Abstract
The order Piroplasmida encompasses tick-borne pathogens of veterinary and medical importance positioned in two main families: Babesiidae and Theileriidae. Even though previous studies carried out in Brazil recorded the occurrence of piroplasmid species circulating in small mammals, 18S RNA gene sequences were only partially sequenced, preventing the assessment of their phylogenetic positioning. The current study aimed to detect and characterize, using morphological, molecular, and bioinformatic approaches, piroplasmids from wild mammals and associated ticks sampled in Central-Western Brazil. Out of 67 Didelphis albiventris sampled, 22 (16.4%) were positive for piroplasmids by PCR. In contrast, none of the 48 small rodents and 14 capybaras (Hydrochoerus hydrochaeris) was PCR-positive. Four Amblyomma dubitatum ticks—one from Rattus rattus, one from H. hydrochaeris, and two from D. albiventris—out of 114 Amblyomma spp. DNA samples were positive for piroplasmids by PCR. The phylogenetic inference performed using the near-complete 18S rRNA gene positioned the putative novel piroplasmid species detected in D. albiventris and associated A. dubitatum ticks near to Babesia sensu lato clade (Western group—cluster III) and distant from the Australian marsupial-associated piroplasms. Phylogenetic inferences based on two additional molecular markers, namely hsp-70 and cox-1, supported the near-complete 18S rRNA gene phylogenetic inference. Finally, the partial 18S rRNA gene sequences detected in ticks from rodents (R. rattus and H. hydrochaeris) showed 97.2–99.4% identity with the Piroplasmida previously detected in a capybara from Brazil, raising evidence that a still uncharacterized piroplasmid species has been identified in the capybara, the largest rodent species from South America.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental changes, such as deforestation and urbanization, caused by anthropic actions may alter the transmission dynamics of vector-borne agents, precipitating the emergence of infectious diseases and the spillover of pathogens from humans and domestic animals to wildlife and vice versa (Chomel 2007; Price et al. 2019). In urban and peri-urban areas, the frequency of contact between humans, their pets, and wildlife ranges from occasional encounters to permanently sharing sites, hence increasing the chance of pathogens spillover (Mackenstedt et al. 2015).
Piroplasmids, haemoprotozoan parasites belonging to the order Piroplasmida (Phylum Aplicomplexa), encompass tick-borne pathogens of veterinary and medical importance distributed worldwide. These organisms are positioned into two main families (Babesiidae and Theileriidae) and taxonomically grouped into three main genera: Babesia, Theileria, and Cytauxzoon (Schnittger et al. 2012; Jalovecka et al. 2018; 2019).
Traditional parasitological methods such as microscopy are insufficient for identification and classification of piroplasmids found in wild vertebrate hosts. In contrast, molecular approaches have overcome some issues in the piroplasmids taxonomy (Schnittger et al. 2012; Jalovecka et al. 2019). Currently, based on molecular and phylogenetic data, ten clades of piroplasmids are distinguished (Jalovecka et al. 2019).
In contrast to studies carried out in Brazil targeting piroplasmids in domestic animals, the data regarding this group of protozoans in wild mammals is still incipient. For instance, Criado-Fornelio et al. (2009) detected a Piroplasmida genotype—showing 90% of identity with Theileria equi—in one out of 14 (7.14%) capybaras (H. hydrochaeris) sampled in Pelotas, State of Rio Grande do Sul, extreme south of Brazil. In addition, Wolf et al. (2017) reported two Piroplasmida genotypes in wild rodents (Thrichomys pachyurus [27.2%; 3/11]) and marsupials (Monodelphis domestica [50%; 1/2]) trapped in Poconé municipality, State of Mato Grosso, Central-Western Brazil. In the Amazon forest, Soares et al. (2017) reported Theileria spp. in one agouti (Dasyprocta sp. [n = 2]) and four lowland pacas (Cuniculus paca [n = 32]). In addition, the authors detected Piroplasmida DNA in one common opossum (Didelphis marsupialis [n = 19]). Besides, Sousa et al. (2018) reported two Piroplasmida genotypes closely related to Babesia vogeli (6.5%; 5/77) and T. equi (1.3%; 1/77) in wild rodents (Trychomis fosteri) in Pantanal biome. Recently, Colle et al. (2019) reported a Piroplasmida genotype in two out of 31 (6.45%) D. marsupialis trapped in Sinop, State of Mato Grosso. This Piroplasmida genotype was identical to that one previously detected in common opossum sampled in Pará State, Northern Brazil (Soares et al. 2017).
Although some studies have been carried out aiming at performing the molecular characterization of piroplasmids in rodents and marsupials in Brazil, all of them were performed using a partial fragment of the 18S rRNA gene, precluding the accurate phylogenetic positioning of the detected piroplasmid species.
In light of the current scenario, the present study aimed to (i) investigate the occurrence and molecularly characterize piroplasmids in Rodentia and Didelphiomorphia mammals and their associated ticks in urban and urban forest fragments from Brazil; and (ii) morphologically and molecularly characterize a piroplasmid species infecting D. albiventris from Central-Western Brazil, by light microscopy and phylogenetic assessment based on three different molecular markers.
Material and methods
Ethical statement
The animal captures were in accordance with the licenses obtained from the “Instituto Chico Mendes de Conservação da Biodiversidade” (license number 56912–2), Imasul (license number 001/2017) and endorsed by the Ethics Committee of FCAV/UNESP under the number: 01952/18.
Study sites, mammals trapping, and blood and ectoparasite sampling
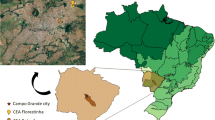
Between May 2017 and August 2018, 105 mammals belonging to four different species were sampled in different sites of Campo Grande municipality (− 20° 42′ 30″ S, − 54° 61′ 60″ W), State of Mato Grosso do Sul, Central-Western Brazil. In Campo Grande, 48 small rodents (Rattus rattus [n = 39] and Mus musculus [n = 9]) were trapped in urban areas (four sites) and urban forest fragments (four sites). Additionally, 14 capybaras (H. hydrochaeris) and 43 marsupials (D. albiventris) were trapped in three and six urban forest fragments, respectively, in Campo Grande. All capture procedures and blood sample collection were performed as previously described (Nantes et al. 2019; Gonçalves et al.2020).
The sampled animals were checked for the presence of ticks. Once collected, the arthropods were placed in microtubes containing absolute ethanol (Merck®) and maintained at − 20 °C until morphological identification and DNA extraction. The morphological identification was performed using previously described taxonomic keys (Onofrio et al. 2005; Martins et al. 2010; Linard et al. 2014; Anholt et al. 2014; Pereira et al. 2017).
Additionally, between May 2019 and January 2020, 24 blood samples from marsupials (D. albiventris) were obtained from a rescue center (“Centro de Triagem de Animais Silvestres do Distrito Federal”—CETAS–DF) in the Federal Disctrict. The animals were from urban areas of Brasilia (15° 47′ 38″ S 47° 52′ 58″ O), Distrito Federal (DF), and were sampled by convenience, independently of age, gender, or clinical status.
Giemsa-stained blood smears
Blood smears were performed using peripheral blood samples collected from wild Rodentia and Didelphiomorphia mammals, fixed with methanol (Merck®, Darmstadt, Germany) and stained with Giemsa (Giemsa stain, modified, Sigma-Aldrich®, St. Louis, MO, USA). The blood smears were then observed at 1000 × magnification and images of the parasites recorded using a Olympus BX-43 microscope coupled to a camera (Olympus DP73). The piroplasmid dimensions detected in D. albiventris’ blood smears were measured using the Olympus CellSens™ microscope imaging standard software.
DNA extraction and quality assessment
DNA was extracted from 10 mg of each small rodent spleen tissue, and from 200 µL of each blood sample from marsupials and capybaras, using the DNeasy® Blood & Tissue Kit (Qiagen®, Valencia, California, USA), according to the manufacturer’s instructions. Furthermore, the sampled arthropods were submitted to DNA extraction individually and/or in pools of up to three tick nymphs or seven tick larvae from the same host.
In order to discard the presence of PCR inhibitors, all extracted mammal DNA samples were used as a template in an internal control PCR targeting the mammal gapdh gene (Birkenheuer et al. 2003). Likewise, all arthropod DNA samples were submitted to an internal control PCR assay targeting the 16S rRNA (Black and Piesman 1994). Internal control-PCR positive samples were submitted to a nested PCR assay targeting a fragment of the 18S rRNA gene of piroplasmids.
Molecular detection and characterization of piroplasmids in mammals and associated ticks
Firstly, DNA samples were screened for piroplasmids DNA using a nested PCR assay targeting a small fragment (~ 800 bp) of the 18S rRNA gene as previously described (Jefferies et al. 2007). Additionally, the positive samples were subjected to further molecular characterization using conventional PCR assays targeting seven molecular markers, namely near-complete 18S rRNA gene (1500 bp—Greay et al. 2018), cox1 (~ 800 bp—Corduneanu et al. 2017), hsp70 (~ 700 bp—Soares et al. 2011), β-tubulin (600 bp—Zamoto et al. 2004), ITS1 (~ 450 bp BROWN et al. 2009), cytB (~ 1 kb—Barbosa et al. 2019), and cox3 (~ 600 bp Barbosa et al. 2019). Additionally, a qPCR assay targeting a lsu region (130 bp) was performed (Qurollo et al. 2017). Conventional PCR assays were carried out in 25 µL reaction volume containing 10 × PCR buffer, 1.0 mM MgCl2, 0.8 mM deoxynucleotide triphosphate (dNTPs) mixture, 1.5 U Taq DNA Polymerase (Life Technologies®), 0.3 µM of each primer, and 5 µl of DNA—used as a template.
A qPCR assay was carried out for marsupial blood samples DNA using the primers B-lsu-F (ACCTGTCAARTTCCTTCACTAAMTT) and B-lsu-R (TCTTAACCCAACTCACGTACCA) as previously described (Qurollo et al. 2017). Briefly, the amplification reaction was performed using the CFX96 thermal cycler (Bio-Rad, CA, USA) real-time system. The qPCR assays were performed with a final volume of 10 µL containing 5 µl of 2xqPCR SYBRBIO (PCR Biosystems™, London, UK), 0.6 μM of each primer, and 1 μL of each DNA sample. The amplification protocol used was as follows: 3 min at 98 °C, followed by 40 cycles of 15 s at 98 °C, 15 s at 60 °C, and 15 s at 72 °C. The melting curves were acquired using 0.5 °C steps, withholds of 5 s, from 65 to 88 °C. The results were assessed through observation of amplification curves using a CFX96 thermal cycler. Serial dilutions were performed with the aim of constructing standard curves with different concentrations of Gblock DNA (Gblock; Integrated DNA Technologies, USA) (2.0 × 107 to 2.0 × 100 copies/μL), which encoded a 135-bp B. bovis Isu fragment.
DNA of B. bovis (Matos et al. 2017) and ultra-pure water were used as positive and non-template controls, respectively, in all (q)PCR assays.
The 18S rRNA gene (large fragment) positive amplicons were submitted to pGEM-T Easy vector cloning (Promega® Madison, WI, USA), following the manufacturer’s recommendations. One clone from each positive sample was selected for sequencing, according to the blue/while colonies system. Finally, the identified clones were submitted to plasmid DNA extraction using the Illustra® PlasmidPrep Mini Spin Kit (GE Healthcare, Buckinghamshire, UK) and to a PCR assay targeting the multiple cloning sites of the pGEM T-Easy plasmid (Lau et al. 2013).
The obtained amplicons were purified using the EXOSAP-IT® (Applied Biosystems) and submitted to sequencing in an automatic sequencer (ABI Prism 310 Genetic Analyser—Applied Biosystem/Perkin Elmer). Consensus sequences were obtained by Phred-Phrap program with Phred quality score established at ≥ 20 (Ewing et al. 1998).
BLAST, phylogenetic analyses, and generation of distance matrices
Identity, query coverage, and e-values were assessed by BLASTn tool (using default parameters), available in the NCBI GenBank database (Altschul et al. 1990). The obtained sequences were aligned with other sequences retrieved from GenBank using MAFFT software, version 7 (Katoh et al. 2019), using default gap penalty on EMBL-EBI analysis tools (Madeira et al. 2019). The model “best of fit” was selected by the program jModelTest2 (version 2.1.6) on 11 XSEDE19, under the Akaike information criterion (AIC) (Darriba et al. 2012). The Bayesian inference (BI) analysis was performed for both large and short 18S rRNA gene sequences with MrBayes 3.1.2. (Ronquist and Huelsenbeck 2003). Markov chain Monte Carlo (MCMC) simulations were run for 107 generations with a sampling frequency of every 100 generations and a burn-in of 25% using the CIPRES Science Gateway (Miller et al. 2010). The number of generations was selected based on the value of the average standard deviation of split frequencies (< 0.02, MrBayes version 3.2 Manual) (Ronquist and Teslenko 2012). Maximum likelihood tree inference was performed with IQ-TREE software (Trifinopoulos et al. 2016) for hsp-70 and cox1 sequences. The phylogenetic tree edition and rooting (outgroup) were performed using the Treegraph 2.0 beta software. The genetic distances were calculated using the p-distance method in MEGA X using the models previously selected in the phylogenetic analysis.
Results
Ticks and DNA extraction quality
Ticks were obtained from 5 out of 48 (10.4%) small rodents sampled in Campo Grande, MS. Moreover, 71.4% (10/14) of sampled capybaras were infested by ticks. Likewise, ticks were observed in 14 out of 43 (32.5%) of the trapped D. albiventris in Campo Grande. The identification of the tick species sampled is shown in Table 1. Ticks were not collected from D. albiventris sampled in Brasilia.
All but three of the tick samples and all of the mammal blood/tissue samples were positive in PCR assays targeting endogenous genes. The three tick samples collected from capybaras negative in arthropod-16S rRNA PCR assay were excluded from subsequent analyses.
Microscopic detection of Piroplasmids
Intra-erythrocytic oval ring–shaped organisms similar to piroplasmid merozoites were detected in blood smears from four and six D. albiventris from Campo Grande and Brasilia, respectively. These merozoites were encircled by basophilic-staining membrane of variable width around a less densely stained pale center. Also, this peripheral basophilic-staining membrane frequently showed more than a single dense chromatin. Merozoites in different stages of development ranged from 1.33 to 3.22 µm (mean = 2.25 ± 0.48) in length, and 1.07 to 2.63 µm (mean = 1.92 ± 0.53) in width (Fig. 1). Intra-erythrocytic structures resembling piroplasmids were neither found in capybaras nor in small rodents’ blood smears.
Occurrence of piroplasmids DNA in synanthropic mammals and ticks
None of 48 small rodents sampled in Campo Grande were positive in the nPCR assay for piroplasmids targeting a small fragment (~ 750 bp) of the 18S rRNA gene. On the other hand, one A. dubitatum nymph collected from a specimen of R. rattus was positive in the nPCR for piroplasmids targeting 18S rRNA gene. The amplified sequence (~ 750 bp) shared 99.4% identity (E-value = 0.0; query-coverage = 98%) with Babesia sp. (EF222255) detected in a capybara captured in Rio Grande do Sul, Southern Brazil. Similarly, none out of 14 capybara-DNA samples showed positivity in the nPCR assay. Among the 77 tick-DNA samples obtained from capybaras, one female adult specimen of A. dubitatum collected from a capybara was positive for piroplasmids. The sequence (~ 750 bp) shared 97.2% identity (E-value = 0.0; query-coverage = 100%) to Babesia sp. (EF222255) previously reported in a capybara.
In addition, 11 out of 43 (25.5%) D. albiventris sampled in Campo Grande were positive to piroplasmids in the nPCR targeting the 18S rRNA gene. Two (nymphs) out of 28 (7.14%) A. dubitatum samples obtained from D. albiventris were also positive. Likewise, 11 out of 24 (48.5%) D. albiventris sampled in Brasília were positive to piroplasmids targeting the 18S rRNA gene region in the screening protocol (S1 Table).
Molecular characterization of the detected piroplasmids
Aiming to investigate the identity of the detected piroplasmids, the positive samples were submitted to additional PCR assays targeting seven different molecular markers. One (obtained from D. albiventris) out of four 18S rRNA-A. dubitatum positive samples was also positive in a PCR assay targeting a large fragment of the 18S rRNA gene. Unfortunately, these tick DNA-samples were negative in the PCR assays targeting the other additional molecular targets, except for the tick-DNA samples obtained from D. albiventris (n = 2) and R. rattus (n = 1) that were positive in the qPCR assay targeting the lsu region. Another large fragment of 18S rRNA gene was obtained from a D. albiventris DNA blood sample. In addition, three hsp70 and two cox1 sequences were amplified and sequenced from D. albiventris. Out of the 43 and 24 D. albiventris from Campo Grande and Brasilia tested, six and 12 were positive, respectively, in the qPCR targeting lsu region (S1 Table). The range of the melting temperature for Isu fragment ranged from 75 to 75.5 °C. Despite attempts—three times—sequences were not obtained for the other target genic regions.
Phylogenetic analyses
Two 18S rRNA gene large sequences were successfully obtained, one detected in a D. albiventris and another one in an A. dubitatum tick. The ML analysis based on an alignment of the ~ 1.3 kb 18S rRNA gene positioned these piroplasmid sequences in an exclusive clade, near to cluster III (B. duncani and B. conrade) previously established by Jalovecka et al. (2019), but with a low bootstrap value (42%) (Fig. 2). In addition, genetic distance ranging from 3.5 to 7.2% was observed between the amplified sequences and those belonging to cluster III (S2 Table). These findings support the assumption that the amplified piroplasmid sequences represent a distinct yet unknown species that likely does not belong to clade III.
Phylogenetic tree based on an alignment of 1.3 kb of 18S rRNA sequences using maximum likelihood (ML) with GTR + I + G evolutionary model. Bootstrap supports were estimated by 1000 replicates and are presented at nodes. Sequences of piroplasmids detected in the present study are highlighted in bold. Cardiosporidium cionae was used as outgroup
Three hsp-70 DNA sequences were successfully amplified and used in the phylogenetic analyses. Similar to the 18S rRNA phylogenetic analysis, the two sequences obtained from D. albiventris captured in Brasília and one sequence amplified in D. albiventris from Campo Grande were positioned in an exclusive clade, near to Babesia (WA1–DQ007005)—formally Babesia duncani—in ML analysis but with a low support (50%) (Fig. 3). Despite the phylogenetic positioning, the amplified sequences showed a relatively high genetic distance (15%) when compared to the abovementioned B. duncani sequence (S3 Table).
Phylogenetic tree based on an alignment of 575 bp of hsp-70 sequences using maximum likelihood (ML) with GTR evolutionary model. Bootstrap supports were estimated by 1000 replicates and are presented at nodes. Sequences of piroplasmids detected in the present study are highlighted in bold. Plasmodium falciparum was used as outgroup
Additionally, two cox-1 DNA sequences were obtained from D. albiventris trapped in Brasília and submitted to ML analyses. Similar to previous trees, the two sequences were grouped near to the cluster III (Babesia conradae), but supported by a low bootstrap value (48%) (Fig. 4). Genetic distances were observed between the amplified cox-1 sequences and B. conradae (group III—33.1%) as well as Theileria sensu stricto (group IX—30.2 to 31.2%) species (S4 Table).
Phylogenetic tree based on an alignment of 708 bp of cox1 sequences translated into amino acids using maximum likelihood (ML) with TVM evolutionary model. Bootstrap supports were estimated by 1000 replicates and are presented at nodes. Sequences of piroplasmids detected in the present study are highlighted in bold. Plasmodium falciparum was used as outgroup
Due to inconsistency in the phylogenetic tree based on small fragment of the 18S rRNA gene, only genetic distances were assessed for this target. Genetic distances ranging from 0 to 0.8% at the 18S rRNA gene were observed between the amplified piroplasmid sequences in Didelphis spp. and associated ticks in the present study and those previously detected in Didelphis spp. from Brazil (S5 Table), suggesting they represent the same species. Conversely, evolutionary distances of 8.8 to 9.7% were observed between the sequences detected in marsupials in the present study and those detected in Australian marsupials (S5 Table), evidencing they are distinct species. Additionally, two piroplasmid 18S rRNA gene sequences amplified from A. dubitatum ticks—from capybara and another one from the black rat (R. rattus)—grouped with a Babesia 18S rRNA gene sequence previously detected in a capybara sampled in southern Brazil. Genetic distance ranging from 0.8 to 3.4% was observed between these amplified haplotypes and that one reported in a capybara from Brazil, suggesting they may represent a novel species.
Discussion
In the present study, we report the occurrence and molecular characterization of a Piroplasmida species circulating in marsupials and their ticks from Brazil. In addition, piroplasmid sequences showing 97.2 to 99.4% identity with a sequence previously detected in a capybara from south Brazil was detected in A. dubitatum ticks collected from H. hydrochaeris and R. rattus, respectively. These results expand our knowledge on the phylogenetic positioning of piroplasmids from marsupials and add novel epidemiological data related to these hemoparasites in Brazil.
Herein, 34.3% (23/67) of all D. albiventris analyzed were found positive for piroplasmids. This prevalence was substantially higher than those previously reported in synanthropic and wild marsupials sampled in Brazil (0–5.8%) (Wolf et al. 2016; Soares et al. 2017; de Sousa et al. 2018; Colle et al. 2019). This finding may be attributed to different aspects, including but not limited to the marsupial species analyzed, sampling sites, fauna composition, environmental conservation status, presence of ectoparasites, and PCR assay used in the screening. Although previous studies carried out in Brazil have sampled different marsupial species, only in a single case a species not belonging to the genus Didelphis (Monodelphis—Wolf et al. 2016) was positive for Piroplasmida, suggesting a potential specificity of this piroplasm to the genus Didelphis (Serra Freire 1979). However, we should keep in mind that species of Didelphis are more often captured and studied because it is a synanthropic animal and abundant in anthropized areas whereas Monodelphis comprises small marsupials with low densities that inhabit pristine areas (Olifiers et al. 2005; Herrera et al. 2007).
Opposite to studies performed in Brazil that detected piroplasmid DNA in small rodents (Wolf et al. 2016; de Sousa et al. 2018), no piroplasmid DNA was found in samples of the black rat. Similar results were reported regarding the capybara DNA samples analyzed. Thus, the role of these rodents as reservoirs for Piroplasmida in Brazil may be limited. However, since few rodent samples were obtained and screened for piroplasmid DNA, these results should be analyzed with caution. Moreover, once piroplasm DNA was detected in A. dubitatum ticks collected from R. rattus and capybaras, the role of these mammals in the epidemiological cycle of this piroplasm must be further addressed.
Morphological identification of piroplasmids (previously named as Nuttallia brasiliensis/Theileria brasiliensis/Babesia ernestoi, and currently Babesia brasiliensis) in marsupials circulating in Brazil was performed virtually 100 years ago (Regendanz and Kikuth 1928). However, the molecular characterization of this Piroplasmida species has not been performed, precluding a robust comparison with the piroplasmids detected in the current study. More recently, the molecular detection of piroplasmids infecting marsupials has been performed in central-western and Northern Brazil (Wolf et al. 2016; Soares et al. 2017; Colle et al. 2019). However, the abovementioned studies targeted a small fragment of piroplasm-18S rRNA gene, precluding an accurate phylogenetic positioning of the piroplasmid species infecting Didelphis spp.
Unlike previous studies performed in Brazil, the molecular characterization of the piroplasmid species infecting South American marsupials and associated ticks is based on the near-complete 18S rRNA, cox-1, and hsp-70 genes. The phylogenetic inference based on a large fragment of the 18S rRNA gene showed that the Piroplasmida detected in D. albiventris was positioned near to Babesia sensu lato (Western group—cluster III, comprising the species B. duncani, B. lengau, and B. conrade) as a sister taxon but with low support (42%). However, the sequences detected in the present study showed a considerable genetic distance from Western group, the most closely related Piroplasmida species. Phylogenetic reconstructions using two mitochondrial genes (cox-1 and hsp-70) were carried out using the piroplasmid sequences detected in marsupials from the present study. These analyses, associated to pairwise distances observed among cox-1 and hsp-70 sequences, provided supporting evidence for the uniqueness of this piroplasmid and corroborate the phylogenetic position as determined by 18S RNA gene analysis of this protozoan species.
These findings demonstrated that the piroplasmids circulating in marsupials from Australia and Brazil are genetically distant, which is likely expected due to the origin, dispersion, and evolutionary history of these animals (Mitchell et al. 2015). The Australian marsupials are phylogenetically and immunologically more closely related between them than they are to any American marsupial. This finding suggest that they originated from a single ancestral stock that would have reached Australia in the beginning of the Tertiary (64 mya), coming from South America via Antarctica (Clements, 1968; Svartman, 2009). Thus, the piroplasmids lineages may have been isolated with their hosts in Australia, providing a most recent date of origin for the piroplasmids recorded in Australia, i.e., 64 mya.
BLAST analysis of a short 18S rRNA sequences (~ 750 bp) identified in A. dubitatum ticks collected from R. rattus and H. hydrochaeris showed 97.2–99.4% identity with a Piroplasmida previously detected in capybaras from south Brazil. Since little is known about this piroplasmid, further studies using molecular and morphological approaches are necessary to better characterize this protozoan. Considering that A. dubitatum is a three-host tick and R. rattus and H. hydrochaeris were captured in the same locality, it is possible that the specimen of A. dubitatum containing DNA of a capybara-associated piroplasmid might have previously acquired the parasite from capybaras found in that region.
Finally, since A. dubitatum is a tick species commonly identified in marsupials (Massini et al. 2019; Ueno et al. 2020) and capybaras (Queirogas et al. 2012) from Brazil, the role of this arthropod in the capybara and marsupial-associated piroplasmids’ life cycles must be further investigated.
Conclusions
The present study showed the occurrence and characterized a putative novel piroplasmid species infecting D. albiventris and associated ticks in Central-Western Brazil. Accordingly, the near-complete 18S rRNA-based phylogenetic inference suggests that although the analyzed piroplasmid sequence seems to be most closely related with the Western group – cluster III, it cannot be associated with any of the recognized ten clades, supporting that it is a novel species. Phylogenetic inferences based on two additional molecular markers, namely hsp-70 and cox-1, supported the near-complete 18S rRNA gene phylogenetic inference. Furthermore, a partial 18S rRNA gene sequence detected in A. dubitatum ticks collected from R. rattus and H. hydrochaeris suggests that a still non-characterized piroplasmid species occurs in the largest rodent species from South America.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Anholt H, Himsworth C, Rothenburger J, Proctor H, Patrick DM (2014) Ear mange mites (Notoedres muris) in black and Norway rats (Rattus rattus and Rattus norvegicus) from inner-city Vancouver. Canada J Wildl Dis 5:104–108. https://doi.org/10.7589/2013-02-046
Barbosa AD, Austen J, Portas TJ, Amigo JÁ, Ahlstrom LA, Oskam CL, Irwin PJ (2019) Sequence analyses at mitochondrial and nuclear loci reveal a novel Theileria sp and AID in the phylogenetic resolution of piroplasms from Australian marsupials and ticks. PloS one. 12:e0225822. https://doi.org/10.1371/journal.pone.0225822
Black W, Piesman J (1994) Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA 91:10034–10038. https://doi.org/10.1073/pnas.91.21.10034
Birkenheuer AJ, Levy MG, Breitschwerdt EB (2003) Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. J Clin Microbiol 41:4172–4177. https://doi.org/10.1128/JCM.41.9.4172-4177.2003
Brown HM, Berghaus RD, Latimer KS, Britt JO, Rakich PM, Peterson DS (2009) Genetic variability of Cytauxzoon felis from 88 infected domestic cats in Arkansas and Georgia. J Vet Diagn Invest 21:59–63. https://doi.org/10.1177/104063870902100109
Chomel BB, Belotto A, Meslin FX (2007) Wildlife, exotic pets, and emerging zoonoses. Emerg Infect Dis 13:6–11. https://doi.org/10.3201/eid1301.060480
Clements WA (1968) Origin and early evolution of marsupials. Evolution 22:1–18. https://doi.org/10.1111/j.1558-5646.1968.tb03444.x
Corduneanu A, Hrazdilová K, Sándor AD, Matei IA, Ionică AM, Barti L, Ciocănău MA, Măntoiu DȘ, Coroiu I, Hornok S, Fuehrer HP, Leitner N, Bagó Z, Stefke K, Modrý D, Mihalca AD (2017) Babesia vesperuginis, a neglected piroplasmid: new host and geographical records, and phylogenetic relations. Parasit Vectors 10(1):598. https://doi.org/10.1186/s13071-017-2536-3
Criado-Fornelio A, Buling A, Asenzo G, Benitez D, Florin-Christensen M, Gonzalez-Oliva A, Torina A (2009) Development of fluorogenic probe-based PCR assays for the detection and quantification of bovine piroplasmids. Vet Parasitol 6:200–206. https://doi.org/10.1016/j.vetpar.2009.03.040
Colle AC, Mendonça RFBD, Maia MO, Freitas LDC, Witter R, Marcili A, Pacheco RDC (2019) Molecular survey of tick-borne pathogens in small mammals from Brazilian Amazonia. Rev Bras Parasitol Vet 12:592–604. https://doi.org/10.1590/s1984-29612019086
De Sousa KCM, Fernandes MP, Herrera HM, Freschi CR, Machado RZ, André MR (2018) Diversity of piroplasmids among wild and domestic mammals and ectoparasites in Pantanal wetland. Brazil Ticks Tick-Borne Dis 9:245–253. https://doi.org/10.1016/j.ttbdis.2017.09.010
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772–772. https://doi.org/10.1038/nmeth.2109
Ewing B, Green P (1998) Basecalling of automated sequencer traces using phred. II Error Probabilities Genome Res 8:186–194. https://doi.org/10.1101/gr.8.3.186
Gonçalves LR, Teixeira MMG, Rodrigues AC, Mendes NS, Matos CA, Pereira CL, André MR (2018) Molecular detection of Bartonella species and haemoplasmas in wild African buffalo (Syncerus caffer) in Mozambique. Africa Parasitol Open 4:e15. https://doi.org/10.1017/pao.2018.10
Gonçalves LR, Herrera HM, Nantes WAG, Santos FM, de Oliveira Porfírio GE, Barreto WTG, Mariano LC (2020) Genetic diversity and lack of molecular evidence for hemoplasma cross-species transmission between wild and synanthropic mammals from Central-Western Brazil. Acta Trop 203:105303. https://doi.org/10.1016/j.actatropica.2019.105303
Greay TL, Zahedi A, Krige A, Owens JM, Rees RL, Ryan UM, Oskam CL, Irwin PJ (2018) Endemic, exotic and novel apicomplexan parasites detected during a national study of ticks from companion animals in Australia. Parasite Vector 11:197. https://doi.org/10.1186/s13071-018-2775-y
Herrera HM, Rademaker V, Abreu UG, D’Andrea PS, Jansen AM (2007) Variables that modulate the spatial distribution of Trypanosoma cruzi and Trypanosoma evansi in the Brazilian Pantanal. Acta Trop 102:55–62. https://doi.org/10.1016/j.actatropica.2007.03.001
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. https://doi.org/10.1093/molbev/msj030
Jalovecka M, Hajdusek O, Sojka D, Kopacek P, Malandrin L (2018) The complexity of piroplasms life cycles. Front Cell Infect Microbiol 248:8. https://doi.org/10.3389/fcimb.2018.00248
Jalovecka M, Sojka D, Ascencio M, Schnittger L (2019) Babesia life cycle–when phylogeny meets biology. Trends Parasitol 13:356–368. https://doi.org/10.1016/j.pt.2019.01.007
Jefferies R, Ryan UM, Irwin PJ (2007) PCR–RFLP for the detection and differentiation of the canine piroplasm species and its use with filter paper-based technologies. Vet Parasitol 144:20–27. https://doi.org/10.1016/j.vetpar.2006.09.022
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–11. https://doi.org/10.1093/bib/bbx108
Lau BT, Malkus P, Paulsson J (2013) New quantitative methods for measuring plasmid loss rates reveal unexpected stability. Plasmid 9:353–361. https://doi.org/10.1016/j.plasmid.2013.07.007
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. https://doi.org/10.1093/bioinformatics/btp187
Linardi PM, Beaucournu JC, De Avelar DM, Belaz S (2014) Notes on the genus Tunga (Siphonaptera: Tungidae) II–neosomes, morphology, classification, and other taxonomic notes. Parasite 21:68. https://doi.org/10.1051/parasite/2014067.DOI:10.1051/parasite/2014067
Mackenstedt U, Jenkins D, Romig T (2015) The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int J Parasitol Parasites Wildl 8:71–79. https://doi.org/10.1016/j.ijppaw.2015.01.006
Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R (2019) The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. https://doi.org/10.1093/nar/gkz268
Massini PF, Drozino RN, Otomura FH, Mongruel ACB, Valente JDM, Toledo MJO, Martins TF, Vidotto O, Vieira TSWJV, Vieira RFDC (2019) Detection of Hemotropic Mycoplasma sp. in white-eared opossums (Didelphis albiventris) from Southern Brazil. Rev Bras Parasitol Vet 28:797–801. https://doi.org/10.1590/s1984-29612019058
Matos CA, Gonçalves LR, Alvarez DO, Freschi CR, Silva JBD, Val-Moraes SP, Mendes NS, André MR, Machado RZ (2017) Longitudinal evaluation of humoral immune response and merozoite surface antigen diversity in calves naturally infected with Babesia bovis, in São Paulo. Brazil Rev Bras Parasitol Vet 26:479–449. https://doi.org/10.1590/s1984-29612017069
Martins TF, Onofrio VC, Barros-Battesti DM, Labruna MB (2010) Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks and Tick-Borne Dis 24:75–99. https://doi.org/10.1016/j.ttbdis.2010.03.002
Miller MA, Pfeiffer W, Schwartz T (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments workshop (GCE). 11: EBO-S21501. Ieee. 10.4137%2FEBO.S21501
Mitchell P (2015) Did disease constrain the spread of domestic dogs (Canis familiaris) into Sub-Saharan Africa? Azania 50:92–135. https://doi.org/10.1080/0067270X.2015.1006441
Nantes WAG, Barreto WTG, Santos FM, de Macedo GC, Rucco AC, Assis WO Porfírio GEO, de Andrade GB, Jansen AM, Herrera HM (2019) The Influence of parasitism by Trypanosoma cruzi in the hematological parameters of the white ear opossum (Didelphis albiventris) from Campo Grande, Mato Grosso Do Sul, Brazil. Int J Parsitol Parasites Wildl 9:16–20. https://doi.org/10.1016/j.ijppaw.2019.03.015
Olifiers N, Gentile R, Fiszon JT (2005) Relation between small-mammal species composition and anthropic variables in the Brazilian Atlantic Forest. Braz J Biol 65:495–501. https://doi.org/10.1590/S1519-69842005000300015
Onofrio VC, Venzal JM, Pinter A, Szabó MPJ (2005) Família Ixodidae: características gerais, comentários e chave para gêneros. In: BarrosBattesti DM, Árzua M, Bechara GH (ed), Carrapatos de Importância MédicoVeterinária da Região Neotropical: Um guia ilustrado para identificação de espécies, (1ª ed.). Integrated Consortium on Ticks and Tick-borne Diseases-ICTTD, São Paulo, p 29-39
Oosthuizen MC, Allsopp BA, Troskie M, Collins NE, Penzhorn BL (2009) Identification of novel Babesia and Theileria species in South African giraffe (Giraffa camelopardalis, Linnaeus, 1758) and roan antelope (Hippotragus equinus, Desmarest 1804). Vet Parasitol 163:39–46. https://doi.org/10.1016/j.vetpar.2009.03.045
Pereira JS, Martins TF, Muñoz-Leal S, Lopes MG, Labruna MB, Paiva KAR, Ahid SM (2017) Infestation by ticks Argasidae and Ixodidae on small wild mammals at the Experimental Station Rafael Fernandes, Mossoró/RN. Brazil Braz Vet Res 7:741–748. https://doi.org/10.1590/s0100-736x2017000700015
Price SJ, Leung WTM, Owen CJ, Puschendorf R, Sergeant C, Cunningham AA, Balloux F, Garner TWJ, Nichols RA (2019) Effects of historic and projected climate change on the range and impacts of an emerging wildlife disease. Glob Chang Biol 12:2648–2660. https://doi.org/10.1111/gcb.14651
Queirogas VL, Claro KD, Nascimento ART, Szabó MPJ (2012) Capybaras and ticks in the urban areas of Uberlândia, Minas Gerais, Brazil: ecological aspects for the epidemiology of tick-borne diseases. Exp Appl Acarol 57:75–82
Qurollo BA, Archer NR, Schreeg ME, Marr HS, Birkenheuer AJ, Haney KN, Thomas BS, Breitschwerdt EB (2017) Improved molecular detection of Babesia infections in animals using a novel quantitative real-time PCR diagnostic assay targeting mitochondrial DNA. Parasit Vectors 10:1–13. https://doi.org/10.1186/s13071-017-2064-1
Regendanz P, Kikuth W (1928) Sur un parasite du sang des “Quica” (Metachirus quica) Nuttallia brasiliensis n. sp., et influence de la rate sur les infections latentes du sang. C. R. H Seanc Mem Soc De Biol 98:1567–1567
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Ronquist F, Teslenko M, van der Mark P, Ayres D L, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Serra Freire da, NM (1979) Babesia ernestoi n. sp., in Didelphis marsupialis L., 1758 and D. albiventris Lund, 1841, in Brazil. Zentralbl Veterinärmed b 26:614–629. https://doi.org/10.1111/j.1439-0450.1979.tb00855.x
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA (2012) Babesia: a world emerging. Infect Genet Evol 21:1788–1809. https://doi.org/10.1016/j.meegid.2012.07.004
Soares JF, Girotto A, Brandão PE, Da Silva AS, França RT, Lopes STA, Labruna MB (2011) Detection and molecular characterization of a canine piroplasm from Brazil. Vet Parasitol 180:203–208. https://doi.org/10.1016/j.vetpar.2011.03.024
Soares HS, Marcili A, Barbieri AR, Minervino AH, Moreira TR, Gennari SM, Labruna MB (2017) Novel piroplasmid and Hepatozoon organisms infecting the wildlife of two regions of the Brazilian Amazon. Int J Parasitol Parasit Wildl 6:115–121. https://doi.org/10.1016/j.ijppaw.2017.05.002
Svartman M (2009) American marsupials chromosomes: why study them? Genet Mol Biol 32:675–687. https://doi.org/10.1590/S1415-47572009005000084
Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. https://doi.org/10.1093/nar/gkw256
Ueno TEH, Cutolo AA, Martins TF, Moraes-Filho J, Azevedo SSD, Labruna MB (2020) Rickettsial infection in equids, opossums and ticks in the municipality of Monte Mor, state of São Paulo, Brazil. Rev Bras Parasitol Vet 29:e015420. https://doi.org/10.1590/s1984-29612020073
Wolf RF, Aragona M, Muñoz-Leal S, Pinto LB, Melo ALT, Braga IA, Costa JS, Martins TF, Marcili A, Pacheco RC, Labruna MB, Aguiar DM (2017) Novel Babesia and Hepatozoon agents infecting non-volant small mammals in the Brazilian Pantanal, with the first record of the ticks Ornithodoros guaporensis in Brazil. Ticks Tick-Borne Dis 7:449–456. https://doi.org/10.1016/j.ttbdis.2016.01.005
Zamoto A, Tsuji M, Wei Q, Cho SH, Shin EH, Kim TS, Leonova GN, Hagiwara K, Asakawa M, Kariwa H, Takashima I, Ishihara C (2004) Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic diversity in the beta-tubulin gene sequences. J Vet Med Sci 66(7):785–792. https://doi.org/10.1292/jvms.66.785
Acknowledgements
This work was financially supported by FAPESP (Foundation for Research Support of the State of São Paulo—process numbers 2018/02753-0 and 2020/12037-0), FUNDECT (Foundation for Support to the Development of Education, Science and Technology of the State of Mato Grosso do Sul, Case 59/300.187/2016), and CNPq (National Council for Scientific and Technological Development) for the Productivity Grant to M. R. A. (CNPq Process #302420/2017-7) and H. M. H. (CNPq Process #308768/2017-5). T. B. B. and M. A. D. received scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). The authors are especially thankful to the InsanaHuna Research Group (www.insanahuna.com) for the fieldwork support and to the reviewers whose suggestions significantly improved the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Daniel K Howe
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
S1 Table
Occurrence of Piroplasmida DNA in the marsupials, rats, capybaras and their related ticks through different target genes (XLSX 11 KB)
S2 Table
Pairwise genetic distances matrix among Piroplasmida clusters and those detected in the present study at the large 18S rRNA fragment (~1.3 kb). Pairwise genetic distances (%) were obtained using the p-distance method in MEGA X (XLSX 17 KB)
S3 Table
Pairwise genetic distances matrix among Piroplasmida clusters and those detected in the present study at the hsp-70 fragment (~700 bp). Pairwise genetic distances (%) were obtained using the p-distance method in MEGA X. (XLSX 14 KB)
S4 Table
Pairwise genetic distances matrix among Piroplasmida clusters and those detected in the present study at the cox-1 fragment (~800 bp). Pairwise genetic distances (%) were obtained using the p-distance method in MEGA X. (XLSX 14 KB)
S5 Table
Pairwise genetic distances matrix among Piroplasmida clusters and those detected in the present study at the small 18S rRNA fragment (~750 bp). Pairwise genetic distances (%) were obtained using the p-distance method in MEGA X (XLSX 19 KB)
Rights and permissions
About this article
Cite this article
Gonçalves, L.R., Paludo, G., Bisol, T.B. et al. Molecular detection of piroplasmids in synanthropic rodents, marsupials, and associated ticks from Brazil, with phylogenetic inference of a putative novel Babesia sp. from white-eared opossum (Didelphis albiventris). Parasitol Res 120, 3537–3546 (2021). https://doi.org/10.1007/s00436-021-07284-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07284-8