Abstract
Clinostomum Leidy, 1856 (Trematoda: Clinostomidae) is a cosmopolitan, zoonotic genus of fluke that has been poorly studied in an Australian setting. Following previous reports of reservoir fish in Australian fish ponds being heavily infected with Clinostomum metacercaria, the current study was conducted to determine the specific identity of Clinostomum sp. in inland Australia, by examining and characterizing parasites collected from a potential definitive host, cormorants. A total of 33 parasite specimens belonging to the genus Clinostomum were collected from two cormorants (little black cormorants, Phalacrocorax sulcirostris) that were collected from the Narrandera Fisheries Research Centre, New South Wales, at the same locality where metacercaria of Clinostomum sp. have been reported in fish. All specimens in our study were immature adults. Clinostomum specimens with similar morphology have been identified as C. complanatum in the past, based on their morphological characteristics. However, phylogenetic analyses based on the ITS sequence data in the present study suggest they are the same as the Clinostomum sp. previously reported from carp gudgeons (Hypseleotris spp.) from the same farm, and distinct from C. complanatum. The ITS sequences obtained from the specimens in the present study were most similar to those belonging to C. phalacrocoracis (never reported in Australia). Our specimens formed a distinct clade on the phylogenetic tree and their specific identity awaits until fully mature specimens are described in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Clinostomum Leidy, 1856, belonging to the family Clinostomidae Lühe, 1901, is a cosmopolitan parasite with reports occurring in all regions of the world (Locke et al. 2015). Over 50 species make up the genus, of which four have been reported in Australia (Table 1). Of these four species, C. complanatum is a cosmopolitan parasite, while the other three are endemic to Australia. All previous reports of Clinostomum spp. in Australia are either from Queensland or from Tweed Heads, New South Wales, near the border with Queensland (Table 1). Recently, there have been reports of unidentified metacercaria of Clinostomum in the Murrumbidgee Catchment Area in inland Australia (Rochat et al. 2020; Shamsi et al. 2021).
Globally, through the use of molecular studies, a clear separation of species has been recognized between continents and various geographical regions (Caffara et al. 2011, 2017; Pérez-Ponce De León et al. 2016). Due to the high degree of morphological intraspecific variability and interspecific similarities, and the more recent addition of molecular analysis in species identification, Clinostomum has undergone several taxonomic revisions (Ukoli 1966; Yamaguti 1971; Gustinelli et al. 2010; Locke et al. 2011; Sereno-Uribe et al. 2013). To date, few morphological traits have been identified to reliably differentiate between species for use in morphological speciation; traits that have been identified include the anterior extent of vitellarium, the presence of lateral evaginations in the uterine sac, gonad location in the body, testicular shape, genital pore location relative to gonads, and cirrus pouch location relative to other organs (Ukoli 1966). Most morphological descriptions within the literature are based on adult samples that possess these mature reproductive systems (Ukoli 1966; Locke et al. 2011; Sereno-Uribe et al. 2013). However, Caffara et al. (2020) argued that metacercaria of Clinostomum could be used for species identification, especially when combined with molecular data. However, morphological differences between the different stages hinder the ability to link them together (Ukoli 1966; Locke et al. 2011; Sereno-Uribe et al. 2013). Therefore, it is preferred that morphological speciation is carried out with molecular support to ensure increased accuracy in identifying Clinostomum species. Of the recognized species, 18 have been genetically sequenced: C. complanatum, C. cutaneum, C. heluans, C. phalacrocoracis, C. marginatum, C. attenuatum, C. detruncatum, C. philippinensis, C. poteae, C. tataxumui, C. tilapiae, C. ukoli, C. caffarae, C. arquus, C. cichlidorum, C. brieni, C. sinensis, and C. album (Gustinelli et al. 2010; Caffara et al. 2011, 2014a, 2014b, 2017, 2020; Locke et al. 2015, 2019; Acosta et al. 2016; Rosser et al. 2018; Briosio-Aguilar et al. 2019; Sokolov and Gordeev 2020; Won et al. 2020). In Australia, unidentified Clinostomum metacercaria have been collected from a firetail gudgeon (Hypseleotris galii) in Brisbane (Olson et al. 2003; Nolan and Cribb 2005) and from Hypseleotris spp. in New South Wales (Rochat et al. 2020; Shamsi et al. 2021).
Clinostomum has low host specificity and an indirect life cycle that can infect a diversity of hosts, damaging and impairing them and, in severe cases, resulting in death (Shamsi et al. 2013; Sutili et al. 2014; Aghlmandi et al. 2018; Montes et al. 2020). The life cycle includes two intermediate hosts, first an aquatic gastropod and then either a freshwater fish or amphibian, and a definitive host, typically a piscivorous bird or potentially a larger freshwater fish (Matthews and Cribb 1998; Kanev et al. 2002; Sutili et al. 2014; Locke et al. 2015; Wang et al. 2017). Several gastropod species have been reported globally as first intermediate host species; however, no such reports have occurred in Australia. Globally, secondary intermediate hosts have been shown to include both wild and farmed freshwater fish and amphibians (Cameron 1945; Anonymous 2001a; Wang et al. 2017). Within Australia, only a few species of fish have been reported with Clinostomum metacercaria infections (Anonymous 2001a; Nolan and Cribb 2004; Rochat et al. 2020; Shamsi et al. 2021). Piscivorous birds are the most common definitive hosts for Clinostomum species. In Australia, this group of birds includes Ardeiform birds, cormorants, and pelicans (Matthews and Cribb 1998), all from Queensland. The lack of reports on Australian host species, especially outside of Queensland, could be simply due to no expertise in other states/territories and/or no investigations being conducted as it has been the case for some other parasite studies in the country (Shamsi 2021). There is, therefore, the potential for Clinostomum to be present within gastropods, fish, amphibian, and piscivorous bird populations within Australia that are yet to be reported.
Clinostomum spp. are also important due to their zoonotic significance. Accidental human infection can occur with the ingestion of infected raw fish (Tiewchaloern et al. 1999; Park et al. 2009; Hara et al. 2014; Sutili et al. 2014) contaminated with metacercariae, resulting in metacercarial excystment in the stomach and migration to the oral cavity or oesophagus where they cause laryngitis and/or pharyngitis, resulting in throat discomfort. Cases of Clinostomum zoonosis have only been reported in countries where eating raw fish is common and no such cases, as of yet, have been reported in Australia. It is not clear whether this is due to a lack of knowledge about the parasite, or poor documentation and recording as has been the case for other fish-borne diseases in Australia (Shamsi and Sheorey 2018), or because no zoonotic cases have occurred within the country. With the growing multicultural diversity and increasing consumption of fish products within Australia (Mosby 2018), it is quite possible that such cases may occur in the future.
Although this cosmopolitan parasite has been studied in some detail in other countries, there is limited research on it within an Australian setting (Matthews and Cribb 1998). What little research has been done focuses on adult forms of Clinostomum within definitive host species in wild populations. Therefore, there is a lack of knowledge on the range of potential intermediate hosts of Clinostomum and its potential occurrence in aquaculture systems within Australia. In other countries, it is known that Clinostomum infections within fisheries can cause significant economic losses in fish farms (Soler-Jiménez et al. 2017). Fish infected with Clinostomum metacercariae demonstrate changes in feeding behaviour and habits (Aghlmandi et al. 2018), resulting in poor growth (Soler-Jiménez et al. 2017). The encystment of the metacercaria results in an unappealing visual appearance of the host as they develop yellow pus-like cysts (Cameron 1945). Consequently, these fish are disposed of and not sent to market, further increasing losses due to infection. Parasite migration and attachment within the host have been shown to cause considerable damage to viscera and musculature, adversely affecting vital organ function and in severe cases, with a high enough burden, this can result in mortality and further loss of production to the fishery (Shareef and Abidi 2012; Aghlmandi et al. 2018).
In Australia, investigations of native freshwater fish within the Murray Darling Basin showed an infection of Clinostomum sp. (metacercarial stage) within a population of gudgeons coexisting as undesirable fish in Murray cod and silver perch farms (Rochat et al. 2020; Shamsi et al. 2021). Therefore, the present study was conducted to determine the specific identity of Clinostomum species by attempting to collect adult Clinostomum from piscivorous birds in the region and characterize them.
Materials and methods
Parasite collection
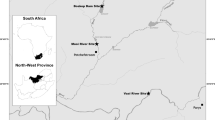
Parasites were collected from little black cormorants, Phalacrocorax sulcirostris (n = 2) from the Narrandera Fisheries Research Centre, New South Wales, at the same locality where Clinostomum sp. have been reported in fish (Rochat et al. 2020; Shamsi et al. 2021). The permit to collect the birds was held by the fishery. The birds were stored frozen before being thawed and examined for parasites.
Parasite identification
Morphological identification
Specimens were rehydrated in distilled water, stained with ACETO-ORCEIN, then dehydrated through a series of ethanol concentrations, cleared with xylene and mounted on slides in Canada Balsam. The mounted specimens were left for a week to allow the Canada Balsam to set before being examined under a light microscope.
Morphological characterization was performed under a light microscope for each of the mounted trematodes using an eyepiece micrometre. Measurements were taken following Matthews and Cribb (1998). The ovary and uterus were examined for the presence of eggs to determine the maturity of the specimen. Trematode samples were morphologically identified by comparison with morphological descriptions from previous research and key interspecies morphological differences (Ukoli 1966; Kanev et al. 2002). All specimens were deposited in the South Australian Museum (Accession number: AHC 36874 and 3687436875).
Genetic characterization
Representative parasite specimens from each cormorant were selected for molecular analysis. Two small wedges of tissue were cut from their lateral border, caudal to the reproductive organs, ensuring no vital morphology for species identification was damaged. These wedges of tissue were placed individually in autoclaved Eppendorf tubes for DNA extraction. The tubes were stored in a freezer until DNA extraction. Extraction was carried out using the Qiagen DNeasy kit (QIAGEN, Germany), according to the manufacturer’s instructions and modified version in Shamsi et al. (2019). The internal transcribed spacers (ITS) region of nuclear ribosomal DNA was picked for molecular species identification (Mesquita et al. 2020). The entire ITS region was amplified by PCR using the D1 (F) 5′-AGGAATTCCTGGTAAGTGCAAG-3′ (forward) and D2 (R) 5′-CGTTACTGAGGGAATCCTGG-3′ (reverse) primers as published in Hillis and Dixon (1991). Two microliters of extracted DNA was directly added to a PCR mixture containing 13.25 µl of nuclease free water, 5 µl of 5 × buffer (green), 2.5 µl of 25 mM MgCl2, 1 µl of 10 µM dNTP, 0.5 µl of 10 µM each primer, and 0.25 µl of Taq polymerase (5 unit/µl Promega, USA) for a total volume of 25 µl (n = 1). PCR were carried on a C1000 touch thermocycler (Biorad). Cycling conditions were: 95 °C for 2 min (initial denaturing), 40 cycles of 95 °C for 30 s (denaturing), 58 °C for 30 s (annealing), and 72 °C for 1 min (extension) followed by final extension at 72 °C for 10 min. The PCR products from this were run through 1.5% TAE agarose gel electrophoresis with SYBR safe dye (Invitrogen, Australia). Samples that were found to be positive of sufficient strength were sent to Australian Genome Research Facility for Sanger sequencing. Sequence quality was checked using SeqMan v8.0 (DNASTAR).
ITS sequences of other well identified Clinostomum species from published works were obtained from GenBank for phylogenetic analyses (Table 2). Sequences were aligned with Clustal W programme built in Bioedit (Hall 1999), manually checked and trimmed according to the shortest sequence. Alignment gaps were excluded for analyses. Pairwise genetic distances, shown as percentage of difference, were calculated using MEGA X (Kumar et al. 2018). Euclinostomum heterostomum (KP721430), which is also a member of the family Clinostomidae, was used as the outgroup. The phylogeny of selected sequences was calculated using MrBayes 3.2 using the GTR + G model as suggested by jModelTest 2 (Darriba et al. 2012). The Markov chain Monte Carlo algorithm was run for 2,000,000 generations until the average standard deviation was less than 0.005. The tree was visualized using Figtree v 1.4.3 (Rambaut 2014).
Results
A total of 33 Clinostomum were found in the stomachs of examined birds. One bird was infected with 26 Clinostomum sp. and the other bird was infected with 7. All parasites were identified as immature Clinostomum based on body size and shape, presence of an oral collar, location of the anterior testis in posterior end of middle third of body and posterior testis in anterior end of posterior third of body, the size and shape of the reproductive organs, position of the genital pore right of the midline level with the middle of the anterior testis, and the location of the cirrus pouch. A description of the immature adult specimens (n = 18) is provided (Fig. 1):
Body elongated, rounded ends, slightly convex dorsally, width generally uniform throughout, although widest in region of reproductive organs in some specimens. Oral collar present. Oral sucker medium sized. Ventral surface covered with large “tubercules” anterior to ventral sucker; fine “tubercules” around oral collar. Pharynx absent. Oesophagus present with bifurcation anterior to ventral sucker. Oesophageal bulb well-developed. Intestinal ceaca thin, run posteriorly from oesophageal bulb to posterior end of body. Caeca diverticula small, commence posterior to ventral sucker. Excretory system difficult to ascertain. Ventral sucker large, muscular, at least twice the size of oral sucker. Anterior testis in posterior region of middle third of body, offset to left of midline, roughly triangular in shape with concave posterior border and anterior apex. Posterior testis in anterior region of posterior third of body, situated on midline, relatively similar in size to anterior testis, triangular with posterior apex and concave anterior border. In some specimens, clear space surrounding testes. Testes location not obvious. Cirrus sac between anterior testis and intestinal caeca on right side. Genital pore right of midline, level with middle of anterior testis. Ovary small, circular, posterior-medial to cirrus sac and slightly overlies posterior border. No vitellarium present. Uterus sac thin, tubular structure, running midline from divergence anterior to anterior testis to just posterior to ventral sucker. No eggs present in uterus. Eighteen specimens were randomly selected for measurements of the taxonomically important features. Morphometrics of specimens examined in present study are provided in Table 3.
Molecular Analyses
A sequence of the ITS region was obtained for one immature Clinostomum from each bird (GenBank accession numbers: MT446440 and MT446441). Sequences of 1338 bp were aligned against all available Clinostomum species in GenBank (Table 2). The sequences of immature Clinostomum sp. from cormorants in the present study were identical with sequences of the metacercaria of Clinostomum sp. from carp gudgeon (GenBank accession number: MT446431-39) and grouped together in the Bayesian inferred phylogenetic tree with 100% branch support forming a clade distinct from any other Clinostomum species (Fig. 2).
Discussion
Sequences of the ITS region were identical between immature Clinostomum specimens found in the stomach of cormorants in the present study and the Clinostomum metacercaria previously reported and characterized in carp gudgeons from the same geographical location (Rochat et al. 2020; Shamsi et al. 2021), suggesting they belong to the same species. However, since the specimens of Clinostomum were immature in our study, the specific description of the species and designation of a name remain on hold until mature adults are found and described in the future. Adult Clinostomum are found in the anterior part of the digestive system, including the mouth area, which has not been examined in the present study. The metacercaria collected from the fish all have very yellow bodies (i.e., yellow grub), but the ones found in the birds, including those in the present study, generally are either white or have reduced amounts of yellow, which suggests what we found were developing in the examined cormorants. Matthews and Cribb (1998) reported four species of clinostomids from a variety of bird hosts and suggested there appears to be a lack of recognizable host-specificity within the community of fish-eating water birds, with the exception for C. australiense, since mature worms have been recovered only from Pelecanus conspicillatus, whereas immature worms have been recovered from a number of species of birds.
Morphologically, the specimens collected from cormorants are closest to C. hornum, based on the features highlighted by Caffara et al. (2020) to differentiate between Clinostomum species metacercaria. Although the description of C. hornum is of an adult (Matthews and Cribb 1998) and the specimens collected in this study were immature adults, the reproductive structures were at approximately the same stage of development as the metacercaria reported in Shamsi et al. (2021). Specifically, the overall body measurements were very similar with overlap in body size, sucker, and reproductive structure dimensions (Table 3). The anterior testis is triangular and offset to the left, which was a characteristic distinguishing C. hornum from the other Australian Clinostomum species (Matthews and Cribb 1998).
None of the Australian Clinostomum species that have been morphologically identified from the adult stage have been sequenced. Thus, there was no corresponding sequences that matched the Clinostomum specimens collected from cormorants in this study. The 18S and 28S sequences of metacercaria from carp gudgeons in NSW were found to be identical with the 18S and 28S sequences of an unidentified Clinostomum sp. metacercaria from H. galii from the Brisbane area (Shamsi et al. 2021). There was no available ITS sequence for comparison against the cormorant specimens, but given the 100% match with the NSW metacercaria in this study, it is most likely that the Brisbane metacercaria are the same species. However, this interpretation should be taken with caution, as the ITS region has been shown to have high intraspecies variation (Locke et al. 2015) and future molecular analysis should look at using other regions such as the COX1 to improve accuracy. However, without a corresponding sequence from an identified mature adult, and to prevent confusing the taxonomy of Clinostomum even further, we cannot confirm the identity of the Clinostomum collected from the cormorant in NSW as C. hornum. If the specimens are confirmed as C. hornum with further research, this will extend the distribution of that species from Townsville in north Queensland (Nicoll 1914; Matthews and Cribb 1998), through to the Murrumbidgee area of southern New South Wales (Rochat et al. 2020; Shamsi et al. 2021; this study).
Of the sequences that are available for comparison, the cormorant specimens were found to be closest to C. phalacrocoracis. Morphological comparison of the Clinostomum sp. in the present study against C. phalacrocoracis (Table 4) showed very little overlap in the measurements or structure of the reproductive organs (Caffara et al. 2014a, b). Clinostomum phalacrocoracis has been reported in Israel (Caffara et al. 2014b), Africa (Peirce and Din 1970; Zhokhov and Morozova 2020), and South America (Acosta et al. 2016), but never in Australia. The genetic divergence between the immature Clinostomum found in the present study and C. phalacrocoracis (0.019) is greater than the interspecies divergence between C. phalacrocoracis and other species, for example C. cutaneum (0.013), calculated in our study (Table 5), so it is unlikely that samples in the current study are indeed C. phalacrocoracis.
Indeed, Caffara et al. (2020) argued that comparison of Clinostomum species between geographical regions was unnecessary as, apart from C. complanatum, there is little evidence of transcontinental distributions of Clinostomum. The phylogenetic results obtained in this study appear to support this, with all specimens grouping according to the geographical regions of collection. For example, specimens collected from the Americas (C. heluans, C. poteae, C. marginatum, C. album, and C. tataxumi), specimens collected from Africa and the Middle East (C. phalacrocoracis, C. cutaneum, and C. complanatum), and the specimens collected from Australia (Clinostomum sp. collected from gudgeons and cormorants) all grouped into separate clades. Caffara et al. (2019) found a similar pattern for the species collected from the Americas, although the pattern for species collected from Africa and Asia was not as clear-cut. However, the lack of sequences for most Clinostomum species, and especially for C. complanatum from different geographical regions, means that these results should be treated with caution.
The position of C. brieni in the ITS phylogenetic tree of this study differed from the results found by Caffara et al. (2019), where C. brieni was placed within the African/Asian group of species. Caffara et al. (2019) used their results to justify the placement of C. brieni within Clinostomum, rather than in Clinostomoides, in which it was originally described. Further research needs to be undertaken on this species, across its range and using a variety of sequences, to finalize its placement within the genera.
Although this study was unable to confirm the identity of the species of Clinostomum infecting cormorants and gudgeons from southern Australia, it has shown, through molecular sequences, that the species is distinct from other species that have been sequenced. However, it does highlight the need for further research on parasites of Australian aquatic birds.
Bayesian inferred phylogenetic tree of ITS region for sequences obtained in this study, and sequences of Clinostomum spp. and Clinostomoides brieni obtained from GenBank. The latter species has been considered as Clinostomum brieni by Caffara et al. 2019. Euclinostomum heterostomum was used as an outgroup. Posterior probabilities (>90%) were listed above the branches. Asterisks denote sequences from this study
References
Acosta AA, Caffara M, Fioravanti ML, Utsunomia R, Zago AC, Franceschini L, da Silva RJ (2016) Morphological and molecular characterization of Clinostomumdetruncatum (Trematoda Clinostomidae) metacercariae infecting Synbranchusmarmoratus. J Parasitol 102(1):151–156
Aghlmandi F, Habibi F, Afraii MA, Abdoli A, Shamsi S (2018) Infection with metacercaria of Clinostomumcomplanatum (Trematoda Clinostomidae) in freshwater fishes from Southern Caspian Sea Basin. Revue De Médecine Vétérinaire 7:147–151
Anonymous (2001a) Possible eye damage associated with fluke infections in murray cod (Maccullochella peeli) Queensland Government Department of Primary Industries Fish Disease 2:4–5
Anonymous (2001b) Possible eye damage associated with fluke infections in Murray cod (Maccullochella peeli). Queensland Government Department of Primary Industries Fish Disease Report 2 (July)
Briosio-Aguilar R, García-Varela M, Hernández-Mena D, Rubio-Godoy M, De León GP-P (2019) Morphological and molecular characterization of an enigmatic clinostomid trematode (Digenea Clinostomidae) parasitic as metacercariae in the body cavity of freshwater fishes (Cichlidae) across Middle America. J Helminthol 93(4):461–474
Caffara M, Locke SA, Gustinelli A, Marcogliese DJ, Fioravanti ML (2011) Morphological and molecular differentiation of Clinostomum complanatum and Clinostomum marginatum (Digenea: Clinostomidae) metacercariae and adults. The Journal of Parasitology 97(5):884–891
Caffara M, Bruni G, Paoletti C, Gustinelli A, Fioravanti M (2014a) Metacercariae of Clinostomumcomplanatum (Trematod Digenea) in European newts Trituruscarnifex and Lissotriton vulgaris (Caudata Salamandridae). J Helminthol 88(3):278–285
Caffara M, Davidovich N, Falk R, Smirnov M, Ofek T, Cummings D, Gustinelli A, Fioravanti ML (2014b) Redescription of Clinostomumphalacrocoracis metacercariae (Digenea Clinostomidae) in cichlids from Lake Kinneret. Israel Parasite. https://doi.org/10.1051/parasite/2014034
Caffara M, Locke SA, Cristanini C, Davidovich N, Markovich MP, Fioravanti ML (2016) A combined morphometric and molecular approach to identifying metacercariae of Euclinostomumheterostomum (Digenea Clinostomidae). J Parasitol 102(2):239–248
Caffara M, Locke SA, Echi PC, Halajian A, Benini D, Luus-Powell WJ, Tavakol S, Fioravanti ML (2017) A morphological and molecular study of Clinostomid metacercariae from African fish with a redescription of Clinostomumtilapiae. Parasitology 144(11):1519–1529
Caffara M, Locke SA, Halajian A, Luus-Powell WJ, Benini D, Tedesco P, Kasembele GK, Fioravanti ML (2019) Molecular data show Clinostomoides Dollfus, 1950 is a junior synonym of Clinostomum Leidy, 1856, with redescription of metacercariae of Clinostomum brieni n. comb. Parasitology 146(6):805–813
Caffara M, Locke SA, Echi PC, Halajian A, Luus-Powell WJ, Benini D, Tedesco P, Fioravanti ML (2020) A new species of Clinostomum Leidy 1856 based on molecular and morphological analysis of metacercariae from African siluriform fishes. Parasitol Res 119(3):885–892
Cameron T (1945) Fish-carried parasites in Canada (1) Parasites carried by fresh-water fish. Can J Comp Med Vet Sci 9(9):245
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2 more models, new heuristics and parallel computing. Nat Methods 9(8):772–772
Dzikowski R, Levy MG, Poore MF, Flowers JR, Paperna I (2004) Clinostomumcomplanatum and Clinostomummarginatum (Rudolphi, 1819) (Digenea Clinostomidae) are separate species based on differences in ribosomal DNA. J Parasitol 90(2):413–414
Gustinelli A, Caffara M, Florio D, Otachi EO, Wathuta EM, Fioravanti ML (2010) First description of the adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey herons Ardea cinerea L. and a redescription of the metacercaria from the Nile tilapia Oreochromis niloticus niloticus (L.) in Kenya. Syst Parasitol 76(1):39–51 https://doi.org/10.1007/s11230-010-9231-5
Hall TA (1999) BioEdit a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hara H, Miyauchi Y, Tahara S, Yamashita H (2014) Human laryngitis caused by Clinostomumcomplanatum. Nagoya J Med Sci 76(1–2):181
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q R Biol 66(4):411–453
Ingram BA, Gavine FM, Lawson P (2005) Fish health management guidelines for farmed Murray cod. Department of Primary Industries
Kanev I, Radev V, Fried B (2002) Family Clinostomidae Lühe, 1901. In: Gibson DI, Jones A, Bray RA (eds) Keys to the Trematoda, vol 1 CAB International and the Natural History Museum, Wallingford, pp 113–120
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Locke SA, McLaughlin JD, Lapierre AR, Johnson PT, Marcogliese DJ (2011) Linking larvae and adults of Apharyngostrigeacornu, Hysteromorphatriloba, and Alariamustelae (Diplostomoidea Digenea) using molecular data. J Parasitol 97(5):846–851
Locke SA, Caffara M, Marcogliese DJ, Fioravanti ML (2015) A large-scale molecular survey of Clinostomum (Digenea, Clinostomidae). Zoolog Scr 44(2):203–217
Locke SA, Caffara M, Barčák D, Sonko P, Tedesco P, Fioravanti ML, Li W (2019) A new species of Clinostomum Leidy, 1856 in East Asia based on genomic and morphological data. Parasitol Res 118(12):3253–3265
Matthews D, Cribb TH (1998) Digenetic trematodes of the genus Clinostomum Leidy, 1856 (Digenea: Clinostomidae) from birds of Queensland, Australia, including C. wilsoni n. sp. from Egretta intermedia. Systematic Parasitology 39(3):199–208
Mawson PM, Angel M, Edmonds SJ (1986) A checklist of helminths from Australian birds. Records of the South Australian Museum 19(15):219–325
Mesquita SG, Rodrigues-Luiz GF, Reis-Cunha JL, Cardoso MS, De Mendonça CLF, Bueno LL, Fujiwara RT, Pinto HA, Caldeira RL, Bartholomeu DC (2020) A multiplex PCR protocol for rapid differential identification of four families of trematodes with medical and veterinary importance transmitted by Biomphalaria Preston, 1910 snails. Acta Tropica 211:105655
Montes M, Plaul S, Croci Y, Waldbillig M, Ferrari W, Topa E, Martorelli S (2020) Pathology associated with three new Clinostomum metacercariae from Argentina with morphological and DNA barcode identification. Journal of Helminthology 94
Mosby D (2018) Australian fisheries and aquaculture statistics. ABARES, Canberra
Nolan MJ, Cribb TH (2004) The life cycle of Paracardicoloides yamagutii Martin, 1974 (Digene Sanguinicolidae). Folia Parasitol 51(4):320–326
Nolan MJ, Cribb TH (2005) The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv Parasitol 60:101–163
Olson P, Cribb T, Tkach V, Bray R, Littlewood D (2003) Phylogeny and classification of the Digenea (Platyhelminthes Trematoda). Int J Parasitol 33(7):733–755
Park CW, Kim JS, Joo HS, Kim J (2009) A Human case of Clinostomum complanatum infection in Korea. Korean J Parasitol 47(4):401–404. https://doi.org/10.3347/kjp.2009.47.4.401
Peirce M, Din N (1970) Two new hosts for Clinostomumphalacrocoracis Dubois, 1931 (Trematoda), from Uganda. J Parasitol 56(3):489–489
Pérez-Ponce De León G, Garcãa-Varela MD, Pinacho-Pinacho C, Sereno-Uribe AL, Poulin R (2016) Species delimitation in trematodes using DNA sequences middle-American Clinostomum as a case study. Parasitology 143(13):1773
Rambaut A (2014) FigTree v1.4.2, a graphical viewer of phylogenetic trees. http://tree.bio.ed.ac.uk/software/figtree/
Rochat EC, Blasco-Costa I, Scholz T, Unmack PJ (2020) High diversity of metazoan parasites in carp gudgeons (Eleotridae: Hypseleotris spp.) from Eastern Australia. Journal of Helminthology 94:e146. https://doi.org/10.1017/S0022149X20000280
Rosser TG, Alberson NR, Woodyard ET, Cunningham FL, Pote LM, Griffin MJ (2017) Clinostomum album n. sp. and Clinostomum marginatum (Rudolphi, 1819), parasites of the great egret Ardea alba L. from Mississippi, USA. Systematic Parasitology 94(1):35–49
Rosser TG, Baumgartner WA, Alberson NR, Noto TW, Woodyard ET, King DT, Wise DJ, Griffin MJ (2018) Clinostomum poteae n. sp.(Digenea: Clinostomidae), in the trachea of a double-crested cormorant Phalacrocorax auritus Lesson, 1831 and molecular data linking the life-cycle stages of Clinostomum album Rosser, Alberson, Woodyard, Cunningham, Pote & Griffin, 2017 in Mississippi, USA. Systematic Parasitology 95(6):543–566
Sereno-Uribe AL, Pinacho-Pinacho CD, García-Varela M, de León GP-P (2013) Using mitochondrial and ribosomal DNA sequences to test the taxonomic validity of Clinostomumcomplanatum Rudolphi, 1814 in fish-eating birds and freshwater fishes in Mexico, with the description of a new species. Parasitol Res 112(8):2855–2870
Shamsi S, Halajian A, Tavakol S, Mortazavi P, Boulton J (2013) Pathogenicity of Clinostomumcomplanatum (Digenea Clinostomidae) in piscivorous birds. Res Vet Sci 95(2):537–539. https://doi.org/10.1016/j.rvsc.2013.06.018
Shamsi S, Dang M, Zhu X, Nowak B (2019) Genetic and morphological characterization of Mawsonascaris vulvolacinata n. sp. (Nematoda: Anisakidae) and associated histopathology in a wild caught cowtail stingray, Pastinachus ater. Journal of Fish Diseases 42(7):1047–1056. https://doi.org/10.1111/jfd.13016
Shamsi S, Sheorey H (2018) Seafood-borne parasitic diseases in Australia: are they rare or underdiagnosed?. Intern Med J 48(5):591–596. https://doi.org/10.1111/imj.13786
Shamsi S (2021) The occurrence of Anisakis spp. in Australian waters: Past, present and future trends. Parasitology Research. https://doi.org/10.1007/s00436-021-07243-3
Shamsi S, Day S, Zhu X, McLellan M, Barton DP, Dang M, Nowak BF (2021) Wild fish as reservoirs of parasites on Australian Murray Cod farms. Aquaculture:736584 https://doi.org/10.1016/j.aquaculture.2021.736584
Shareef PAA, Abidi S (2012) Incidence and histopathology of encysted progenetic metacercaria of Clinostomum complanatum (Digenea Clinostomidae) in Channa punctatus and its development in experimental host. Asian Pac J Trop Biomed 2(6):421–426. https://doi.org/10.1016/s2221-1691(12)60068-9
Sokolov SG, Gordeev II (2020) Molecular and morphological characterisation of flatworm larvae parasitising on fish in Cat Tien National Park, Vietnam. Nature Conservation Research 5:19–30. https://doi.org/10.24189/ncr.2020.039
Soler-Jiménez L, Paredes-Trujillo A, Vidal-Martínez V (2017) Helminth parasites of finfish commercial aquaculture in Latin America. J Helminthol 91(2):110
Sutili FJ, Gressler LT, de Pelegrini LFV (2014) Clinostomum complanatum (Trematoda, Digenea): a parasite of birds and fishes with zoonotic potential in southern Brazil. A Review. Revista Brasileira de Higiene e Sanidade Animal: RBHSA 8(1):99–114
Tiewchaloern S, Udomkijdecha S, Suvouttho S, Chunchamsri K, Waikagul J (1999) Clinostomum trematode from human eye. Southeast Asian J Trop Med Public Health 30(2):382–384
Ukoli F (1966) On Clinostomum tilapiae n. sp., and C. phalacrocoracis Dubois, 1931 from Ghana, and a discussion of the systematics of the genus Clinostomum Leidy, 1856. Journal of Helminthology 40(1-2):187–214
Wang M-L, Chen H-Y, Shih H-H (2017) Occurrence and distribution of yellow grub trematodes (Clinostomumcomplanatum) infection in Taiwan. Parasitol Res 116(6):1761–1771
Won EJ, Lee YJ, Kim M-J, Chai J-Y, Na B-K, Sohn W-M (2020) Morphological and molecular characteristics of clinostomid metacercariae from Korea and Myanmar. Korean J Parasitol 58(6):635
Yamaguti S (1971) Synopsis of digenetic trematodes of vertebrates. Keigaku Publishing Co, Tokyo, Japan
Zhokhov A, Morozova D (2020) Clinostomid metacercariae (Clinostomidae Lühe, 1901) in Fishes of Lake Tana (Ethiopia). Inland Water Biology 13(2):279–290
Funding
This study was financially supported by Charles Sturt University (grant no: A516-828–66770 awarded to SS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shamsi, S., Barton, D.P., Day, S. et al. Characterization of Clinostomum sp. (Trematoda: Clinostomidae) infecting cormorants in south-eastern Australia. Parasitol Res 120, 2793–2803 (2021). https://doi.org/10.1007/s00436-021-07246-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07246-0