Abstract
A tissue culture independent in-planta transformation technique is an alternate option to generate transgenics in crop cultivars recalcitrant for regeneration. The T0 plants generated by in-planta technique are chimeric hence very large number of T1 seeds needs to be screened to identify the putative transformants. Besides, low transformation efficiency and stable integration is another lacuna, because the T0 seedling explants are not subjected for selection. To overcome these constraints, a modified in-planta technique was developed in this study to generate gain-in-function activation tagged mutants in the background of a recalcitrant rice genotype, KMP-153. A binary vector was developed with three glyphosate selectable marker genes and 4X CaMV 35S enhancer elements for developing rice plants. Simultaneous expression of Glyphosate insensitive CP4-EPSPS with two other glyphosate degrading enzymes, PsAKR1 (Aldo–Keto reductases) and GO (Glycine Oxidase) substantially improved tolerance up to 100 ppm of glyphosate in the seedling bioassay system. Selecting the seedling explants and T1 generation seeds at higher concentration (100 ppm) of glyphosate resulted in recovering fewer transformants but with stable integration and expression of all the transgenes compared to those screened at lower levels of glyphosate. Further, T2 generation plants from these transgenic lines grown in contained nethouse conditions were tolerant when sprayed up to 1500 ppm concentrations of glyphosate. These transgenics showed substantial lower levels of shikimic acid compared to wild type plants treated with glyphosate signifies the expression of the transgenes and less inhibition of shikimic acid pathway by glyphosate. The proteins extracted from the transgenic plants showed significant degradation of glyphosate. The stable integration was confirmed through flanking sequence information and event specific PCR information. Our results clearly demonstrate that the modified in-planta technique is a potential transformation protocol to generate stable transformants in rice cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is grown under diverse agro climatic conditions. Several biotic and abiotic factors affect the growth and development and ultimately the productivity. Genetic enhancement of the different plant mechanisms and traits has been the major emphasis for improving the adaptation of rice to different biotic and abiotic stresses and also to improve the yield. However, required genetic variability for specific traits may not exist among the germplasm accessions within the species. Therefore, to introduce the traits of our interest across the species transgenics emerged as a potential option. Transgenic plants in different crop species are developed mainly by particle bombardment (Iida et al. 1991) or Agrobacterium mediated transformation (Gelvin 2003). Agrobacterium mediated gene transfer, is one of the most common rice transformation methods, and has been extensively used for developing transgenic rice to study gene function and also to improve agricultural traits like resistance to disease and insect pest, tolerance to drought and salt and higher quality and yield. The in vitro transformation protocols include the transformation of individual plant cells followed by regeneration of whole plants from those transformed cells (Kumria et al. 2001). However, the major limitation in this approach has been development of robust regeneration protocol which is often a limitation in many crop species including rice. In rice, Indica varieties are more recalcitrant for regeneration through tissue culture when compared to the Japonica varieties (Nishimura et al. 2006).
Even though, regeneration and transformation protocols have been developed in rice, many of these protocols are genotype dependent and often not suitable for many genotypes especially indica cultivars. To tackle these problems in recalcitrant genotypes of rice, methods to eliminate the regeneration steps have been followed. These are broadly called as in-planta transformation protocols. High throughput transformation methods that avoid tissue culture have been developed in Arabidopsis initially (Azipiroz-Leehan and Feldmann 1997). The Agrobacterium mediated vacuum infiltration method has been widely adopted to generate transgenics. Subsequently in-planta transformation techniques transforming different plant tissues also have been developed for several crop species. In most of the studies Agrobacterium is directed towards either the apical meristem or the meristem of axillary buds. One such viable in-planta transformation protocol has also been standardized for several crops (Rohini and Rao 2000a, b, 2001). The strategy essentially involves in-planta inoculation of embryo axis of germinated seeds and allowing them to grow into seedlings ex vitro. These in-planta transformation protocols are advantageous over the other methods because they do not involve regeneration procedures and therefore the tissue culture-induced somaclonal variations can be avoided. The first in-planta transformation has been standardized in rice by Supartana et al. (2005). Subsequently an in-planta transformation technique has been employed to generate stable transformants in several recent studies (Babitha et al. 2013).
Transgenic technology became an important tool not only to improve traits of our interest but also to generate genetic variability by developing insertional mutants and gain-in function activation tagged mutants. These insertional mutants and activation tagged mutants in Arabidopsis and also in rice are being extensively used for functional validation of the genes. These genetic resources immensely contributed in gene discovery and their functional characterization. Besides generating genetic resources for functional validation, gain in function activation tagged mutants can generate variants altering several agronomic traits. Therefore, this approach is widely used to generate desirable mutants in elite germplasm lines or varieties. One of the major limitations in generating these potential genetic resources is lack of high through put transformation protocols. Though transposition based mutagenesis approach provided a solution to generate large number of insertional and activation tagged mutants (Shaohong et al. 2008; An et al. 2003; Manimaran et al. 2017), in this approach several insertions occur in a genome and hence identifying the target gene that is associated with altered phenotype by forward genetic approaches is difficult and time consuming. Therefore, high throughput transformation protocol to generate large number of transformants in species with poor regeneration efficiency has phenomenal relevance.
With this background the in-planta transformation technology has been extensively used to generate insertional mutagenesis in Arabidopsis. Besides, the in-planta transformation technique, where Agrobacterium is directed towards the emerging plumule is being used to generate stable transformants even in rice (Supartana et al. 2005; Ratanasut et al. 2017). In such an approach T0 plants are chimeric and hence large number of T1 generation need to be characterized initially by using a selection media and subsequently by PCR analysis. Further in this approach there is no selection of infected seedling explants, therefore several T0 plants can be non-transformed plants. Further the transformation efficiency is also very less because several T1 seeds from these T0 plants are screened to identify transformants. Though the in-planta transformation technology is a potential approach to develop transgenics, there is a need to improve transformation efficiency with stable integration of transgenes by exposing to the suitable selection agents. In this study we describe a modified in-planta technique where in agro infected seedling explants are subjected for selection pressure and subsequently screening the T1 seedlings at high concentrations of selection agent. By adopting this technique we generated stable gain-in function activation tagged mutants in recalcitrant rice genotype KMP-153.
Materials and methods
Plant material
Seeds of rice genotype KMP-153 were procured from a senior rice breeder, at Zonal Agricultural Research station, V. C. farm Mandya. Seeds were surface sterilized with 1% Bavastin for 15 min, rinsed with distilled water to remove the traces of Bavastin. Overnight soaked in distilled water and then seeds were kept for germination in petriplates in dark at 28 °C. Seeds initiating the germination were used as seedling explants for transformation.
Transformation and recovery of transformants
We followed a modified multisite gateway cloning LR recombination strategy to construct binary vector (Vemanna et al. 2013). Three genes, CP4EPSP, Aldo–keto reductase (PsAKR1) and Glycine Oxidase (GO) were sub-cloned along with 4xCaMV35S enhancer sequences. Initially the glycine oxidase was cloned to IM1.1 vector under RBCS promoter, RBCS terminator sequences and subsequently the entire gene cassette was subcloned into gateway entry vectors pGATE L1-L4 at ASCI and PacI restriction site. The EPSPS genes was cloned into pRT101 2×35S promoter and whole gene cassette with promoter and terminator was digested and subcloned to pGATE R4-R3 at HindIII, EcoRI restriction sites. A Multimerized copy of 35S enhancer fragment was sub-cloned into the entry vector at pGATE L3-L2 at HindIII and EcoRI site (Karimi et. al. 2002). The destination vector pi12GW was horbouringAKR1 gene driven by RBCS promoter. The resulting recombinant entry vectors (pGATEL1:2x35S∷EPSPS::PolyAL2, pGATER4:RBCSP::GO::RBCSTR3 and pGATEL3:4x35S::L2) were subjected to recombination reaction with modified destination vector pi12GW which is harboring PsAKR1 in the ratio of 1:1:1:1.5(v/v/v/v) in a 15 μl reaction mix containing 2 μl of LR clonase II plus enzyme mix (Invitrogen, USA). The reaction was performed at 25 °C overnight and the reaction was terminated by adding Proteinase K (1 μl). The recombined product was used to transform E. coli (DH5α) and recombinant clones were selected on spectinomycin (50 mg l−1). Subsequently the construct was transferred to Agrobacterium competent cells and confirmed the integration by PCR.
Agrobacterium tumefaciens strain (EHA105) harboring the binary vector pi12GW harboring AKR1 + EPSPS + GO + 4X35S enhancer was used for transformation. The vector contains the PsAKR1 gene driven by RBCS promoter, EPSPS gene driven by 2X CaMV35S promoter and the GO gene driven by the RBCS promoter. Agrobacterium was grown overnight at 28 °C in 25 ml of LB medium (pH 7.0) containing 50 μg ml−1 Spectinomycin and Rifampicin. The bacterial culture was later resuspended in 100 ml of Winans’ AB minimal medium (Winans et al, 1988) (pH 5.2) and grown for 18 h. For vir gene induction treatment, filter sterilized wounded tobacco leaf extract was added to the Agrobacterium suspension in Winans’ AB medium 5 h before infection (Yang et al. 1996). Tobacco leaf extract was prepared by crushing 10 g of leaf tissue in 10 ml distilled water and added to Agrobacterium suspension in Winans’ AB medium. The seedlings with plumule just emerging were pricked at the apical meristem of the axis region with a sterile needle and infected/co-cultivated by immersion in the suspension of Agrobacterium for 1 h and 45 min. Following infection-co-cultivation, the seedlings were washed briefly with sterile water and blotted on autoclaved blotting paper to remove the excess water allowed to grow for 48 h. The seedlings survived from infection shock were screened in autoclaved quartz sand media containing different concentrations of glyphosate (100, 80 and 60 ppm). The same glyphosate concentrations were maintained in the plastic bowls by gravimetrically replenishing the water lost by evaporation and transpiration for a period of 7 days. The autotrophically grown seedlings which survived after a week were transferred to earthen pots and the recovered plants grown to maturity in the contained green house facility. Seeds were advanced to next generations.
Insitu sand culture screening of T1 transformants against different concentrations of glyphosate
In vivo screening of T1 seeds against glyphosate to select stable transformants, known number of 2 days old germinated seeds from each T0 plant were transferred to sand culture media with different concentration of glyphosate and allowed to grow under an autotrophic period for a period of 10 days. The seedlings with normal robust growth were considered as stable transformants. These seedlings were transferred to the pots and grown to maturity in the controlled greenhouse condition.
Molecular analyses of the putative transgenic plants
Tissues from the progeny plants were analyzed for the presence of the introduced genes. Genomic DNA was isolated following the procedure of CTAB by Doyle and Doyle (1990) from fresh leaf tissue of the greenhouse grown T1 and T2 generations that was used for polymerase chain reaction (PCR) and Flanking sequence analysis. PCR was performed to amplify 425 bp EPSPS gene fragment, a 596 bp GO gene and a 583 bp PsAKR1 gene fragment. PCR was initiated by a hot start at 95 °C for 5 min followed by 30 cycles of 1 min at 94 °C, 45 s at 58 °C and 1 min at 72 °C. Annealing temperature was at 58 °C for all three gene fragment. The products were separated on a 0.8% agarose gel.
Response of T2 generation activation tagged mutants to glyphosate spray
The glyphosate spray test was performed as described by Zhao et al. (2011) with few modifications. The T2 generation gain-in function activation tagged mutants were grown in net house. For each transgenic T2 generation lines, 20 plants were maintained. On 35th DAS, one set of the plants were sprayed with 1000 ppm and other set was sprayed with 1500 ppm. The tolerance levels of different activation tagged mutant lines were assessed based on survival and visual injury and chlorotic symptoms (Campbell et al. 1976; Reddy et al. 2004; Zobiole et al. 2012).
Shikimic acid quantification
The shikimic acid content was determined by RP-HPLC (LC- 20AD, Shimadzu, Japan) in the glyphosate treated transgenics, wild type and non-treated wild type plants. The LC separation involved RP-C18 column (250 mm * 4.6 mm, 5 µm) and the detection wavelength was 235 nm using SPD-M 20A photodiode array detector. The mobile phase used was 20% acetonitrile, 80%, (0.1%) trifluro acetic acid (TFA) in an isocratic mode with the injection volume of 20 µl and a flow rate of 0.5 ml/min. The leaves were ground well to fine powder using liq.N2; 100 mg of tissue was treated with 4 ml of 0.01 M H2SO4 and incubated on a rotary shaker water bath for 2 h at 50 °C. The samples were cooled down to room temperature and 1 ml of 0.4 M NaHCO3 was added and spin at 4000 g for 20 min at 4 °C. The supernatant was filtered through Whatman filter paper and the extract was lyophilized and dissolved in methanol to get higher concentration. 20 µl of this extract was loaded to estimate the levels of shikimic acid (Zelaya et al. 2011). The shikimic acid (99% HPLC grade, sigma Aldrich Inc., USA) dissolved in methanol was used to prepare standard curve. The concentration of shikimic acid in the extract was determined based on the standard curve of shikimic acid.
Cucumber seedling assay
Cucumber seedling assay is one of the indirect methods of assessing the expression of transgenes. Two of the three genes co-expressed in the transgenic plants detoxify the glyphosate (Ramu et al. 2017) to non- toxic metabolites. To assess the expression and function of GO and AKR1, crude protein was extracted from the leaves of PCR confirmed glyphosate tolerant (1500 ppm sprayed) plants. The protein content was quantified and known amount of crude protein from each plant was incubated with 750 ppm of glyphosate for 3 h and subsequently the assay mixture was diluted and cucumber assay was performed. Germinated seeds were allowed to grown in diluted assay mixture and the growth readings were recorded.
Flanking sequence analysis by TAIL-PCR
Genomic sequences flanking the T-DNA insertions were amplified according to Liu et al. (1995, 2007), with minor modification of 15 supercycles (consisting of two high-stringency cycles and one low stringency cycle) in the secondary reaction, and 15 super cycles in the tertiary reaction instead of 20 reduced-stringency cycles were performed. 50 ng of genomic DNA was used as template DNA. We designed three nested and target-specific primers: GSP1, GSP2 and GSP3. Each of these primers was used in combination with six arbitrary degenerate primers: AD1 and AD2. Tertiary reaction TAIL-PCR products were purified by the Gel Extraction Kit (Fermentas gene JET™), cloned into pTZ 57 R/T easy vector (Promega, USA) and sequenced. The integration was reconfirmed by amplifying the gene in the inserted region.
Results and discussion
In rice genotypes recalcitrant for regeneration, alternate option has been to develop tissue culture independent transformation protocols. In-planta transformation technique is one of the widely adopted approaches, and development of stable transformants using this technique has been reported in several crop species (Keshamma et al. 2008; Babitha et al. 2015). The in-planta transformation technique is high throughput and large number of insertion and activation tagged mutants can be generated in the background of a genotype which is less amenable for in vitro tissue culture mediated transformation. In this technique primary transformants i.e., seedling explants are not subjected for selection pressure, hence several T0 plants are non-transformed. From this context, to minimize the escapes in T0 the explants have to be subjected to selection pressure. Since the T0 plants are chimeric, the putative transformants are identified in this technique by screening large number of T1 seeds. Further, autotrophically grown T1 seedlings are screened in selection media in greenhouse conditions, therefore antibiotic selection markers are not suitable and hence herbicide selection markers are used. In this regard the primary pre-requisite is to develop robust screening protocols with herbicide selection markers to identify the stable transformants. In view of this, the major emphasis of this study is to develop a suitable binary vector with glyphosate resistant marker genes to achieve adequate tolerance against glyphosate concentrations and also to detoxify it.
Development of suitable activation tagging vector to generate gain in function mutants in KMP-153
Activation tagging involves introduction of a T-DNA containing regulatory sequences such as the enhancer of the CaMV 35S promoter randomly into a plant genome to enhance expression of nearby genes along with strong screenable marker, which potentially resulted in a gain-in-function phenotype. The success of generating the mutants by using gain-in-function activation tagging mainly depends on developing a suitable vector with specific enhancers and suitable selectable markers.
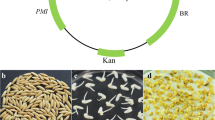
CaMV enhancers have been widely used as transcriptional activator to develop activation tagging lines. In the present study we used four copies of 35S enhancer to enhance the expression of neighboring genes near the site of integration. In addition to the enhancer element, for the selection of transformants against glyphosate three genes were cloned into the T-DNA. Co-expression of these three genes CP4EPSPS, AKR1, GO not only brings about tolerance against glyphosate but also detoxifies the glyphosate with minimal residual effect. CP4EPSPS has been shown to enhance glyphosate tolerance because the binding of glyphosate to CP4EPSPS negligible and hence the shikimic acid pathway did not alter further (Schönbrunn et al. 2001). AKR1 has been demonstrated as a glyphosate detoxifying enzyme (Ramu et al. 2017; Cheo et al. 2004). Besides, the other purpose is to manage reactive carbonyl compounds likely to be generated while screening transformants with glyphosate. The mutated GO from Bacillus subtilis has been shown to detoxify glyphosate (Nicolia et al. 2014). We followed a modified, multisite gateway cloning strategy to construct binary vector. Initially AKR1, CP4EPSPS and GO genes were cloned into the entry vectors followed by LR reaction to clone all the genes into destination vector (Magnani et al. 2006). The recombinant binary vector (pKi12GW) harboring four gene cassettes (RBCS P:: AKR1:: RBCST, 2x35S∷EPSPS::PolyA∷RBCSP::GO::RBCST and 4x35S:) was transformed to Agrobacterium tumefaciens (strain EHA105) by electroporation. The recombinant Agrobacterium was selected on spectinomycin (50 mg l−1) and rifampicin (10 mg l−1) and positive colonies were screened by PCR using gene-specific primers (S1). The three genes transferred from entry vectors and also the presence of AKR1 was further confirmed by restriction analysis (S2). Results confirm the presence of all four gene cassette in the destination vector. The confirmed recombinant vector pi12GW with all the four genes (Fig. 1) was used to develop activation tagged mutants in rice by in-planta transformation technique.
In this study the objective is to generate variants altering agronomic traits by developing gain-in function activation tagged mutants in an elite germplasm accession, KMP-153 with superior drought adoptive traits. The KMP-153 is highly recalcitrant for regeneration and hence a modified in-planta transformation technique was employed to generate transformants. In in-planta transformation technology since all the cells of epical meristem may not be transformed during infection, the seedling explants are not subjected for selection process and the surviving seedlings after infection are directly transformed into the pots and developed into T0 plants. In this process often several infected explants without transformation also developed as T0 plants. Further, in this technique the transgene integration is random into few cells of already differentiated cells. Therefore T0 plants will be chimeric and stable integration can be seen only in the T1 generation.
The modified in-planta transformation method developed in this study follows the same steps involved in the conventional in-planta transformation methods except subjecting the infected seedling explants for plant selection pressure. The infected seedling explants were subjected to selection pressure by transforming to sterile sand culture media with different concentrations of glyphosate under aseptic conditions. The seedlings were allowed to grow for a week in the selection media and surviving seedlings were transferred to pots and developed as T0 plants. The seedlings grown in selection media survives only when reasonable number of cells of the epical meristem was transformed. Non-transformed seedling explants and apical meristem with few transformed cells will not survive in the selection media. By this modified protocol we can avoid advancing the non-transformed seedlings to the next generation. Further, the seedling explants survived at higher selection pressure may have stable integration and expression of the transgenes. Therefore in this study the seedling explants after infections were subjected for selection at different concentrations of glyphosate. The selection of the putative transformants was done at different stringency levels to arrive at optimum concentrations for achieving stable integration of all the transgenes. One set of the seedling explants were transferred to sand culture selection media with 100 ppm of glyphosate and the other two sets were transferred to 80 and 60 ppm of glyphosate. The seedlings are allowed to grow for 7 days and the surviving seedlings were transformed to pots and grown to maturity in contained greenhouse conditions. The response of seedling explants was significantly different to different concentrations of glyphosate in the selection media (Table 1). At 100 ppm of glyphosate in the selection media only 12% of the seedlings survived, whereas, at 80 and 60 ppm survival percent was 22.5 and 24.4. The results clearly indicate that at higher selection pressure the number of putative transformants generated was very low and it was relatively higher when selected at lower concentrations of glyphosate.
Characterization of the T1 putative transformants at higher glyphosate concentration identifies stable transformants
The T0 putative transformants generated from seedling explants subjected for selection at different concentrations viz., 100, 80 and 60 ppm were harvested and maintained separately. The T1 seeds obtained from T0 plants generated by screening the explants at 100 ppm were challenged with 100 ppm of glyphosate in the in situ screening technique adopted in this study. Similarly the T1 seeds obtained from T0 plants selected against 80 and 60 ppm were challenged with respective concentrations. The response of T1 generation activation tagged transformants to different concentrations of glyphosate in selection media clearly demonstrates that the percent of seedlings survived and established was relatively less at 100 ppm when compared to 80 and 60 ppm (Table 2). Seedlings survived after screening at 100 ppm was only 70% whereas, it was 84% at 80 ppm and almost 96.6% at 60 ppm glyphosate concentrations. Only 62% of the seedlings were established in pots when screening was done at 100 ppm of glyphosate, whereas, 91.3% of plants were established when screened at 60 ppm glyphosate. The recovery growth of seedlings survived after screening in glyphosate was relatively less when glyphosate concentration was 100 ppm compared to the seedlings recovered at 80 and 60 ppm (Fig. 2a). The established plants in the pots with normal phenotype were chosen for further molecular characterization. In these selected plants the integration of all the genes AKR1, CP4EPSPS and GO was assessed by PCR analysis. The number of plants with stable integration of all the three genes differed amongst the plants selected at different glyphosate concentrations. Out of 45 T1 plants chosen from 100 ppm selection background all are PCR positives for all the three genes (Fig. 2b, e(i)). However, when the screening was done at 80 and 60 ppm, only 79% and 50% of the plants showed the integration of all the three genes (Fig. 2c, d, e(ii, iii)).
Screening of T1 generation putative transformants at 100, 80 and 60 ppm of glyphosate and confirmation of the transgenes integration by PCR analysis. T1 seeds from T0 plants developed from seedling explants selected at different concentrations were germinated in sand culture media either with 100, 80 or 60 ppm of glyphosate. Surviving seedlings with robust growth were subsequently transferred to pots and developed into T1 plants. In these tolerant plants integration of all the transgenes was analyzed by the PCR. Based on PCR analysis the number of plants having one, two or all the three genes was computed. a Response of wild type and T1 seeds from putative transformants screened at different concentrations of glyphosate. PCR analysis of transformants survived at 100, 80 and 60 ppm of glyphosate respectively (b–d). PCR analysis was done using respective gene specific primers. 425 bp amplicon size confirms the stable integration of CP4EPSPS (i) gene in the transformed lines. Similarly 596 bp and 583 bp amplified product showed the stable integration of GO (ii) and AKR1 (iii) gene in the genome. e Number of plants showing the integration of one, two or all the three genes in the plants selected at 100 (i), 80 (ii) and 60 ppm (iii) of glyphosate respectively
The seedlings screened at higher concentrations of the glyphosate showed less survival percentage and subsequent establishment in the pots. However, in these plants stable integration of all the three genes was noticed. This clearly indicates that adequate expression of all the three genes is essential for the seedling survival at higher concentration of glyphosate. Whereas when the seedlings were screened at 60 ppm, more than 90% of the seedlings survived. But only 50% of plants showed integration of all the three genes. Survived plants showed integration of any one or two genes. It is likely that the seedlings in which one or two genes were integrated and expressed also survived at 60 ppm of glyphosate concentration. In this study to increase the robustness of screening against glyphosate we co-expressed the different genes. CP4EPSPS, GO, AKR1 involved in glyphosate tolerance. CP4EPSPS was cloned from Agrobacterium sp. strain CP4 (Baerson et al. 2002; Barry et al. 1992; Deng et al. 2014; Padgette et al. 1995). In CP4EPSPS the glyphosate target amino acid is changed from Alanine to Arginine and hence the glyphosate doesn’t have the binding affinity to EPSPS (Schönbrunn et al. 2001). Transgenic plants expressing CP4EPSPS are shown to be highly tolerant to glyphosate in several crop species (Johanns and Wiyatt 2005) and many of commercially grown transgenics express CP4EPSPS. Recent studies have clearly demonstrated that AKR1detoxify the glyphosate and AKR1 can be used as a selectable marker to screen transformants against glyphosate (Ramu et al. 2017). Leaf explants from AKR1 tobacco transgenic plants showed high regeneration in vitro in presence of glyphosate in the media. In this study we also co-expressed a mutated gene of GO from Bacillus subtilis that detoxify the glyphosate (Zhan et al. 2013). The author clearly demonstrated that the mutant form of GO detoxifies glyphosate. The custom synthesized GO was also shown to impart the glyphosate tolerance in a recent study by Ramu et al. (2017). The data clearly suggests that these three genes can be used as potential selectable markers against glyphosate to identify the putative transformants. Co-expression of these three genes substantially improved the tolerance of seedling explants and also T1 seedlings to higher concentrations of glyphosate. Further the results also suggest that, selection at higher stringency may result in identifying the transformants not only with stable integration of all the three genes but with higher expression of the transgenes. Therefore it is necessary to develop a suitable selectable marker for high stringency selection pressure and also to arrive at optimum concentration of glyphosate both for selection and also for screening the putative transformants to identify transgenics with stable integration. Though the percent survival of the seedlings was low, most of the survived transformants showed integration of all the three genes.
Response of activation tagged mutants for foliar spray in the field conditions
To further validate the stability of transgenes in the subsequent generations, the T2 plants were characterized for herbicide tolerance under field grown conditions. Based on the response to herbicide concentrations and robustness of the phenotype, 50 T1 lines were selected from all the three screening backgrounds. Out of 50 lines selected 5 are from T1 plants screened at 100 ppm, 21 are from 80 ppm, 24 are from 60 ppm. A subset of all the 50 lines was initially screened for glyphosate tolerance in the insitu screening technique. The lines selected at 100 ppm background were screened at 100 and accordingly the others either at 80 or 60 ppm of glyphosate. In all the lines more than 80% of the seedlings survived in the respective concentrations of glyphosate. These selected lines were established in the net house under soil grown conditions (Fig. 3a). One subset of field grown plants from these lines was sprayed with 1000 ppm and another set with 1500 ppm of glyphosate on 35th day after sowing (DAS)and the number of lines which showed susceptible and tolerant phenotypes were recorded. When the lines were sprayed with 1000 ppm the five selected lines from 100 ppm screening protocol showed complete tolerance whereas, the lines selected from 80 ppm showed only 85.71% survival and the lines selected from 60 ppm screening showed only 50% survival (Fig. 3b, d). However, when the plants were sprayed with 1500 ppm the number of plants survived decreased substantially. For instance out of the 5 lines selected from the 100 ppm screening protocol only 3 lines survived which amounts 60% of survival, whereas the lines selected from 80 and 60 ppm protocol the percentage of surviving lines was 28.27% and 12.25% respectively (Fig. 3c, e). The lines survived at 1500 ppm were further analyzed for the presence of all the transgenes by PCR analysis. All the selected plants showed amplification of desired fragment in the PCR analysis indicating the presence of all the transgenes (Fig. 3f).
Response of T2 transformants to foliar spray of 1000 and 1500 ppm glyphosate. 50 T1 lines were selected for the study out of which 5 are selected from 100 ppmbglyphosate selection pressure, 21 are from 80 ppm and 24 are from 60 ppm. Two sets of seeds from selected T1 lines were raised in the contained nethouse condition. One set of the plants were sprayed with 1000 ppm of glyphosate and other with 1500 ppm of glyphosate on 35th day after sowing. The number of tolerant lines were scored based on toxicity symptoms due to glyphosate spray. Integration of transgenes was assessed in the plants survived at 1500 ppm glyphosate spraying concentrations by PCR. a Overview of the plants in the net house. b Response of T2 transformants for 1000 ppm glyphosate. c Response of selected T2 transformants for 1500 ppm glyphosate. The number of plants survived after spraying with 1000 (d) and 1500 ppm (e) of glyphosate in lines identified from 100, 80, and 60 ppm glyphosate screening concentrations. f PCR confirmations of inserted genes in T2 rice transformants. PCR analysis was done using respective gene specific primers. 425 bp amplicon size confirms the stable integration of CP4EPSPS (i) gene in the transformed lines. Similarly 583 bp and 596 bp amplified product showed the stable integration of AKR1 (ii) and GO (iii) gene in the genome. Wt is wild type. M—1 kb ladder, B—blank, P—plasmid
Glyphosate degradation by PsAKR and GO reduced shikimic acid levels in transgenic lines
The target protein of the glyphosate is EPSPS which catalyzes the reaction of PEP and 3-phosphoshikimate into 5-enolpyruvilshikimate-3-phosphate (Franz et al. 1997). Inhibition of EPSPS results in the accumulation of shikimic acid. Shikimic acid was quantified both from glyphosate treated and non-treated wild type plants on 7th day after spray. In wild type plants treated with glyphosate, shikimic acid levels were significantly higher when compared to transgenics and untreated wild type. There is no significant variation in levels of shikimic acid amongst the transgenic plants selected. The gain-in-function activation tagging mutants showed resistance after spraying with glyphosate showed lower levels of shikimic acid (Fig. 4b–e). This could be due to functionality of the co-expressed genes. The glyphosate does not bind to the modified CP4EPSPS and hence, catalytic activity of EPSPS is not affected. The other two genes co-expressed PsAKR1 and GO detoxifies the glyphosate and hence the EPSPS enzyme activity is not affected. The results confirm that EPSPS activity in transgenic plants is not affected by the glyphosate, therefore, it can be concluded that the transgenes are stably integrated and expressed (Fig. 4).
Shikimic acid levels in wild type and transgenic plants sprayed with glyphosate. Leaves from transgenic lines and wild type were collected 7 days after glyphosate spray and the shikimic acid was extracted and quantified by HPLC at 6.8 retention time. Peaks in the graphs representing the content of shikimic acid levels at 6.8 retention time a Shikimic acid standard, b wild type untreated, c wild type treated, d transgenic line 16-108 and e transgenic line 25-5. f Shikimic acid levels in few transgenic lines which survived at 1500 ppm of glyphosate spray
Protein extracted from transgenic plants degraded the glyphosate and rescued cucumber seedlings
To assess whether transgenic plant proteins has the ability to detoxify the glyphosate we adapted cucumber seedling experiment. Previously we had showed that PsAKR1 and GO proteins expressed in the E. coli rescued cucumber seedling from glyphosate effect (Ramu et al. 2017). GO cleaves the glyphosate into glyoxylate and AMPA (Zhan et al. 2013; Nicolia et al. 2014), whereas, PsAKR1 cleaves the glyphosate into sarcosine and inorganic phosphate and subsequently formaldehyde and glycine (Fitzgibbon and Braymer 1990). Crude protein extracted from four transgenic rice lines and wildtype plants was incubated with 750 ppm of glyphosate for 3 h. Subsequently, appropriately diluted protein solution was used for cucumber seedlings assay (Bradford 1976) (Fig. 5a). The seedlings treated with protein assay solution from transgenics showed relatively higher growth compared to wild type plants. The seedling shoot length was as low as 0.5 cm when incubated in protein solution from wild type plants whereas, from transgenic plants it ranged from 2 to 2.5 cm. Similar response was observed with regards to root length and seedling weight (Fig. 5b). These results clearly demonstrate that in the selected T2 transgenic lines stable integration and enhanced expression of transgenes lead to detoxification of glyphosate and hence more growth in cucumber seedlings.
Crude protein from PCR positive stable activation tagged mutants rescued growth of the cucumber seedlings treated with glyphosate. Crude protein was extracted from glyphosate transgenic lines and then incubated with 750 ppm of glyphosate for 3 h, subsequently the crude extract with glyphosate was diluted and spread on the petri- dishes and uniformly germinated cucumber seedlings were incubated for 72 h and growth was recorded. a Photograph showing the phenotype of cucumber seedlings grown in protein extracted from wild type and transgenic plants. b Shoot length, root length and fresh weight of the seedlings at the end of 72 h
Flanking sequence information and development of event specific PCR
To confirm stable integration of the transgene in rice plants, flanking sequence analysis was done to identify the site of integration. The TAIL PCR technique (Liu and Whittier 1995) was adopted for one of the transgenic events (17-8) (S3). The flanking sequence in the rice genome was identified and transgene integration was located on chromosome 11 at 12123278 to 12123485 bp region (Fig. 6a). One of the objectives of generating flanking sequence information is to generate event specific PCR to identify the transgenic line and also for subsequent characterization (Fig. 6b). Primers designed on the T-DNA region and plant genomic region amplified expected amplicon. The event specific PCR clearly demonstrates integration of the transgene.
Flanking sequence analysis was done to confirm the integration of transgenes in the genome. Genomic DNA was isolated from transgenic line 17-8 and used as a template for TAIL PCR. AD primers AD1 and AD2 and three nested gene specific primers were used in series of primary, secondary and tertiary amplification. Expected band in the tertiary amplification was eluted and sequenced. The sequence obtained is blasted against rice genome sequence to find the integration site in the genome. Event specific primers were designed near to site of integration and event specific amplification was carried using flanking region specific primer and T-DNA specific primer. Two other transgenic lines were also analyzed for PCR as a negative control. a Sequence homology with chromosome 11. b Event specific amplification was observed only with 17-8 (Lane 2) when amplified along with 16-108 (Lane 1) and 25-5 (Lane 3)
Modified in-planta technique a potential option to generate stable transformants in recalcitrant rice cultivar
The modified in-planta transformation technique developed in this study was quite efficient and robust in identifying true transformants by eliminating the escapes and with stable integration of all the transgenes. In this approach we completely eliminated the non- transformed T0 plants. To impose higher stringency selection pressure with glyphosate we adopted a strategy to co-express genes with diverse mode of action against glyphosate. Our study provided evidence that co-expression of CP4EPSPS which is insensitive to glyphosate and two glyphosate detoxifying enzymes is a potential option not only to improve the tolerance but with least residual levels. The relevance of this approach has been demonstrated in earlier studies in tobacco and rice by Ramu et al. (2017). The novelty of approach is the selection of primary transformants and also T1 generation seedlings at different concentrations of glyphosate. Our results demonstrated that screening at higher concentrations though substantially decreased the number of putative transformants survived and recovered, those plants which survived showed the integration and expression of all the transgenes. We provided proof of concept in this study that it is essential to co-express desired genes imparting tolerance against glyphosate for efficient screening at high stringency of glyphosate levels. The response of the selected transgenics in the T2 generation to higher concentrations of glyphosate spray and stable expression of all the three genes in tolerant lines support these findings. Lower levels of shikimic acid observed in these transgenics after glyphosate spray further confirms the expression of functional proteins to sustain the EPSPS activity. The invivo studies assessing the recovery growth of cucumber seedlings with crude extract from plants tolerant to glyphosate, further, confirmed the adequate expression of the transgene in these selected transgenic plants. We also confirm the stable integration by flanking sequence information and by generating event specific PCR. Finally our results clearly demonstrate that the modified in-planta technique is a potential transformation protocol to generate stable transformants in rice cultivars.
References
An, S., Park, S., Jeong, D.-H., Lee, D.-Y., Kang, H.-G., Yu, J.-H., et al. (2003). Generation and analysis of end sequence database for T-DNA tagging lines in rice. Genetics, Genomics, and Molecular Evolution.. https://doi.org/10.1104/pp.103.030478.
Azipiroz-Leehan, R., & Feldmann, K. A. (1997). T-DNA insertion mutagenesis in Arabidopsis: Going back and forth. Trends in Genetics,13, 152–156.
Babitha, K. C., Ramu, S. V., Pruthvi, V., Mahesh, P., Nataraja, K. N., & Udayakumar, M. (2013). Co-expression of AtbHLH17 and AtWRKY28 confers resistance to abiotic stress in Arabidopsis. Transgenic Research, 22(2), 327–341.
Babitha, K. C., Vemanna, R. S., Nataraja, K. N., & Udayakumar, M. (2015). Over expression of EcbHLH57 transcription factor from Eleusine coracana L. in tobacco confers tolerance to salt, oxidative and drought stress. PLoS ONE,10(9), e0137098. https://doi.org/10.1371/journal.pone.0137098.
Baerson, S. R., Rodriguez, D. J., Tran, M., Feng, Y., Biest, N. A., & Dill, G. M. (2002). Glyphosate-resistant goosegrass. Identification of a mutation in the target enzyme 5-enolpyruvyl shikimate-3-phosphate synthase. Plant Physiology,129, 1265–1275. https://doi.org/10.1104/pp.001560.
Barry, G., Kishore, G., Padgette, S. R., Taylor, M. L., Kolacz, K., Weldon, M., et al. (1992). Inhibitors of amino acid biosynthesis: Strategies for imparting glyphosate tolerance to crop plants (pp. 139–145). Madison, WI: American Society of Plant Physiologists.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry,72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3.
Campbell, W. F., Evans, J. O., & Reed, S. C. (1976). Effects of glyphosate on chloroplast ultra structure of quack grass mesophyll cells. Weed Science,24(1), 22–25. https://doi.org/10.1017/S0043174500065346.
Cheo, D. L., Titus, S. A., Byrd, D. R. N., Hartley, J. L., Temple, G. F., & Brasch, M. A. (2004). Concerted assembly and cloning of multiple DNA segments using in vitro site-specific recombination: Functional analysis of multi-segment expression clones. Genome Research,14(10B), 2111–2120. https://doi.org/10.1104/pp.97.4.1585.
Deng, L. H., Weng, L. S., & Xiao, G. Y. (2014). Optimization of Epsps gene and development of double herbicide tolerant transgenic PGMS rice. Journal of Agricultural Science and Technology,16, 217–228.
Doyle, J. J., & Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Scientific Research,12(1), 13–15.
Fitzgibbon, J. E., & Braymer, H. D. (1990). Cloning of a gene from Pseudomonas sp. strain PG2982 conferring increased glyphosate resistance. Applied and Environmental Microbiology,56(11), 3382–3388.
Franz, J. E., Mao, M. K., & Sikorski, J. A. (1997). Glyphosate: A unique global pesticide. Washington, DC: American Chemical Society.
Gelvin, S. B. (2003). Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiology and Moecularl Biology Reviews,67(1), 16–37. https://doi.org/10.1128/MMBR.
Iida, A., Yamashita, T., Yamada, Y., & Morikawa, H. (1991). Efficiency of particle-bombardment-mediated transformation is influenced by cell cycle stage in synchronized cultured cells of tobacco. Plant Physiology,97, 1585–1587.
Johanns, M., & Wiyatt, S. D. (Eds.). (2005). National agriculture statistics service. Acreage. Washington, DC: U.S. Department of Agriculture.
Karimi, M., Inzé, D., & Depicker, A. (2002). GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science,7, 193–195.
Keshamma, E., Rohini, S., Rao, K. S., Madhusudhan, B., & Udayakumar, M. (2008). Tissue culture-independent in-planta transformation strategy: An Agrobacterium tumefaciens-mediated gene transfer method to overcome recalcitrance in cotton (Gossypium hirsutum L.). The Journal of Cotton Science,12, 264–272.
Kumria, R., Waie, B., & Rajam, M. V. (2001). Plant regeneration from transformed embryogenic callus of an elite indica rice via agrobacterium. Plant Cell, Tissue and Organ Culture,67(1), 63–71.
Liu, Y. G., & Chen, Y. (2007). High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques, 43(5), 649–650, 652, 654.
Liu, Y. G., Mitsukawa, N., Oosumi, T., & Whittier, R. F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant Journal,8(3), 457–463. https://doi.org/10.1046/j.1365-313x.1995.08030457.x.
Liu, Y. G., & Whittier, R. F. (1995). Thermal asymmetric interlaced PCR: Automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics,25(3), 674–681. https://doi.org/10.1016/0888-7543(95)80010-J.
Magnani, E., Bartling, L., & Hake, S. (2006). From gateway to multi site gateway in one recombination event. BMC Molecular Biology,6(7), 46. https://doi.org/10.1186/1471-2199-7-46.
Manimaran, P., Venkata Reddy, S., Moin, M., Raghurami Reddy, M., PoliYugandhar, S. S., Mohanraj, S., et al. (2017). Activation-tagging in indica rice identifies a novel transcription factor subunit, NF-YC13 associated with salt tolerance. Scientific Reports. https://doi.org/10.1038/s41598-017-10022-9.
Nicolia, A., Ferradini, N., Molla, G., Biagetti, E., Pollegioni, L., Veronesi, F., et al. (2014). Expression of an evolved engineered variant of a bacterial glycine oxidase leads to glyphosate resistance in alfalfa. Journal of Biotechnology,184, 201–208. https://doi.org/10.1016/j.jbiotec.2014.05.020.
Nishimura, A., Aichi, I., & Matsuoka, M. (2006). A protocol for Agrobacterium-mediated transformation in rice. Nature Protocols,1(6), 2796–2802.
Padgette, S. R., Kolacz, K. H., Delannay, X., Re, D. B., LaVallee, B. J., Tinius, C. N., et al. (1995). Development, identification, and characterization of a glyphosate-tolerant soybean line. Crop Science,35, 1451–1461.
Qu, S., Desai, A., Wing, R., & Sundaresan, V. (2008). A versatile transposon-based activation tag vector system for functional genomics in cereals and other monocot plants. Plant Physiology. https://doi.org/10.1104/pp.107.111427.
Ramu, S. V., Amaranatha Reddy, V., Easwaran, M., Babitha, K. C., Rao, H., Ghanti, K., et al. (2017). Aldo-ketoreductase enzymes detoxify glyphosate and improve herbicide resistance in plants. Plant Biotechnology Journal,15(7), 794–804. https://doi.org/10.1111/pbi.12632.
Ratanasut, K., Rod-In, W., & Sujipuli, K. (2017). In-planta agrobacterium-mediated transformation of rice. Rice Science,24(3), 181–186. https://doi.org/10.1016/j.rsci.2016.11.001.
Reddy, K. N., Rimando, A. M., & Duke, S. O. (2004). Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. Journal of Agricultural and Food Chemistry,52, 5139–5143. https://doi.org/10.1021/jf049605v.
Rohini, V. K., & SankaraRao, K. (2000a). Transformation of peanut (Arachis hypogeae L.): A non-tissue culture based approach for generating transgenic plants. Plant Science,150, 41–49.
Rohini, V. K., & SankaraRao, K. (2000b). Embryo transformation, a practical approach for realizing transgenic plants of safflower (Carthamus tinctorius L.). Annals of Botany,86(5), 1043–1049.
Rohini, V. K., & SankaraRao, K. (2001). Transformation of peanut (Arachis hypogaea L) with tobacco chitinase gene: Variable response of transformants to leaf spot disease. Plant Science,160(5), 883–892.
Schönbrunn, E., Eschenburg, S., Shuttleworth, W. A., Schloss, J. V., Amrhein, N., Evans, J. N., et al. (2001). Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. PNAS,98(4), 1376–1380. https://doi.org/10.1073/pnas.98.4.1376.
Supartana, P., Shimizu, T., Shioiri, H., Nogawa, M., Nozue, M., & Kojim, M. (2005). Development of simple and efficient in-planta transformation method for rice (Oryza sativa L) using Agrobacterium tumefaciens. Journal of Bioscience and Bioengineering,100(4), 391–397. https://doi.org/10.1263/jbb.100.391.
Vemanna, R. S., Chandrashekar, B. K., Rao, H. H., Sathyanarayanagupta, S. K., Sarangi, K. S., Nataraja, K. N., et al. (2013). A modified multisite Gateway cloning strategy for consolidation of genes in plants. Molecular Biotechnology, 53(2), 129–138.
Winans, S. C., Kerstetter, R. A., & Nester, E. W. (1988). Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. Journal of Bacteriology,170(9), 4047–4054.
Yang, T., Lin, C., & Kain, S. R. (1996). Optimized codon usage and chromophore mutations provide enhanced sensitivity with the green fluorescent protein. Nucleic Acids Research,24(22), 4592–4593. https://doi.org/10.1016/j.fgb.2015.03.022.
Zelaya, I. A., Anderson, J. A. H., Owen, M., & Landes, R. D. (2011). Evaluation of spectrophotometric and HPLC methods for shikimic acid determination in plants: Models in glyphosate-resistant and -susceptible crops. Journal of Agricultural and Food Chemistry,59(6), 2202–2212. https://doi.org/10.1021/jf1043426.
Zhan, T., Zhang, K., Chen, Y., Lin, Y., Wu, G., Zhang, L., et al. (2013). Improving glyphosate oxidation activity of glycine oxidase from Bacillus cereus by directed evolution. PLoS ONE,8(11), 79175. https://doi.org/10.1371/journal.pone.0079175.
Zhao, T., Lin, C., & Shen, Z. (2011). Development of transgenic glyphosate-resistant rice with G6 gene encoding 5-enolpyruvylshikimate-3phosphate synthase. Agricultural Sciences in China,10(9), 1307–1312. https://doi.org/10.1016/s1671-2927(11)60123-5.
Zobiole, L. H. S., Kremer, R. J., Oliveira, R. S., & Constantin, J. (2012). Glyphosate effects on photosynthesis, nutrient accumulation, and nodulation in glyphosate-resistant soybean. Journal of Plant Nutrition and Soil Science,175, 319–330. https://doi.org/10.1002/jpln.201000434.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Basavaraju, S.N., Lakshmikanth, R.Y. & Makarla, U. A modified in-planta transformation technique to generate stable gain-in function transformants in a recalcitrant indica rice genotype. Plant Physiol. Rep. 25, 231–244 (2020). https://doi.org/10.1007/s40502-020-00517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-020-00517-5