Abstract
A single Agrobacterium strain harbouring two binary plasmids was successfully used for the first time to develop a marker-free transgenic rice of improved nutritional value. Sixty-eight T0 co-transformants were obtained in three indica rice cultivars—two popular high-yielding Bangladeshi varieties (BR28 and BR29), and one high-iron rice cultivar (IR68144). Marker-free lines were obtained from 14 out of 24 selected co-transformants screened in the T1 generation. The accumulation of total carotenoids in polished T2 rice seeds of the primary transgenic VPBR29-17-37 reached levels of up to 3.0 μg/g, with the level of β-carotene reaching 1.8 μg/g. In the cultivars BR28 and IR68144, total carotenoid levels in the transformants reached 2.0 μg/g of polished rice seeds. The levels of lutein and other carotenoids in the seeds were also significantly enhanced. T1 plants obtained from primary transgenics with simple gene-integration patterns tended to have a lower carotenoid content than the original parental lines. This study describes the development of marker-free transgenic rice lines containing high levels of carotenoids, and addresses the relationship between the rearrangement of transgenes and the presence of metabolic end products in transgenic rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids, a broad class of isoprenoid-derived pigments, serve as photoprotectants, anti-oxidants, and as precursors of abscisic acid. These pigments are synthesized by all photosynthetic, and many non-photosynthetic, organisms (Naik et al. 2003). Vertebrates cannot synthesize carotenoids de novo, and depend entirely on dietary sources for their supply. β-Carotene and lutein are the most important carotenoids in human nutrition. The 40-carbon compound β-carotene (also known as provitamin A) is the main precursor of the retenoids: retinol, vitamin A and retinoic acid. Lutein, a derivative of α-carotene, provides protection against age-related degeneration of the macula lutea in the retina (Krinsky et al. 2003). Although high levels of β-carotene and lutein have been reported in some rice germplasms (Tan et al. 2005), these pigments are found mainly in the aleurone layer of the seed, and are removed by polishing, leaving only negligible amounts in the rice grain. Genetic engineering has tremendous potential to overcome malnutrition and vitamin A deficiency (VAD), especially in developing countries. The reported accumulation of β-carotene in rice endosperm following insertion of crtI (phytoene desaturase), psy (phytoene synthase) and lyc (lycopene cyclase) genes into japonica and indica rice varieties (Ye et al. 2000; K. Datta et al. 2003a; Hoa et al. 2003) has greatly raised hopes that VAD can be overcome.
Carotenoid biosynthesis is a complex trait and is influenced by environmental stresses such as drought, salinity and high temperature (see Demmig-Adams and Adams 2002, and references therein). To study the carotenoid biosynthetic pathway in transgenic plants would require collection of a considerable number of independent transgenic events, which in turn requires an effective selection/marker system. However, selectable marker genes are themselves often undesirable, and represent an extra load on the host genome once the final product is developed. To simplify the regulatory process, and to improve consumer acceptance of genetically modified (GM) crops, the ability to dispense with sequences that serve no purpose in the final product is highly desirable. The development of marker-free crops will help to raise confidence in the use of GM crops amongst consumers and farmers, as well as addressing the anti-GMO (Geneticaly modified organisms) stance of environmentalists and consumer organizations.
In recent years, several means of producing marker-free transgenic plants have been developed. Transposon-mediated elimination of selectable markers in transgenic rice expressing Bt endotoxin, where the marker is excised in the T1 generation, was reported by Cotsaftis et al. (2002). Techniques using intrachromosomal homologous recombination (Zubko et al. 2000), and site-specific recombination such as the Cre/LoxP (Odell et al. 1990) and FLP/FRT (Lloyd and Davis 1994) systems have also been developed for the excision of marker genes. In these cases, the marker gene is flanked by the target sequence for a specific DNA recombinase, and can be removed from the genome of the transgenic plant by the introduction of the corresponding recombinase gene by conventional crossing. The main disadvantages of these recombinase-mediated systems are the low efficiency of DNA recombination, and the time-consuming crossing process. Co-transformation of marker and target genes, with subsequent excision of the marker gene in the T1 generation by genetic segregation, is an alternative way to develop marker-free transgenic plants. Because of its simplicity, co-transformation is the method of choice for excision of selectable markers for seed-bearing, short-duration crops like rice, and is independent of the method of gene delivery. Biolistic transformation (Tu et al. 2003; Rao et al. 2003) and Agrobacterium-mediated transformation (Komari et al. 1996) can both be used, although the latter has the advantage of generating transgenics with simple gene-integration patterns and small numbers of transgenes, and is more cost-effective.
Depicker et al. (1985) demonstrated the generation of marker-free plants by using the simplest method of co-transformation, i.e. using two Agrobacterium strains with one binary plasmid in each. Although simple, this co-transformation method has its limitations, such as lower efficiency compared to single-strain transformation (Depicker et al. 1985). The high frequency with which the two T-DNAs become linked is also a disadvantage (Poirier et al. 2000). An advanced system of co-transformation using multiple T-DNAs in one binary plasmid, with segregation of marker gene and gene of interest in the succeeding generation, was described by Komari et al. (1996). Although this method gives a high frequency of co-transformation and segregation of the selectable marker, the vector used is very large, and is not suitable for routine cloning of genes of interest. A single binary vector with two right borders in one T-DNA (Lu et al. 2001; Huang et al. 2004) has also been used to achieve a high efficiency of co-transformation and marker segregation. However, these systems require specialized cloning vectors, and the size of the vector could be a limiting factor, especially when two or more genes of interest need to be cloned.
The ‘two binary plasmids in one Agrobacterium strain’ approach, which requires no specialized binary vector for co-transformation, and allows segregation of the selectable marker, is the method of choice when more than two genes need to be transferred for product development. Here we report the first use of this system in rice for the development of a genetically engineered product of commercial value. The goal of this study was the development of marker-free transgenic lines of indica rice (starting from two popular varieties as well as a high-iron rice cultivar) with enhanced accumulation of β-carotene, lutein, and other carotenoids in polished rice endosperm. Two genes coding for enzymes of the carotenoid biosynthetic pathway were inserted into the rice genome: psy for phytoene synthase (from Narcissus pseudonarcissus; Schledz et al. 1996), driven by the endosperm-specific Glutelin promoter, and crtI for phytoene desaturase (from Erwinia uredovora; Misawa et al. 1993), linked to the CaMV 35S promoter and fused to the coding sequence for the transit peptide of Rubisco.
Materials and methods
Construction of transformation vectors
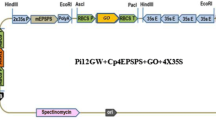
The binary plasmid pCacar was obtained from Dr. Peter Beyer (University of Freiburg, Germany). The selection marker on pCacar, pmi (phosphomannose isomerase), is flanked by recognition sequences for the restriction enzyme XhoI. The binary plasmid pCacar has four recognition sequences for XhoI. To excise the pmi gene from pCacar, the plasmid was partially digested with XhoI, re-ligated, and transformed into the Escherichia coli strain XL1Blue (Invitrogen, Carlsbad, CA, USA). Colonies were selected on LB medium containing chloramphenicol (25 mg/l). Plasmid DNAs was isolated from 40 colonies and digested with XhoI to identify clones that lacked the 1,186-bp coding sequence of the pmi gene. The vector lacking the pmi gene was designated pNCacar (see Fig. 1). The following genes are present in the T-DNA of the pNCacar plasmid: psy (phytoene synthase) under the control of the endosperm-specific Glutelin promoter, and crtI (phytoene desaturase) fused to the Official Reading Frame (ORF for the Rubisco transit peptide sequence under the control of the CaMV 35S promoter.
Partial map of the binary plasmid pNCacar containing the phytoene synthase (psy gene under the control of the endosperm-specific Glutelin promoter (Gt1) and the nos terminator (nosT), and the phytoene desaturase (crtI) gene with 35S CaMV promoter (35S P), nos terminator and pea Rubisco transit peptide sequence (TP). Restriction sites used for cloning are indicated. BL Left border, BR right border
pTok233 (Hiei et al. 1994) is a super-binary vector with an extra copy of the virulence genes with the T-DNA. The T-DNA carries three genes: two selectable marker genes, nptII and hph (which confer resistance to kanamycin and hygromycin, respectively) and one scorable marker gene (reporter gene), gus.
Transfer of both binary vectors into one Agrobacterium strain
The Agrobacterium strain LBA4404 was transformed with the super-binary vector pTok233 using a freeze–thaw transformation method (Hellens et al. 2000). Kanamycin (nptII) was used for transformant selection. The LBA4404 (pTok233) strain was then transformed with the second binary vector, pNCacar, using the same freeze–thaw method, and applying chloramphenicol (25 mg/l) and kanamycin (50 mg/l) as selection agents. A single doubly resistant colony was selected and plasmids were isolated to confirm the presence of both plasmids in this strain. All genes present on pTok233 and pNCacar could be amplified using stringent PCR conditions (data not shown).
Plant transformation
Embryogenic calli were generated on MS+2,4-D (2 mg/l) medium (Murashige and Skoog 1962) from scutellum of immature embryos. Embryogenic calli (3–4 weeks old; 3–4 mm2) of the indica rice varieties BR28, BR29 and IR68144 were incubated for 30 min with an overnight culture (OD600=0.5–0.8) of Agrobacterium (LBA4404/pNCacar/pTok233). Calli were then blotted on sterile filter paper and transferred to co-cultivation medium [MS+2,4-D (2 mg/l) and acetosyringone (200 μM)] and incubated in the dark at 28°C for 3 days. This was followed by three successive selection cycles of 3 weeks each on MS+2,4-D (2 mg/l) + hygromycin (50 mg/l). The calli were then transferred to regeneration medium [MS with BAP (2.5 mg/l)] as described earlier (Datta et al. 1990, 2000; Vasconcelos et al. 2003) with some modifications. Regenerated rice plants were transferred to a special greenhouse for transgenics (containment facilities). GUS staining of T0 plants was carried out using the method described by Jefferson et al. (1987).
Polymerase Chain Reaction
DNA was extracted from leaf fragments (2 cm2) using the modified protocol described by Edward et al. (1991). Polymerase chain reactions (PCRs) were carried out for crtI, psy, hph, and gus genes using standard PCR conditions as follows: initial denaturation at 94°C for 5 min, followed by 32 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min. A final extension at 72°C for 10 min was included to polish the ends of PCR products. The primer sequences used were: psy F (5′-TGGTGGTTGCGATATTACGA-3′), psy R (5′-ACCTTCCCAGTGAACACGTC-3′), crtI F (5′-GGTCGGGCTTATGTCTACGA-3′), crtI R (5′-ATACGGTCGCGTAGTTTGG-3′), hph F (5′-CCTGAACTCACCGCGACG-3′), hph R (5′-AAGACCAATGCGGAGCATATAC-3′), gus F (5′-AATTGATCAGCGTTGGTGG-3′) and gus R (5′-GGTGTAGAGCATTACGCTGC-3′).
Southern hybridization
High-molecular-weight genomic DNA was extracted using the procedure described by Datta et al. (2003a, 2003b). Genomic DNA was digested with EcoRI and fractionated on a 0.8% agarose gel by electrophoresis in TAE-buffer at 20 V for 20 h. Southern hybridization was carried out as described by Sambrook et al. (1989). PCR probes of 1 and 0.8 kb were used for hybridization to the crtI and psy genes, respectively. The blots were then stripped with 0.5% SDS and rehybridized with an 0.8-kb PCR probe specific for the hph gene.
Transgene inheritance and screening for marker-free plants
Of 68 transgenic lines containing both marker and target genes, 24 were selected for segregation analysis in T1. Lines were selected based on a carotenoid content of more than 0.5 μg/g. T1 progenies of selected lines were screened by histochemical staining for gus expression, and by PCR to detect the hph, crtI, and psy genes. All plants that were negative for GUS staining but PCR-positive for hph were analyzed for gus by PCR. Marker-free T1 progenies were confirmed by Southern analysis.
HPLC analysis of carotenes
Polished rice seeds were ground to a fine powder, and 500 mg of rice powder was soaked in 1 ml water and incubated at 50°C for 20 min, followed by the addition of 2 ml of acetone. The mixture was then vortexed and incubated for a further 20 min. The samples were centrifuged for 5 min at 1,000 rpm, and the supernatants were transferred to clean tubes. This procedure was repeated three times, or until the rice-seed powder became completely white. Half a volume of petroleum ether was added to the total supernatant, mixed thoroughly, and water was then added for phase separation. The upper layer was removed and dried in a Speed vac (Maxi Dryer Plus, Heto, Allerod, Denmark). Carotenoids were dissolved in 1.0 ml of ether, and the absorbance was measured at 450 nm in a spectrophotometer. The solution was evaporated to dryness, and the solute was re-dissolved in 100 μl of acetone; a 40-μl aliquot of this sample was then used for HPLC analysis. HPLC analysis was performed using a Waters Alliance 2690 Separation Module (Waters Corporation, Milford, MA, USA) equipped with a Waters 996 photodiode array detector, Waters 474 scanning fluorescence detector, and Waters Millennium32 Chromatography Manager. Samples were loaded on a Waters YMC Carotenoid column (4.6′250 m, 5 μm) after passing them through a guard column containing the same material (4.0′10 mm, 5 μm), and eluted using solvents A (acetonitrile-tetrahydrofuran-water, 10 : 4 : 6) and B (acetonitrile–tetrahydrofuran–water, 10 : 8.8 : 1.2). The column was developed with 100% solution A for the first 3 min, then a linear gradient to 100% solution B was applied over a period of 7 min, then 100% solution B was pumped through the column for 20 min. Peak identification was based on the retention time, the main absorption maxima, and spectrum shape in comparison with the corresponding standards subjected to the same chromatographic conditions. Lutein and β-carotene standards were obtained from Sigma (St. Louis, Mo, USA). All other chemicals were obtained from J.T. Baker (Phillipsburg, NJ, USA).
Results
Vector construction and development of a single Agrobacterium strain carrying two binary plasmids
The T-DNA of the binary plasmid pNCacar carries two genes for carotenoid biosynthesis, i.e. psy (under the control of the endosperm-specific Glutelin promoter) and crtI (with the 35S CaMV promoter and the Rubisco transit peptide) (Fig. 1). The Agrobacterium strain LBA4404 was first transformed with the binary plasmid pTok233 carrying the selectable marker gene hph and the gus reporter gene. pNCacar was then transferred into the LBA4404 (pTok233 strain), resulting in an Agrobacterium strain carrying two binary plasmids, i.e. LBA4404 (pTok233, pNCacar).
Transformation of rice and analysis of T0 transformants
The popular high-yielding Bangladeshi indica rice varieties BR28 and BR29, and the high-iron indica rice cultivar IR68144, were transformed using Agrobacterium strain LBA4404 (pTok233, pNCacar). Of the 124 transformants obtained, 56 contained only the marker genes hph and gus, while 68 carried the carotenoid genes psy and crtI together with the marker genes (Table 1). The presence of the marker genes was confirmed by staining for GUS activity, and the presence of the genes psy and crtI was confirmed by PCR and Southern analysis. All putative transformants that grew on hygromycin-containing selection medium were found to contain both hph and gus transgenes.
Segregation analysis and identification of marker-free transgenic plants in the T1 generation
The T1 progenies of 24 independent transgenic (T0) founders (selected based on the total carotenoid content in polished seeds) were analyzed in order to identify the plants that had lost the marker genes; 14 of the 24 lines showed segregation of marker genes (hph and gus). The carotenoid genes crtI and psy could be amplified by PCR from the marker-free progeny, while no amplification of the marker gene hph was observed (Fig. 2). All PCR-positive, marker-free transgenic T1 progenies were confirmed by Southern analysis (Fig. 3). Four types of linkage were found in this analysis (H represents the marker genes hph and gus; C represents carotenoid biosynthetic genes, psy and crtI): (H–C), (H, H–C, C), (H, H–C) and (H–C, C). The H–C class was rare, while 50% of transgenic events showed the (H, H–C, C) pattern (Table 2). Various patterns of gene integration were found, both simple and re-arranged. Based on the analysis of T1 plants, most of the transgenic founders had integrated the marker genes at multiple loci, whereas the T-DNA with the carotenoid genes of interest showed single-locus integration in most transgenics (Table 3).
Southern analysis of electrophoretically fractionated, EcoRI-digested genomic DNA isolated from T1 transgenic progenies of transgenic founders VPBR29-9 and VPBR29-32 identified as crtI-positive by PCR, using a crtI probe (upper panel). The same blot re-probed with hph is shown in the lower panel. Progeny designated by bold numbers are marker-free
Integration of truncated T-DNAs and reduced gus expression in the T1 generation
Integration of truncated T-DNA was observed in the case of the vectors carrying carotenoid biosynthesis genes as well as marker genes. In one IR68144- and two BR29-derived plants, the crtI gene, but not psy, was detected (Fig. 4). A different transgenic BR29 plant had retained the gus gene but lost hph (Table 4). Fewer plants showed GUS activity (as assessed by staining) than integration of the gus gene. This was found to be the case in 13 out of 24 transgenic plants analyzed in the T1 generation (Table 2).
Southern analysis of truncated T-DNA integration in T1 progenies of transgenic founders VPBR29-9 and VPBR29-31. In the two boxed lines, the crtI gene is present but the psy gene is absent (arrow), although both genes were originally present on the same T-DNA. P, plasmid-positive control; NT, non-transgenic control
Estimation of the carotenoid content of polished rice seeds in the T0 generation
Carotenoid accumulation in polished rice seeds could be assessed directly by visual examination of the seeds (Fig. 5). The carotenoids were identified by HPLC analysis (Fig. 6) and quantified spectrophotometrically at 450 nm (Fig. 7). Most of the transgenic plants contained less than 1 μg of total carotenoids per gram of polished rice seeds, while a few lines contained 2–3 μg/g. One transgenic plant, VPBR29-16, contained 3.128 μg of total carotenoid per gram of T0 polished rice seeds, the highest value obtained (Table 5). Based on a β-carotene calibration curve, the highest β-carotene content in polished T0 seeds (1.808 μg/g) was found in plant VPBR29-35. The lutein content ranged between 0.085 and 0.284 μg/g in the three cultivars transformed (see Table 6 and Fig. 7). Other carotenoids found in the transgenic rice seeds were cryptoxanthin and α-carotene. It was also noted that the tocopherol content (detected by its fluorescence emission at 330 nm upon excitation at 290 nm) was 2- to 3-fold higher in transgenic as compared to non-transgenic seeds.
a–c Accumulation of carotenoids in polished rice seeds of transgenic rice lines. a Transgenic BR29-17-37 (right) in comparison with the non-transgenic BR29 (left). b, c Variation in carotenoid accumulation among the T1 progenies of transgenic founder VPBR29-17 with a rearranged gene-integration pattern (b) and VPBR29-16 with a simple gene integration pattern (c)
Gene integration patterns and differences in carotenoid expression levels
All 24 transgenic founders examined for marker-free status were also studied for gene-integration pattern, as well as for the expression of carotenoid genes in the T0 and T1 generations. Both simple and rearranged types of gene-integration patterns were observed in the T0 generation (with a few exceptions representing truncated gene integrations). Simple gene-integration patterns (single band) detected in the T0 generation were maintained in T1, while rearranged patterns in T0 (2–3 bands) subsequently became either more complex, or were resolved into a mixture of simple and rearranged gene-integration patterns. Wide variation was observed in the level of carotenoid expression in polished rice seeds of T1 progenies of transgenic founders that exhibited rearranged gene-integration patterns in T0. Surprisingly, in all T0 transgenic plants with a simple gene-integration pattern, the level of carotenoid gene expression was not retained in the T1 generation; in most such cases, carotenoid accumulation was lower than in the T0 plant. However, enhanced carotenoid levels were observed in most of the T1 progenies of plants showing rearranged gene integration at T0 (Table 5).
Discussion
Co-transformation with two binary plasmids carried by one Agrobacterium strain was successfully used to develop co-transformants of three elite indica rice cultivars, BR28 and BR29 (popular Bangladeshi high-yield varieties) and IR68144 (high iron rice). The efficiency of co-transformation was 53.90%, which is similar to the previously reported values for this system (Daley et al. 1998), as well as the ’two T-DNAs in one binary plasmid system’ of co-transformation (Komari et al. 1996). The present system represents an improvement over the two Agrobacterium strain system of co-transformation described by DeBlock and Debrouwer (1991) and Poirier et al. (2000). Using advanced vector systems, recent studies on the development of marker-free transgenic crops have achieved co-transformation efficiencies of 70–90% (Miller et al. 2002; Breitler et al. 2004), but these systems have the disadvantage that they require specialized binary vectors.
Over 50% of the co-transformants obtained with both systems showed segregation of the marker gene (Komari et al. 1996; Daley et al. 1998). These values were based on the phenotypic expression of both the selectable marker gene and a reporter gene, but in such cases a gene may be present but not be expressed. Here, we studied the segregation of the selectable marker based on the presence of the gene, and found that the selectable marker segregated away from the genes of interest in 58.33% of co-transformants. In 13 out of 24 transgenic founders studied in the T1 generation, the gus reporter gene, though present, was not expressed. This could be due to gene silencing, gene rearrangement, or integration of a truncated gene in another locus. Integration of truncated T-DNA is a common phenomenon in Agrobacterium-mediated transformation (Vain et al. 2003); indeed, in the T1 generation, we observed four cases in which a truncated T-DNA had been integrated at one of the loci (Table 7). In two of these, the hph gene was missing from the T-DNA carrying the marker genes, while in another the psy gene had been deleted from the T-DNA containing the carotenoid genes (Fig. 4).
The type of Agrobacterium strain used for insertion of the T-DNA can influence transgene integration into the plant genome (DeBlock and Debrouwer 1991). Nopaline-derived Agrobacterium strains favour insertion of multiple T-DNAs at genetically linked loci; while octopine-derived strains favour integration at unlinked loci (Breitler et al. 2004). Here, we used the octopine-derived strain LBA4404, and this could explain the high frequency of unlinked markers obtained in the T1 generation. Based on chi square values, it was found that most of the T1 transgenic events studied showed single-locus integration of T-DNA carrying carotenoid genes, while the marker gene T-DNA integrated at multiple loci. However, the sample size used for T1 analysis was too small to allow any general conclusions to be drawn.
Earlier studies have reported the accumulation of 1–1.6 μg/g of total carotenoids in polished rice seeds of indica as well as japonica rice cultivars (Ye et al. 2000; Datta K et al. 2003a; Hoa et al. 2003). Based on the recommended daily allowance (RDA), this may not be enough to meet the daily requirement for pro-vitamin A. The main aim of this study was to develop a marker-free transgenic rice with enhanced accumulation of carotenoids in the rice seeds. Here, we have achieved accumulation of more than 3.0 μg/g total carotenoids in T2 polished rice seeds in transgenic rice cultivar BR29, with about 2 μg/g in BR28 and IR68144. The highest accumulation of β-carotene (1.812 μg/g in polished T2 rice seeds) was found in plants derived from the transgenic founder VPBR29-17-37. The level of β-carotene did not always represent a constant proportion of the total carotenoid content (Fig. 7). This phenomenon has also been reported in potato (Ducreux et al. 2004). However, β-carotene always represented the major component of the total carotenoids, followed by lutein and other carotenoids. A possible reason for the high levels of β-carotene could be that the activity of β-carotene hydroxylases becomes rate limiting as the carotenogenic flux increases, resulting in a metabolic bottleneck. Accumulation of α-carotene in polished rice seeds was found to be very low when compared with β-carotene; enzyme activities on this branch of the carotenoid pathway are thus probably not rate limiting. Upon stimulation of carotenogenesis, the tocopherol content in potato increased (Romer et al. 2002; Ducreux et al. 2004). Similarly, expression of two genes of the carotenoid biosynthetic pathway, psy and crtI, in rice endosperm, results in a 2- to 3-fold increase in tocopherol levels in polished transgenic rice seeds as compared to the control seeds. Of the 68 co-transformants obtained, 24 showed considerable accumulation of carotenoids, while the remaining 44 showed poor to negligible accumulation of carotenoids in the polished seeds. Since accumulation of the final product is dependent on the proper coordination of all enzymes in the metabolic pathway, one cannot reasonably expect to generate valuable transgenic plants after a few transgenic events. Large numbers of transgenic events need to be developed to select the best phenotype based on product accumulation as well as agronomic performance.
Gene rearrangement is very commonly observed in Agrobacterium-mediated transformation and cannot be correlated with gene expression. Stable and heritable expression of transgenic Bt rice with a rearranged gene integration pattern has been observed over eight generations (Datta et al. 2003a, 2003b). Due to the complex character of the carotenoid biosynthetic pathway, four to five generations will be required stabilize gene expression. The performance of transgenic plants in different agronomic and climatic conditions also needs to be studied. T1 progenies of transgenic events with simple gene-integration patterns did not maintain the level of carotenoid gene expression found in the T0 generation; in most cases the amount of product declined, with a marked variation in carotenoid expression among T1 progenies. In cases where the genes were re-arranged during integration, wide variation in carotenoid expression was observed among T1 progenies and, in some progenies, the carotenoid content was even found to be higher than that in the parents. By using the skills of rice breeders together with carotenoid analysts, the plants that combine best stable high carotenoid accumulation (in polished rice seeds) and superior agronomic performance could be selected prior to releasing the genetically engineered products to the public.
Reasonable levels of β-carotene and lutein accumulation have been reported in transgenic tomato and potato (Romer et al. 2000; Ducreux et al. 2004). However, considering the importance of rice as the staple food of more than half the world’s population (mainly in developing countries), any improvement in the content of β-carotene and other carotenoids in polished rice seeds will directly benefit developing countries in helping to prevent VAD. The carotenoids in polished rice seeds should not be the sole source of vitamin A, but increased levels could prove a useful supplement. Furthermore, enhanced accumulation of carotenoids in the high-iron rice cultivar IR68144 could help simultaneously to combat both VAD and iron-deficiency anaemia.
References
Breitler J, Meynard D, Boxtel J, Royer M, Bonnot F, Cambillau L, Guiderdoni E (2004) A novel two T-DNA binary vector allows efficient generation of marker-free transgenic plants in three elite cultivars of rice (Oryza sativa L). Transgenic Res 13:271–278
Cotsaftis O, Sallaud C, Breitler JC, Meynard D, Greco R, Pereira A, Guiderdoni E (2002) Transposon-mediated generation of T-DNA and maker free rice plants expressing a Bt endotoxin gene. Mol Breed 10:165–180
Daley M, Knauf VC, Summerfelt KR, Turner JC (1998) Co-transformation with one Agrobacterium tumefaciens strain containing two binary plasmids as a method for producing marker-free transgenic plants. Plant Cell Rep 17:489–496
Datta SK, Peterhans A, Datta K, Potrykus I (1990) Genetically engineered fertile Indica-rice plants recovered from protoplasts. Biotechnology 8:736–740
Datta K, Koukolíková-Nicola Z, Baisakh N, Oliva N, Datta SK (2000) Agrobacterium-mediated engineering for sheath blight resistance of indica rice cultivars from different ecosystems. Theor Appl Genet 100:832–839
Datta K, Baisakh N, Oliva N, Torrizo L, Abrigo E, Tan J, Rai M, Rehana S, Al-Babili S, Beyer P, Potrykus I, Datta S (2003a) Bioengineered ’ golden’ indica rice cultivar with β-carotene metabolism in the endosperm with hygromycin and mannose selection systems. Plant Biotechnol J 1:81–90
Datta SK, Chandel G, Tu J, Baisakh N, Datta K (2003b) Engineering of Bt transgenic rice for insect pest protection. In: Metz M (ed) Bacillus thuringienesis: a cornerstone of modern agriculture (Part III). Food Products, Binghamton, pp 77–91
DeBlock M, DeBrouwer D (1991) 2 T-DNAs co-transformed into Brassica napus by double Agrobacterium tumefaciens infection are mainly integrated at the same locus. Theor Appl Genet 82:257–263
Demmig-Adams B, Adams WW (2002) Antioxidants in photosynthesis and human nutrition. Science 298:2149–2153
Depicker A, Herman L, Jacobs S, Schell J, van Montagu M (1985) Frequency of simultaneous transformation with different T-DNAs and their relevance to the A grobacterium-plant cell interaction. Mol Gen Genet 201:477–484
Ducreux LJM, Morris WL, Hedley PE, Sheperd T, Davies HV, Millam S, Taylor MA (2005) Metabolic engineering of high carotenoid potato tubers containing enhanced level of β-carotene and lutein. J Expt Bot 56:81 – 89
Edward K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Hellens R, Mullineaux P, Klee H (2000) A guide to Agrobacterium binary Ti vectors. Trends Plant Sci 5:446–451
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–228
Hoa TTC, Al-Babili S, Schaub P, Potrykus I, Beyer P (2003) Golden indica and japonica rice lines amenable to deregulation. Plant Physiol 133:161–169
Huang S, Gilbertson LA, Adams TH, Malloy K, Reisenbigler EK, Birr DH, Snyder MW, Zhang Q, Luethy MH (2004) Generation of marker free transgenic maize by regular two right-border Agrobacterium transformation vectors. Transgenic Res 13:451–461
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vector carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10:165–174
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23:171–201
Lloyd AM, Davis RW (1994) Functional expression of the yeast FLP/FRT site specific recombination system in Nicotiana tabacum. Mol Gen Genet 242:653–657
Lu H-J, Zhou X-R, Gong Z-X, Upadhyaya NM (2001) Generation of selectable marker free transgenic rice using double right-border vectors. Aust J Plant Physiol 28:241–248
Miller M, Tagliani L, Wang N, Berka B, Bidney D, Zhao Z-Y (2002) High frequency transgene segregation in co-transformed maize plants using an Agrobacterium tumefaciens 2 T-DNA binary system. Transgenic Res 11:381–396
Misawa N, Yamano S, Linden H, DeFelip MR, Lucas M, Ikenaga H, Sandmann G (1993) Functional expression of Erwinia uredovora carotenoid biosynthesis gene crtI in transgenic plants showing an increase of β-carotene biosynthesis activity and resistance to bleaching herbicide norflurazon. Plant J 4:833–840
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:474–497
Naik PS, Chanemougasoudharam A, Paul Khurana SM, Kalloo G (2003) Genetic manipulation of carotenoid pathway in higher plants. Curr Sci 85:1423–1430
Odell J, Caimi P, Sauer B, Russell S (1990) Site directed recombination in the genome of transgenic tobacco. Mol Gen Genet 223:369–378
Poirier Y, Ventre G, Nawrath C (2000) High-frequency linkage of co-expressing T-DNA in transgenic Arabidopsis thaliana transformed by vacuum-infiltration of Agrobacterium tumefaciens. Theor Appl Genet 100:487–493
Rao R, Abrigo E, Rai M, Oliva N, Datta K, Datta SK (2003) Marker-free Bt transgenic rice. Rice Genet Newslett 20:51–53
Romer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley P (2000) Elevation of provitamin A content of transgenic tomato plants. Nat Biotechnol 18:666–669
Romer S, Lubeck J, Kauder F, Steiger S, Adomat C, Sandmann G (2002) Genetic engineering of zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab Eng 4:263–272
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory, Cold Spring Harbor
Schledz M, Al-Babili S, von Lintig J, Haubruck H, Rabbani S, Kleinig H, Beyer P (1996) Phytoene synthase from Narcissus pseudonarcissus: functional expression, galactolipid requirement, topological distribution in chromoplasts and induction during flowering. Arch Biochem Biophys 385:4–12
Tan J, Baisakh N, Oliva N, Parkhi V, Rai M, Oliva N, Torrizo L, Datta K, Datta SK (2005) The screening of rice germplasm, including those transgenic rice lines which accumulate β-carotene in their polished seeds, for their carotenoid profile. Int J Food Sci Technol 40:563–569
Tu J, Datta K, Oliva N, Zhang G, Xu C, Khush GS, Zhang Q, Datta SK (2003) Site-independently integrated transgenes in the elite restorer rice line Minghui 63 allow removal of a selectable marker from the gene of interest by self-segregation. Plant Biotechnol J 1:155–165
Vain P, Afolabi A, Worland B, Snape W (2003) Transgene behavior in populations of rice plants transformed using a new dual binary vector system: pGreen/pSoup. Theor Appl Genet 107:201–217
Vasconcelos M, Datta K, Oliva N, Khalekuzzaman M, Torrizo L, Krishnan S, Oliveira M, Goto F, Datta SK (2003) Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci 64:371–378
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:302–305
Zubko E, Scutt C, Meyer P (2000) Intrachromosomal recombination between attP regions as a tool to remove selectable marker genes from tobacco transgenes. Nat Biotechnol 18:442–445
Acknowledgements
Financial support from USAID and the Rockefeller Foundation is acknowledged. Thanks are due to Syngenta for an international collaborative programme. The work has been carried out in compliance with the current laws governing genetic experimentation in the country concerned.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hagemann
Rights and permissions
About this article
Cite this article
Parkhi, V., Rai, M., Tan, J. et al. Molecular characterization of marker-free transgenic lines of indica rice that accumulate carotenoids in seed endosperm. Mol Genet Genomics 274, 325–336 (2005). https://doi.org/10.1007/s00438-005-0030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-0030-7