Abstract
The vacuolar H+-ATPases (V-ATPases) are ATP-dependent proton pumps responsible for acidification of intracellular compartments in eukaryotic cells. To investigate the functional roles of the V-ATPase in Schizosaccharomyces pombe, the gene vma1 encoding subunit A or vma3 encoding subunit c was disrupted. Both deletion mutants lost the capacity for vacuolar acidification in vivo, and showed sensitivity to neutral pH or high concentrations of divalent cations including Ca2+. The delivery of FM4-64 to the vacuolar membrane and accumulation of Lucifer Yellow CH were strongly inhibited in the vma1 and vma3 mutants. Moreover, deletion of the S. pombe vma1 + or vma3 + gene resulted in pleiotropic phenotypes consistent with lack of vacuolar acidification, including the missorting of vacuolar carboxypeptidase Y, abnormal vacuole morphology, and mating defects. These findings suggest that V-ATPase is essential for endocytosis, ion and pH homeostasis, and for intracellular targeting of vacuolar proteins and vacuolar biogenesis in S. pombe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vacuolar-type proton-translocating ATPases (V-ATPases) are found in all eukaryotic cells, and acidify a number of different organelles, including lysosomes, endosomes, the Golgi apparatus, secretory vesicles and clathrin-coated vesicles (Forgac 1989). V-ATPases have been shown to play key roles in diverse physiological processes such as receptor-mediated endocytosis, protein degradation and processing, protein sorting and targeting, coupled transport of small molecules and ions across the vacuolar membrane (Anraku et al. 1992; Stevens and Forgac 1997), and membrane fusion of vesicles (Peters et al. 2001).
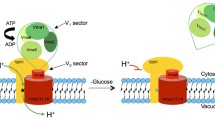
V-ATPases from fungi, plants and animals share a high degree of structural similarity. Like the F1F0-ATPases in mitochondria, V-ATPases are large multimeric enzymes consisting of more than 10 different subunits, and can be functionally divided into two structurally distinct domains. The catalytic V1 domain consists of eight subunits (A–H), whereas the V0 domain contains five subunits (a, d, c, c’, c”) (Stevens and Forgac 1997). The V1 subcomplex is assembled onto the V0 subcomplex, which is composed of both peripheral and integral membrane proteins, resulting in the formation of a functional enzyme complex (Kane et al. 1999; Kane and Parra 2000).
In the budding yeast Saccharomyces cerevisiae, at least 13 subunits are required for V-ATPase function (Graham et al. 2000; Arata et al. 2002). All of the V-ATPase subunit genes have been identified in S. cerevisiae. With the exception of VPH1 and STV1, which encode homologous subunit a proteins of the V0 sector (Manolson et al. 1992, 1994), all are present as single-copy genes in the yeast genome. The nucleotide-binding sites are located in the A and B subunits of the V1 sector, and the catalytic sites are found primarily in the A subunits encoded by VMA1/TFP1 (Hirata et al. 1990). Proton translocation is postulated to occur at the interface between the A subunit and the ring of proteolipid subunits (c, c’, c”) encoded by VMA3, VMA11 and VMA16 (Kawasaki-Nishi et al. 2003). Disruption of each of the genes encoding essential V-ATPase subunits (except for VPH1 and STV1) results in the same phenotype: the mutant yeast cells cannot grow at a pH higher than 7.0 (Nelson and Nelson 1990; Yamashiro et al. 1990) and are sensitive to low and high concentrations of calcium (Ohya et al. 1986, 1991) or heavy metals such as Zn2+, Mn2+ and Cu2+ (Bachhawat et al. 1993; Eide et al. 1993; Ramsey and Gadd 1997; Szczypka et al. 1997) in the culture medium. Vacuoles in such mutants are not acidified (Nelson and Nelson 1990; Yamashiro et al. 1990), and cytosolic Ca2+ levels are elevated (Ohya et al. 1991).
The fission yeast Schizosaccharomyces pombe, which is taxonomically and evolutionarily distant from the budding yeast (Russell and Nurse 1986), is genetically and physiologically well characterized. We have recently isolated and characterized an S. pombe mutant which is deficient for vacuolar function, and has a deletion in the vps33 + gene (Iwaki et al. 2003). Although vps33Δ cells are viable at low temperatures, the severity of the vps33 phenotype demonstrates that the presence of a normal vacuole, and correct targeting of cellular material to it, are essential for fission yeast cells (Iwaki et al. 2003). Several genes encoding subunits of V-ATPase ( vma1 + for subunit A; vma2 + for subunit B; vma3 + for the c subunit) have been cloned and sequenced (Toyama et al. 1991; Ghislain and Bowman 1992). Comparison of the S. cerevisiae genes for V-ATPase subunits with the genome sequence of fission yeast enabled us to identify the other V-ATPase subunit genes in S. pombe, and revealed that all of these are present in single copies (our unpublished results). However, little is known about the function of V-ATPases in S. pombe.
To elucidate the functional role of the V-ATPase in S. pombe, we have constructed disruptants for the vma1 + and vma3 + genes. The phenotypes of these vma deletion mutants demonstrate that vacuole acidification by V-ATPase is essential for intracellular ion homeostasis, mating and sporulation, and for endocytosis, vacuolar biogenesis and protein transport to the vacuole.
Materials and methods
Strains, media, and materials
The E. coli strain XL1-blue (Stratagene) was used for all cloning procedures. The wild-type S. pombe strain ARC039 ( h - leu1-32 ura4-C190T) was obtained from Dr. Y. Giga-Hama (Asahi Glass Co., Yokohama, Japan), while KJ100-7B ( h 90 leu1-32 ura4) was obtained from Dr. K. Tanaka (Tokyo University). The mutants cpy1Δ ( h + leu1-32 his2 ura4-D18 ade6-M216 cpy1::ura4 +) and vps34Δ ( h + leu1-32 ura4-D18 ade6-M216 vps34::ura4 +) were constructed as described previously (Takegawa et al. 1995; Tabuchi et al. 1997). Standard rich medium (YES or YPD), synthetic minimal medium (MM), and sporulation medium (MEA) for S. pombe cells were used (Moreno et al. 1991). Restriction enzymes and other DNA-modifying enzymes were purchased from either Takara Shuzo (Kyoto, Japan) or New England BioLabs (Beverly, Mass., USA). The Expres35S Protein Labeling Mix (NEG-072) for protein labeling was from NEN Life Science Products, and FM4-64 was obtained from Molecular Probes (Eugene, Ore., USA). All other chemicals were from Sigma Chemical or Wako Pure Chemicals Co. (Osaka, Japan).
Genetic methods and plasmid construction
The general genetic methods used have been described previously (Alfa et al. 1993). S. pombe cells were transformed by electroporation as detailed elsewhere (Suga et al. 2000; Suga and Hatakeyama 2001). The construction of pREP41-Hmt1-GFP was described in a previous report (Iwaki et al. 2003). The hmt1 cDNA was amplified and cloned into pTN197, which was derived from pREP41 (Nakamura et al. 2001). The plasmid pTN381 (GFP-psy1) was obtained from Dr. T. Nakamura (Osaka City University).
Gene disruptions
The vma1 + locus was disrupted in the wild-type strain by replacing an internal vma1 gene fragment with the S. pombe ura4 gene. In order to clone the vma1 gene from chromosomal DNA of S. pombe, two oligonucleotides, sense (5′-GGTTTGACACAATTCTGCATCAAACATGC-3′) and antisense (5′-CGCGATTTAATGTGCAAAATTACTTAGGAC-3′), were designed based on the genomic vma1 sequence (SPAC343.05) in GeneDB (Sanger Centre, Hinxton, UK; available at http://www.genedb.org/genedb/pombe/index.jsp). A fragment of 2.6 kb was recovered, and ligated into the vector pGEM-T EASY (Promega). An internal Hin dIII fragment in the cloned vma1 gene was then replaced by the 1.6-kb ura4 + cassette (Grimm et al. 1988). A linearized DNA fragment harboring this disrupted vma1 gene was used for transformation of wild-type haploid ARC039 cells. Transformants were plated on selective MM-Ura medium, and colonies in which the resident vma1 gene had been replaced were identified by PCR.
The S. pombe vma3 gene (SPAC1B3.14) was amplified from chromosomal DNA by PCR using appropriate primers (5′-GGAAAAACCCGATATCCGGTAGTCAAGG-3′ and 5′-TCAATACCAATGTGCCGATGCCTTTAGACG-3′); the Kpn I-BglII fragment within the vma3 ORF was removed, and replaced with the ura4 + gene cassette. A linearized DNA fragment carrying this disrupted vma3 gene was used for transformation of wild-type strains, and the disruption was verified by PCR.
Staining of vacuoles and fluorescence microscopy
Vacuoles were labeled with FM4-64 as described previously (Iwaki et al. 2003). Acidic compartments were stained with quinacrine as reported by Roberts et al. (1991). Log-phase yeast cells were harvested, resuspended in 0.5 ml of YPD buffered with 50 mM Na2HPO4 (pH 7.6) containing 200 μM quinacrine, and incubated at room temperature for 5 min. Cells were sedimented at 10,000× g for 5 s, washed once with 0.5 ml of 2% glucose buffered with 50 mM Na2HPO4 (pH 7.6), and resuspended in the same solution. Samples were viewed immediately with a fluorescence microscope. Fluid-phase endocytosis was by observed fluorescence microscopy after cells had been treated with Lucifer Yellow CH (Sigma). The procedure for staining with Lucifer Yellow has been described by Murray and Johnson (2001). Briefly, cells were grown to exponential phase in YES medium, and collected by centrifugation. Cells were incubated at 27°C for 60 min in 0.5 ml of YES medium containing 5 mg/ml of Lucifer Yellow. After washing of the cells, dye that had accumulated in the vacuole was visualized by fluorescent microscopy. Stained cells were observed with a fluorescence microscope (Model BX-60; Olympus, Tokyo, Japan) equipped with a U-MGFPHQ filter set (Olympus) for quinacrine, Lucifer Yellow CH and GFP, and a U-MWIG filter set (Olympus) for FM4-64. Images were captured with a Sensys Cooled CCD camera using MetaMorph (Roper Scientific, San Diego, Calif., USA) and were saved as Adobe Photoshop files on a Macintosh G4 computer.
Pulse-chase analysis and immunoblot analysis of the S. pombe CPY
For analyses of CPY processing, cells were pulse-labeled with Expres35S Protein Labeling Mix (NEN) for 15 min at 30°C, and chased at the same temperature for given periods. Immunoprecipitation of CPY was performed using a rabbit polyclonal antibody raised against S. pombe Cpy1p as described previously (Tabuchi et al. 1997). For the CPY colony-blot assay, freshly grown spots were replicated onto a nitrocellulose filter and incubated for 2 days; the assay was then performed as reported elsewhere (Cheng et al. 2002).
Assay for mating efficiency
For assay of mating efficiency, S. pombe cells were grown on YES medium, and then transferred to MEA plates. These were incubated for 3 days, and the cultures were examined under a microscope, in order to count the numbers of zygotes and spores. The mating efficiency (ME) was calculated with the formula ME=(2Z+2A+0.5S)÷(H+2Z+2A+0.5S), where H is the number of haploid cells, Z the number of zygotes; A the number of asci, and S the number of free spores.
Results
Vacuole acidification does not occur in fission yeast mutants deleted for V-ATPase genes
Comparison of the S. cerevisiae subunit A (Vma1p) protein sequence with subunit A from other organisms indicates overall conservation except within the VDE (Vma1-derived element) (Table 1). VDE is a 50-kDa segment that self-splices from the middle of the 119-kDa precursor protein (Hirata et al. 1990; Kane et al. 1990). The VDE is a specific endonuclease that can cleave this gene during meiosis (Gimble and Thorner 1992). To our knowledge, this self-splicing segment has been described in only two organisms, S. cerevisiae and Candida tropicalis (Kane et al. 1990; Gu et al. 1993). The sequence of subunit c is also conserved (Table 1). The amino acid sequence of the predicted product of S. pombe vma3 + shares 57% identity with the S. cerevisiae Vma3p sequence.
To investigate the functional role of V-ATPase in fission yeast, we constructed deletion-mutants for vma1 + (Ghislain and Bowman 1992) and vma3 + (Toyama et al. 1992). We sought to determine the physiological consequences of loss of V-ATPase function within defined time parameters by studying vacuolar acidification using quinacrine fluorescence. Quinacrine is a weakly basic dye that accumulates in acidic compartments in response to proton gradients, and is frequently used to assess the state of vacuolar acidification (Umemoto et al. 1990; Roberts et al. 1991; Morano and Klionsky 1994). The wild-type strain as a control, vma1 -disrupted ( vma1Δ) cells and vma3 -disrupted cells ( vma3Δ) were treated with quinacrine. Wild-type cells showed strong fluorescence localized to the vacuoles (Fig. 1A). The vma1Δ and vma3Δ mutants did not show vacuolar fluorescence, indicating that the vacuole could not be acidified in these disruptants (Fig. 1A).
General effects of disruption of V-ATPase genes. A Quinacrine staining of acidic compartments. Wild-type (WT), vma1Δ and vma3Δ cells were incubated with 200 μM quinacrine for 5 min at room temperature and washed as described in Materials and methods. Cells were then viewed immediately using either Nomarski optics or a fluorescence microscope. B Gene disruptants are sensitive to elevated pH and CaCl2. Wild-type (WT), vma1Δ and vma3Δ cells were grown at 30°C for 3 days on YES plates containing 100 mM CaCl2 or supplemented with 50 mM MOPS (pH 7.0) and adjusted to pH 7.0 with NaOH
Disruption of S. cerevisiae VMA1/TFP1 or VMA3 affects cell growth at neutral pH (Nelson and Nelson 1990; Yamashiro et al. 1990) and confers sensitivity to divalent cations, such as Ca2+, Cu2+ and Zn2+ (Ohya et al. 1986, 1991; Bachhawat et al. 1993; Eide et al. 1993; Ramsey and Gadd 1997; Szczypka et al. 1997). A vmaA disruptant of Aspergillus oryzae, which lacks a catalytic subunit of the vacuolar H+-ATPase, also displays a pH-dependent growth defect (Kuroki et al. 2002). Similarly, a vma-1 mutant of Neurospora crassa, which is deficient for another V-ATPase catalytic subunit, cannot grow in medium buffered to pH 7.0 or above, or in medium supplemented with Zn2+ (Bowman et al. 2000). We therefore examined the ability of our mutants to grow at neutral pH. We found that S. pombe vma1Δ and vma3Δ cells grew poorly at pH 7.0 (Fig. 1B) and could not grow at pH 7.5 (data not shown). Compared to the wild-type strain, vma1Δ and vma3Δ also showed strong sensitivity to 100 mM CaCl2 (Fig. 1B), 1 mM CuCl2 (or CuSO4) and 5 mM ZnCl2 (data not shown).
The morphology of vacuoles in vma mutants of S. cerevisiae and Ashbya gossyppii has been reported to be normal (Yamashiro et al. 1990; Förster et al. 1999). Smaller or fragmented vacuoles were observed in the vmaA -disrupted strain of A. oryzae grown at pH 8.5 (Kuroki et al. 2002). Vacuoles in vma-1 mutant strains of N. crassa were irregular, often misshapen, and frequently multilamellar (Bowman et al. 2000). As determined by Nomarski optics (see Figs. 1 and 2), vma1Δ and vma3Δ cells showed abnormally large vacuoles. This analysis strongly suggested that vacuolar morphology was significantly altered by inactivation of V-ATPase in S. pombe.
Effects of disruption of V-ATPase genes disruption on endocytosis. A Endocytosis of the fluorescent endocytic marker FM4-64 into the vacuole is inhibited in V-ATPase disruption mutants. Wild-type (WT), vma1Δ, vma3Δ, vps34Δ, and ste12Δ cells were stained with FM4-64 as described in Materials and methods, and photographed after a 90-min chase in YES medium. The panels on the left show views in Nomarski optics; the right panels show FM4-64 fluorescence in the same cells. B Accumulation of Lucifer Yellow CH is reduced in V-ATPase disruption mutants. Wild-type (WT), vma1Δ and vma3Δ cells were incubated for 60 min at 28°C in YES medium containing Lucifer Yellow (5 mg/ml). The washed cells were viewed with a fluorescence microscope ( right) and with Nomarski optics ( left)
Endocytosis is affected in V-ATPase mutants
FM4-64 is a lipophilic styryl dye, and has been used as a marker for the endocytic pathway and for vacuoles in budding yeast (Vida and Emr 1995). We used this vital dye to study the vacuolar morphology in V-ATPase mutants. Wild-type, vma1Δ and vma3Δ cells were exposed to FM4-64 for 30 min, and then subjected to a 90-min chase in YES medium. Numerous vacuoles were visible in the wild-type strain. In vma1Δ and vma3Δ cells, intermediate compartments were stained (Fig. 2A), and no vacuole staining was observed after an additional 30- to 60-min chase (data not shown). FM4-64 has been shown to be endocytosed via a route involving the prevacuolar compartment (Vida and Emr 1995), and therefore the stained structures in vma1Δ and vma3Δ cells may be prevacuolar compartments. In contrast, vacuoles were stained under the same conditions in the mutants vps34Δ (Takegawa et al. 1995) and ste12Δ (Morishita and Shimoda 2000), which have large vacuoles. These observations demonstrate that the vacuolar membrane staining with FM4-64 does not correlate with an aberrant vacuolar morphology and that V-ATPase is involved in endocytosis.
Nonspecific fluid-phase endocytosis in yeast cells can be easily monitored microscopically using the membrane-impermeable fluorescent dye Lucifer Yellow CH. In S. cerevisiae, the vma1 deletion strain shows a marked reduction in the fluid-phase uptake of Lucifer Yellow (Liu et al. 1997), and the vma3 disruption strain is completely unable to take up the dye (Umemoto et al. 1990). To determine whether the V-ATPase mutants of S. pombe exhibit blocks in fluid-phase endocytosis, the accumulation of Lucifer Yellow was examined. Vacuoles of wild-type cells were seen as fluorescence-positive compartments as a consequence of endocytosis (Fig. 2B). There were no such brightly fluorescent compartments in either the vma1Δ cells or the vma3Δ cells. This observation suggests that the acidified vacuolar compartment is required for endocytosis in S. pombe cells.
Effects of disruption of genes for V-ATPase subunits on the delivery of vacuolar proteins
As shown above, the disruption of a V-ATPase subunit gene, either vma1 or vma3, causes loss of vacuolar acidification, and affects endocytosis and vacuolar morphology. We therefore examined whether this type of acidification-defective vacuole can function serve as a target for vacuolar proteases or vacuolar membrane proteins. In S. cerevisiae, a kinetic delay in the processing of carboxypeptidase Y (CPY) occurs (Umemoto et al. 1990; Klionsky et al. 1992; Morano and Klionsky 1994), and missorting of CPY into the periplasmic space and/or medium takes place, in V-ATPase mutants (Umemoto et al. 1990; Klionsky et al. 1992; Bonangelino et al. 2002). We examined the sorting of S. pombe carboxypeptidase Y (SpCpy1p) in the vma1Δ and vma3Δ strains. During the initial 15 min of labeling, the ER- and Golgi-specific precursor form (proCPY) and a small amount of the vacuole-specific mature form (mCPY) were produced in the wild-type cells. After a 30-min chase, proCPY is almost completely converted to the mature form (Fig. 3A; Tabuchi et al. 1997). The vma1Δ and vma3Δ mutants, in contrast, were defective in processing SpCpy1p. After a 30-min chase, much less proCPY had been converted into mCPY in the mutant than in the wild-type cells. To confirm the missorting of SpCPY to the cell surface in these mutants, we employed a CPY colony blot assay that directly tests cells for secretion of SpCpy1p. In wild-type cells, SpCpy1p is efficiently sorted to the vacuoles, and is therefore not detected by the assay. In contrast, the vma1Δ and vma3Δ cells showed secretion of SpCPY (Fig. 3B). These results indicate that V-ATPase activity is required for delivery of SpCpy1p to the vacuole in S. pombe.
Disruption of genes for V-ATPase subunits results in mislocalization of the S. pombe CPY protein. A Processing of SpCPY in vivo. Wild-type (WT), vma1Δ and vma3Δ cells were pulse-labeled with Express-35S label for 15 min at 30°C and chased for 30 min. The immunoprecipitates were fractionated on an SDS-10% polyacrylamide gel. The autoradiograms of the fixed, dried gels are shown. The positions of proCPY (110 kDa) and mature CPY (mCPY: 32 kDa) are indicated. B Filter immunoblot for the detection of secreted SpCPY. The indicated strains were grown on MM plates in contact with the nitrocellulose filter at 28°C for 3 days. The filter was processed for immunoblotting using a rabbit polyclonal antibody against Cpy1p. cpy1Δ was used as a negative control and vps34Δ was used as a positive control for mis-sorting of Cpy1p
A marker protein, Hmt1-GFP, was used as a probe to monitor the delivery of vacuolar membrane proteins (Iwaki et al. 2003). The hmt1 + gene encodes an ABC-type transporter protein required for cadmium tolerance (Ortiz et al. 1992). Fission yeast responds to cadmium stress by inducing the synthesis of phytochelatins, a family of glutathione-related peptides having the structure (γ-Glu-Cys) n -Gly (where n=2–11) (Cobbett 2000). The Hmt1 protein is located in the vacuolar membrane, and is responsible for the transport of phytochelatins and phytochelatin-Cd complexes into the vacuole (Ortiz et al. 1992, 1995). In wild-type cells, Hmt1-GFP fluorescence was found in the vacuolar membrane as described previously (Fig. 4, Iwaki et al. 2003). Comparison of fluorescence patterns of Hmt1-GFP with the corresponding Nomarski images showed that Hmt1-GFP resides predominantly on the vacuolar membrane in vma1Δ and vma3Δ cells (Fig. 4). In agreement with these observations, vma1Δ and vma3Δ cells were tolerant to 100 μM CdCl2 (data not shown). These results suggest that the vacuolar membrane protein Hmt1p is correctly transported to the vacuoles; however, the sorting of the soluble vacuolar protein CPY is significantly inhibited because the intracellular compartment is not adequately acidified in V-ATPase mutants of S. pombe.
Intracellular localization of Hmt1-GFP. Wild-type (WT), vma1Δ and vma3Δ cells were grown in MM-leu medium supplemented with thiamine (5µg/ml) at 28°C. The cells were transferred to MM-leu without thiamine medium for 16 h, and then incubated with water for 2 h to induce vacuolar fusion. The Hmt1-GFP fusion protein was visualized using Nomarski optics and fluorescence microscopy
Mating efficiency is reduced in the vma3Δ mutant
In A. gossypii, the vma1 gene disruption mutant fails to form generative spores (Förster et al. 1999). The vma-1 mutant strains of N. crassa can not produce conidia, asexual spores, or perithecia (Bowman et al. 2000). The fission yeast exists as two mating types, h + and h -. When haploid cells of opposite types are shifted to a nitrogen-free medium they conjugate to form a diploid zygote, which then undergoes meiosis and sporulation (Egel 1989). To assess the influence of the defect in vacuolar acidification on these processes in S. pombe, a vma3 disruption mutant was derived from a homothallic strain, which was then streaked onto MEA medium and observed by microscopy. After incubation for 3 days, the mating efficiency (ME) of the wild-type h 90 cells was 71.1%, and that of the vma3Δ was 53.7% (Fig. 5A). Hence the vma3Δ cells were not sterile under these conditions. When homothallic strains were incubated in the MEL medium for 24 h under aerobic conditions, vma3Δ cells did not aggregate, and only a few mated diploids were observed (ME=9.3%). Under the same conditions, wild-type cells formed large aggregates including diploids, spore-containing zygotes and haploid cells. In this case, the ME was 30.5%.
Mating and sporulation behavior of the homothallic strain with a disrupted vma3 gene ( A) and intracellular localization of Psy1-GFP ( B). A Wild-type KJ100-7B ( h 90 leu1-32 ura4) and vma3Δ ( h 90 leu1-32 ura4 vma3::ura4 +) cells were cultured on ME plates at 28°C for 3 days, and then observed using Nomarski optics. B Intracellular localization of Psy1-GFP in homothallic strains. Wild-type (WT) KJ100-7B and vma3Δ cells were grown in MM-leu medium at 28°C overnight, then observed using Nomarski optics and fluorescence microscopy
Furthermore, the distribution of GFP-tagged syntaxin-like protein Psy1p was examined. In the wild-type strain, the fluorescence was found to be distributed in the plasma membrane in vegetative cells, and in the forespore membrane in mature spores (Fig. 5B, Nakamura et al. 2001). The same pattern was observed in the vma3Δ cells (Fig. 5B). These results suggest that the V-ATPase takes part in the mating of fission yeast cells, but the delivery of Psy1p to the forespore membrane is not inhibited in vma3Δ cells.
Discussion
Genes encoding V-ATPase subunits have been identified in a variety of organisms, including humans, mice, Drosophila melanogaster , Caenorhabditis elegans , higher plants and fungi. Deletion, or interference with the expression, of some of these genes resulted in a lethal phenotype in animals and Dictyostelium (Davies et al. 1996; Xie et al. 1996; Dow et al. 1997; Oka and Futai 2000; Pujol et al. 2001; Choi et al. 2003). Inactivation of V-ATPase subunits may be responsible for the lack of this enzyme activity in specific cells, such as osteoclasts in mammal and H-shaped excretory cells in C. elegans, thereby affecting their function and leading to embryonic or larval lethal phenotypes (Oka and Futai 2000; Scimeca et al. 2000; Pujol et al. 2001; Choi et al. 2003). In contrast, gene disruptions in fungi were not lethal but did cause a number of growth defects (Nelson and Nelson 1990; Yamashiro et al. 1990; Ohya et al. 1986, 1991; Bachhawat et al. 1993; Eide et al. 1993; Ramsey and Gadd 1997; Szczypka et al. 1997; Förster et al. 1999; Bowman et al. 2000; Kuroki et al. 2002).
In this study, we have investigated the functional and structural roles of the V-ATPase in vacuolar acidification and protein sorting to the vacuole in S. pombe, based on the functional analysis of two disruption mutants, vma1Δ and vma3Δ. We found that neither vma1 + nor vma3 + is essential for growth, but both are indispensable for vacuolar acidification in vivo.
Phenotypes of vmaΔ mutants in S. pombe
Deletion of either of these two V-ATPase subunits had pleiotropic effects on the growth of S. pombe cells. We found that vma1Δ cells and vma3Δ cells of S. pombe showed nearly the same phenotypes. In S. cerevisiae, cells lacking a V0 subunit fail to incorporate any V-ATPase subunits into the vacuolar membrane; however, the V1 domain is stable in the cytosol (Kane et al. 1992, 1999). Loss of one V0 subunit destabilizes the other V0 subunits (Kane et al. 1992) and an assembled V0 domain is required to recruit the V1 domain to the membrane. S. pombe vma3Δ cells might not express the V1 subunits in the vacuole, resulting in loss of function of the V-ATPase. We are currently examining the localization of the V1 domain in vma3Δ cells to clarify the assembly of V-ATPase in this mutant.
The vma1Δ and vma3Δ cells showed severe pH sensitivity and did not grow at pH 7.0 or more. The basis for pH sensitivity has been investigated in S. cerevisiae vma mutants. A role for endocytosis in acidification is supported by the observation that blocking of endocytosis in a V-ATPase-deficient strain results in cell death (Munn and Riezman 1994). When the cells are grown at basic pH, fluid-phase endocytosis may be insufficient to acidify endocytic compartments in the absence of V-ATPase activity. However, an NH4 +-dependent system has been reported to be responsible for vacuolar acidification in V-ATPase-deficient yeast (Plant et al. 1999). Rich growth media contain high concentrations of ammonium in equilibrium with the unprotonated form (ammonia) at basic pH. After reaching the vacuole, ammonia is protonated, thereby elevating the vacuolar pH.
Growth of the V-ATPase mutants of S. pombe was inhibited by the divalent cations Zn2+ and Cu2+. It has been reported that the endoplasmic reticulum transporter Zhf1p and the zinc metallothionein Zym1p contribute to zinc homeostasis in fission yeast by sequestering surplus zinc in vivo (Borrelly et al. 2002; Clemens et al. 2002). These two proteins are major determinants of zinc tolerance in fission yeast, but the sensitivity to Zn2+ of the vacuole-less mutant vps33Δ (Iwaki et al. 2003) and V-ATPase mutants indicates that vacuoles are also involved in zinc homeostasis. Recently, a vacuolar copper transporter, Ctr6p, was identified in fission yeast, although it was suggested that Ctr6p serves to mobilize stored copper from the vacuole to the cytosol under conditions in which copper is scarce (Bellemare et al. 2002). Which of these divalent cations are stored in the intracellular compartments (including vacuoles) of S. pombe, and how they are sequestered, has not been elucidated, but these results indicate that the V-ATPase is critical for storage and/or detoxification of Zn2+ and Cu2+.
In addition, high concentrations of Ca2+ in medium inhibited the growth of the vma1Δ and vma3Δ strains of S. pombe, as in the case of the corresponding S. cerevisiae mutants (Ohya et al. 1986, 1991). Tight control of cytosolic Ca2+ concentrations in budding yeast is achieved primarily via a vacuolar Ca2+/H+ exchanger (Vcx1p) driven by V-ATPase, and a vacuolar Ca2+ pump (Pmc1p; Förster and Kane 2000), and V-ATPase cooperates with calcineurin to regulate the concentration of free Ca2+ in the cytosol (Tanida et al. 1995). The sequestering of Ca2+ in fission yeast is carried out by the Ca2+/H+ antiporter(s) of intracellular membranes, and no Ca2+-ATPase activity has been detected; however, genes for putative Ca2+-ATPases have been found in the genome sequence of fission yeast (Okorokov et al. 2001). Our results suggest that acidification of intracellular compartments by V-ATPase has a significant effect on Ca2+ homeostasis mediated by Ca2+/H+ antiporters in S. pombe.
Vacuolar morphology is abnormal in vma mutants
The most obvious and pronounced effects of disruption of vma1 or vma3 in S. pombe are on vacuolar morphology. The enlarged vacuoles seen in V-ATPase mutant strains of S. pombe are similar to those observed in vps34Δ and ste12 cells (Takegawa et al. 1995; Morishita and Shimoda 2000) (Fig. 2). The S. pombe Vps34p encodes a phosphatidylinositol (PtdIns) 3-kinase, and the vps34Δ strain lacks PtdIns 3-kinase activity and shows a defect in vacuolar protein transport in S. pombe (Takegawa et al. 1995; Tabuchi et al. 1997). Therefore, the Vps34p/PtdIns 3-kinase facilitates anterograde protein transport from the Golgi to the vacuole through the regulated synthesis of PtdIns(3)P. The ste12 + gene has been cloned and was found to be homologous to the S. cerevisiae FAB1 gene (Yamamoto et al. 1995), which was originally identified in studies on mutants that displayed aberrant chromosomal segregation (Morishita et al. 2002). In cells lacking Ste12p, PtdIns(3,5)P2 is undetectable, and this result implies that the ste12 + gene encodes a PtdIns(3)P 5-kinase, which synthesizes PtdIns(3,5)P2 from PtdIns(3)P (Morishita et al. 2002). Increases in vacuole surface area in S. pombe vps34 and ste12 mutants may be a consequence of defects in the turnover or efflux of the vacuolar membrane. The precise role of vacuolar acidification in vacuolar membrane turnover has yet to be determined, and we are examining the PtdIns 3-kinase activity and the level of PtdIns(3)P and PtdIns(3,5)P2 in vma1 and vma3 mutant cells.
During the fusion of vesicles, V0 sectors from opposing membranes form complexes by docking and bilayer fusion (Peters et al. 2001; Nishi and Forgac 2002). The vma3Δ mutant of S. pombe was expected to be an ideal model for studying the role of the V0 sector in vacuolar fusion in vivo, since hypotonic stress caused a transitory fusion of vacuoles in S. pombe, affecting the volume and number of vacuoles (Bone et al. 1998). However, when the vma3Δ mutant was exposed to hypotonic stress, the volume and numbers of vacuoles were indistinguishable from those seen by Nomarski optics under normal conditions (data not shown). The large vacuoles found in vma3Δ mutants suggest that V0 sectors are not involved in vacuolar fusion in S. pombe cells.
Effects of the inactivation of V-ATPase on membrane trafficking in S. pombe
The S. cerevisiae V-ATPase plays a role in membrane trafficking from endosomes to vacuoles (Stevens and Forgac 1997). Targeting of newly synthesized vacuolar enzymes from the Golgi to the vacuole is dependent on vacuolar acidification. S. cerevisiae vma strains were found to secrete significant amounts of newly synthesized vacuolar proteases, including CPY (Umemoto et al. 1990; Klionsky et al. 1992, Bonangelino et al. 2002). In S. cerevisiae, the targeting of CPY requires its binding to the CPY receptor Vps10p in the Golgi, followed by delivery via vesicles to an endosomal compartment (Marcusson et al. 1994; Cooper and Stevens 1996). Within this compartment, the low pH is hypothesized to activate release of CPY into the lumen, and recycling of Vps10p back to the trans -Golgi (Marcusson et al. 1994; Cooper and Stevens 1996). Neutralization of this compartment results in the saturation of receptors with ligand, and the secretion of vacuolar enzymes via a secretory pathway. In this study, we have shown that S. pombe V-ATPase mutants showed a delay in the transport of SpCPY and also secreted SpCPY. The mechanism required for moving SpCPY to the vacuole is similar to that noted for CPY in S. cerevisiae (Takegawa et al. 2003). Comparison of the S. cerevisiae VPS10 gene with the fission yeast genome sequence has identified a homologous gene (SPBC16C6.06) (Takegawa et al. 2003). Characterization of this vps10 homologue and the distribution of Vps10p in vma1 and vma3 mutants should reveal the mechanism used for the sorting of vacuolar proteins in S. pombe.
In S. cerevisiae, vma mutants are defective in endocytosis (Umemoto et al. 1990; Liu et al. 1997; Perzov et al. 2002). Although the vacuolar delivery of internalized FM4-64 was strongly inhibited in V-ATPase mutants of S. pombe, Hmt1-GFP was transported normally to the vacuolar membrane in these cells. The delivery of Hmt1p to the vacuoles is not well understood, but this observation may indicate that the sorting pathway for Hmt1p is distinct from the sorting pathway for SpCPY and the endocytic process. In addition, the distribution of Psy1-GFP in the vma3Δ mutant strain was normal (Fig. 5). In the wild-type strain, Psy1-GFP fluorescence was found to be distributed in the plasma membrane in vegetative cells, and in the forespore membrane in mature spores (Nakamura et al. 2001). Two possible explanations for the localization of Psy1p were proposed. One is that Psy1p on the plasma membrane is degraded at metaphase II, and the Psy1p synthesized de novo is exclusively transported to forespore membranes via the conventional ER/Golgi pathway. The other explanation is that the plasma membrane Psy1p is internalized by endocytosis and transported to the forespore membrane. Our results showed that vma3Δ cells are defective in endocytosis, but transport of Psy1p to the forespore membrane is normal. This finding suggests that Psy1-GFP is incorporated into the forespore membrane not by endocytosis from the plasma membrane, but by transport of newly synthesized protein via the normal ER/Golgi pathway.
Mating efficiency is affected in the vma3Δ strain
Mating efficiency is reduced in the S. pombe vma3Δ mutant, suggesting that V-ATPase is vital for mating in fission yeast. We have three possible explanations for this mild defect in mating. First, missorting of vacuolar proteases may diminish the activity of vacuolar proteases which are required for the nitrogen starvation-induced protein degradation that accompanies sporulation. In S. cerevisiae, vma mutants secrete precursor forms of CPY and proteinase A (Klionsky et al. 1992). It may be possible for fission yeast vma mutants to secrete vacuolar proteases—Isp6p and other unidentified proteases. A change in vacuolar pH may be partly responsible for the reduced activity in vacuoles. Second, the defect may be due to reduced endocytosis. The role of endocytosis in sporulation remains completely unknown; however, some endocytosis mutants show a defect in sporulation in budding yeast (Whitacre et al. 2001). These mutants also have a defect in the actin cytoskeleton. Therefore, the third possible explanation is that the large vacuoles in V-ATPase mutants disturb the polarization of the cytoskeleton. The cytoskeletal changes that accompany conjugation in fission yeast have been described, with cortical actin patches moving to the point of cell extension (Petersen et al. 1998). However, when vma mutants of fission yeast were grown in rich medium, the actin distribution seemed to be almost the same as in wild-type cells (data not shown). Thus, this third explanation seems less plausible than the first two.
References
Alfa C, Fantes P, Hyams J, McLoed M, Warbrick E (1993) Experiments with fission yeast: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Anraku Y, Hirata R, Wada Y, Ohya Y (1992) Molecular genetics of the yeast vacuolar H+-ATPase. J Exp Biol 172:67–81
Arata Y, Nishi T, Kawasaki-Nishi S, Shao E, Wilkens S, Forgac M (2002) Structure, subunit function and the regulation of coated vesicle and yeast vacuolar (H+)-ATPases. Biophys Biochim Acta 1555:71–74
Bachhawat AK, Manolson MF, Murdock DG, Garman JD, Jones EW (1993) The VPH2 gene encodes a 25-kDa protein required for activation of the yeast vacuolar H(+) ATPase. Yeast 9:175–184
Bellemare DR, Shaner L, Morano KA, Beaudoin J, Langlois R, Labbé S (2002) Ctr6, a vacuolar membrane copper transporter in Schizosaccharomyces pombe. J Biol Chem 277:46676–46686
Bonangelino CJ, Chavez, EM, Bonifacino JS (2002) Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol Biol Cell 13:2486–2501
Bone N, Millar JB, Toda T, Armstrong J (1998) Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr Biol 8:135–144
Borrelly GPM, Harrison MD, Robinson AK, Cox SG, Robinson NJ, Whitehall SK (2002) Surplus zinc is handled by Zym1 metallothionein and Zhf endoplasmic reticulum transporter in Schizosaccharomyces pombe. J Biol Chem 277:30394–30400
Bowman EJ, Kendle R, Bowman BJ (2000) Disruption of vma-1, the gene encoding the catalytic subunit of the vacuolar H+-ATPase, causes severe morphological changes in Neurospora crassa. J Biol Chem 175:167–176
Cheng H, Sugiura R, Wu W, Fujita M, Lu Y, Sio SO, Kawai R, Takegawa K, Shuntoh H, Kuno T (2002) Role of the Rab GTP-binding protein Ypt3 in the fission yeast exocytic pathway, and its connection to calcineurin function. Mol Biol Cell 13:2963–2976
Choi KY, Ji YJ Dhakal BK, Yu J-R, Cho C, Song WK, Ahnn J (2003) Vacuolar-type H+-ATPase E subunit is required for embryogenesis and yolk transfer in Caenorhabditis elegans. Gene 311:13–23
Clemens S, Bloss T, Vess C, Neumann D, Nies DH, zur Nieden U (2002) A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J Biol Chem 277:18215–18221
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Cooper AA, Stevens TH (1996) Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol 133:529–542
Davies SA, Goodwin SF, Kelly DC, Wang Z, Sozen MA, Kaiser K, Dow JAT (1996) Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J Biol Chem 271:30677–30684
Dow JAT, Davies SA, Guo Y, Graham S, Finbow M, Kaiser K (1997) Molecular genetic analysis of V-ATPase function in Drosophila melanogaster. J Exp Biol 200:237–245
Egel R (1989) Mating type genes, meiosis, and sporulation. In: Nasim A, Young P, Johnson BF (eds) Molecular biology of the fission Yeast. Academic Press, New York; pp 31–73
Eide DJ, Bridgham JT, Zhao Z, Mattoon JR (1993) The vacuolar H+-ATPase of Saccharomyces cerevisiae is required for efficient copper detoxification, mitochondrial function, and iron metabolism. Mol Gen Genet 241:447–456
Forgac M (1989) Structure and function of the vacuolar class of ATP-driven proton pumps. Physiol Rev 69:765–796
Förster C, Kane PM (2000) Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J Biol Chem 275:38245–38253
Förster C, Santos MA, Ruffert S, Krämer R, Revuelta JL (1999) Physiological consequence of disruption of the VMA1 gene in the riboflavin overproducer Ashbya gossypii. J Biol Chem 274:9442–9448
Ghislain M, Bowman EJ (1992) Sequence of the genes encoding subunits A and B of the vacuolar H+-ATPase of Schizosacchaaromyces pombe. Yeast 8:791–799
Gimble FS, Thorner J (1992) Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae. Nature 357:301–306
Graham LA, Powell B, Stevens TH (2000) Composition and assembly of the yeast vacuolar H+-ATPase complex. J Exp Biol 203:61–70
Grimm C, Kohli J, Murray J, Maundrell K (1988) Genetic engineeering of Schizosaccharomyces pombe—a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet 215:81–86
Gu HH, Xu J, Gallagher M, Dean GE (1993) Peptide splicing in the vacuolar ATPase subunit A from Candida tropicalis. J Biol Chem 268:7372–7381
Hirata R, Ohsumi Y, Nakano A, Kawasaki H, Suzuki K, Anraku Y (1990) Molecular structure of a gene, VMA1 , encoding the catalytic subunit of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem 265:6726–6733
Iwaki T, Osawa F, Onishi M, Koga T, Fujita Y, Hosomi A, Tanaka N, Fukui Y, Takegawa K (2003) Characterization of vps33 +, a gene required for vacuolar biogenesis and protein sorting in Schizosaccharomyces pombe. Yeast 20:845–855
Kane PM, Parra KJ (2000) Assembly and regulation of the yeast vacuolar H+-ATPase. J Exp Biol 203:81–87
Kane PM, Yamashiro CT, Wolczyk DF, Neff N, Goebl M, Stevens TH (1990) Protein splicing converts the yeast TFP1 gene product to the 69-kDa subunit of the vacuolar H+-adenosine triphosphatase. Science 250:651–657
Kane PM, Kuehn MC, Howard-Stevenson I, Stevens TH (1992) Assembly and targeting of peripheral and integral membrane subunits of the yeast vacuolar H+-ATPase. J Biol Chem 267:447–454
Kane PM, Tarsio M, Liu J (1999) Early steps in assembly of the yeast vacuolar H+-ATPase. J Biol Chem 274:17275–17283
Kawasaki-Nishi S, Nishi T, Forgac M (2003) Proton trasnlocation driven by ATP hydrolysis in V-ATPase. FEBS Lett 545:76–85
Klionsky DJ, Nelson H, Nelson N (1992) Compartment acidification is required for efficient sorting of proteins to the vacuole in Saccharomyces cerevisiae. J Biol Chem 267:3416–3422
Kuroki Y, Juvvadi PR, Arioka M, Nakajima H, Kitamoto K (2002) Cloning and characterization of vmaA, the gene encoding a 69-kDa catalytic subunit of the vacuolar H+-ATPase during alkaline pH mediated growth of Aspergillus oryzae. FEMS Microbiol Lett 209:277–282
Liu Q, Leng X-H, Newman PR, Vasilyeva E, Kane PM, Forgac M (1997) Site-directed mutagenesis of the yeast V-ATPase A subunit. J Biol Chem 272:11750–11756
Manolson MF, Proteau D, Preston RA, Stenbit A, Roberts BT, Hoyt MA, Preuss D, Mulholland J, Botstein D, Jones EW (1992) The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J Biol Chem 267:14294–14303
Manolson MF, Wu B, Proteau D, Taillon BE, Roberts BT, Hoyt MA, Jones EW (1994) STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vph1p. J Biol Chem 269:14064–14074
Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD (1994) The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77:579–586
Morano KA, Klionsky DJ (1994) Differential effects of compartment deacidification on the targeting of membrane and soluble proteins to the vacuole in yeast. J Cell Sci 107:2813–2824
Moreno S, Klar A, Nurse P (1990) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194:795–823
Morishita M, Shimoda C (2000) Positioning of medial actin rings affected by eccentrically located nuclei in a fission yeast mutant having large vacuoles. FEMS Microbiol Lett 188:63–67
Morishita M, Morimoto F, Kitamura K, Koga T, Fukui Y, Maekawa H, Yamashita I, Shimoda C (2002) Phosphatidylinositol 3-phosphate 5-kinase is required for the cellular response to nutritional strarvation and mating pheromone signals in Schizosaccharomyces pombe. Genes Cells 7:199-215
Munn AL, Riezman H (1994) Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: idetification of six new END genes. J Cell Biol 127:373–386
Murray JM, Johnson DI (2001) The Cdc42p GTPase and its regulators of Nrf1p and Scd1p are involved in endocytic trafficking in the fission yeast Schizosaccharomyces pombe. J Biol Chem 276:3004–3009
Nakamura T, Nakamura-Kubo M, Hirata A, Shimoda C (2001) The Schizosaccharomyces pombe spo3 + gene is required for assembly of the forespore membrane and genetically interacts with psy1 +-encoding syntaxin-like protein. Mol Biol Cell 12:3955–3972
Nelson H, Nelson N (1990) Disruption of genes encoding subunits of yeast vacuolar H+-ATPase causes conditional lethality. Proc Natl Acad Sci USA 87:3503–3507
Nishi T, Forgac M (2002) The vacuolar (H+)-ATPases—Nature’s most versatile proton pumps. Nature Rev 3:94–103
Oka T, Futai M (2000) Requirement of V-ATPase for ovulation and embryogenesis in Caenorhabditis elegans. J Biol Chem 275:29556–29561
Okorokov LA, Silva FE, Façanha ALO (2001) Ca2+ and H+ homeostasis in fission yeast: a role of Ca2+/H+ exchange and distinct V-H+-ATPases of the secretory pathway organelles. FEBS Lett 505:321–324
Ohya Y, Ohsumi Y, Anraku Y (1986) Isolation and characterization of Ca2+-sensitive mutants of Saccharomyces cerevisiae. J Gen Microbiol 132:979–988
Ohya Y, Umemoto N, Tanida I, Ohta A, Iida H, Anraku Y (1991) Calcium-sensitive cls mutants of Saccharomyces cerevisiae showing a Pet- phenotype are ascribable to defects of vacuolar membrane H+-ATPase activity. J Biol Chem 266:13971–13977
Ortiz DF, Kreppel L, Speiser DM, Scheel G, McDonaldo G, Ow DW (1992) Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J 11:3491–3499
Ortiz DF, Ruscitti T, McCue KF, Ow DW (1995) Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem 270:4721–4728
Perzov N, Padler-Karavani V, Nelson H, Nelson N (2002) Characterization of yeast V-ATPase mutants lacking Vph1p or Stv1p and the effect on endocytosis. J Exp Biol 205:1209–1219
Peters C, Bayer MJ, Bühler S, Anderson JS, Mann M, Mayer A (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409:581–588
Petersen J, Nielsen O, Egel R, Hagan IM (1998) F-actin distribution and function during sexual differentiation in Schizosaccharomyces pombe. J Cell Sci 111:867–876
Plant PJ, Manolson MF, Grinstein S, Demaurex N (1999) Alternative mechanisms of vacuolar acidification in H+-ATPase-deficient yeast. J Biol Chem 274:37270–37279
Pujol N, Bonnerot C, Ewbank JJ, Kohara Y, Thierry-Mieg D (2001) The Caenorhabditis elegans unc-32 gene encodes alternative forms of a vacuolar ATPase a subunit. J Biol Chem 276:11913–11921
Ramsay LM, Gadd GM (1997) Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol Lett 152:293–298
Roberts CJ, Raymond CK, Yamashiro CT, Stevens TH (1991) Methods for studying the yeast vacuole. In: Guthrie C, Fink GR (eds) Guide to yeast genetics and molecular biology. Academic Press, San Diego, pp 644–661
Russell P, Nurse P (1986) Schizosaccharomyces pombe and Saccharomyces cerevisiae: a look at yeasts divided. Cell 45:781–782
Scimeca J-C, Franchi A, Trojani C, Parrinello H, Grosgeorge J, Robert C, Jaillon O, Poirier C, Gaudray P, Carle GF (2000) The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic ( oc/oc) mutants. Bone 26:207–213
Stevens TH, Forgac M (1997) Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol 13:779–808
Suga M, Hatakeyama T (2001) High efficiency transformation of Schizosaccharomyces pombe pretreated with thiol compounds by electroporation. Yeast 18:1015–1021
Suga M, Isobe M, Hatakeyama T (2000) Cryopreservation of competent intact yeast cells for efficient electroporation. Yeast 16:889–896
Szczypka M, Zhu Z, Silar P, Thiele DJ (1997) Saccharomyces cerevisiae mutants altered in vacuole function are defective in copper detoxification and iron-responsive gene transcription. Yeast 13:1423–1435
Tabuchi M, Iwaihara O, Ohtani Y, Ohuchi N, Sakurai J, Morita T, Iwahara S, Takegawa K (1997) Vacuolar protein sorting in fission yeast: cloning, biosynthesis, transport, and processing of carboxypeptidase Y from Schizosaccharomyces pombe. J Bacteriol 179:4179–4189
Takegawa K, DeWald DB, Emr SD (1995) Schizosaccharomyces pombe Vps34p, a phosphatidylinositol-specific PI 3-kinase essential for normal cell growth and vacuole morphology. J Cell Sci 108:3745–3756
Takegawa K, Tokutomi S, Bhuiyan MSA, Tabuchi M, Fujita Y, Iwaki T, Utsumi S, Tanaka N (2003) Heterologous expression and characterization of Schizosaccharomyces pombe vacuolar carboxypeptidase Y in Saccharomyces cerevisiae. Curr Genet 42:252–259
Tanida I, Hasegawa A, Iida H, Ohya Y, Anraku Y (1995) Cooperation of calcineurin and vacuolar H+-ATPase in intracellular Ca2+ homeostasis of yeast cells. J Biol Chem 270:10113–10119
Toyama R, Goldstein DJ, Schlegel R, Dhar R (1991) A genomic sequence of the Schizosaccharomyces pombe 16 kDa vacuolar H+-ATPase. Yeast 7:989–991
Umemoto N, Yoshihisa T, Hiratat R, Anraku Y (1990) Roles of the VMA3 gene product, subunit c of the vacuolar membrane H+-ATPase on vacuolar acidification and protein transport. A study with VMA3 -disrupted mutants of Saccharomyces cerevisiae. J Biol Chem 265:18447–18453
Vida TA, Emr SD (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779–792
Whitacre JL, Davis DA, Toenjes KA, Brower SM, Adams AEM (2001) Generation of an isogenic collection of yeast actin mutants and identification of three interrelated phenotypes. Genetics 157:533–543
Xie Y, Coukell MB, Gombos Z (1996) Antisense RNA inhibition of the putative vacuolar H+-ATPase proteolipid of Dictyostelium reduces intracellular Ca2+ transport and cell viability. J Cell Sci 109:489–497
Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D (1995) Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell 6:525–539
Yamashiro CT, Kane PM, Wolczyk DF, Preston RA, Stevens TH (1990) Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol Cell Biol 10:3737–3749
Acknowledgements
We are grateful to Dr. Yuko Giga-Hama for providing the S. pombe strain and to Dr. Taro Nakamura for providing the plasmids. This work was partly supported by the Project for Development of a Technological Infrastructure for Industrial Bioprocesses on R&D of New Industrial Science and Technology Frontiers by the Ministry of Economics, Trade and Industry (METI) and by a fellowship from the New Energy and Industrial Technology Development Organization (NEDO)
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Johnston
Rights and permissions
About this article
Cite this article
Iwaki, T., Goa, T., Tanaka, N. et al. Characterization of Schizosaccharomyces pombe mutants defective in vacuolar acidification and protein sorting. Mol Genet Genomics 271, 197–207 (2004). https://doi.org/10.1007/s00438-003-0971-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-003-0971-7