (Doganlar et al. 1997; Moretti et al. 2002). However, another approach is to isolate the Mi-3 gene from S. peruvianum by a positional cloning strategy and to introduce the gene into cultivated tomato by plant transformation. Both approaches require additional information on the genetic position of Mi-3. Here, we report the fine-scale genetic mapping of Mi-3 and the development of a tomato DNA contig that spans this locus.
Materials and methods
Plant materials
Two S. peruvianum populations were used in this study. The S. peruvianum population VWP2×4 consists of progeny from a cross between two plants VWP2 and VWP4, which were previously found to have the genotypes Mi-3/mi-3 and mi-3/mi-3, respectively (Yaghoobi et al. 1995). Crossing of sibling plants heterozygous for Mi-3 from the VWP2×4 population produced a second S. peruvianum population, referred to in this paper as a pseudo-F2 population. These Mi-3/mi-3 sibling plants were identified as heterozygotes by testing their resistance to nematodes and by marker analysis and were crossed to each other. Genotyping of the linked marker loci NR14 and TG180 on chromosome 12 (Fig. 1), together with resistance tests on the plants, identified Mi-3-homozygotes. These homozygotes were crossed to each other and additional seeds of this genotype were obtained in fertile combinations.
Nematode assays
M. incognita 557R is a virulent strain that can reproduce on tomato plants carrying the Mi-1 gene, but does not reproduce on S. peruvianum plants with Mi-3 (Yaghoobi et al. 1995). The M. javanica strain VW4 is avirulent on tomato plants with Mi-1. VW4 can reproduce on tomato lines with Mi-1 at 32°C, but does not reproduce on S. peruvianum plants carrying the Mi-3 gene at either temperature.
For nematode resistance assays, six well-rooted cuttings were established for each plant in sterile river sand as previously described (Yaghoobi et al. 1995). Three cuttings of each plant were infected with M. incognita 557R and maintained for 6 weeks at 27°C in a growth chamber. The other three rooted cuttings were infected with M. javanica strain VW4 and were maintained at 32°C in a growth chamber. Each plant was infected with 3,000 second-stage juveniles (VW4) or 10,000 eggs (557R). After 6 weeks, the plants were uprooted and the egg masses were stained and counted as previously described (Yaghoobi et al. 1995). A plant was designated as resistant if it harbored 20 or fewer egg masses and as susceptible if more than 20 egg masses were present. The S. lycopersicum cultivars VFNT cherry (Mi-1/Mi-1) and UC82 (mi-1/mi-1) were used as controls for nematode infection.
Marker development and scoring
Different marker types were used to characterize the VWP2X4 progeny. TG180, TG68 and TG 263 were scored as RFLP markers and NR14 and NR18 were scored as CAPS (cleaved amplified polymorphic sequence) and RAPD (randomly amplified polymorphic DNA) markers, respectively (Yaghoobi et al. 1995). To characterize the pseudo-F2population, key markers were converted to a format that could be screened by PCR, as follows (Table 1). The NR18-specific RAPD fragment was cloned into the TA cloning vector pCR2.1 (Invitrogen, Carlsbad, CA) and the sequence of the insert was determined. Plasmid-bearing clones harboring the RFLP markers TG180 and TG263 (Tanksley et al. 1992) were also sequenced. Specific primers were prepared for all three markers that could amplify DNA of the parental lines VWP2 and VWP4 (Table 1).
All PCRs were carried out in a GeneAmp PCR system 9700 thermocycler (Applied Biosystems, Foster City, CA). Each reaction contained ~50 ng of template DNA; incubation conditions have been described elsewhere (Yaghoobi et al. 1995). Amplification products were resolved by electrophoresis on 1% agarose gels.
Identification and analysis of recombinants in the pseudo F2 population
Seeds of the pseudo-F2 population were subjected to a 2–3 day cold shock to overcome dormancy, then placed in 10% bleach for 15 min and washed five to six times with sterile water. Individual seeds were germinated in small plastic pots containing greenhouse potting soil covered with a layer of vermiculite. Two leaves were taken from young seedlings for DNA extraction following the rapid method described by Edwards et al. (1991). The DNA was resuspended in 30 μl of 1×TE buffer and 2.5 μl of a 1/10 dilution was used in a PCR assay. DNA samples were screened for the Mi-3 flanking markers TG180 and TG263 to identify plants in which recombination events had occurred. Recombinant individuals were transferred into larger pots and large-scale DNA preparations were carried out as described previously (Yaghoobi et al. 1995).
S. lycopersicum library screening and contig construction
Seven high-density filters corresponding to clones in a BAC library of the S. lycopersicum cultivar Heinz 1706 were obtained from the Clemson University Genomics Institute (CUGI, Clemson, SC). This library represents approximately15 genome equivalents and has an average insert size of about 114 kb (Budiman et al. 2000). The probes used to screen the BAC library were created and used as follows. Using PCR primers for a marker of interest, a fragment was amplified from homozygous Mi-3 plant DNA, gel purified using either Prep-a-Gene DNA purification kit (Bio-Rad, Hercules, CA) or QIAquick Gel Extraction kit (Qiagen, Valencia, CA) and used to probe the filters. The probe DNA was labeled with 32P using a mega-prime DNA labeling kit (Amersham Pharmacia Biotech, Piscataway, NJ). Hybridization, washing and clone identification were carried out as described in Budiman et al. (2000). In most cases only a subset of the high-density filters was screened with each probe. BAC clones identified by the probe were obtained from CUGI and BAC DNA was prepared following an alkaline-lysis protocol (Budiman et al. 2000) using Qiagen plasmid preparation kits. NotI-digested DNA from the positive BACs was analyzed on a CHEF Mapper apparatus (Bio-Rad) to determine insert sizes. HindIII-digested DNA from positive BACs was fractionated on an agarose gel for fingerprinting and determination of overlaps. Southern hybridization was used to examine the overlaps and to develop the contig.
BAC-ends were isolated as PCR fragments essentially as described by Marek and Shoemaker (1997), then gel purified and sub-cloned into the Topo-TA cloning vector (Invitrogen). DNA sequences of the BAC-ends were used to develop specific oligonucleotide primers (Table 2). DNA fragments were amplified from VWP2, VWP4 and a plant homozygous for Mi3/Mi3 with these primers. If a size polymorphism was observed between parental lines, primers were used to directly score recombinants in the pseudo-F2 population. If amplified fragments were monomorphic in size, they were digested with an array of restriction enzymes to identify any polymorphism (Table 1).
Results
Analysis of the VWP2×4 population
In our previous analysis of 48 progeny of the S. peruvianum population VWP2×4, we were not able to genetically separate the Mi-3 locus from the markers TG180 and NR14 (Yaghoobi et al. 1995). The closest markers to Mi-3 on one side were the unresolved markers TG263 and NR18, and no closely linked markers were identified on the other side. To increase the resolution of our map and to identify Mi-3-flanking markers, we examined 133 additional plants from the VWP2×4 population. Plants were assayed both for resistance to the Mi-1-virulent nematode strain 557R at 27°C and for resistance to the Mi-1-avirulent strain VW4 at 32°C. In most cases, resistance and susceptibility were correlated in the two assays. In the few cases where the scoring differed between the two assays, the discrepancy could be explained by a small or unhealthy root system on plants in one assay but not the other. Thus certain plants appeared resistant, even though our genetic markers and the other assay predicted a susceptible response. Additional cuttings of these plants were re-tested and found to be susceptible. We therefore concluded that the two resistance assays were correlated for all samples, which is consistent with the interpretation that a single, dominant gene controls resistance to the Mi-1-infecting nematode strain 557R at 27°C and the resistance to strain VW4 at 32°C. Out of 133 plants tested from the VWP2×4 population, 69 were scored as resistant and 64 as susceptible, in agreement with the expected 1:1 segregation ratio based on chi-square statistics (0.70>P>0.50). The same 133 plants were scored with the DNA markers TG180, TG263, TG68, NR18 and NR14 (Table 2). Segregation ratios for these loci were in agreement with the expected 1:1 ratios (0.90>P>0.70 for TG180, NR18 and NR14, and 0.95>P>0.90 for TG263 and TG68). Recombinants that separated markers NR14 and TG180 from each other and from Mi-3 resistance were identified, and the most likely map order placed these markers telomeric to Mi-3 . By combining the data for our previous sample of 48 plants with those for the 133 plants in this study, we produced a genetic map of the Mi-3 region with closely linked flanking markers (Fig. 1).
Identification of recombinants in a pseudo-F2 population
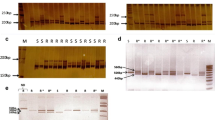
To aid in screening for additional recombinants, the markers TG180, TG263 and NR18 were converted to a PCR format (Table 1). TG180 and TG263 showed a length polymorphism between the parental lines, and progeny could be scored directly with these codominant markers (Fig. 2). NR18 was monomorphic between the two parental lines, but was converted into a polymorphic CAPS marker with the restriction enzyme Bsu36I and used in this manner to genotype the progeny (Fig. 2).
a, b Co-dominant PCR-based markers used to identify recombinants. The numbers 1 and 2 on the left (arrowheads) indicate the fragment position for each marker allele. For each marker the allele associated with resistance (Mi-3) is designated Allele 1 and that associated with the susceptible locus (mi-3) is designated Allele 2. a Fragment length polymorphisms detected after amplification with primers for TG180 and TG263 (Table 1). Each lane contains DNA from a separate, representative plant in the F2 population. b Patterns obtained from selected recombinants between markers TG180 and TG263 after amplification using PCR primers developed from RAPD marker NR18 (Table 1). The first lane shows the undigested 1.2 kb PCR fragment. The remaining lanes show Bsu36I-digested PCR products. The fragment derived from allele 2 is cleaved once by this enzyme (giving 0.65- and 0.55-kb products) whereas allele 1 is not cut (1.2 kb). The lane marked M contained a 1 kb ladder DNA marker (Invitrogen)
We were not able to generate a true F2 population due to the complex incompatibility system in S. peruvianum (Rivers et al. 1993; Kowyama et al. 1994). However, by crossing heterozygous (Mi-3/mi3) sib plants from the VWP2×4 population, we produced a pseudo-F2 population. We screened 390 individuals from this population with the flanking markers TG180 and TG263 to identify plants with recombination events near Mi-3. Sixty-nine recombinant plants were identified and scored for alleles of the marker NR18 (Table 3). Of these, 48 had undergone a recombination event between markers TG180 and NR18, while 23 plants had a recombination event between NR18 and TG263, including two double recombinants. Three cuttings each of the 69 plants were tested for Mi-3 resistance (Table 3). Plants scored as resistance had egg mass numbers ranging from 0 to 15, while susceptible plant roots all had >58 egg masses. The ratio of resistant to susceptible plants was consistent with 3:1 segregation (0.90>P>0.70).
We produced seeds from plants that were homozygous for the marker alleles flanking Mi-3, and thus were presumably homozygous for Mi-3 as well. Forty of these Mi-3-homozygotes were tested for resistance to strain 557R at 27°C and for resistance to M. javanica strain VW4 at 32°C. No egg masses were found on any of these plants, indicating a high level of resistance. In comparison the average egg mass number on forty individual heterozygous (Mi-3/mi-3) plants from the VWP2×4 population was 1.8 (range 0–20) with strain 557R at 27°C and 4.4 (range 0–20) for the same plants tested with VW4 at 32°C. The observed copy number effect for this gene suggests that the Mi-3 allele is incompletely dominant.
Chromosome walking and BAC contig construction
A S. lycopersicum BAC library was screened with the markers NR14, TG180, and NR18 and hybridizing clones were identified. Since these markers had previously been shown in RFLP analyses to hybridize to more than one genetic locus, it was expected that some of the BAC clones would not be linked to the Mi-3 locus. BAC clones were tested for PCR amplification with locus-specific primers corresponding to each marker. Positive BACs were digested with HindIII, and digestion patterns were compared to those of genomic DNA after hybridization with each marker. The markers TG180 and NR14 identified three BACs (224E15, 193F20 and 206H3) with the expected hybridization patterns, and each of these BACs carried both NR14 and TG180 (Fig. 3). The marker NR18 identified BAC 276B2 from the library.
S. lycopersicum BAC contig spanning the Mi-3 region. The thick horizontal line represents the region of chromosome 12 spanning the Mi-3 gene in S. peruvianum. The telomer is to the left and the centromere to the right, as indicated by the arrows. Markers listed below the thick line are from BAC-end probes (except for TG180, NR14 and NR18). The numbers indicated above the line are the numbers of recombinants from the pseudo-F2 population in each interval. The thin horizontal lines represent BACs spanning the Mi-3 region with left (L) and right (R) ends indicated. Numbers in parentheses are estimates of BAC-insert size as determined on a CHEF gel system. The six BACs marked with asterisks were used to estimate contig length
A chromosome-walking strategy was followed to develop a complete DNA contig spanning the Mi-3 region between the markers NR14/TG180 and NR18. DNA sequence from cloned BAC-insert ends was used to develop markers for re-screening the library. Marker order and genetic distances were determined with recombinants from theVWP2×4 and pseudo-F2 populations. The BAC-end marker E15R mapped centromeric to TG180 and closer to Mi-3, while E15L mapped telomeric to TG180 and NR14, allowing us to orient the BAC (Fig. 3). Marker E15R was used to re-screen the library and identified three BACs, 95D9, 48P18 and 45N22. PCR analysis, HindIII fingerprinting and Southern analyses were carried out to determine the extent of overlap of the BACs. The BAC-ends D9L, P18L and N22R all mapped centromeric to E15R. N22R co-segregated with Mi-3 in all 181 progenies of the segregating VWP2×4 population. We were not able to genetically map the BAC-end markers H3L and F20R due to a lack of polymorphism, but we were able to determine their relative positions based on overlap analysis.
On the centromeric side, B2L mapped closer to Mi-3 locus than did NR18 (Fig. 3), and this probe identified four BACs: 55I2, 90K24, 57J5 and 228J18. The markers J5R and J18L both mapped telomeric to B2L and both identified BAC 179I24. The BAC-end I24R mapped closer to Mi-3 and was used to identify BACs 274D1 and 271P13. The BAC-end D1R could not be resolved from I24R by recombinants, while P13L mapped closer to the Mi-3 locus. P13L identified BAC 175H8. H8L hybridized to BACs 271P13, 274D1 and 179I24, indicating that it was farther from the Mi-3 locus. We were not able to genetically map the right end of 175H8 due to a lack of polymorphism, but an H8R probe identified BAC 265L16 (Fig. 3).
The probe D9L identified BAC 260P22. The BAC-end P22L was separated from Mi-3 by only three recombination events. N22R, the marker cosegregating with Mi-3, identified BAC 127E21 from the library (Fig. 3). E21R mapped 1.85 cM telomeric to Mi-3, while E21L mapped about 0.175 cM centromeric from the Mi-3 locus. Therefore, the ends of BAC 127E21 flank the Mi-3 locus (Fig. 3). The marker E21L identified BACs 234F6 and 248K13 (not shown in Fig. 3). The BAC-end F6L mapped centromeric to both Mi-3 locus and E21L. We identified another BAC, 81L14, using F6L as a probe. BACs 81L14 and 265L16 overlapped and completed the DNA contig spanning the region from TG180 to NR18. Using both PCR and Southern hybridization analysis it was determined that L16R is on BAC 81L14 and L14L is on BAC 265L16 (Fig. 3).
Discussion
Our data indicate that a single locus, Mi-3, confers resistance to Mi-1-virulent nematodes at 27°C and to Mi-1-avirulent nematode strains at 32°C (heat-stable resistance). However, Veremis and Roberts (1996) suggested that the two phenotypes were controlled by linked genes, Mi-3 and Mi-5. Possible explanations for the discrepancy with our results include differences in the backgrounds of the parental lines or resistance assay procedures. Whereas our cross involved resistant and susceptible S. peruvianum plants, Veremis and Roberts (1996) used a bridge line, EPP-1, which is a complex hybrid of S. peruvianum and S. lycopersicum, as their susceptible parent. Segregation of modifiers of Mi-3 activity, possibly due to S. lycopersicum genes, may explain the discrepancies between their results and ours. Our assays also suggest that nematode resistance is more effective in plants that are homozygous for Mi-3 than in heterozygotes, though both homozygotes and heterozygotes display strong resistance.
We present here a fine-scale map of the Mi-3 gene in S. peruvianum. The flanking markers TG180 and NR18 define a genetic distance of about 6.07 cM. We identified 48 recombinants in this region in the pseudo-F2 population and 12 recombinants from the VWP2×4 population, yielding an average resolution of about 0.1 cM per recombination event. We have identified a marker that cosegregates with Mi-3 and flanking markers within 0.1–0.2 cM on either side. S. peruvianum is the most variable of the Lycopersicon section of Solanum species in terms of both DNA polymorphisms and phenotype variability, and it is also in a distinct clade from S. lycopersicum (Miller and Tanksley 1990; Marshal et al. 2001). In contrast to S. lycopersicum, S. peruvianum is self incompatible and green-fruited (Rick and Yoder 1988; Parokonny et al. 1997). However, a comparison of the two maps indicates that the marker order is conserved. The genomes of Lycopersicon species are in general highly syntenic (van Ooijen et al. 1994). However, Van Ooijen et al. (1994) have postulated an inversion spanning the region TG180–TG68 in their S. peruvianum cross compared to the S. lycopersicum gene order, although the evidence for this inversion is fairly weak. However, if such an inversion does exist, it may present a problem in introgressing Mi-3 into tomato using conventional breeding.
There is also extensive synteny between the genomes of tomato, potato and pepper (Tanksley et al. 1992; Livingstone et al. 1999; Grube et al. 2000). Several other disease resistance genes have been mapped to chromosome 12 of solanaceous plants, including Lv, which confers resistance to powdery mildew in tomato (Chunwongse et al. 1994), Gpa-2, a cyst nematode resistance gene in potato (Roupe van der Voort et al. 1997b), Rx, a virus resistance gene in potato (Ritter et al. 1991), CMV, a virus resistance gene in tomato (Stamova and Chetelat 2000), and Me3 and Me4, which are root-knot nematode resistance genes in pepper (Djian-Caporalino et al. 2001). The potato nematode resistance gene Gpa2 maps 10 cM away from the marker NR14, suggesting that neither Gpa2 nor Rx1, which is in the same gene cluster as Gpa2, is allelic to Mi-3 (Roupe van der Voort et al. 1997b; van der Vossen et al. 2000). However, few common markers have been used to map the R-genes on chromosome 12, and the information available is insufficient to allow us to ascertain whether Mi-3 is allelic to any of these other genes (Grube et al. 2000). Like Mi-3, the Me3 gene of pepper confers resistance against Mi-1-virulent nematode strains and is effective at elevated temperatures (Djian-Caporalino et al. 2001). It will be particularly interesting to determine the positional and genetic relationship between Mi-3 and Me3 once clones of one or both genes are available.
The six overlapping BACs 224E15, 127E21, 81L14, 265L16, 271P13 and 276B2 form a DNA contig spanning the region between the markers NR14 and NR18 that flank the Mi-3 gene (Fig. 3). Taking into account an approximation of the extent of overlaps based on the fingerprinting gels, we estimate that these BACs span a region of about 600 kb. The genetic distance between these markers is about 7.2 cM. From this we can calculate a physical to genetic distance ratio of about 83 kb/cM for the region. However, it should be noted that the physical distance was obtained from a BAC carrying S. lycopersicum DNA, while the genetic distance was obtained from recombination within S. peruvianum. In the current study we found that the markers NR14 and TG180 are 1.1 cM apart in S. peruvianum. Our earlier work indicated that the markers NR14 and TG180 were 6.4 cM apart in an S. lycopersicum×S. pennellii mapping population (Yaghoobi et al. 1995), suggesting an even higher recombination rate. Since, on average, 1 cM in tomato corresponds to about 750 kb (Tanksley et al. 1992), the Mi-3 region appears to lie in an area of the genome with a high recombination rate. The physical to genetic distance ratio varies greatly in different regions of the tomato genome. For example, a ratio of 25 Mb/cM was found for the jointless-2 region, which maps near the centromere of chromosome 12, probably due to the heterochromatic nature of the genomic environment (Budiman et al. 2004), whereas for jointless the corresponding value is <50 kb/cM (Mao et al. 2001). Interestingly, high recombination frequency is also observed near the Cf-4/Cf-9 resistance gene cluster on chromosome 1 (Bonnema et al. 1997).
Although the BAC library used in the construction of the contig is derived from S. lycopersicum and does not contain the resistance allele of the Mi-3 gene, it allowed us to produce new molecular markers and a detailed map of the Mi-3 region in S. peruvianum. The fine genetic map and tightly linked markers identified in this work should be useful to for incorporating Mi-3 by marker-assisted selection. The high recombination rates in the Mi-3 region suggest that linkage drag will not be a serious problem, but that tightly linked markers must be used to monitor introgression. The marker N22R, which cosegregates with Mi-3, and the markers P22L and E21L, which flank Mi-3 and are less than 0.25 cM away, should be suitable markers. Within our S. peruvianum cross, the PCR amplification products differ in size between the parents, allowing the markers to be scored directly without restriction digestion (Table 2). In order to monitor the introgression, it will be necessary to test primers for their ability to distinguish S. peruvianum from S. lycopersicum alleles.
Based on our estimate of physical to genetic distance of 83 kb/cM for this region of the chromosome, we have delimited Mi-3 to a 25–30-kb region. We are now in a very good position to isolate the gene. However, for this we will need to produce a S. peruvianum library containing the Mi-3 gene.
References
Ammati M, Thomason IJ, McKinney HE (1986) Retention of resistance to Meloidogyne incognita in Lycopersicon genotypes at high soil temperature. J Nematol 18:491–495
Ammiraju JSS, Veremis JC, Huang X, Roberts PA, Kaloshian I (2003) The heat-stable, root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor Appl Genet 106:478–484
Bonnema G, Schipper D, van Heusden S, Lindhout P, Zabel P (1997) Tomato chromosome 1: high resolution and physical mapping of the short arm in an interspecific Lycopersicon esculentum×Lycopersicon peruvianum cross. Mol Genet Genomics 253:455–462
Budiman MA, Mao L, Wood TC, Wing RA (2000) A deep-coverage tomato BAC library and prospects toward development of an STC framework for genome sequencing. Genome Res 10:129–136
Budiman MA, Chang S-B, Lee S, Yang TJ, Zhang H-B, de Jong H, Wing RA (2004) Localization of jointlass-2 gene in the centromeric region of tomato chromosome 12 based on high resolution genetic and physical mapping. Theor Appl Genet 108:190–196
Cap GB, Roberts P, Thomason IJ (1993) Inheritance of heat-stable resistance to Meloidogyne incognita in Lycopersicon peruvianum and its relationship to the Mi gene. Theor Appl Genet 85:777–783
Castagnone-Sereno P, Wajnberg E, Bongiovanni M, Leroy F, Dalmasso A (1994) Genetic variation in Meloidogyne incognita virulence against the tomato Mi resistance gene. Evidence from isofemale line selection studies. Theor Appl Genet 88:749–753
Chunwongse J, Bunn TB, Crossman C, Jiang J, Tanksley SD (1994) Chromosomal localization and molecular-marker tagging of powdery mildew resistance gene (Lv) in tomato. Theor Appl Genet 89:76–79
Djian-Caporalino C, Pijarowski L, Fazari A, Samson M, Gaveau L, O’Byrne C, Lefebvre V, Caranta C, Palloix A, Abad P (2001) High-resolution genetic mapping of pepper (Capsicum annuum L.) resistance loci Me3 and Me4 conferring heat-stable resistance root-knot nematodes (Meloidogyne spp.). Theor Appl Genet 103:592–600
Doganlar S, Frary A, Tanksley SD (1997) Production of interspecific F1 hybrids, BC1, BC2 and BC3 populations between Lycopersicon esculentum and two accessions of Lycopersicon peruvianum carrying root-knot nematode resistance genes. Euphytica 95:203–207
Dropkin VH (1969) The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: reversal by temperature. Phytopathology 59:1632–1637
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Grube RC, Radwanski ER, Jahn M (2000) Comparative genetics of disease resistance within Solonaceae. Genetics 155:873–887
Holzman OV (1965) Effects of soil temperature on resistance of tomato to root-knot nematode (Meloidogyne incognita). Phytopathology 55:990–992
Huang X, McGiffen M, Kaloshian I (2004) Reproduction of Mi-virulent Meloidogyne incognita isolates on Lycopersicon spp. J Nematol 36:69–75
Kaloshian I, Williamson VM, Miyano G, Lawn DA, Westerdahl BB (1996) Resistance-breaking nematodes in California tomatoes. Calif Agric 50:18–19
Kowyama Y, Kunz C, Lewis I, Newbigin E, Clarke AE, Anderson MA (1994) Self-compatibility in a Lycopersicon peruvianum variant (LA2157) is associated with a lack of style S-RNase activity. Theor Appl Genet 88:859–864
Livingstone KD, Lackney VK, Blauth JR, van Wijk R, Jahn MK (1999) Genome mapping in Capsicum and the evolution of genome structure in the Solanaceae. Genetics 152:1183–1202
Mao L, Begum D, Goff SA, Wing RA (2001) Sequence and analysis of the tomato JOINTLESS locus. Plant Physiol 126:1331–1340
Marek LF, Shoemaker RC (1997) BAC contig development by fingerprint analysis in soybean. Genome 40:420–427
Marshal JA, Knapp S, Davey MR, Power JB, Cocking EC, Bennett MD, Cox AV (2001) Molecular systematics of Solanum section Lycopersicum Lycopersicon) using the nuclear ITS rDNA region. Theor Appl Genet 103:1216–1222
Medina-Filho HP, Stevens MA (1980) Tomato breeding for nematode resistance: survey of resistance varieties for horticultural characteristics and genotype of acid phosphatase. Acta Hortic 100:383–391
Miller JC, Tanksley SD (1990) RFLP analysis of phylogenetic relationships and genetic variation in the genus Lycopersicon. Theor Appl Genet 80:437–448
Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root-knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10:1307–1319
Moretti A, Bongiovanni M, Castagnone-Sereno P, Caranta C (2002) Introgression of resistance against Mi1-virulent Meloidogyne spp. from Lycopersicon peruvianum into L. esculentum. Tomato Genet Coop Rep 52:21–23
Nombela G, Williamson VM, Muniz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe Int 16:645–649
Parokonny AS, Marshal JA, Bennett MD, Cocking EC, Davey MR, Power JB (1997) Homoeologous pairing and recombination in backcross derivatives of tomato somatic hybrids (Lycopersicon esculentum + L. peruvianum). Theor appl Genet 94:713–723
Rick CM, Yoder JI (1988) Classical and molecular genetics of tomato: highlights and perspectives. Annu Rev Genet 22:281–300
Riggs RD, Winstead CR (1959) Studies on the resistance in tomato to root-knot nematodes and on the occurrence of pathogenic biotypes. Phytopathology 49:716–724
Ritter E, Debener T, Barone A, Salamini F, Gebhardt C (1991) RFLP mapping on potato chromosomes of two genes controlling extreme resistance to potato virus X (PVX). Mol Gen Genet 227:81–85
Rivers BA, Bernatzky R, Robinson SJ, Jahnen-Dechent W (1993) Molecular diversity at the self-incompatibility locus is a salient feature in natural populations of wild tomato (Lycopersicon peruvianum). Mol Gen Genet 238:419–427
Roberts PA, Thomason IJ (1986) Variability in reproduction of isolates of Meloidogyne incognita and M. javanica on resistant tomato genotypes. Plant Dis 70:547–551
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ulman DE, Williamson VM (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci USA 95:9750–9754
Roupe van der Voort J, Wolters P, Folkertsma R, van Zandvoort P, Vinke H, Kanyuka K, Bendahmane A, Jacobsen E, janssen R, Bakker J (1997b) Mapping of the cyst nematode resistance locus Gpa2 in potato using a strategy based on co-migrating AFLP markers. Theor Appl Genet 95:874–880
Smith PG (1944) Embryo culture of a tomato species hybrid. Proc Am Soc Hortic Sci 44:413–416
Stamova BS, Chetelat RT (2000) Inheritance and genetic mapping of cucumber mosaic virus resistance introgressed from Lycopersicon chilense into tomato. Theor Appl Genet 101:527–537
Tanksley SD et al (1992) High-density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141–1160
Van Ooijen JW, Sandbrink JM, Vrielink M, Verkerk R, Zabel P, Lindhout P (1994) An RFLP linkage map of Lycopersicon peruvianum. Theor Appl Genet 89:1007–1013
Van der Vossen EAG, Rouppe van der Voort JNAM, Kanyuka K, Bendahamane A, Sandbrink H, Baulcombe DC, Bakker J, Stiekema WJ, Klein-Lankhorst RM (2000) Homologues of a single resistance-gene cluster in potato confer resistance to distinct pathogens: a virus and a nematode. Plant J 23:567–576
Veremis JC, Roberts PA (1996) Relationship between Meloidogyne incognita resistance gene in Lycopersicon peruvianum differentiated by heat sensitivity and nematode virulence. Theor Appl Genet 93:950–959
Veremis JC, van Heusden AW, Roberts PA (1999) Mapping a novel heat-stable resistance to Meloidogyne in Lycopersicon peruvianum. Theor Appl Genet 98:274–280
Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, Hogers R, Frijters A, Groenendijk J, Diergaarde P, Reijans M, Rierens-Onstenk J, de Both M, Peleman J, Liharska T, Hontelez J, Zabeau M (1998) The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat Biotechnol 16:1365–1369
Williamson VM (1998) Root-knot nematode resistance genes in tomato and their potential for future use. Annu Rev Phytopathol 36:277–293
Yaghoobi J, Kaloshian I, Wen Y, Williamson VM (1995) Mapping a new nematode resistance locus in Lycopersicon peruvianum. Theor Appl Genet 91:457–464
Acknowledgements
We would like to thank R. Peng, E. Westbrook and W. Pudlo for technical support and S. King for developing the pseudo-F2 population. We thank S. Seah for critical reading of the manuscript. This work was supported by funds from: the National Science Foundation Cooperative Agreement BIR-8920216 to the Center for Engineering Plants for Resistance against Pathogens, an NSF Science and Technology Center; United States Department of Agriculture National Research Initiative Award 2003-00996; the University of California BioSTAR Program and the DNA Plant Technology Corporation. All experiments described in this paper comply with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. McCombie
Rights and permissions
About this article
Cite this article
Yaghoobi, J., Yates, J.L. & Williamson, V.M. Fine mapping of the nematode resistance gene Mi-3 in Solanum peruvianum and construction of a S. lycopersicum DNA contig spanning the locus. Mol Genet Genomics 274, 60–69 (2005). https://doi.org/10.1007/s00438-005-1149-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-1149-2